Abstract
Significance: Delayed and nonhealing wounds pose a health, economic, and social problem worldwide. For decades, the conventional wisdom pointed to growth factors as the driving force of wound healing and granted them a center stage for therapeutic development. To date, few have obtained US FDA approvals or shown clinical effectiveness and safety.
Critical Issue: Wound closure is the initial and most critical step during wound healing. Closing chronic wounds to shut down continued infection is the primary and likely the only achievable goal at the clinic in the foreseeable future. The critical question here is to identify the factor(s) in wounded tissues that drives the initial wound closure.
Recent Advances: We made an unexpected discovery of the secreted form of heat shock protein-90alpha (Hsp90α) for promoting skin cell motility, reepithelialization, and wound closure. Secreted Hsp90α possesses unique properties to remain functional under the hostile wound environment that compromises conventional growth factors' effectiveness. Through the common lipoprotein receptor-related protein-1 cell surface receptor and activation of the Akt signaling pathway, topical application of human recombinant Hsp90α protein greatly accelerates excision, burn, and diabetic skin wound closure in rodent and porcine models.
Future Directions: In almost all cells, the 2–3% of their total proteins (∼7,000 per cell) are Hsp90 (α and β), a long unraveled puzzle. Our new finding of Hsp90 secretion in wounded tissues suggests that the stockpile of Hsp90α by all cells is to rapidly supply the need for extracellular Hsp90α to repair damaged tissues. We propose that keratinocytes at the wound edge secrete Hsp90α that leads the reepithelialization process to close the wound.

Wei Li, PhD
Scope and Significance
Skin wounds include chronic wounds (venous stasis, pressure, and diabetic ulcers) and acute (traumatic, surgical, and burn) wounds. The care for chronic wounds costs the United States ∼$11 billion/year. There is a lack of effective treatments for 95% of chronic wounds. Traumatic, surgical, and burn wounds can also cause significant clinical problems, including life-threatening consequences, if they fail to heal by the primary (first) intention on time. There is a pressing need for new and effective FDA-approved treatments for chronic or acute or both wounds.
Translational Relevance
A therapeutic entity of heat shock protein-90alpha (Hsp90α), a 115 amino acid fragment called F-5, has completed preclinical studies in normal and diabetic rodent and porcine wound models and showed robust effects, including less host inflammatory response, decreased cell death/apoptosis, accelerated wound reepithelialization, better organized dermis, and increased angiogenesis. F-5 has entered its industrial developmental phase as a new topical treatment for diabetic wounds and beyond. The outcomes of F-5 treatment of human wounds remain to be seen.
Introduction
Due to natural selection and survival of the fittest, wild animals must heal skin wounds quickly or die. Similarly, most human beings are well programmed to heal skin wounds. Nevertheless, even today, skin wound care represents a major clinical conundrum, which is not only expensive but also poses serious health, economic, and social problems worldwide due to a paucity of effective treatments. The central issue of what constitutes the natural driving force of skin wound closure, the early and most critical phase of wound healing, remains unanswered. In the latter part of the last century, a great deal of research was done to find the right factor (or cocktail of factors) that would heal chronic wounds. Growth factors, such as epidermal growth factor and nerve growth factor, are called such because they have mitogenic potential to stimulate cell division. Growth factors can also be promotility factors, that is, have a distinct ability independent from their mitogenic activity to stimulate target cell migration, the critical event during the early phase of wound healing. Therefore, since the discovery of growth factors (in the early 70s), the conventional wisdom was that they represented the key driving force in skin wound healing.1–4 To date, this unproved theory remains as the mainstream understanding. However, despite the establishment of many start-up companies, experimental studies, and numerous clinical trials to determine the therapeutic potential of exogenous growth factors for wound healing in humans, to this day, we only have one FDA-approved growth factor, the recombinant PDGF-BB (bercaplermin gel, Regranex™).5–9 Regranex has had limited clinical use due to its modest efficacy, high cost, and a black box warning to cause cancer.8,9 These outcomes of growth factor clinical trials surprised many in the field and raised questions about the conventional wisdom that growth factors are the driving force of wound closure.10
Hurdles That Have Faced Growth Factors in Wound Healing
For many years our laboratory has studied signal transduction by growth factors, their receptors, and intracellular signaling networks. We also strongly believed that growth factors play a central role in promoting wound closure until we made a surprising observation. While many researchers regard fetal bovine serum (FBS) and human serum (HS) entirely functionally exchangeable, we formally compared the effects of FBS and HS on migration and proliferation of the three major human skin cell types: keratinocytes, dermal fibroblasts, and microvascular endothelial cells. Our decision to carry out these experiments was for a simple fact that human cells are never in contact with FBS in reality. In addition, HS is the rich source of growth factors and the main soluble ingredients in wounded human skin. We found that while FBS (widely used for culturing human cells by the entire research community) stimulated proliferation and migration of all three human skin cell types, HS blocked proliferation of all the human cell types and migration of the dermal fibroblast and endothelial cell. HS only promoted human keratinocyte migration. We went on to discover that it was the TGFβ3 in HS that selectively inhibited the actions of growth factors, such as TGFα, PDGF-BB, and VEGF in HS, and the expression levels of the type II TGFβ receptor determined the selective inhibition of the dermal fibroblast and endothelial cell, but not epidermal keratinocyte, migration11 (This finding was chosen for Editor's Choice, Science, 312:162, 2006, for Skte, Science 329:tw112, 2006, and for CMC Gateway, Nature, May 11, 2006). These data strongly argue against growth factors as the main driving force during the early phase of wound healing.
