Abstract
Heightened inflammation and immune activation are associated with lower bone mineral density (BMD) and lean body mass (LBM) among HIV-infected persons. We hypothesized that a reduction in inflammation with rosuvastatin would be associated with improvements in BMD and LBM. HIV-infected participants on stable antiretroviral therapy without statin indication and with heightened immune activation (≥19% CD8+CD38+HLA-DR+ T cells) or inflammation (hsCRP ≥2 mg/liter) were randomized to rosuvastatin 10 mg daily or placebo for 96 weeks. Among 72 participants randomized to rosuvastatin and 75 to placebo, there were no significant differences in the relative changes in BMD (p > 0.29) or in fat (p ≥ 0.19). A trend toward increased LBM (p = 0.059) was seen in the rosuvastatin arm without differences in creatinine kinase or self-reported physical activity (p ≥ 0.10). In a multivariable regression model, rosuvastatin was associated with a significant positive effect on LBM after adjusting for age, sex, race, smoking status, and detectable HIV-1 viral load. Higher baseline sCD163 correlated with increases in LBM from weeks 0 to 96 (p = 0.023); greater changes in total and leg lean mass were seen among statin users with higher compared to lower baseline IP-10 levels (LBM 1.8 vs. −0.3%; p = 0.028 and leg lean mass 2.9 vs. −1.7%; p = 0.012). Rosuvastatin is associated with an absence of toxicity on BMD and a potential benefit on LBM over 96 weeks of therapy. The preservation of LBM in the rosuvastatin arm over the 2 years of the study is of major clinical relevance in delaying loss of muscle mass with aging.
Introduction
Despite effective antiretroviral therapy (ART), many inflammation and immune activation markers persist at higher levels in HIV-infected compared to HIV-uninfected persons.1,2 This low-grade but persistent inflammation is associated with a greater incidence of comorbid conditions and, in some studies, a greater risk of mortality.1,3,4 The antiinflammatory effect of HMG-CoA reductase inhibitors, or statins, has led to a growing interest in the use of statins to attenuate the low-grade inflammation and immune activation observed in treated HIV infection.5–7 In addition to reductions in inflammation, cardiovascular events, and mortality, statins have been shown to have a beneficial effect on bone mineral density (BMD)8,9 and lean body mass (LBM)10,11 in some studies of older, HIV-uninfected cohorts. With the heightened inflammation and activation and lower BMD and LBM seen among HIV-infected persons, we hypothesized that rosuvastatin therapy would be associated with an improvement in BMD and gain in LBM compared to placebo. We recently published the interim week 48 results of the changes in BMD, LBM, and fat mass.12 Briefly, at 48 weeks, we observed modest relative increases in trochanter and total hip BMD in the statin arm that were significantly greater than placebo, a trend toward increased leg lean mass that did not reach statistical significance, and no significant difference in total body, trunk, and limb fat changes between arms. Here we present the final, week 96 results with results that differed from those of the interim analyses.
Materials and Methods
Study design and participants
The SATURN-HIV (Stopping Atherosclerosis and Treating Unhealthy bone with RosuvastatiN in HIV) study is a randomized, double-blinded, placebo-controlled trial designed to measure the impact of daily rosuvastatin at 10 mg on cardiovascular disease and skeletal health, as previously described.5,13–15 This report details the results of the completed BMD, fat, and LBM changes from baseline to week 96. Enrollment occurred between March 2011 and August 2012. Eligible participants were HIV-infected adults ≥18 years of age with a fasting low-density lipoprotein (LDL) cholesterol of ≤3.37 mmol/liter (130 mg/dl) and either a high sensitivity C-reactive protein (hsCRP) level of ≥19.05 nmol/liter (2 mg/liter) and/or ≥19% activated CD8+ T cells (CD8+CD38+HLA-DR+). Additional eligibility criteria included receipt of stable ART for ≥12 weeks with cumulative ART duration of ≥6 months, HIV-1 RNA ≤1,000 copies/ml, and no history of fragility fractures. Participants were excluded for an active or chronic inflammatory condition (besides HIV), prior myocardial infarction, pregnancy/lactation, receipt of systemic chemotherapy or steroids, diabetes mellitus or uncontrolled thyroid disease, or use of anabolic agents, growth hormone, >81 mg aspirin daily, bisphosphonates, or teriparatide. Supplements including vitamin D were permitted, but participants were instructed not to change supplement doses or frequency. The study is registered on clinicaltrials.gov (NCT01218802) and was approved by the Institutional Review Board of University Hospitals Case Medical Center (Cleveland, OH). Written informed consent was provided by all participants.
