Abstract
Bone formation, a highly regulated developmental process, involves osteoblast differentiation, which is controlled by different important transcription factors. Recent evidence has suggested possible negative regulation of inhibitors of growth (ING) 1b on the osteoblast marker expression. The aim of this study is to examine the detailed mechanism by which the activity of ING1b inhibits osteoblast differentiation. In the current study, we investigated the function and mechanism by which ING1b inhibits osteoblast differentiation using C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts. Real-time polymerase chain reaction and Western blotting showed that ING1b was decreased during osteoblast differentiation and ING1b overexpression markedly decreased alkaline phosphatase (ALP) activity, runt-related transcription factor 2 (Runx2) expression, and collagen type 1 synthesis, whereas ING1b silencing significantly upregulated ALP activity, Runx2 expression, and collagen type 1 synthesis. Further studies indicated that ING1b suppressed the expression of peroxisome proliferator-activated receptor (PPAR)-β/δ in a hypoxia-inducible factor (HIF) 1α-dependent manner, while ING1b silencing significantly increased the expression of PPAR-β/δ and HIF1α. Moreover, PPAR-β/δ or HIF1α silencing significantly inhibited ALP activity, Runx2 expression, and collagen type 1 synthesis. These results demonstrated that ING1b is an important regulator of osteoblast differentiation and suppresses PPAR-β/δ. Our study may provide additional insight into osteoblast differentiation and offer a potential new molecular target for osteoporosis.
Introduction
Osteoporosis, a representative age-associated human disease, is characterized by decreased quality and density of the skeleton with aging (NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, 2001). Evidence has suggested that the disturbed regulation of the bone remodeling process is the main cause of osteoporosis (Eriksen et al., 1994). Recently, osteoblasts and osteocytes have been implicated in the regulation of bone remodeling (Mullender et al., 1996; Chen et al., 2012). The activities of different signaling pathways that affect the osteoblast-specific gene expression may contribute to differentiation of osteoblasts that originated from mesenchymal stem cells (Park et al., 2010). A robust body of recent literature showed that a number of transcription factors controlled osteoblast differentiation and function, such as runt-related transcription factor 2 (Runx2), Osterix, ATF4, members of the AP1 family, CREB, and FOXO1 (Wei et al., 2012). Moreover, some nuclear proteins, such as STAT1 (Kim et al., 2003) and Twist (Bialek et al., 2004), also affect osteoblast differentiation through interaction with transcription factors such as Runx2.
The nature of the cellular and molecular mechanisms of osteoblast differentiation remains poorly understood. A recent study found that inhibitors of growth (ING) 1b may decrease osteoblast marker expression, including Runx2 (Bigot et al., 2015). ING1, a member of the ING family, has been described as a type II tumor suppressor that can regulate cell growth, apoptosis, chromatin remodeling, senescence, cell cycle regulation, and DNA repair (Garkavtsev et al., 1998; Guérillon et al., 2013). The human ING1 gene contains three exons and is located at chromosome 13q33–34 (Garkavtsev et al., 1997; Feng et al., 2002). ING1 has three isoforms: ING1a (a 47-kDa protein), ING1b (an alternatively spliced 33-kDa protein), and ING1c (a 24-kDa protein from an internal initiation site) (Garkavtsev, 1999; Saito et al., 2000). It is now well established that overexpression of ING1b inhibits cell growth and promotes apoptosis, and ING1b is upregulated during the apoptosis process (Garkavtsev et al., 1996; Helbing et al., 1997; Vieyra et al., 2002).