Using PDGF-BB as an example, Cheng et al. continued the study and reported three potential hurdles for conventional growth factor trials in wound healing. First, skin wound closure requires the coordinated migration of epidermal keratinocytes, dermal fibroblasts, and microvascular endothelial cells. PDGF-BB only engages one of these cell types, namely dermal fibroblasts, because there are no PDGF receptors (α or β) on keratinocytes or endothelial cells.12 Second, the presence of TGFβ (particularly TGFβ3 that is absent in intact skin and appears in wounds) nullifies the biological activities of PDGF-BB.12–14 This finding provides an explanation for why PDGF-BB in Regranex needs to be several thousand fold higher in concentration (∼100 μg/mL) than its steady-state levels in human circulation (5–30 ng/mL). The excessive PDGF-BB applied to the wound could travel to other parts of the human body through circulation, causing unwanted consequences. Third, growth factors' biological effects are compromised under pathological conditions, such as hyperglycemia of diabetes.12 As schematically summarized in Figure 1, these problems are inherent in all conventional growth factors. Taken together, it would be prudent to keep in mind these three salient principles regarding the biology of how skin wounds heal when investigators and pharmaceutical companies attempt to develop other wound healing agents. These authors argued that these defects in growth factors are the possible reasons for how many previous growth factor trials failed to obtain FDA approval. An ideal wound healing agent would have the following properties: (1) it would promote equally the motility of human keratinocytes (for reepithelialization), human dermal fibroblasts (for fibroplasia and creation of a neodermis), and human dermal microvascular endothelial cells (for angiogenesis); (2) its activity resists the inhibition by TGFβ in the wound bed; (3) it would remain active in both a normoglycemic and hyperglycemic microenvironment; and (4) it is a motility-promoting factor, but not a mitogenic factor that could cause increased cancer occurrence.10
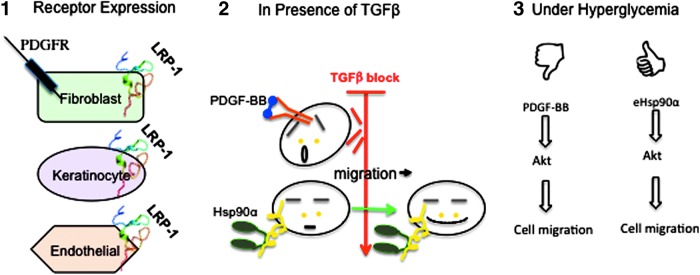
Figure 1.
Advantages for the heat shock protein-90alpha (Hsp90α) lipoprotein receptor-related protein-1 (LRP-1) signaling over growth factor signaling in promoting wound healing. (1) The expression of a growth factor receptor is often restricted to limited skin cell types, whereas the Hsp90 receptor, LRP-1, is ubiquitously expressed in all skin cell types. (2) Growth factor-stimulated cell proliferation and cell migration are inhibited by the copresence of TGFβ in the wound environment. Hsp90α-induced cell migration is resistant to TGFβ inhibition. (3) Growth factors do not work well under hyperglycemia. Hsp90α stimulates skin cell migration even under hyperglycemia. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Keratinocyte-Secreted Hsp90α Carries Unique Properties to Effectively Promote Acute and Diabetic Wound Closure
If the wound closure-promoting factor(s) does not come from growth factors in circulation, where would it come from and what would it be? The most plausible place is from the secretion of the keratinocytes and the resident dermal fibroblasts at the wound edge in response to injury-generated stress signals, such as hypoxia and TGFα.15,16 This factor must satisfy as being a common promotility factor for all human skin cell types and being able to override TGFβ3 inhibition. As expected, Cheng et al. detected a strong such common promotility activity in serum-free conditioned medium of keratinocytes pretreated with TGFα (only appears in wounded skin) or hypoxia. A series of FPLC chromatography from conditioned medium of ∼5×108 primary human keratinocytes with cell motility assays as the readouts allowed them to locate the full promotility activity, by total surprise, to secreted Hsp90α protein. They directly verified this finding by demonstrating that recombinant human Hsp90α, but not Hsp90β, alone could fully replace the promotility activity in the conditioned medium and neutralizing anti-Hsp90α antibody completely eliminated the activity from the conditioned medium.17,18 Most importantly, recombinant Hsp90α is a common promotility factor for all the three major types of human skin cells.12,18 Second, even in the presence of TGFβ3, Hsp90α remains equally effective to promote migration of all three types of human skin cells. Third, recombinant Hsp90α rescues migration of the cells cultured under hyperglycemia.12 We believe that secreted Hsp90α promotes diabetic wound healing by bypassing the hyperglycemia-caused HIF-1α downregulation and jumpstarting migration of the cells that otherwise cannot respond to the environmental hypoxia. In preclinical wound healing models, topical application of recombinant Hsp90α proteins shortened the time of 1×1 cm full-thickness acute wound closure from 2 weeks to 10 days in nude mice and from 35 to ∼15 days in db/db (diabetic) mice. In comparison, Regranex (PDGF-BB) treatment showed little improvement on acute excisional wound healing and shortened the time for diabetic wound closure from 35 to 28 days.12 Similarly, topical application of human recombinant Hsp90α protein strongly promoted wound closure of full-thickness excision wounds in healthy and diabetic pigs.19
Based on these findings, we propose a new model for how keratinocyte-secreted Hsp90α drives wound reepithelialization and closure. This new model shows that before injury, secreted Hsp90α and TGFβ3 levels remain minimal in intact skin (Step 0). When skin is wounded, keratinocytes start to migrate laterally across the wound possibly induced by acute hypoxia in the wound bed. At this early stage, dermal fibroblasts and dermal microvascular endothelial cells at the wound edge are unable to migrate into the wound due to the presence of TGFβ3 (Step 1). The migrating keratinocytes start to secrete Hsp90α, and once secreted, Hsp90α reaches the threshold concentration of 100 nM, the dermal cells are recruited inwardly into the wound from the surrounding wound edge, despite TGFβ3 (Step 2). The migrated keratinocytes resurface the wound, the recruited dermal fibroblasts remodel the wound, and the moved-in dermal microvascular endothelial cells rebuild new blood vessels (Step 3). The dermal remodeling and neovascularization processes would take many months to complete. Taken together, it is the keratinocyte-secreted Hsp90α instead of serum growth factors that is responsible for leading wound reepithelialization and closure.