Clinical assessments
Demographics and medical and HIV treatment history were obtained by self-report and confirmed by medical records. The targeted physical examination included height and weight measurements; body mass index (BMI) was calculated as kg/m2. Blood samples were collected after a 12-h fasting period. Minutes per 2 weeks of physical activity was collected by the AIDS Clinical Trials Group Physical Activity Assessment.16
Dual-energy X-ray absorptiometry
Dual-energy X-ray absorptiometry (DXA) of the whole body, lumbar spine (L1–4), and left hip was performed in an anteroposterior view using the Lunar Prodigy Advance machine (GE Healthcare, Madison, WI). Peripheral fat depot (limb fat) and central fat depots (trunk fat) from whole body DXA were used in the analysis. Total LBM was defined as fat-free, bone-free mass as measured by DXA, with leg LBM that in the lower extremities only. Technicians used the same machine on the same subject throughout the study. DXA scans were read at Case Medical Center by an experienced radiologist blinded to study information. Osteopenia was defined by a t-score ≤ −1 and osteoporosis by a t-score ≤ −2.5 at either the total hip or lumbar spine.17
Measurement of soluble markers in plasma and serum
Concentrations of interleukin-6 (IL-6), interferon gamma-inducible protein 10 (IP-10 or CXCL10), soluble tumor necrosis factor receptor (sTNFR)-1 and 2, soluble vascular cell adhesion molecule-1 (sVCAM), and intracellular adhesion molecule-1 (sICAM) were determined by quantitative sandwich ELISAs (R&D Systems, Minneapolis, MN). Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) concentrations were measured with an ELISA (PLAC Test; diaDexus, South San Francisco, CA). Hs-CRP concentration was determined by particle enhanced immunonepholometric assays on a BNII nephelometer (Siemens, Munich, Germany). Serum levels of soluble (s) CD14 and sCD163 were measured as markers of monocyte immune activation using Quantikine ELISA kits (R&D Systems). The interassay and intraassay coefficients of variance were <5% and <9%, respectively.
Statistical analysis
This was a prespecified, preplanned analysis to assess changes from baseline to 96 weeks in BMD, fat, and LBM and was powered accordingly for change in spine BMD. Continuous measures were described by medians and interquartile ranges and nominal variables with frequencies and percentages. Nominal variables were compared using χ2 analysis or Fisher's exact test. For between-group and within-group comparisons (at baseline and baseline to 48 week changes), normally distributed variables were compared using the t test or paired t test, respectively; nonnormally distributed variables were compared using the Wilcoxon rank-sum test or the signed-rank test, respectively. Correlations were assessed using Spearman's nonparametric rho. A multivariable linear regression model including age, sex, race, smoking status, and HIV-1 RNA above/below the limit of detection was constructed to examine the effect of covariates on the association between statin and relative LBM change. No adjustments were made for multiple analyses. Analyses were performed with SAS version 9.2 and 9.4 (SAS Institute, Cary, NC).
Results
One-hundred and forty-seven HIV-infected persons enrolled and were assigned to receive rosuvastatin (72 participants) or placebo (75 participants). The median age was 47 years, the median BMI was 26.7 kg/m2, and the majority of participants were male, African American, smokers, and taking tenofovir-containing ART regimens. The treatment and placebo groups were similar in demographic and clinical characteristics (all p ≥ 0.18; Table 1).
Table 1.