Bigot et al. (2015) showed that ING1b negatively regulates hypoxia-inducible factor-1α (HIF1α) protein levels in adipose-derived stromal cells. The transcription factor HIF1 consists of a heterodimer of HIF1α and HIF1β (Semenza, 2003). HIF1α is a major regulator of the cellular response to hypoxia. Hypoxia can inhibit the bone-forming capacity, growth, and differentiation of rat osteoblasts (Utting et al., 2006). Hiraga et al. (2007) showed that HIF overexpression by adenovirus significantly inhibited BMP2-induced osteoblast differentiation and osteocalcin mRNA expression in C3H10T1/2 cells. Numerous studies have demonstrated that HIF 1α facilitates recruitment and differentiation of multipotent human mesenchymal stromal cells (Wagegg et al., 2012). Furthermore, HIF1α causes enhanced bone modeling through activation of VEGF in endochondral ossification (Wang et al., 2007). Because ING1b can inhibit HIF1α expression, we hypothesized that ING1b may participate in osteoblast differentiation through regulation of the level of HIF1α. To approach this, we studied the effects of ING1b in osteoblast proliferation and differentiation. Our results indicate that osteoblast growth and differentiation are inhibited by ING1b overexpression. ING1b inhibits peroxisome proliferator-activated receptor (PPAR)-β/δ expression, and this inhibition is dependent on the level of HIF1α. This reveals a new molecular mechanism through which ING1b inhibits differentiation in osteoblasts.
Materials and Methods
Cell culture
C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts were provided by ATCC and cultured as described in Park et al., 2010. C3H10T1/2 mesenchymal stem cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. MC3T3-E1 mouse preosteoblastic cells were maintained in α-Minimal Essential Medium supplemented with FBS and 1% penicillin/streptomycin. Cells were all cultured in a humidified atmosphere at 37°C. To induce osteoblast differentiation, cells were transferred to a differentiation medium that contained 10% FBS, 50 μg/mL ascorbic acid (Sigma), and 10 mM β-glycerophosphate (Sigma). The differentiation medium was replaced every 2 days.
Transfections
Stably transfected C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts were produced using the empty pcDNA 3.1 vector and the pcDNA 3.1 ING1b vector (encoding ING1b) according to the method described previously (Bigot et al., 2015) and were then selected with G418.
ING1b, PPAR-β/δ, and HIF1α silencing was performed according to the method described previously (Bigot et al., 2015). ING1b siRNA (Life Technologies), PPAR-β/δ siRNA (Life Technologies), and HIF1α siRNA (Santa Cruz Biotechnology, CliniSciences) were used. Experiments were performed with the Lipofectamine RNAiMAX Transfection Reagent (Life Technologies) according to the manufacturer's instructions.
Measurements of alkaline phosphatase activity
After transfection with ING1b or HIF1α, C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts were cultured in an osteogenic differentiation medium for 3, 5, 7, and 9 days. Cells were lysed using a commercial cell lysis solution according to the manufacturer's instructions. The alkaline phosphatase (ALP) activity assay was performed using a commercial Alkaline Phosphatase Detection Kit (Nanjing Jiancheng Bioengineering Institute). Cell lysates were incubated with ALP assay working solution (containing 1 mM pNPP). The absorbance of the samples was determined by using an ELISA plate reader at 405 nm (Fung et al., 2014).
Quantitative real-time polymerase chain reaction
Total RNA was extracted using the RNeasy Kit (Qiagen) according to the protocol provided by the manufacturer. cDNA was synthesized using the Superscript II reverse transcriptase (Life Technologies). The level of ING1b, HIF1α, and PPAR-β/δ gene expression was quantified using SYBR Green Master Mix (Life Technologies). The relative gene expression was normalized to β-actin.