Unlike growth factors, secreted Hsp90α is solely a promotility factor and does not promote cell proliferation.17,18 The unique property of Hsp90α makes physiological sense. Keratinocyte migration occurs a few hours following the skin injury, whereas the inward migration of dermal cells is not detected until several days later.2 Second, migrating cells cannot proliferate at the same time and vice versa. Third, cell migration precedes cell proliferation during wound healing. In addition, any attempt by on-site growth factors to stimulate proliferation of the cells will be inhibited by TGFβ3 that only appears when skin is wounded.11,12 Then, when and where does cell proliferation take place in the wounded skin? Taking the above three observations into consideration, we believe that cells around the wound bed are unable to proliferate, due to the presence of TGFβ. Instead, after the cells at the wound edge have migrated toward the wound bed, they left an empty space between themselves and the cells behind them in the unwounded area where TGFβ3 is low or undetectable, causing a temporary loss of cell–cell contact inhibition. It is the cells behind the front migrating cells that start to proliferate after losing contact inhibition. Therefore, the role of cell proliferation in wound healing is to refill the space generated by the front migrating cells. This model of migration then proliferation is summarized in Figure 2. The stimuli of cell proliferation likely come from the plasma growth factors diffused from surrounding unwounded blood vessels. When the initial wound closure is completed, many other factors (including growth factors) will participate in the remaining long (months) and tedious wound remodeling and tissue homeostasis processes.
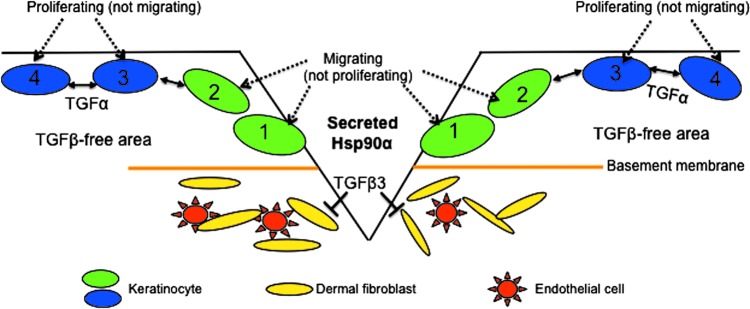
Figure 2.
Cell migration precedes cell proliferation during wound healing. Secreted Hsp90α-induced migration of keratinocytes (green) at the wound edge creates loss of contact inhibition of the cells behind the front migrating cells. The keratinocytes (blue) behind the wound edge then use growth factors, such as TGFα, around them to proliferate and fill the newly created space. Please note that the reason TGFα is able to promote keratinocyte proliferation is due to the lack of TGFβ in the unwounded area of the skin.
Secreted Hsp90α is No Chaperone
Hsp90 family proteins are expressed in almost all cell and tissue types studied. Quantitatively, the levels of Hsp90α are ten and hundred fold higher than each of the 7,000 other proteins present in the same cell. It remains to be studied why cells in our body stockpile Hsp90 proteins in such an unusually large quantity. The decade long theory of chaperones for Hsp90 proteins can no longer fully explain this irony. Most interestingly, Hsp90α-null mice developed normally, providing direct evidence strongly against it being a critical intracellular chaperone that controls cell survival, growth, and transformation.21,22 The N-terminal ATP binding and ATPase of Hsp90α are known as the regulator of its chaperone function. Focusing on the promotility activity of Hsp90α, Cheng et al. carried out site-directed mutagenesis studies to address the question of whether ATPase also determines the extracellular function of Hsp90α. The authors found that three ATPase mutant proteins of Hsp90α retained a full promotility activity as the wild-type Hsp90α. Then, they used sequential deletion mutagenesis to narrow down the promotility domain to a 115 amino acid fragment (aa-236 to aa-350) called F-5, between the linker region (LR) and the middle (M) domain of human Hsp90α. The 115 amino acid sequence of F-5 is highly conserved during evolution.10 Recombinant F-5 peptide promoted skin cell migration in vitro and wound healing in vivo as effectively as the full-length Hsp90α-wt.12,18 These findings demonstrate that the N-terminal ATPase domain and the C-terminal dimerization and cofactor-binding domain are dispensable for secreted Hsp90α to promote cell migration. The structure and function requirements for intracellular Hsp90α and extracellular Hsp90α are schematically depicted in Figure 3.
