Abstract
The complement system is an ancient pattern recognition system that becomes activated during all stages of HIV infection. Previous studies have shown that C5a can enhance the infection of monocyte-derived macrophages and T cells indirectly through the production of interleukin (IL)-6 and tumor necrosis factor (TNF)-α and the attraction of dendritic cells. C5a exerts its multiple biologic functions mainly through activation of C5a receptor 1 (C5aR1). Here, we assessed the role of C5aR1 as an enhancer of CCR5-mediated HIV infection. We determined CCR5 and C5aR1 heterodimer formation in myeloid cells and the impact of C5aR1 blockade on HIV entry and genomic integration. C5aR1/CCR5 heterodimer formation was identified by immunoprecipitation and western blotting. THP-1 cells and monocyte-derived macrophages (MDM) were infected by R5 laboratory strains or HIV pseudotyped for the vesicular stomatitis virus (VSV) envelope. Levels of integrated HIV were measured by quantitative PCR after targeting of C5aR1 by a C5aR antagonist, neutralizing C5aR1 monoclonal antibody (mAb) or hC5a. C5aR1 was also silenced by specific siRNA prior to viral entry. We found that C5aR1 forms heterodimers with the HIV coreceptor CCR5 in myeloid cells. Targeting C5aR1 significantly decreased integration by R5 viruses but not by VSV-pseudotyped viruses, suggesting that C5aR1 is critical for viral entry. The level of inhibition achieved with C5aR1-blocking reagents was comparable to that of CCR5 antagonists. Mechanistically, C5aR1 targeting decreased CCR5 expression. MDM from CCR5Δ32 homozygous subjects expressed levels of C5aR1 similar to CCR5 WT individuals, suggesting that mere C5aR1 expression is not sufficient for HIV infection. HIV appeared to preferentially enter THP-1 cells expressing high levels of both C5aR1 and CCR5. Targeted reduction of C5aR1 expression in such cells reduced HIV infection by ∼50%. Our data thus suggest that C5aR1 acts as an enhancer of CCR5-mediated HIV entry into macrophages, the targeting of which may prove useful to reduce HIV infection by R5 strains.
Introduction
The complement system is an ancient danger sensing system that can kill microbial invaders directly or indirectly by attracting and activating innate immune cells. Complement also instructs such cells to drive adaptive immune responses aimed at destructing microbes. Although complement is activated during all stages of HIV infection,1 the virus has found several ways to not only escape complement-mediated killing but to exploit the system for increased infection and reproduction.2,3 The complement system recognizes HIV gp41 and gp120 through direct binding of C1q or mannose-binding lectin (MBL) driving the activation of either the classical and/or the lectin pathway.2,4 After seroconversion, immune complexes significantly amplify complement activation. However, HIV is enveloped with host cell-derived complement regulators such as CD55 and CD46, which prevent efficient complement-mediated lysis of virus particles. Furthermore, gp41 and gp120 can bind the complement regulator protein factor H in the circulation, additionally promoting a nonactivator surface.2
The coating of HIV with C3 fragments allows the virus to bind to different complement receptors, such as CR1 (CD35), CR2 (CD21), CR3 (CD11b/CD18), and CR4 (CD11c/CD18). CR1 is widely expressed on erythrocytes and C3b-coated HIV uses CR1 on erythrocytes to spread into tissues, where degradation of C3b into iC3b and C3d,g allows the virus to infect a wide spectrum of CR2-, CR3-, and CR4-expressing cells such as B cells, macrophages, and dendritic cells (DCs).5–7 B cells that bind C3d,g-coated virus particles may disseminate and transmit infectious virus to activated T cells.8 Furthermore, follicular DCs that bind C3d-opsonized HIV through CR2 provide an important extracellular HIV reservoir in germinal centers.9
Less is known about the role of the cleavage fragment of C5, C5a, which is generated when HIV activates complement at mucosal surfaces. C5a serves as a potent chemoattractant for immature DCs and macrophages, which are recruited to sites of HIV entry.10 C5a can bind to two receptors, C5aR1 (CD88) and C5aR2 (C5L2), both of which belong to the large group of seven-membrane spanning receptors. While C5aR can couple to several G-proteins such as Gαi2, Gαi3, and Gαq, C5aR2 is uncoupled from G-proteins due to a mutation in the highly conserved DRY motif in the third transmembrane domain. C5aR1 is expressed on the cell surface whereas most of C5aR2 is expressed intracellularly.11 Previous studies have shown that C5a can enhance the infection of MDM and T cells indirectly through the production of interleukin (IL)-6 and tumor necrosis factor (TNF)-α and the attraction of DCs.10,12 Complement-mediated enhancement of HIV infection was also described for CD4 cells.13
Interestingly, C5aR1 forms heterodimers with the chemokine receptor CCR5, a major coreceptor for viral entry for R5 strains14 in a rat basophilic leukemia cell line.15 CCR5 cell surface levels are highly variable in the human population and their regulation has been shown to depend on multiple factors, including polymorphisms in the CCR5 promoter and the expression of its chemokine ligands.16,17 Furthermore, CCR5 forms heteromers with CXCR4 and CCR2 in activated T cells or monocytes. Importantly, small molecule antagonists of either receptor partially cross-inhibit the binding of chemokines to the associated receptors, thereby blunting their functional responses.18 Regulation of CCR5 function or surface expression through heterodimerization could be of importance, because low CCR5 and high chemokine levels are associated with slower HIV-1 disease progression rates.19,20 Also, variation in CCR5 levels has implications for the development of resistance to CCR5 inhibitors.21
As macrophages express high levels of both molecules, we hypothesized that heterodimerization of CCR5 and C5aR could also occur in these cells, and that it could affect HIV entry. In this study, we show that C5aR1 and CCR5 form heterodimers in the macrophage cell line THP-1. Furthermore, we found that HIV targets C5aR1 for cell entry/fusion into MDM, as specific blockade or knock-down of C5aR1 resulted in a significant reduction of HIV entry and integration. Importantly, our findings indicate that HIV preferentially entered THP-1 cells expressing high levels of both C5aR1 and CCR5. Our findings thus suggest that C5aR1 serves as an enhancer of CCR5-mediated HIV entry into macrophages.