Western blot analysis
Total proteins were extracted from MC3T3-E1 preosteoblasts using the lysis buffer (50 mM Tris, pH 7.5; 150 mM NaCl; 1% Tween 20; 0.2% Nonidet P-40; 1 mM phenylmethylsulfonyl fluoride; 500 mM NaF; 1 mM Na3VO4; 10 μg/mL aprotinin; 2 μg/mL pepstatin A; and 10 μg/mL leupeptin) (Park et al., 2010). Proteins were separated by 12% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a polyvinylidene difluoride membrane (Millipore). Five percent nonfat milk was used to block the membrane for 1 h at room temperature. The membrane was then incubated overnight at 4°C with a rabbit anti-mouse ING1b polyclonal antibody (dilution: 1:1000, catalog number: ABIN2486913; Antibodies online.com), rabbit anti-mouse HIF1α polyclonal antibody (dilution: 1:200, catalog number: sc-10790; Santa Cruz Biotechnology, Inc.), rabbit anti-mouse PPAR-β/δ polyclonal antibody (dilution: 1:200, catalog number: sc-7197; Santa Cruz Biotechnology, Inc.), rabbit anti-mouse runx2 polyclonal antibody (dilution: 1:200, catalog number: sc-10758; Santa Cruz Biotechnology, Inc.), or anti-β-actin monoclonal antibody (dilution: 1:200, catalog number: sc-130656; Santa Cruz Biotechnology, Inc.). HRP-conjugated anti-rabbit IgG (Santa Cruz Biotechnology, Inc.) was added to blots and incubated for 1 h at room temperature. Bound proteins were measured using an ECL Chemiluminescence Kit (Santa Cruz Biotechnology, Inc.).
Measurement of collagen type 1
Collagen type 1 synthesis in MC3T3-E1 preosteoblasts was determined according to the previously described method (Reffitt et al., 2003). The synthesis of collagen type 1 was measured by quantification of the level of the carboxy-terminal propeptide of type 1 procollagen (CICP) in the culture medium using the Prolagen-C Kit (Metra Biosystems Ltd.).
Statistical analysis
For statistical analysis, data were processed by SPSS13.0 and analyzed by ANOVA followed by the Student's t-test. p < 0.05 was considered statistically significant. All data are shown as mean ± SD of five separate experiments.
Results
ING1b expression is reduced during osteoblast differentiation
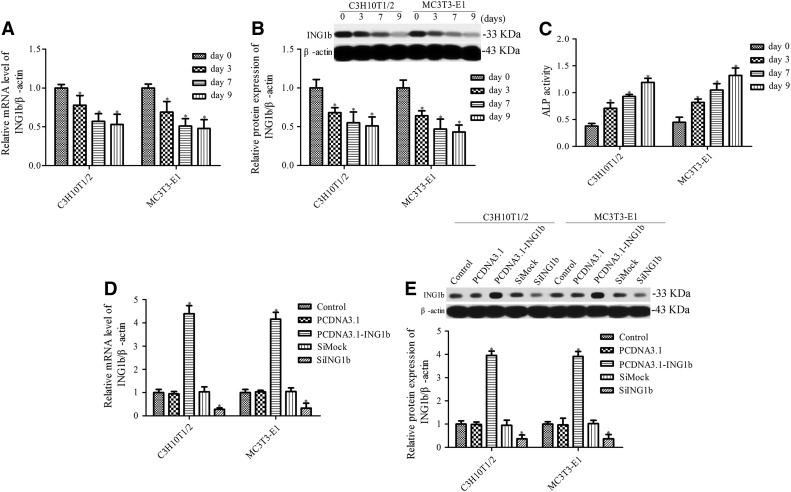
Initially, we aimed to evaluate the expression of ING1b in C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblast cells after osteoblast differentiation. Quantitative real-time polymerase chain reaction (qRT-PCR) and Western blotting were used to detect the level of ING1b mRNA and protein, respectively. ING1b expression was significantly decreased after osteoblast differentiation (Fig. 1A, B), suggesting that ING1b may play a vital function in osteoblast differentiation. The activity of differentiation marker ALP was also determined, and the results indicated that along with osteoblast differentiation, ALP activity was significant increased compared with the control group (Fig. 1C). To determine the function of ING1b in osteoblast differentiation, we generated C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts cells with overexpressed or silenced ING1b using pcDNA 3.1 ING1b or ING1b siRNA, respectively (Fig. 1D, E).
FIG. 1.