Figure 3.
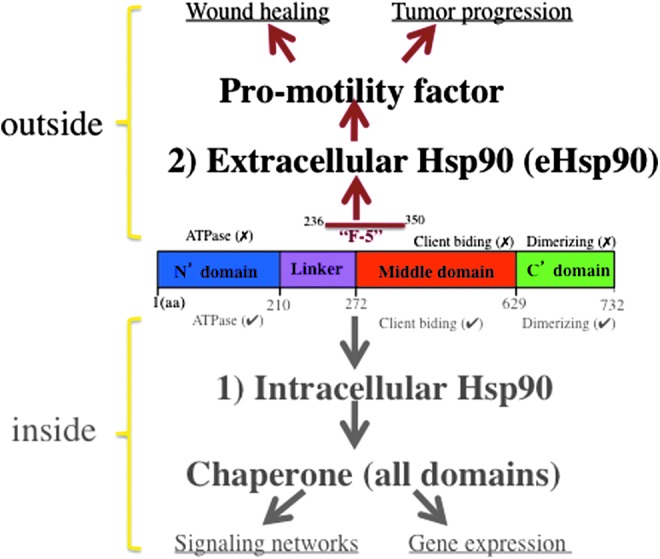
Two evolutionarily distinct functions for Hsp90 family proteins. Intracellular Hsp90, especially Hsp90β, is a chaperone that requires its intrinsic ATPase activity. Its purpose is to maintain the signaling networks in working condition. Extracellular Hsp90, especially Hsp90α, is a promotility factor that uses the F-5 region in an ATPase-independent manner. Its purpose is to help repair damaged tissue. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Mechanism of Action by Secreted Hsp90α
It is clear now that extracellular Hsp90α acts as a ligand that binds to the cell surface receptor, the low-density lipoprotein receptor-related protein-1 (LRP-1, also identified as α2-macroglobulin receptor, CD91, and TGFβR-V), to trigger cross membrane signal transduction.18 LRP-1 is detected in almost all normal cell types studied, including the three major cell types that are involved in skin wound healing, keratinocytes, dermal fibroblasts, and dermal microvascular endothelial cells.23–25 LRP-1 belongs to a family of at least seven members related to the LDL receptor. Deletion of the LRP-1 gene leads to embryonic lethality in mice.26 LRP-1 binds a variety of extracellular molecules, including lipoproteins, proteases and their inhibitors, ECMs, heat shock proteins, and growth factors. Structurally, LRP-1 consists of a 515-kDa extracellular subunit and a membrane-anchoring 85-kDa subunit, which are formed from proteolytic products of a common 600-kDa precursor.23 Cheng et al. reported that LRP-1 receptor mediates secreted Hsp90-stimulated human skin cell migration in vitro and wound healing in vivo. Their studies showed that (i) neutralizing antibodies against the LRP-1 ligand-binding domain blocked recombinant Hsp90-induced cell migration; (ii) downregulation of LRP-1 completely blocked Hsp90α-stimulated keratinocyte and dermal fibroblast cell migration; and (iii) reintroducing LRP-1 (minireceptor) rescued the motility defect. Furthermore, blocking ligand-binding domains of LRP-1 by RAP (receptor-associated protein) dramatically delayed wound healing in mice.18 The exact relationship between LRP-1 and the Hsp90 family proteins has recently been worked out. Jayaprakash et al. showed that under environmental stress cues, such as hypoxia and nutrient paucity, Hsp90α and Hsp90β work around the surface LRP-1 to promote wound healing. Specifically, Hsp90β binds to the cytoplasmic tail of LRP-1 and stabilizes the receptor at the cell surface. In contrast, Hsp90α does not bind LRP-1 from inside the cell, instead it is secreted into the extracellular environment where it binds and triggers LRP-1 receptor signaling to promote cell motility, leading to wound closure.27 This novel finding is summarized in Figure 4. The authors argue that this hypoxia and nutrient paucity-responding mechanism applies broadly to other injured noncutaneous tissues as well.
Figure 4.