Baseline Characteristics of the Study Population
| Characteristic | Statin (n = 72) | Placebo (n = 75) |
|---|---|---|
| Demographics | ||
| Age (years) | 45.6 (41.1–51.4) | 46.9 (39.2–53.6) |
| Male | 58 (81%) | 57 (76%) |
| African American | 51 (71%) | 52 (69%) |
| Current smoker | 43 (60%) | 54 (72%) |
| HIV-related characteristics | ||
| CD4+ lymphocyte count (cells ×108/ liter) | 6.1 (4.4–8.5) | 6.3 (4.0–8.5) |
| Nadir CD4+ T cell count (cells ×108/ liter) | 1.7 (0.84–3.1) | 1.9 (0.89–2.8) |
| HIV-1 RNA <50 copies/ml | 56 (78%) | 58 (77%) |
| Antiretroviral therapy duration (months) | 63 (37–119) | 71 (39–116) |
| Current protease inhibitor- containing regimen | 36 (50%) | 36 (48%) |
| Current tenofovir- containing regimen | 64 (89) | 66 (88) |
| Hepatitis C | 5 (7%) | 7 (9%) |
| Clinical characteristics | ||
| Body mass index (kg/m2) | 26.6 (23.4–30.0) | 27.2 (23.5–30.5) |
| Hip T-score <-1 | 18 (25%) | 17 (22%) |
| Lumbar spine T-score <-1 | 19 (26%) | 13 (17%) |
| Fat mass (kg) | 23.3 (14.3, 32.0) | 22.4 (14.0, 32.2) |
| Lean body mass (kg) | 56.3 (50.6, 61.9) | 57.2 (48.9, 61.3) |
| LDL (mmol/liter) | 2.49 (1.97, 2.77) | 2.51 (1.99, 3.13) |
Values presented as median (25th, 75th percentile) or number (%).
Twenty-eight participants withdrew prior to the week 96 analysis, including nine in the rosuvastatin arm and 19 in the placebo arm. Eleven subjects were lost to follow-up; two moved from the area and two were incarcerated: these participants could not be evaluated for safety evaluations. Participants who withdrew or were lost to follow-up were more likely to be female (p = 0.013); other baseline characteristics were not significantly different. Twenty (71%) were smokers and 23 (82%) were taking tenofovir-containing ART regimens. Three cases of myalgias without rhabdomyolysis (two placebo, one rosuvastatin) were reported within the first 48 weeks12; no further cases occurred between week 48 and 96.
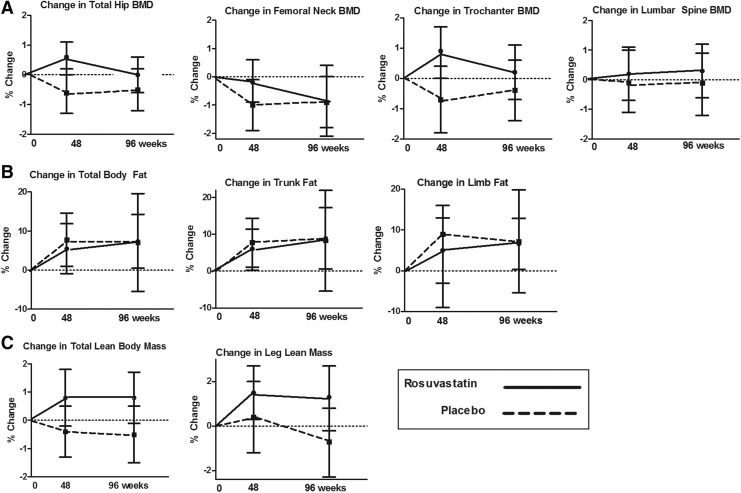
Relative changes in BMD from baseline to week 96 were not significantly different between the rosuvastatin and placebo arms at any site including total hip (p = 0.52), femoral neck (p = 0.68), trochanter (p = 0.29), or lumbar spine BMD (p = 0.89; Fig. 1A). Total body, trunk, and limb fat increased in both the rosuvastatin and placebo arm from weeks 0 to 96; these changes were not significantly different between groups (p = 0.28, 0.19, and 0.41, respectively; Fig. 1B).
FIG. 1.
Relative changes in bone mineral density (BMD) and the total hip, femoral neck, trochanter, and lumbar spine (A); relative changes in total body, trunk, and limb fat (B); relative changes in total lean body mass and leg lean mass (C). Changes in the rosuvastatin arm are indicated by circles at weeks 48 and 96 with a solid connecting line; the placebo arm is indicated by squares and a dashed connecting line.