Establishment of cell lines transfected with pcDNA3.1-ING1b or ING1b siRNA. Analysis of ING1b mRNA and protein expression during osteoblast differentiation of C3H10T1/2 mesenchymal stem cells (A) and MC3T3-E1 preosteoblasts (B). Cells were cultured in an osteogenic differentiation medium, and ING1b expression was measured at days 0, 3, 7, and 9 after osteogenic differentiation induction. (C) ALP activity was measured during osteoblast differentiation of C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts using an ALP activity kit. *p < 0.05 versus day 0. Detection of ING1b expression in cells transfected with pcDNA3.1-ING1b or ING1b siRNA by RT-PCR (D) and Western blotting (E). *p < 0.05 versus control. ALP, alkaline phosphatase; ING, inhibitors of growth. RT-PCR, real-time polymerase chain reaction.
ING1b mediates ALP activity, Runx2 expression, and collagen type 1 synthesis
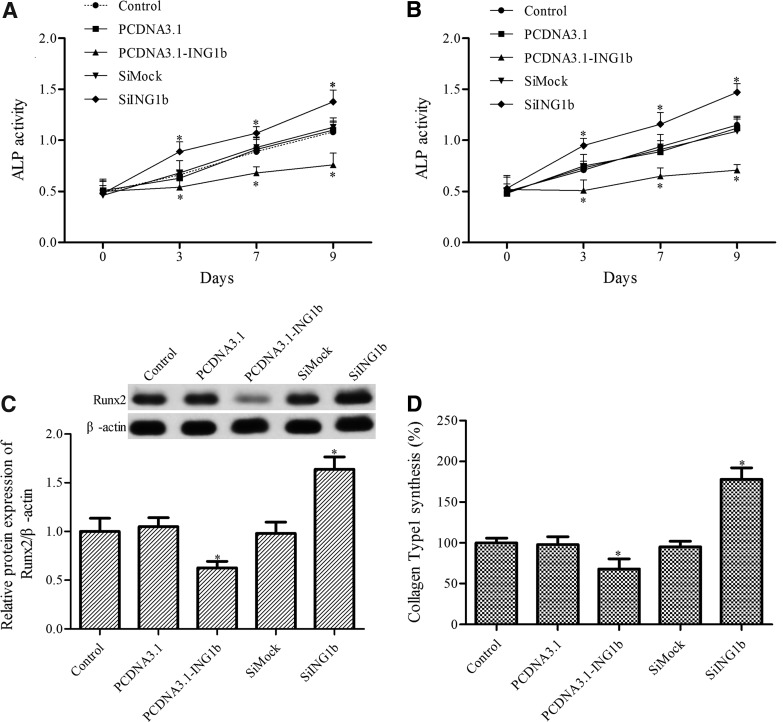
To detect the effect of ING1b overexpression and silencing on osteoblast differentiation, the activity of ALP was measured. ING1b overexpression markedly inhibited the ALP activity in both C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblast cells during osteoblast differentiation, whereas ING1b silencing increased ALP activity (Fig. 2A, B).
FIG. 2.
The role of ING1b in osteoblast differentiation. ALP activity was measured in pcDNA3.1-IG1b- or SiING1b-transfected C3H10T1/2 mesenchymal stem cells (A) and MC3T3-E1 preosteoblasts (B) after osteoblast differentiation using an ALP activity kit. (C) Western blotting was used to determine the level of Runx2 in MC3T3-E1 preosteoblasts after osteoblast differentiation. (D) The synthesis of collagen type 1 in MC3T3-E1 preosteoblasts after osteoblast differentiation was measured using the Prolagen-C kit. *p < 0.05 versus control. Runx2, runt-related transcription factor 2.
Runx2 and collagen type 1, two master regulators of skeletogenesis, can regulate the expression of several downstream genes associated with osteoblast differentiation (Ducy et al., 1997; Komori et al., 1997). Thus, we next determined the effect of ING1b overexpression or silencing on the level of Runx2 and collagen type 1 in MC3T3-E1 cells. Western blotting showed that ING1b overexpression inhibited Runx2 expression, whereas its silencing increased the level of Runx2 (Fig. 2C). ING1b overexpression also upregulated the synthesis of collagen type 1, and ING1b silencing downregulated the level of collagen type 1 (Fig. 2D).