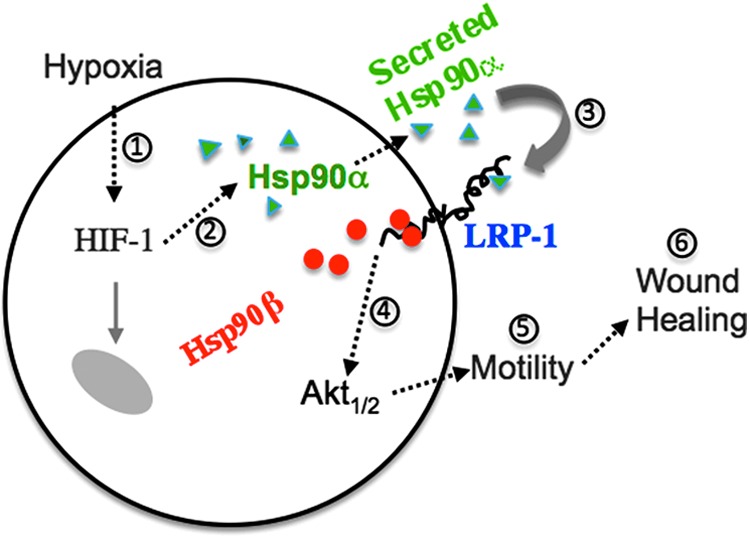
Hsp90α and Hsp90β together operate a tissue repair mechanism. Schematically, when tissue is damaged, acute hypoxia triggers cells in the wound edge to secrete Hsp90α. Intracellular Hsp90β stabilizes LRP-1 and the secreted Hsp90α binds and signals through the LRP-1 receptor to promote cell migration and wound healing. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Following binding to LRP-1, Hsp90α triggers a unique transmembrane signaling to activate downstream pathways inside the cell. Tsen et al. provided direct evidence for the long debated claim that secreted Hsp90α is a bona fide signaling molecule rather than a chaperone. They showed that extracellular Hsp90α binds to the subdomain II in the extracellular part of LRP-1 (it has totally four subdomains). The Hsp90α-binding signal crosses the plasma membrane through LRP-1 and exits at the NPVY, but not NPTY, site in the cytoplasmic tail of LRP-1, leading to, through unknown mediator(s), the serine-473, but not threonine-308, phosphorylation in Akt kinases. The activation of Akt1 and Akt2 is critical, since Akt1 and Akt2 knockdown blocked Hsp90α signaling to promote cell motility in vitro. More convincingly, Akt1 and Akt2 knockout mice showed impaired wound healing that could not be corrected by supplementation with recombinant Hsp90α.28 A schematic representation of this signaling mechanism is shown in Figure 5. Besides LRP-1, it was reported that extracellular Hsp90 binds and activates other secreted motility-promoting factors, such as secreted MMP2, in a manner that seems to be similar to the chaperone–client protein interactions.29,30 The critical evidence that specific inhibition of (secreted) MMP2 (most MMP inhibitors are toxic to cells) blocks Hsp90-stimulated cell motility remains unavailable.
Figure 5.
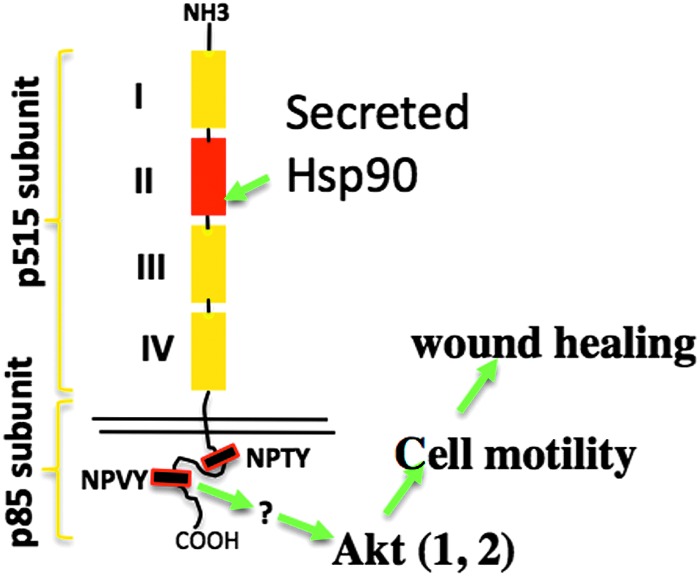
Extracellular Hsp90 signals through the LRP-1 receptor to promote reepithelialization during wound healing. Akt is essential for extracellular Hsp90α signaling to promote cell migration and wound healing. How LRP-1 mediates Hsp90α signaling to other three pathways and the function of these pathways in Hsp90α-induced wound healing remain to be investigated. The question marks indicate that intermediate activators of the pathways listed remain unclear. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Newly Standardized Acute and Diabetic Pig Wound Healing Models
Live animals remain as the most useful method to evaluate potential wound healing therapeutics for humans. Among the current animal models, it is broadly accepted that pig skin wound healing are closest to those in humans and therefore pigs are regarded as the right model for wound healing studies.31 Pigs and human beings are both tight-skin animals and close skin wounds by driving the lateral migration of keratinocytes from the wound edge to across the wound bed, the process called reepithelialization. Pig is also an effective model for wound therapeutic studies because a large number of replicate wounds (10–14 on each side) can be created in the same pig. In randomized preclinical studies, a high concordance of the results between pig and man has been reported.31–34
O'Brien et al. recently made great efforts to standardize the procedures of how to create pig skin wounds, how to compare placebo and treatment, and why previous studies gave hard to reproduce results. In addition, they demonstrated for the first time when a delay in wound closure occurs in STZ-treated pigs. They made several new findings. First, along the torso area where the wounds are created, due to differences, even within a distance of a few centimeters, in the elasticity, thickness, and hair follicle density from top to bottom and left to right, the wound closure rates were significantly different. Instead, they showed that a pair of wounds created on the opposite sides of the torso, but at corresponding locations, underwent a similar rate of healing. Therefore, they suggested that the treatment and control should use wounds at the corresponding spot, but on opposite sides of the pig body.19 This new model is depicted in Figure 6. Based on the new procedures, these authors went further to systematically analyze previously reported STZ-induced diabetic pig models. Unfortunately, they were unable to detect significant delay in wound closure by following the procedures of those studies.35–39 O'Brien et al. concluded that