The mean LBM increased by 0.8% (95% CI 0.5, 0.9%; p = 0.091) in the rosuvastatin arm and decreased slightly in the placebo arm (−0.5%; −1.5, 0.5%; p = 0.35); differences between arms did not reach statistical significance (p = 0.059; Fig. 1C). Changes in leg LBM were also not significantly different between groups (p = 0.12; Fig. 1C). No significant correlation was detected between changes in weeks 48 or 96 leg LBM and changes in total hip BMD (all r ≤ 0.11, all p ≥ 0.26). CPK increased by 23.5% (−8.5, 55.6%; p = 0.15) in the rosuvastatin arm compared to 11.9% (−3.4, 27.3%; p = 0.15) in the placebo arm; between-group differences were not significant (p = 0.91). Both the rosuvastatin (187.1%; 152.4, 218.0%) and placebo (248.2%; 135.5, 360.8%) arms had significant increases in self-reported physical activity (p = 0.017 and <0.0001, respectively), but no significant difference was observed between groups (p = 0.10).
The effects of rosuvastatin on 96 week LBM changes were further investigated in a multivariable linear regression model including age, sex, race, smoking status, and detectable HIV-1 viral load. As shown in Table 2, rosuvastatin was associated with a significant gain in LBM (p = 0.026) and African American race was associated with a loss in LBM (p = 0.014).
Table 2.
Ninety-Six Week Relative Change in Lean Body Mass by Multivariable Regression Models
| Variable of interest | Estimate (%) | 95% confidence interval (%) | p-value |
|---|---|---|---|
| Intercept | 2.77 | −0.7, 6.3 | 0.12 |
| Rosuvastatin | 1.43 | 0.17, 2.7 | 0.026 |
| Age | −0.01 | −0.07, 0.05 | 0.74 |
| Male sex | −1.61 | −3.24, 0.02 | 0.053 |
| African American | −1.71 | −3.07, −0.35 | 0.014 |
| Smoking (current) | −0.95 | −2.26, 0.36 | 0.16 |
| HIV-1 RNA >50 copies/ml (vs. <50) | −0.05 | −1.63, 1.5 | 0.95 |
Lastly, we explored whether the change in LBM was explained by the baseline levels of inflammation and immune activation. Higher baseline levels of sCD163 and a trend toward higher baseline sTNFR-1 correlated with increases in LBM from weeks 0 to 96 (r = 0.21; p = 0.023 and r = 0.16; p = 0.089, respectively) (Table 3). When restricted only to the statin arm, significantly greater changes in total LBM (1.8%; 0.6, 3.0%; p = 0.028) and leg lean mass (2.9%; 0.7, 5.1%; p = 0.012) were seen among participants with higher baseline IP-10 levels (above the median) compared to lower levels (LBM −0.3%; −1.7%, 1.1% and leg mass −0.3%; −2.2, 1.5%).
Table 3.
Correlation Between Baseline Inflammatory Markers and Relative Change in Lean Body Mass Among All Participants
| Inflammatory marker | Correlation coefficient (r) | p-value |
|---|---|---|
| IL-6 | −0.003 | 0.98 |
| Lp-PLA2 | 0.04 | 0.71 |
| IP-10 | 0.08 | 0.37 |
| sCD14 | −0.06 | 0.52 |
| sCD163 | 0.21 | 0.02 |
| sTNFR-1 | 0.16 | 0.09 |
| sTNFR-2 | −0.04 | 0.64 |
| sVCAM | 0.13 | 0.17 |
| sICAM | 0.08 | 0.37 |
| hsCRP | 0.08 | 0.42 |
IL-6, interleukin 6; Lp-PLA2, plasma lipoprotein-associated phospholipase A2; IP-10, interferon gamma-inducible protein 10; sTNFR, soluble tumor necrosis factor receptor; sVCAM, soluble vascular cell adhesion molecule; sICAM, soluble intracellular cell adhesion molecule; hsCRP, high sensitivity C-reactive protein.
Discussion
Here, we present the final results from a 96-week randomized placebo-controlled trial of rosuvastatin to assess its impact on BMD, fat, and LBM among treated HIV-infected adults with normal LDL cholesterol and increased levels of inflammation or immune activation. In contrast to the small, but significantly greater BMD at the hip and trochanter that we previously reported after 48 weeks,7 the BMD in the rosuvastatin arm trended back toward baseline between weeks 48 and 96, and differences were no longer significant between study arms by week 96. Outside of a trend for increased LBM in the rosuvastatin arm, we were unable to detect significant differences in peripheral or central fat, CK, or physical activity between rosuvastatin and placebo.