Materials and Methods
Human myeloid cells
The monocytic cell line THP-1 (ATCC) was differentiated for 24 h with PMA (40 ng/ml). Monocytes were purified from the blood of healthy HIV-uninfected donors (Hoxworth Blood Bank Center, Cincinnati, OH) using CD14 beads (Miltenyi, San Diego, CA) and were differentiated into monocyte-derived macrophages (MDM) by 3-day cultures with GM-CSF (100 UI/ml, Peprotech, Rocky Hill, NJ). In one experiment, monocytes from CCR5Δ32-homozygous uninfected individuals (kindly provided by Dr. R.G. Collman, UPenn CFAR, Philadelphia, PA) were similarly differentiated. Protocols to obtain blood were IRB approved in each institution.
Determination of C5aR and CCR5 expression by flow cytometry or western blot
For flow cytometric analysis, differentiated THP1 and MDM were stained with anti-CCR5 Ab (clone 2D7, BD Pharmingen, San Jose, CA) and anti-C5aR1 (clone S5/1, Santa Cruz Biotech, Dallas, TX) at room temperature for 20 min. Quadrants were set up according to staining with isotype-matched control Abs. To determine the formation of C5aR-CCR5 heterodimers, THP-1 whole cell lysates were prepared using a Triton X-100-based lysis buffer. CCR5 was immunoprecipitated with anti-CCR5 monoclonal antibody (mAb) (T22/8 clone) and agarose beads-conjugated anti-mouse IgG followed by Western blot analysis of CCR5, C5aR, CCR1, and Gαi2. For detection of CCR1 and Gαi2, we used pAbs H-52 and L-5 (Santa Cruz Biotech).
HIV infection of THP-1 or MDM
HIV viruses were prepared from 293T cells transfected with different plasmids (all from the NIH AIDS Research and Reference Reagent Program), using Fugene transfection (Roche) as recommended by the manufacturer. In particular, we used plasmids encoding the R5 YK-JRLF full clone. HIV vesicular stomatitis virus (VSV) pseudotyped virus was obtained by cotransfection of YU2Δenv and VSV-G plasmids. The R5 YK-JRCSF-BlaM-Vpr was obtained by cotransfection of YK-JRCSF and BlaM-Vpr plasmids. After 2 days, the supernatants were harvested and the virus precipitated using polyethyleneglycol. Virus titers were determined using the TZM-bl cell line, as described.22
Differentiated THP1 cells or MDM were cultured at 37°C with C5aR1 blocking reagents for 1 h. These reagents were used at the following concentrations: C5aR antagonist (C5aRA) A8Δ71–7323: 87.8 μM; anti-C5aR1 Ab or isotype-matched control (Hycult Biotech Plymouth Meeting, PA and BD Pharmingen): 1 μg/ml; CCR5 antagonist TAK779 (NIH AIDS Research and Reference Reagent Program): 20 μM; or hC5a (Hycult Biotech): 100 μM. Cells were then infected with YK-JRLF at a multiplicity of infection (MOI) of 1 or 3 for THP-1 and MDM, respectively. After 6 h of infection at 37°C, cells were washed twice with complete RPMI, cultured for an additional 24 h, and then lysed with a mix of TRIS, Tween 20, NP40, and proteinase K. Cell-associated HIV-1 DNA was measured in cell lysates by nested real-Time PCR, as described.24–26 A first amplification round was performed using oligonucleotides that target two Alu sequences and the U3-RU5 sequence of the HIV-1 LTR.
In the same reaction, CD3 primers were also used to precisely determine the number of cells. Following the first amplification, specific primers and a specific labeled probe against the HIV-LTR were used to detect integrated HIV, using real-time PCR (Light Cycler, Roche). In parallel, CD3 primers and SYBR Green were used in another real-time PCR reaction to quantify CD3 expression. ΔCt was then used to calculate the relative expression of HIV, normalized to the CD3 expression. The efficiency of the amplification process was verified using the ACH-2 cell line, i.e., a cell line of human T-lymphocytic leukemia that contains a single copy of LAV strain proviral DNA (NIH AIDS Research and Reference Reagent Program).
Assessment of cytokine and chemokine production in response to HIV infection
MDM were cultured in RPMI1640 medium in the absence or the presence of 10 μg/ml anti-C5aR-antibody S5/1 (Hycult Biotech) for 1 h prior to 24 h stimulation with 3 MOI R5 YK-JRFL or BaL (NIH). As a positive control, cells were stimulated with 100 ng/ml Escherichia coli LPS (SIGMA). Supernatants were collected and analyzed for the production of TNF-α, IL-1ß, IL-6, IL-10, IL-15, and IL-12p40, as well as the production of several chemokines including CXCL8 (IL-8), CCL2 (MCP-1), CCL3 (MIP-1α), CCL4 (MIP-1β), and CCL5 (RANTES) using MILLIPLEX, Multiplex Immunoassay Kit (EMD Millipore, Billerica, MA).
C5aR silencing by siRNA and fusion/entry assay
THP-1 cells were transfected using either C5aR siRNA (60 nM) or control siRNA, containing a scrambled sequence that does not lead to specific degradation of any known cellular mRNA, according to the manufacturer's instructions (Santa Cruz Biotech). After 24 h, cells were treated with PMA (40 ng/ml) for an extra 24 h. Surface levels of CCR5 and C5aR were evaluated by flow cytometry. After PMA treatment, transfected THP-1 cells were infected overnight with YK-JRCSF-BlaM-Vpr (MOI = 1).27 The cells were then loaded with the CCF2-AM dye for 1 h and the fusion was measured 6 h later by flow cytometry. Fluorescent blue cells (measured at 405 nm) were considered infected cells. Uninfected cells loaded with CCF2-AM were used as a negative control.