there was a need to systematically evaluate the critical parameters of diabetes over time and (re-) standardize the diabetic wound healing model in pigs. They followed STZ-injected pigs for up to 4 months by monitoring weekly their body weights, insulin production, plasma glucose levels, and A1c data. Then, they followed full-thickness wound closure by primary intention over time. Their data clearly showed that a significant delay in wound closure only occurred in pigs after STZ injection for at least 45 days with the best outcomes on 90 days, whereas all previous studies used pigs with STZ injection for a maximum of 2 weeks before introducing skin wounds. Finally, they validated this new standardized diabetic pig model by showing (i) effectiveness for an FDA-approved growth factor therapy (on diabetic wounds only); (ii) a robust effect of F-5 on promoting wound closure by stimulating reepithelialization; and (iii) identification of a 27 amino acid peptide, called F-8, as the minimum entity in Hsp90α that is responsible for its wound healing-promoting activity.19
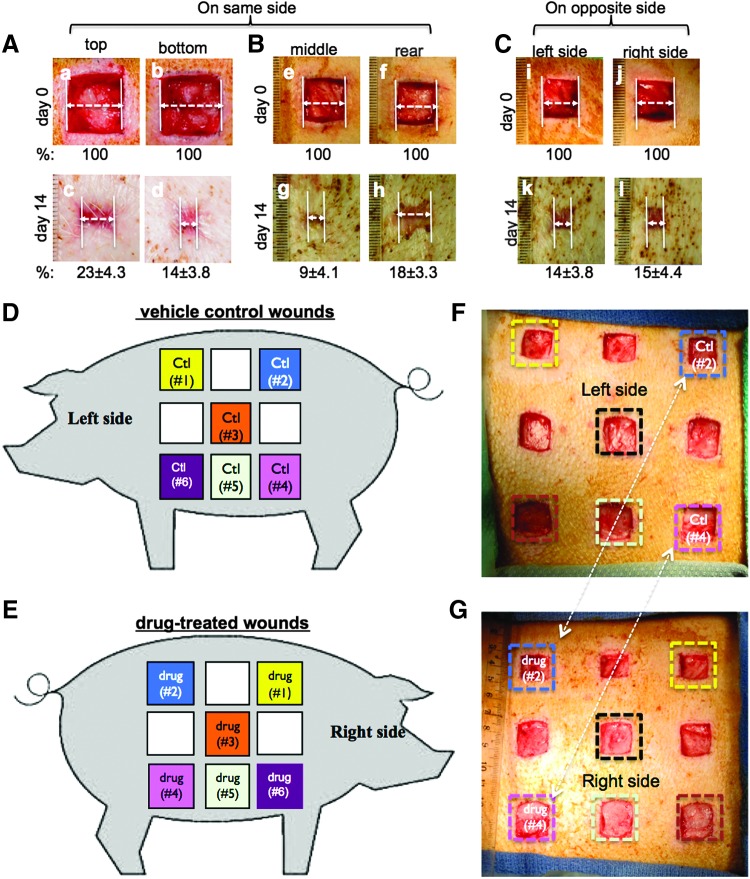
Figure 6.
Newly restandardized acute and diabetic pig wound healing models. (A–C) 2.0×2.0 cm full-thickness wounds were created on the same side of the pigs (n=3), 2.0 cm apart between wounds, and were compared either between the top and the bottom wounds (A) or between the middle and rear wounds (B) or between wounds made at the similar spots, but on the opposite sides of the pig (C). Quantitations (% of healing) were made based on triplicate wounds in each pig and are shown below each of the images. (D, E) A schematic presentation of nine 2.0×2.0 cm (in normal pigs) or 1.5×1.5 cm (in diabetic pigs) full-thickness wounds created on each side of the pig, 2.0 cm apart between wounds. Comparison between treatment and control should be made between two wounds at the similar spots, but on the opposite sides of the pig, as indicated by same color squares. (F, G) Based on the above design, real wounds were created on the two sides of pigs. Treatments versus controls are indicated. Taken from O'Brien et al.19 with permission.
Secreted Hsp90α is a General Tissue Repair Factor
When a tissue environment becomes hostile, such as injuries, cells in the injured tissue immediately downregulate the overall protein synthesis rate and yet selectively upregulate expression of heat shock protein-90 from its already 2–3% of the total cellular proteins up to several fold more. This observation has long been interpreted as a tissue's self-protecting mechanism to cope with the environmental changes by protecting the folding and stability of the existing intracellular proteins. Csermely et al. were among the first to challenge this theory and argued that storage of a single protein in such a large amount only for the purpose of chaperoning would not have been tolerated by evolution. They speculated that those extra Hsp90 proteins must have other functions.40 The recent findings that Hsp90α is unessential for development21 and that secreted Hsp90α promotes wound healing12 suggest that evolution purposely designed the abundant storage of intracellular Hsp90α to supply the extracellular Hsp90α pool in case of tissue damage. Consistently, Hsp90α is rapidly secreted by a variety of cell types in response to extracellular stress signals, including heat, reactive oxygen species, hypoxia, radiation, and UV, just to mention a few.10 This exciting hypothesis can be tested using the recently available Hsp90α-null mice.21,22
Summary
It is clear that Hsp90, especially Hsp90α, has two evolutionarily assigned functions: an intracellular chaperone and an extracellular tissue-repairing factor. Both functions are critical for their host cells to cope with environmental changes, such as tissue damages. Tumor cells take advantage of these functions to gain more invasiveness. A known mediator of Hsp90α secretion is HIF-1α in both normal and tumor cells. LRP-1 is the cell surface receptor that transmits the extracellular Hsp90α signals. More importantly, the discovery of the extracellular Hsp90α role in tissue repair and cancer invasion has revealed a new line of therapeutic interventions. We believe that secreted Hsp90α is crucial for the early phase of wound healing, reepithelialization, and wound closure, whereas growth factors play more important roles in the later phases of wound remodeling, neovascularization, and tissue homeostasis.