On first glance, the results are disappointing. When considering the detrimental reported effects of statins, including myalgias and rhabdomyolysis, cognitive impairment, aminotransferase elevations, and insulin resistance,12,18 however, our results provide reassurance that rosuvastatin did not appear to exacerbate the impairments in BMD, fat distribution, or LBM in HIV-infected persons. Despite the negative BMD results, a few findings are noteworthy. First, the initial increase in BMD at 48 weeks and then stabilization or decline suggests a mechanistic pathway through inflammation and immune activation, as it mirrored the initial decline and then stabilization in several inflammation and immune activation markers.12,19
Second is the association between rosuvastatin and increased 96-week LBM. A recent qualitative review of 25 longitudinal, observation studies of middle-aged to older, HIV-uninfected adults reported a median LBM loss of 0.5% per year in men and 0.4% per year in women20; similar, aged-expected declines were observed in our placebo arm. Although the differences between rosuvastatin and placebo did not reach clinical significance, rosuvastatin was a significant predictor of LBM decline after adjusting for covariates in the multivariable model; the preservation of LBM in the rosuvastatin arm over the 2 years of the study could be of major clinical relevance in delaying loss of muscle mass with aging.
As has been shown in the Health, Aging, and Body Composition study, among older, HIV-uninfected adults, the loss of strength was approximately three times that of the loss in muscle mass.21 As heightened inflammation is a strong predictor for the loss of muscle mass and muscle function,22 those with the greatest inflammation prior to statin would presumably benefit the most. Indeed, participants in the rosuvastatin arm with the highest IP-10 at baseline had the greatest increase in LBM while on therapy. IP-10 is a Th1 chemokine with a pivotal role in inflammatory muscle diseases; IP-10 secretion is induced by tumor necrosis factor (TNF)-α, and further exaggerated by IL-6, both key cytokines in the regulation of muscle mass and function.23
Two rodent studies utilizing a cancer24 or motor neuron disease model25 provide support for the relationship between inflammation and LBM response to statins: compared to placebo, simvastatin and atorvastatin, respectively, were associated with an increase in total body weight, muscle weight, and muscle fiber diameter among diseased rodents; no statin-related changes were seen among healthy rodents. In older adults, statin use was associated with less decline in lower extremity strength among the oldest women in the Women's Health Initiative,10 better chair rise performance among statin-using older veterans,26 and less impairment in gait speed or on a standard performance battery among statin users with peripheral artery disease.11,27 Even among relatively healthy older adults, statin use was associated with improved lower-extremity blood flow28 and greater gain in lean body mass with initiation of resistance exercise training.29 Although we did not obtain an objective measure of muscle function, the changes in LBM suggest that rosuvastatin has a positive effect on muscle function. Whether the effect would continue over the often long-term duration of statin therapy remains clinically important but an unanswered question.
In routine clinical practice and in randomized studies, statins are more often recognized for deleterious effects on muscle, with reported myalgia or weakness occurring in approximately 5–15% of patients, and resulting rarely in rhabdomyolysis.30 Outside of the occurrence of myopathy, however, the effects on muscle mass and physical function are less clear. A large (N = 420) randomized, placebo-controlled trial of high-dose atorvastatin was associated with muscle complaints and decreased physical activity, but no objective change in strength or performance.31,32 In contrast, some observational studies have found lower strength,33 lean mass,33,34 and self-reported or objectively measured physical activity35 among statin users compared to nonusers. A small (N = 37) randomized, but nonblinded trial of overweight or obese adults randomized to exercise training with or without high-dose simvastatin demonstrated a blunted improvement in cardiovascular fitness and skeletal muscle citrate synthase activity, a marker of mitochondrial content, but greater gain in LBM among the participants randomized to high-dose simvastatin with exercise training versus exercise training alone.36 Rosuvastatin was well-tolerated in our study, with similar complaints of myalgias between the rosuvastatin (n = 1) and placebo (n = 2) arms. The muscle safety of rosuvastatin in our study was further substantiated by the lack of statistically significant differences in CK or self-reported physical activity between study arms.