Statistical analysis
Statistical analysis was performed using Prism (GraphPad Software 6). HIV proviral DNA levels were compared using the paired t-tests after normalization. Levels of CCR5 and C5aR1 expression were compared using the paired t-test. p-values of <0.05 were considered as denoting a significant difference.
Results
Human myeloid cells express CCR5 and C5aR1 heterodimers
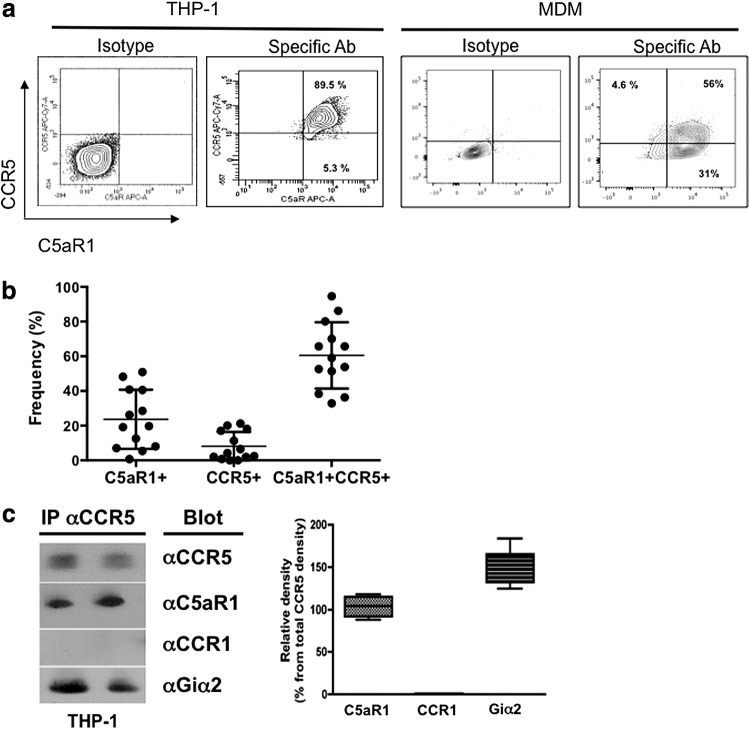
CCR5/C5aR1 heterodimer formation has been described in a rat basophilic leukemia cell line.15 To determine whether the CCR5-C5aR complex plays a role in HIV infection of macrophages, we first analyzed the expression of these two receptors on human myeloid cells. PMA-differentiated THP1 cells are commonly used to model macrophage infection, and these cells express high levels of both C5aR1 and CCR5 (Fig. 1a; left panel), whereas nondifferentiated THP1 express low levels of both receptors (data not shown). Furthermore, as shown in Fig. 1a (right panel) and Fig. 1b, about 60% of human MDM express both C5aR1 and CCR5. About 20% of MDM expressed C5aR1 alone, whereas single expression of CCR5 occurred in about 5% of MDM (Fig. 1b).
FIG. 1.
Formation of CCR5 and C5aR1 heterodimers in human myeloid cells. (a) Flow cytometric analysis of C5aR1 and CCR5 coexpression in differentiated THP-1 cells and monocyte-derived macrophages (MDM). After differentiation, THP1 and MDM were stained for membrane expression with anti-CCR5 and anti-C5aR1 monoclonal antibodies (mAbs) or matched isotype control Abs. Quadrants were set up according to staining with isotype-matched control Abs. Percentages indicate the percentages of gated cells expressing each marker. (b) Frequencies of C5aR1+, CCR5+, or CCR5+C5aR1+ MDM. Data show values from 13 individual donors. Bars show the means ± SEM. (c) CCR5 was immunoprecipitated from THP-1 whole cell lysates followed by Western blot analysis of CCR5, C5aR1, CCR1, and Gαi2 expression. Left panel: two independent samples indicated as lanes A and B, respectively. Right panel: relative abundance of C5aR1, CCR1, and Gαi2 protein as determined by densitometric assessment of the band intensity. Data are the means ± SEM (in percent) of the Western blot signal obtained for each marker in comparison to the CCR5 signal obtained in the same sample (n = 4).
Next, we determined whether CCR5 and C5aR1 are associated in differentiated THP-1. Whole THP-1 cell lysates were immune precipitated with anti-CCR5, followed by Western blotting and using antibodies specific for either CCR5, C5aR1, CCR1, or Gαi2 (as a positive control). As shown in Fig. 1c (showing two representative cell lysates), CCR5 (as well as Gαi2) coprecipitated with C5aR1 in both samples. In contrast, CCR1 did not coprecipitate with CCR5 under these conditions suggesting that CCR5 is associated with C5aR1 and Gαi2, but not with CCR1 in the membrane of differentiated THP-1 cells.