Take-Home Messages.
The secreted form of the naturally occurring molecule, called heat shock protein-90alpha (Hsp90α), represents a previously overlooked wound healing driving force that is responsible for the initial and most critical step of wound healing—wound closure.
Abbreviations and Acronyms
- FBS
fetal bovine serum
- Hsp90α
heat shock protein-90alpha
- HS
human serum
- LR
linker region
- LRP-1
lipoprotein receptor-related protein-1
- M
middle
Acknowledgments
The authors thank many of their previous laboratory colleagues who made contributions to some of the work described in this review. The authors apologize if they failed to acknowledge every publication on secreted Hsp90 while this review was being written. This study was supported by NIH grants, GM066193 and GM067100 (to W. L.).
Author Disclosure and Ghostwriting
University of Southern California has obtained three approved patents on the F-5 fragment of Hsp90α for treatment of skin wounds. The authors listed expressly wrote the content of this article. No ghostwriters were used to write this article.
About the Authors
Wei Li, PhD received a PhD degree in 1991 from Albert Einstein College of Medicine (New York). Following a 2-year postdoctoral fellowship with Dr. Joseph Schlessinger at NYU, he joined the faculty of the University of Chicago as an Assistant Professor in 1993. He was recruited as an Associate Professor to the University of Southern California (USC) in 1999. He rose to Professor in 2006. He is currently the Director of the GMCB Graduate Program at USC. His research focuses on skin wound healing and breast cancer. Ayesha Bhatia graduated with a Masters in Molecular Microbiology and Immunology from USC and is currently a PhD student under Dr. Wei Li at USC, focusing on the role of Hsp90α in mice wound healing. Kathryn O'Brien is currently a postdoctoral fellow in Dr. Wei Li's laboratory and works on establishing mouse and pig models for wound healing. Mei Chen is currently a Professor and also the Director of Research at the Department of Dermatology at USC. David T. Woodley joined USC as Professor and Cochief of the Division of Dermatology in 1999 and currently holds the chair for the Department of Dermatology.
References
- 1.Martin P. Wound healing—aiming for perfect skin regeneration. Science 1997;276:75–81 [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 3.Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003;83:835–870 [DOI] [PubMed] [Google Scholar]
- 4.Grose R, Werner S. Wound healing studies in transgenic and knockout mice. A review. Methods Mol Med 2003;78:191–216 [DOI] [PubMed] [Google Scholar]
- 5.LeGrand EK. Preclinical promise of becaplermin (rhPDGF-BB) in wound healing. Am J Surg 1998;176:48S–54S [DOI] [PubMed] [Google Scholar]
- 6.Steed DL. Clinical evaluation of recombinant human platelet-derived growth factor for the treatment of lower extremity diabetic ulcers. Diabetic Ulcer Study Group. J Vasc Surg 1995;21:71–78; discussion 79–81 [DOI] [PubMed] [Google Scholar]
- 7.Wieman TJ, Smiell JM, Su Y. Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998;21:822–827 [DOI] [PubMed] [Google Scholar]
- 8.Nagai MK, Embil JM. Becaplermin: recombinant platelet derived growth factor, a new treatment for healing diabetic foot ulcers. Expert Opin Biol Ther 2002;2:211–218 [DOI] [PubMed] [Google Scholar]
- 9.Mandracchia VJ, Sanders SM, Frerichs JA. The use of becaplermin (rhPDGF-BB) gel for chronic nonhealing ulcers. A retrospective analysis. Clin Podiatr Med Surg 2001;18:189–209 [PubMed] [Google Scholar]
- 10.Li W, Sahu D, Tsen F. Secreted heat shock protein-90 (Hsp90) in wound healing and cancer. Biochim Biophys Acta 2012;1823:730–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bandyopadhyay B, Fan J, Guan S, et al. A “traffic control” role for TGFbeta3: orchestrating dermal and epidermal cell motility during wound healing. J Cell Biol 2006;172:1093–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng CF, Sahu D, Tsen F, et al. A fragment of secreted Hsp90α carries properties that enable it to accelerate effectively both acute and diabetic wound healing in mice. J Clin Invest 2011;121:4348–4361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grainger DJ, Mosedale DE, Metcalfe JC. TGF-beta in blood: a complex problem. Cytokine Growth Factor Rev 2000;11:133–145 [DOI] [PubMed] [Google Scholar]
- 14.Hering S, Isken F, Janott J, et al. Analysis of TGFbeta3 gene expression and protein levels in human bone and serum. Exp Clin Endocrinol Diabetes 2001;109:107–115 [DOI] [PubMed] [Google Scholar]
- 15.O'Toole EA, Marinkovich MP, Peavey CL, et al. Hypoxia increases human keratinocyte motility on connective tissue. J Clin Invest 1997;100:2881–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Fan J, Chen M, Li W, Woodley DT. Transforming growth factor-alpha: a major human serum factor that promotes human keratinocyte migration. J Invest Dermatol 2006;126:2096–2105 [DOI] [PubMed] [Google Scholar]