Our study is relatively small and limited in generalizability. First, the effects of different statins can differ considerably, thus our results should be considered specific for rosuvastatin and may not be generalizable to other statins. The majority of participants were less than 50 years of age, male, African American, on stable ART, and with a healthier phenotype (without diabetes, and with normal BMD and normal LDL cholesterol), which may have underestimated the effect of statins. Similarly, some inflammatory markers may be higher in women, and the correlations between inflammation and LBM may have been, in part, confounded by sex. However, the double-blinded, randomized assignment to rosuvastatin therapy, eliminating the prescribing bias found in observational studies of statins, and the study duration of nearly 2 years are clear strengths.
In summary, the absence of toxicity on BMD and the potential benefit of rosuvastatin on LBM after 96 weeks of therapy provide some reassurance as to the safety, as well as potential mechanistic insight into the preservation of LBM in an inflammatory state. The heightened risk of cardiovascular disease and cardiovascular disease-related deaths in HIV-infected persons despite effective ART has led to growing interest in developing strategies to prevent cardiovascular disease. A comprehensive understanding of statin risks, including insulin resistance as previously shown in SATURN-HIV,12 and potential cardiovascular and noncardiovascular benefits, including effects on muscle, will inform treatment decisions in HIV, particularly when determining the role of statins in primary prevention.
Acknowledgments
Results of this study were presented in part at the Conference on Retroviruses and Opportunistic Infections 2015, Seattle, WA. This project was supported by the National Institutes of Health [NR012642 to G.A.M. and K23 AG050260 to K.M.E.]. Technical support was provided by the Center for AIDS Research, Case Western Reserve University (P30 AI36219). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Study drug and matching placebo were donated by AstraZeneca.
Author Disclosure Statement
G.A.M. has served as a scientific advisor or speaker for Bristol-Myers Squibb, Gilead, ViiV/GlaxoSmithKline, Pfizer, and ICON and has received research grants from Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences. S.M.D. serves on the DSMB for Johnson & Johnson-sponsored studies.
References
- 1.Kuller LH, Tracy R, Belloso W, et al. : Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med 2008;5(10):e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wada NI, Jacobson LP, Margolick JB, et al. : The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015;29(4):463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sandler NG, Wand H, Roque A, et al. : Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis 2011;203(6):780–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McComsey GA, Kitch D, Sax PE, et al. : Associations of inflammatory markers with AIDS and non-AIDS clinical events after initiation of antiretroviral therapy: AIDS clinical trials group A5224s, a substudy of ACTG A5202. J Acquir Immune Defic Syndr 2014;65(2):167–174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eckard AR, Jiang Y, Debanne SM, et al. : Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis 2014;209(8):1156–1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ganesan A, Crum-Cianflone N, Higgins J, et al. : High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: Results of a double-blind randomized placebo controlled clinical trial. J Infect Dis 2011;203(6):756–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Wit S, Delforge M, Necsoi CV, and Clumeck N: Downregulation of CD38 activation markers by atorvastatin in HIV patients with undetectable viral load. AIDS 2011;25(10):1332–1333 [DOI] [PubMed] [Google Scholar]
- 8.Liu J, Zhu LP, Yang XL, et al. : HMG-CoA reductase inhibitors (statins) and bone mineral density: A meta-analysis. Bone 2013;54(1):151–156 [DOI] [PubMed] [Google Scholar]
- 9.LaCroix AZ, Cauley JA, Pettinger M, et al. : Statin use, clinical fracture, and bone density in postmenopausal women: Results from the Women's Health Initiative Observational Study. Ann Intern Med 2003;139(2):97–104 [DOI] [PubMed] [Google Scholar]
- 10.Gray SL, Aragaki AK, LaMonte MJ, et al. : Statins, angiotensin-converting enzyme inhibitors, and physical performance in older women. J Am Geriatr Soc 2012;60(12):2206–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giri J, McDermott MM, Greenland P, et al. : Statin use and functional decline in patients with and without peripheral arterial disease. J Am Coll Cardiol 2006;47(5):998–1004 [DOI] [PubMed] [Google Scholar]