C5a treatment or C5aR blockade decreases HIV infection of THP-1 cells and primary macrophages
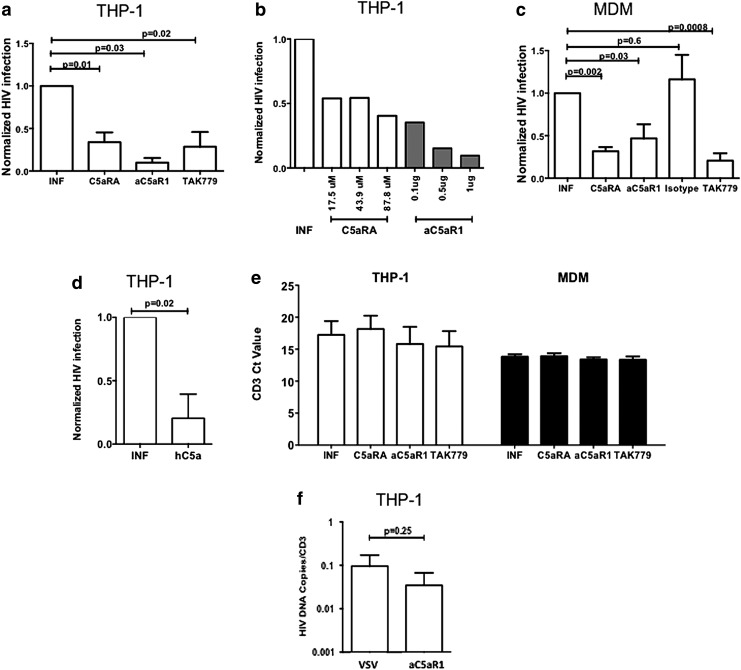
Given that CCR5 and C5aR1 can form heterodimers, we next tested the impact of C5aR1 blockade on HIV infection of differentiated THP-1 cells or MDM by the R5 HIV laboratory strain YK-JRLF. C5aR1 was targeted using the C5aRA A8Δ71–73,23 which blocks the interaction of C5a with C5aR1 and C5aR2. To specifically block C5aR1, we used the C5aR1-neutralizing mAb S5/1 clone.28 Levels of integrated HIV were measured using the Alu-long terminal repeat (Alu-LTR) quantitative nested PCR assay, as described earlier.24,26
As shown in Fig. 2a, treatment by both C5aR1 blocking reagents significantly decreased the level of integrated HIV to levels comparable to those achieved following treatment by the CCR5 antagonist TAK779. C5aRA and anti-C5aR1 mAb led to a similar reduction, suggesting a predominant role for C5aR1. This inhibition was not specific for this particular virus strain, as similar inhibition was observed for the infection with YU2, another R5 laboratory strain (data not shown). This effect was dose dependent, as shown in Fig. 2b. Importantly, similar (>50%) inhibition of HIV integration was observed in primary MDM pretreated by C5aRA or anti-C5aR1 mAb, but not by the isotype-matched Ab (Fig. 2c). Furthermore, we observed a significant reduction of HIV integration when THP-1 cells were prepretreated with C5a (Fig. 2d). None of the reagents caused significant cell death and CD3 levels were comparable in all conditions (Fig. 2e).
FIG. 2.
C5a treatment or C5aR1 blockade decreases HIV infection of THP-1 and MDM but does not decrease infection with vesicular stomatitis virus (VSV)-pseudotyped HIV. (a) Differentiated THP-1 cells were cultured at 37°C with C5aRA (87.4 μM), anti-C5aR1 mAb S5/1, isotype control mAb (both at 1 μg/ml), or TAK779 (20 μM). The cells were infected with YK-JRLF at an MOI = 1 for THP-1 or MOI = 3 for MDM. After 6 h, cells were washed, cultured for another 24 h, and then lysed. Integrated HIV-1 DNA was measured in cell lysates by Alu-RT-PCR. ΔCt was used to calculate the relative expression of HIV, normalized to CD3 expression. Eventually, results were normalized to infection in untreated cells. The graphs show the mean normalized infection levels ± SEM from four independent experiments. (b) C5aRA and C5aR1-specific antibody treatment decreases HIV infection of THP-1 cells in a dose-dependent manner. (c) As in (a), except that MDM were used instead of THP-1 cells. The graph shows the mean normalized infection levels ± SEM of MDM from five individual donors. (d) As in (a), except that THP-1 cells were incubated with hC5a (100 μM) for 1 h. (e) C5aR1 and CCR5 blocking reagents do not affect the number of cells. Differentiated THP-1 cells or MDM were cultured at 37°C with C5aRA, anti-C5aR1 mAb S5/1, or TAK779 and infected with YK-JRLF as described above. CD3 DNA levels were measured in cell lysates by Alu-RT-PCR. The graph shows the mean ± SEM of the cycle threshold (Ct) values from ≥4 independent experiments. (f) Infection of differentiated THP-1 cells with VSV-pseudotyped YU2 in the presence or absence of C5aR1 blockade with C5aR1 mAb (1 mg/ml). Data are the mean values ± SEM from three independent experiments.
To assess the role of C5aR targeting on cytokine production during HIV infection, we infected MDM with YK or BaL HIV strains at an MOI of 3 in the presence or absence of the C5aR1-specific mAb 5S/1 at a concentration 10-fold higher than that required to decrease HIV integration. LPS stimulation was used as the positive control. We determined the production of cytokines and chemokines, including TNF-α, IL-1ß, IL-6, IL-10, IL-15, IL-12p40, CXCL8, and CCL2-5. While LPS treatment induced the production of all of these cytokines and chemokines 24 h after administration (although with different potencies), infection with the two different HIV strains did not result in the production of any of the cytokines or chemokines. Furthermore, we observed no impact of anti-C5aR1 treatment on cytokine or chemokine induction (data not shown).
C5aR affects HIV entry
To determine what step of the HIV life cycle is affected by C5aR1 blockade, we first used an R5 YU2 strain pseudotyped for the VSV envelope, which mediates entry into mammalian cells through CD4 and coreceptor-independent pathways. C5aRA treatment had a minor and nonsignificant effect on infection by this VSV-pseudotyped R5 virus (Fig. 2f). Levels of infection of THP-1 by wt and VSV-pseudotyped viruses were similar (means ± SEM HIV copies/CD3 in infected THP-1 were 0.192 ± 0.12 vs. 0.126 ± 0.12 for wt vs. VSV-pseudotyped viruses, p = 0.81). These data suggest that C5aR1 is critical for HIV entry into THP-1 cells.