- 17.Li W, Li Y, Guan S, et al. Extracellular heat shock protein-90alpha: linking hypoxia to skin cell motility and wound healing. EMBO J 2007;26:1221–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng CF, Fan J, Fedesco M, et al. Transforming growth factor alpha (TGFalpha)-stimulated secretion of HSP90alpha: using the receptor LRP-1/CD91 to promote human skin cell migration against a TGFbeta-rich environment during wound healing. Mol Cell Biol 2008;28:3344–3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Brien K, Bhatia A, Tsen F, et al. Identification of the critical therapeutic entity in secreted Hsp90α that promotes wound healing in newly re-standardized healthy and diabetic pig models. PLoS One 2014;9:e113956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Young JC, Moarefi I, Hartl FU. Hsp90: a specialized but essential protein-folding tool. J Cell Biol 2001;154:267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grad I, Cederroth CR, Walicki J, et al. The molecular chaperone Hsp90α is required for meiotic progression of spermatocytes beyond pachytene in the mouse. PLoS One 2010;5:e15770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Imai T, Kato Y, Kajiwara C, et al. Heat shock protein 90 (HSP90) contributes to cytosolic translocation of extracellular antigen for cross-presentation by dendritic cells. Proc Natl Acad Sci U S A 2011;108:16363–16368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest 2001;108:779–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lillis AP, Mikhailenko I, Strickland DK. Beyond endocytosis: LRP function in cell migration, proliferation and vascular permeability. J Thromb Haemost 2005;3:1884–1893 [DOI] [PubMed] [Google Scholar]
- 25.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev 2008;88:887–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strickland DK, Ashcom JD, Williams S, Burgess WH, Migliorini M, Argraves WS. Sequence identity between the alpha 2-macroglobulin receptor and low density lipoprotein receptor-related protein suggests that this molecule is a multifunctional receptor. J Biol Chem 1990;265:17401–17404 [PubMed] [Google Scholar]
- 27.Jayaprakash P, Dong H, Zou M, et al. Hsp90α and Hsp90β Co-Operate a Stress-Response Mechanism to Cope With Hypoxia and Nutrient Paucity during Wound Healing. J Cell Sci 2015. [Epub ahead of print]; DOI: 10.1242/jcs.166363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsen F, Bhatia A, O'Brien K, et al. Extracellular heat shock protein 90 signals through subdomain II and the NPVY motif of LRP-1 receptor to Akt1 and Akt2: a circuit essential for promoting skin cell migration in vitro and wound healing in vivo. Mol Cell Biol 2013;33:4947–4959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eustace BK, Sakurai T, Stewart JK, et al. Functional proteomic screens reveal an essential extracellular role for hsp90 alpha in cancer cell invasiveness. Nat Cell Biol 2004;6:507–514 [DOI] [PubMed] [Google Scholar]
- 30.Sims JD, McCready J, Jay DG. Extracellular heat shock protein (Hsp)70 and Hsp90α assist in matrix metalloproteinase-2 activation and breast cancer cell migration and invasion. PLoS One 2011;6:e18848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76 [DOI] [PubMed] [Google Scholar]
- 32.Brahmatewari J, Serafini A, Serralta V, Mertz PM, Eaglstein WH. The effects of topical transforming growth factor-beta2 and anti-transforming growth factor-beta2,3 on scarring in pigs. J Cutan Med Surg 2000;4:126–131 [DOI] [PubMed] [Google Scholar]
- 33.Davis SC, Cazzaniga AL, Eaglstein WH, Mertz PM. Over-the-counter topical antimicrobials: effective treatments? Arch Dermatol Res 2005;297:190–195 [DOI] [PubMed] [Google Scholar]
- 34.Lindblad WJ. Considerations for selecting the correct animal model for dermal wound-healing studies. J Biomater Sci Polym Ed 2008;19:1087–1096 [DOI] [PubMed] [Google Scholar]
- 35.Velander P, Theopold C, Hirsch T, et al. Impaired wound healing in an acute diabetic pig model and the effects of local hyperglycemia. Wound Repair Regen 2008;16:288–293 [DOI] [PubMed] [Google Scholar]
- 36.Hirsch T, Spielmann M, Velander P, et al. Insulin-like growth factor-1 gene therapy and cell transplantation in diabetic wounds. J Gene Med 2008;10:1247–1252 [DOI] [PubMed] [Google Scholar]
- 37.Hirsch T, Spielmann M, Zuhaili B, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med 2009;11:220–228 [DOI] [PubMed] [Google Scholar]
- 38.Velander P, Theopold C, Bleiziffer O, et al. Cell suspensions of autologous keratinocytes or autologous fibroblasts accelerate the healing of full thickness skin wounds in a diabetic porcine wound healing model. J Surg Res 2009;157:14–20 [DOI] [PubMed] [Google Scholar]
- 39.Bergmann J, Hackl F, Koyama T, et al. The effect of amnion-derived cellular cytokine solution on the epithelialization of partial-thickness donor site wounds in normal and streptozotocin-induced diabetic swine. Eplasty 2009;9:e49. [PMC free article] [PubMed] [Google Scholar]
- 40.Csermely P, Schnaider T, Soti C, Prohászka Z, Nardai G. The 90-kDa molecular chaperone family: structure, function, and clinical applications. A comprehensive review. Pharmacol Ther 1998;79:129–168 [DOI] [PubMed] [Google Scholar]