- 12.Erlandson KM, Jiang Y, Debanne SM, and McComsey GA: Effects of randomized rosuvastatin compared with placebo on bone and body composition among HIV-infected adults. AIDS 2015;29(2):175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funderburg NT, Jiang Y, Debanne SM, et al. : Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis 2014;58(4):588–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erlandson KM, O'Riordan M, Labbato D, and McComsey GA: Relationships between inflammation, immune activation, and bone health among HIV-infected adults on stable antiretroviral therapy. J Acquir Immune Defic Syndr 2014;65(3):290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longenecker CT, Funderburg NT, Jiang Y, et al. : Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med 2013;14(6):385–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McComsey GA, Kendall MA, Tebas P, et al. : Alendronate with calcium and vitamin D supplementation is safe and effective for the treatment of decreased bone mineral density in HIV. AIDS 2007;21(18):2473–2482 [DOI] [PubMed] [Google Scholar]
- 17.Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organization Tech Rep Ser 1994;843:1–129 [PubMed] [Google Scholar]
- 18.FDA Expands Advice on Statin Risks: U.S. Department of Health and Human Services, U.S. Food and Drug Administration. Accessed February/20/2015 at www.fda.gov/ForConsumers/ConsumerUpdates/ucm293330.htm
- 19.Funderburg NT, Jiang Y, Debanne SM, et al. : Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 2015;68(4):396–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mitchell WK, Williams J, Atherton P, et al. : Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front Physiol 2012;3:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodpaster BH, Park SW, Harris TB, et al. : The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J Gerontol A Biol Sci Med Sci 2006;61(10):1059–1064 [DOI] [PubMed] [Google Scholar]
- 22.Ferrucci L, Penninx BW, Volpato S, et al. : Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002;50(12):1947–1954 [DOI] [PubMed] [Google Scholar]
- 23.Crescioli C, Sottili M, Bonini P, et al. : Inflammatory response in human skeletal muscle cells: CXCL10 as a potential therapeutic target. Eur J Cell Biol 2012;91(2):139–149 [DOI] [PubMed] [Google Scholar]
- 24.Palus S, von Haehling S, Flach VC, et al. : Simvastatin reduces wasting and improves cardiac function as well as outcome in experimental cancer cachexia. Int J Cardiol 2013;168(4):3412–3418 [DOI] [PubMed] [Google Scholar]
- 25.Iwamoto K, Yoshii Y, and Ikeda K: Atorvastatin treatment attenuates motor neuron degeneration in wobbler mice. Amyotroph Lateral Scler 2009;10(5–6):405–409 [DOI] [PubMed] [Google Scholar]
- 26.Agostini JV, Tinetti ME, Han L, et al. : Effects of statin use on muscle strength, cognition, and depressive symptoms in older adults. J Am Geriatr Soc 2007;55(3):420–425 [DOI] [PubMed] [Google Scholar]
- 27.McDermott MM, Guralnik JM, Greenland P, et al. : Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation 2003;107(5):757–761 [DOI] [PubMed] [Google Scholar]
- 28.Parker BA, Capizzi JA, Augeri AL, et al. : Atorvastatin increases exercise leg blood flow in healthy adults. Atherosclerosis 2011;219(2):768–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riechman SE, Andrews RD, Maclean DA, and Sheather S: Statins and dietary and serum cholesterol are associated with increased lean mass following resistance training. J Gerontol A Biol Sci Med Sci 2007;62(10):1164–1171 [DOI] [PubMed] [Google Scholar]
- 30.Thompson PD. and Parker B: Statins, exercise, and exercise training. J Am Coll Cardiol 2013;62(8):715–716 [DOI] [PubMed] [Google Scholar]
- 31.Ballard KD, Parker BA, Capizzi JA, et al. : Increases in creatine kinase with atorvastatin treatment are not associated with decreases in muscular performance. Atherosclerosis 2013;230(1):121–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parker BA, Capizzi JA, Grimaldi AS, et al. : Effect of statins on skeletal muscle function. Circulation 2013;127(1):96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scott D, Blizzard L, Fell J, and Jones G: Statin therapy, muscle function and falls risk in community-dwelling older adults. QJM 2009;102(9):625–633 [DOI] [PubMed] [Google Scholar]
- 34.Dzien A, Winner H, Theurl E, et al. : Fat-free mass and fasting glucose values in patients with and without statin therapy assigned to age groups between <60 and >75 years. Obesity Facts 2013;6(1):9–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DS, Markwardt S, Goeres L, et al. : Statins and physical activity in older men: The osteoporotic fractures in men study. JAMA Intern Med 2014;174(8):1263–1270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mikus CR, Boyle LJ, Borengasser SJ, et al. : Simvastatin impairs exercise training adaptations. J Am Coll Cardiol 2013;62(8):709–714 [DOI] [PMC free article] [PubMed] [Google Scholar]