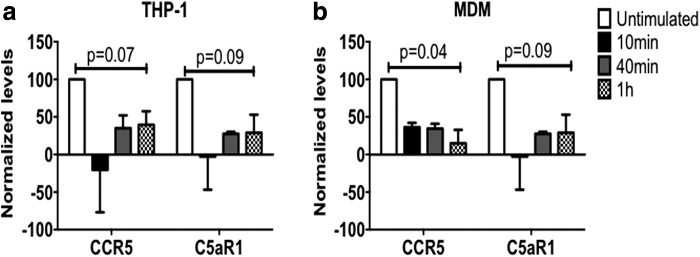
C5aR1 engagement by C5aRA results in a decrease of CCR5 surface expression
In search for mechanisms underlying the C5aR1-mediated infection of MDM and THP-1 cells, we reasoned that the interaction of the C5aR1 blocking reagents might decrease the surface expression of CCR5. We thus determined CCR5 and C5aR1 expression on differentiated THP-1 cells or MDM treated with the C5aRA. Incubation with C5aRA induced down-regulation of C5aR1 surface expression within 10 min on both cell types (Fig. 3). Importantly, CCR5 expression followed the same pattern, suggesting that both receptors were rapidly internalized upon C5aR1 engagement (Fig. 3). Notably, CCR5 remained low 1 h after C5aRA treatment, which is the time when HIV was added to the cultures in the experiments described above (Fig. 2).
FIG. 3.
C5aRA treatment decreases CCR5 expression by THP-1 or MDM. Differentiated THP-1 (a) or MDM (b) were treated with C5aRA (87.4 μM) for the indicated times. CCR5 or C5aR1 expression (MFI) was determined by flow cytometry. The graph shows mean normalized levels (MFI at time 0 was considered as 100% expression) (n = 3).
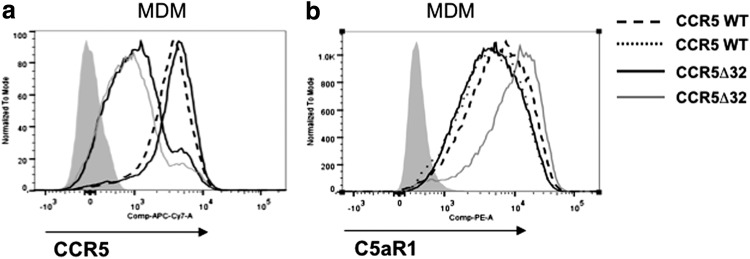
MDM from CCR5 Δ32-homozygous uninfected individuals have long been known to be resistant to R5 HIV infection.29–31 Due to the heterodimerization between CCR5 and C5aR1, we next determined whether the decrease of CCR5 levels in Δ32-homozygous uninfected individuals (Fig. 4a) also affected the expression of C5aR1 (Fig. 4b). Importantly, we found that this is not the case. MDM from Δ32-homozygous subjects expressed levels of C5aR1 similar to those from normal subjects (Fig. 4b). Taken together, these data suggest that blockade of C5aR1 affects HIV entry through the CCR5-dependent pathway. Furthermore, our data imply that C5aR1 alone is not sufficient to mediate efficient viral entry and may act as an enhancer of CCR5-mediated entry.
FIG. 4.
C5aR1 expression is not affected in macrophages from CCR5Δ32-homozygote individuals. Plastic-adherent cells purified from unfrozen peripheral blood mononuclear cells (PBMCs) of two healthy HIV-uninfected and CCR5 WT individuals and two healthy uninfected CCR5Δ32-homozygote individuals were differentiated into MDM. After differentiation, MDM were stained with (a) anti-CCR5 or (b) anti-C5aR1 mAbs. The gray histograms indicate isotype antibody control staining.
C5aR facilitates CCR5-mediated HIV entry into differentiated THP-1 cells
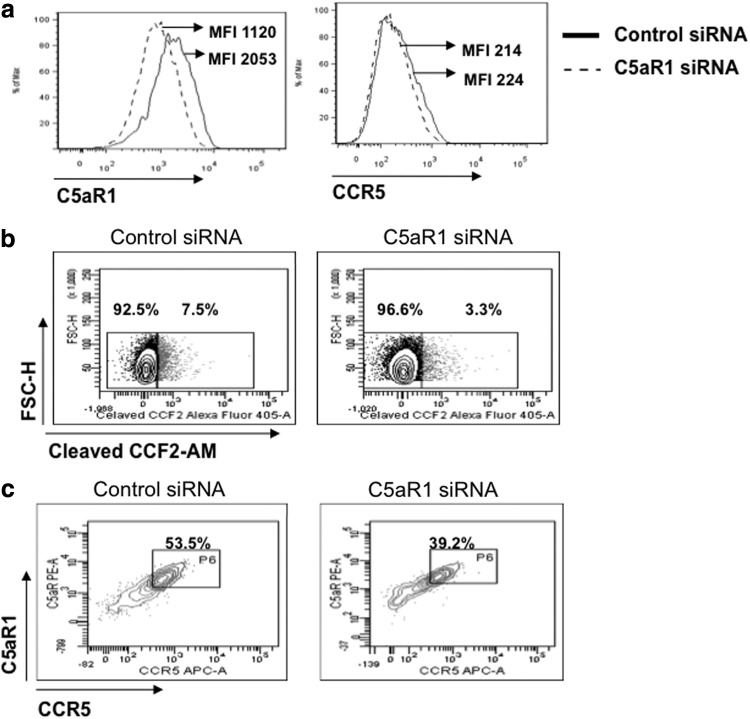
To provide additional evidence for the view that C5aR1 facilitates and increases CCR5-mediated HIV entry, we knocked down C5aR1 expression in differentiated THP-1 cells by an siRNA approach before infection. This C5aR1-siRNA-specific treatment reduced C5aR1 expression by ∼50% (Fig. 5a, left panel) but did not decrease CCR5 levels (Fig. 5a, right panel). We next evaluated viral fusion and entry by a flow cytometry-based assay,27 and observed a similar reduction (∼50%) of HIV entry/fusion, from 7.5% in control siRNA-treated THP-1 cells to 3.3% in THP-1 cells that were silenced for C5aR1 expression using C5aR1-specific siRNA (Fig. 5b, right panel). Reduction of C5aR1 expression in C5aR1-siRNA-treated THP-1 affected the percentage of cells in which HIV entered, but not the level of infection in those cells that were infected, as the intensity of cleaved CCF2-AM was similar in the CCF2-AM+ cells present in control or C5aR1-siRNA-treated THP-1 cells (MFI of 1243 vs. 1443 in experiment one and MFI 1406 vs. 1445 in experiment two). In support of the view that the presence of C5aR1 facilitates HIV entry, the majority of the HIV-infected THP-1 cells were CCR5+C5aR1+ (∼53%), and inhibition of C5aR1 expression reduced viral entry into these double-positive cells (Fig. 5c).
FIG. 5.
Decreased C5aR1 surface expression results in reduced CCR5-mediated HIV infection of THP-1 cells. (a) THP-1 cells were treated with C5aR1 siRNA or control siRNA for 24 h, and then differentiated with PMA for an additional 24 h. Efficiency of C5aR1 silencing was measured by flow cytometry. (b) Transfected cells were loaded with the CCF2-AM dye and infected with YK-JRCSF-BlaM-Vpr. Fusion was measured 6 h later by flow cytometry. Dot plots show the levels of cleaved-CCF2-AM in control siRNA-treated cells (left panel) versus C5aR1 siRNA-transfected cells (right panel). Percentages of positive/negative cells are indicated; the positivity gate was set up based on the fluorescence level in uninfected cells loaded with CCF2-AM. The MFI of cleaved CCF2-AM was measured in both experimental conditions, and was 338 and 247 for control siRNA and C5aR1 siRNA-transfected cells, respectively. The data shown are representative of two independent experiments. (c) Cells gated based on cleaved CCF2-AM fluorescence were assessed for CCR5 and C5aR1 expression. The dot plots represent the frequency of CCR5+C5aR1+ HIV-infected cells in control siRNA-transfected (left panel) versus C5aR1 siRNA-transfected cells (right panel). The data shown are representative of two independent experiments.
Discussion
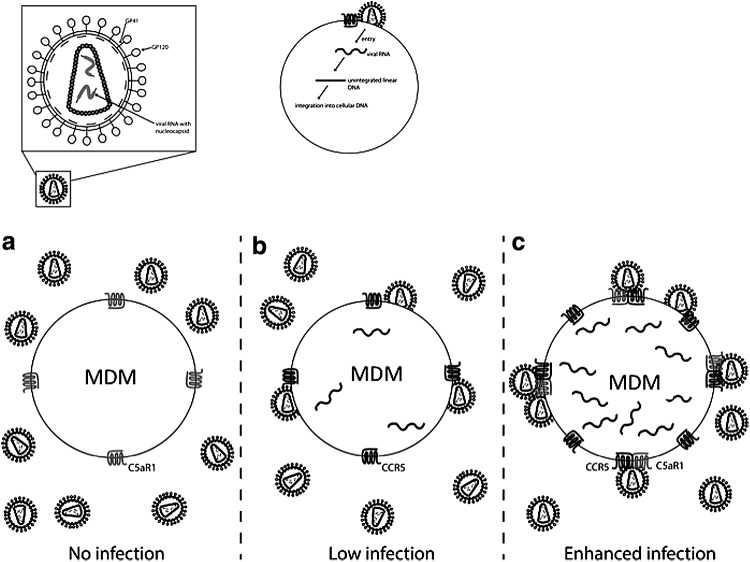
HIV strains associated with mucosal transmission predominantly use CCR5 for entry into target cells. In depth knowledge of CCR5/HIV interactions is therefore fundamental for a better understanding of HIV infection as well as for the development of new therapeutic and preventive approaches. Our data provide evidence that CCR5 and C5aR1 form heterodimers on human myeloid cells. Importantly, we demonstrate for the first time that treatment of differentiated THP-1 cell or MDM with reagents that target C5aR1 impair cellular entry and HIV genomic integration of R5 laboratory strains in vitro, although these processes were not completely blocked. C5aR1 could act either as a novel coreceptor for HIV infection or facilitate CCR5-mediated entry and infection. Our data support the latter view, as we found strong expression of C5aR1 on MDMs from CCR5 Δ32-homozygous individuals who are resistant to HIV infection.29–31 Our data also suggest that HIV preferentially infects myeloid cells that express high levels of both receptors, most likely forming heterodimer complexes (Fig. 6).
FIG. 6.
Model depicting the impact of C5aR1 CCR5 association on HIV infection. (a) When MDM express C5aR1 but lack CCR5, as in homozygous CCR5Δ32 individuals, R5-tropic HIV strain YK-JRCSF does not enter the target cell. (b) MDM become infected by R5-tropic HIV strains when they express CCR5. (c) When C5aR1 and CCR5 are both expressed in MDM, they tend to form heterodimers. Our data indicate that such heterodimer formation is associated with enhanced infection of R5-tropic strains, as the virus seems to preferentially infect cells that express both receptors at high levels.
To the best of our knowledge, our finding that a GPCR (i.e., C5aR1) partners with CCR5 and that this complex appears to facilitate HIV entry is a novel concept. Of note, we have recently shown that C5aR1 cooperates with another GPCR, i.e., bradykinin B2 receptor in Trypanosoma cruzi infection, suggesting that the association of C5aR1 with other GPCRs is a more general phenomenon in host–pathogen interaction.32 However, our study, which was designed as a proof of concept that C5aR/CCR5 heterodimerization could affect HIV entry in myeloid cells, is limited by the fact that we did not study mucosal infection by primary isolates. Macrophages from the mucosa may perform differently than MDM, and additional experiments will be necessary using mucosal macrophages or human mucosal tissue explants to confirm the relevance of this study.
Looking for underlying mechanisms, we observed that C5aR1 targeting results in down-regulation of CCR5 surface expression within 10 min. Cross-desensitization of CCR5 upon C5a or C5adesArg-mediated activation of C5aR1 associated with cointernalization has been described previously.15 Although C5aRA does not promote C5aR1 signaling including C5aR1 phosphorylation,33 it may indirectly affect C5aR1 signaling and internalization by its impact on C5aR2, which couples to ß-arrestin.34 This indirect regulation of C5aR1 internalization could also drive CCR5 cross-internalization through the ß-arrestin pathway. Alternatively, binding of C5aRA to C5aR1 and C5aR2 may result in the aggregation of the CCR5/C5aR1, CCR5/C5aR2, or even a trimeric complex comprising CCR5/C5aR1/C5aR2 that activates a novel signaling pathway not triggered by any of the receptors or a pathway downstream of CCR5. Delineating the exact mechanism by which C5aRA treatment leads to down-regulation of CCR5 surface expression is beyond the scope of the current study and is the focus of future experiments.
In addition to our data suggesting that it could serve as an enhancer of CCR5-driven infection, C5aR1 has previously been shown to enhance HIV infection through two other mechanisms. First, Kacani et al. have shown that preincubation of MDM with C5a for 48 h increased the susceptibility of such cells for HIV infection 40-fold as compared with untreated controls. This effect could be blocked using a C5aR1-specific mAb.12 In search for mechanisms underlying this effect, the authors found that C5a increased TNF-α and IL-6 production from MDM. Furthermore, they have shown that specific targeting of C5aR1, using the same mAb that we have used in our study (S5/1), inhibited the C5a-mediated increase of TNF-α or IL-6, demonstrating that C5aR1 signaling is critical for the effect. TNF-α and IL-6 are known to increase HIV replication and HIV infection of MDM, which, in turn, enhances secretion of such cytokines.35,36 Importantly, IL-6 is also known to increase C5aR1 expression on macrophages.37 Thus, enhanced susceptibility to HIV infection may also result from increased numbers of receptors available for infection.
Second, the same laboratory demonstrated that C5a generated during HIV infection recruits immature DCs and promotes infection of autologous T cells, probably by increasing the infection in DC/T cell aggregates.10 In the past, we have assessed the impact of C5aR targeting in human monocytes on IL-12p70, IL-10, and TNF-α production. C5aRA treatment led to marked and dose-dependent reduction of IL-12p70 and TNF-α production (albeit to a lower extent than IL-12), and reversed IFN-γ suppression of IL-10 production.38 Thus, our previous data support the view that C5a signaling drives a more proinflammatory milieu whereas its absence is associated with an antiinflammatory environment. However, in the present study, the C5aR1-mediated enhancement of early phases of HIV infection does not appear to be driven by changes in cytokine induction.
In summary, our results and the findings of the Stoiber laboratory suggest a biphasic contribution of C5a to HIV infection, i.e., a direct contribution of C5aR1 during the early phase of HIV infection through enhanced CCR5-mediated entry, and an indirect role later on during the infection through the release of proinflammatory cytokines, the attraction of DCs, and the spreading to T cells. Targeting C5aR1 may prove useful as an additional therapeutic arm to block HIV at different phases of infection.
Acknowledgments
We thank the National Institutes of Health AIDS Research and Reference Reagent Program for supplying reagents, cell lines, and HIV viruses. We also thank Dr. R.G. Collman (UPenn CFAR, Philadelphia, PA) for providing us with blood samples from CCR5Δ32-homozygous uninfected individuals and Christian M. Karsten for help with the diagram shown in Fig. 6.
This work was supported by Deutsche Forschungsgemeinschaft (DFG) IRTG 1911 project B1 to J.K. and J.A. and project B6 to C.A.C.
J.A., J.K., and C.A.C. shared supervision of this work.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Stoiber H, Banki Z, Wilflingseder D, and Dierich MP: Complement-HIV interactions during all steps of viral pathogenesis 245. Vaccine 2008;26(24):3046–3054 [DOI] [PubMed] [Google Scholar]
- 2.Huber G, Banki Z, Lengauer S, Stoiber H: Emerging role for complement in HIV infection. Curr Opin HIV AIDS 2011;6(5):419–426 [DOI] [PubMed] [Google Scholar]
- 3.Frank MM, Hester C, and Jiang H: Complement and the control of HIV infection: An evolving story. Curr Opin HIV AIDS 2014;9(3):278–290 [DOI] [PubMed] [Google Scholar]
- 4.Ballegaard V, Haugaard AK, Garred P, et al. : The lectin pathway of complement: Advantage or disadvantage in HIV pathogenesis? Clin Immunol 2014;154(1):13–25 [DOI] [PubMed] [Google Scholar]
- 5.Bajtay Z, Speth C, Erdei A, and Dierich MP: Cutting edge: Productive HIV-1 infection of dendritic cells via complement receptor type 3 (CR3, CD11b/CD18). J Immunol 2004;173(8):4775–4778 [DOI] [PubMed] [Google Scholar]
- 6.Wilflingseder D, Banki Z, Dierich MP, and Stoiber H: Mechanisms promoting dendritic cell-mediated transmission of HIV. Mol Immunol 2005;42(2):229–237 [DOI] [PubMed] [Google Scholar]
- 7.Ellegard R, Crisci E, Burgener A, et al. : Complement opsonization of HIV-1 results in decreased antiviral and inflammatory responses in immature dendritic cells via CR3. J Immunol 2014;193(9):4590–4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moir S, Malaspina A, Li Y, et al. : B cells of HIV-1-infected patients bind virions through CD21-complement interactions and transmit infectious virus to activated T cells. J Exp Med 2000;192(5):637–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banki Z, Kacani L, Rusert P, et al. : Complement dependent trapping of infectious HIV in human lymphoid tissues. AIDS 2005;19(5):481–486 [DOI] [PubMed] [Google Scholar]
- 10.Soederholm A, Banki Z, Wilflingseder D, et al. : HIV-1 induced generation of C5a attracts immature dendritic cells and promotes infection of autologous T cells. Eur J Immunol 2007;37(8):2156–2163 [DOI] [PubMed] [Google Scholar]
- 11.Klos A, Tenner AJ, Johswich KO, et al. : The role of the anaphylatoxins in health and disease. Mol Immunol 2009;46(14):2753–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kacani L, Banki Z, Zwirner J, et al. : C5a and C5a(desArg) enhance the susceptibility of monocyte-derived macrophages to HIV infection. J Immunol 2001;166(5):3410–3415 [DOI] [PubMed] [Google Scholar]
- 13.Nielsen SD, Sorensen AM, Schonning K, et al. : Complement-mediated enhancement of HIV-1 infection in peripheral blood mononuclear cells. Scand J Infect Dis 1997;29(5):447–452 [DOI] [PubMed] [Google Scholar]
- 14.Deng H, Liu R, Ellmeier W, et al. : Identification of a major co-receptor for primary isolates of HIV-1. Nature 1996;381(6584):661–666 [DOI] [PubMed] [Google Scholar]
- 15.Hüttenrauch F, Pollok-Kopp B, and Oppermann M: G protein-coupled receptor kinases promote phosphorylation and beta-arrestin-mediated internalization of CCR5 homo- and hetero-oligomers. J Biol Chem 2005;280(45):37503–37515 [DOI] [PubMed] [Google Scholar]
- 16.Martin MP, Dean M, Smith MW, et al. : Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science 1998;282(5395):1907–1911 [DOI] [PubMed] [Google Scholar]
- 17.Trkola A, Dragic T, Arthos J, et al. : CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature 1996;384(6605):184–187 [DOI] [PubMed] [Google Scholar]
- 18.Sohy D, Yano H, de Nadai P, et al. : Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J Biol Chem 2009;284(45):31270–31279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez E, Kulkarni H, Bolivar H, et al. : The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 2005;307(5714):1434–1440 [DOI] [PubMed] [Google Scholar]
- 20.Reynes J, Portales P, Segondy M, et al. : CD4+ T cell surface CCR5 density as a determining factor of virus load in persons infected with human immunodeficiency virus type 1. J Infect Dis 2000;181(3):927–932 [DOI] [PubMed] [Google Scholar]
- 21.Parker ZF, Iyer SS, Wilen CB, et al. : Transmitted/founder and chronic HIV-1 envelope proteins are distinguished by differential utilization of CCR5. J Virol 2013;87(5):2401–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimpton J. and Emerman M: Detection of replication-competent and pseudotyped human immunodeficiency virus with a sensitive cell line on the basis of activation of an integrated beta-galactosidase gene. J Virol 1992;66(4):2232–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Otto M, Hawlisch H, Monk PN, et al. : C5a mutants are potent antagonists of the C5a receptor (CD88) and of C5L2: Position 69 is the locus that determines agonism or antagonism. J Biol Chem 2004;279(1):142–151 [DOI] [PubMed] [Google Scholar]
- 24.Brussel A. and Sonigo P: Analysis of early human immunodeficiency virus type 1 DNA synthesis by use of a new sensitive assay for quantifying integrated provirus. J Virol 2003;77(18):10119–10124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno-Fernandez ME, Zapata W, Blackard JT, et al. : Human regulatory T cells are targets for human immunodeficiency virus (HIV) infection, and their susceptibility differs depending on the HIV type 1 strain. J Virol 2009;83(24):12925–12933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chomont N, El-Far M, Ancuta P, et al. : HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med 2009;15(8):893–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavrois M, De Noronha C, and Greene WC: A sensitive and specific enzyme-based assay detecting HIV-1 virion fusion in primary T lymphocytes. Nat Biotechnol 2002;20(11):1151–1154 [DOI] [PubMed] [Google Scholar]
- 28.Oppermann M, Raedt U, Hebell T, et al. : Probing the human receptor for C5a anaphylatoxin with site-directed antibodies. Identification of a potential ligand binding site on the NH2-terminal domain. J Immunol 1993;151:3785–3794 [PubMed] [Google Scholar]
- 29.Liu R, Paxton WA, Choe S, et al. : Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell 1996;86(3):367–377 [DOI] [PubMed] [Google Scholar]
- 30.Huang Y, Paxton WA, Wolinsky SM, et al. : The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med 1996;2(11):1240–1243 [DOI] [PubMed] [Google Scholar]
- 31.Dean M, Carrington M, Winkler C, et al. : Genetic restriction of HIV-1 infection and progression to AIDS by a deletion allele of the CKR5 structural gene. Hemophilia Growth and Development Study, Multicenter AIDS Cohort Study, Multicenter Hemophilia Cohort Study, San Francisco City Cohort, ALIVE Study. Science 1996;273(5283):1856–1862 [DOI] [PubMed] [Google Scholar]
- 32.Schmitz V, Almeida LN, Svensjo E, et al. : C5a and bradykinin receptor cross-talk regulates innate and adaptive immunity in Trypanosoma cruzi infection. J Immunol 2014;193(7):3613–3623 [DOI] [PubMed] [Google Scholar]
- 33.Heller T, Hennecke M, Baumann U, et al. : Selection of a C5a receptor antagonist from phage libraries attenuating the inflammatory response in immune complex disease and ischemia/reperfusion injury. J Immunol 1999;163(2):985–994 [PubMed] [Google Scholar]
- 34.Bamberg CE, Mackay CR, Lee H, et al. : The C5a receptor C5L2 is a negative modulator of C5aR mediated signal transduction. J Biol Chem 2009;285(10):7633–7644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffin GE, Leung K, Folks TM, et al. : Activation of HIV gene expression during monocyte differentiation by induction of NF-kappa B. Nature 1989;339(6219):70–73 [DOI] [PubMed] [Google Scholar]
- 36.Mellors JW, Griffith BP, Ortiz MA, et al. : Tumor necrosis factor-alpha/cachectin enhances human immunodeficiency virus type 1 replication in primary macrophages. J Infect Dis 1991;163(1):78–82 [DOI] [PubMed] [Google Scholar]
- 37.Riedemann NC, Neff TA, Guo RF, et al. : Protective effects of IL-6 blockade in sepsis are linked to reduced c5a receptor expression. J Immunol 2003;170(1):503–507 [DOI] [PubMed] [Google Scholar]
- 38.Karp CL, Grupe A, Schadt E, et al. : Identification of complement factor 5 as a susceptibility locus for experimental allergic asthma. Nat Immunol 2000;1(3):221–226 [DOI] [PubMed] [Google Scholar]