ING1b regulates the expression of PPAR-β/δ in MC3T3-E1 cells in a HIF1α-dependent manner
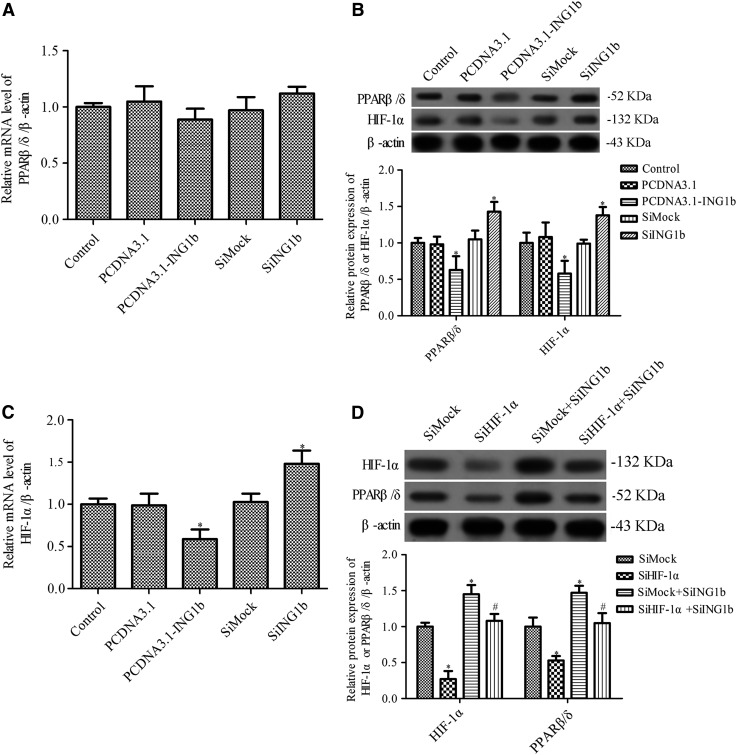
PPAR activity is involved in osteoblastic differentiation (Takano et al., 2012) and hypoxia modulation of HIF1α and PPARs in human glioblastoma stems cells (Galzio et al., 2012). Evidence has also suggested that ING1b negatively regulates HIF1α protein levels in adipose-derived stromal cells in a SUMOylation-dependent mechanism (Bigot et al., 2015). Thus, we hypothesized that ING1b regulates the expression of PPAR-β/δ in MC3T3-E1 cells in a HIF1α-dependent manner. RT-PCR and Western blotting were used to measure the expression of HIF1α and PPAR-β/δ after ING1b overexpression or silencing. ING1b silencing increased the expression of PPAR-β/δ at the protein level, whereas there was no significant change in the PPAR-β/δ mRNA level (Fig. 3A, B). ING1b overexpression significantly downregulated the expression of PPAR-β/δ at the protein level, but not the mRNA level (Fig. 3A, B). The mRNA and protein levels of HIF1α were significantly downregulated or increased by ING1b overexpression or inhibition, respectively (Fig. 3B, C). Furthermore, Western blotting showed that HIF1α siRNA inhibited the expression of PPAR-β/δ and HIF1α in ING1b-silenced cells (Fig. 3D). The results also showed that ING1b silence cannot rescue the expression of PPAR-β/δ after suppression of HIF1α (Fig. 3D). These results suggest that ING1b regulates the expression of PPAR-β/δ in MC3T3-E1 cells in a HIF1α-dependent manner.
FIG. 3.
The effects of ING1b overexpression or silencing on the level of PPAR-β/δ and HIF1α. The mRNA and protein expression of PPAR-β/δ (A, B) and HIF1α (B, C) was determined by RT-PCR and Western blotting, respectively. (D) Western blotting was used to measure the protein expression of PPAR-β/δ and HIF1α in SiHIF1-treated MC3T3-E1 preosteoblasts following osteoblast differentiation. *p < 0.05 versus control or SiMock group, #p < 0.05 versus SiMock+SiING1b group. HIF, hypoxia-inducible factor; PPAR, peroxisome proliferator-activated receptor.
ING1b regulates osteoblast differentiation by targeting PPAR-β/δ in a HIF1α-dependent manner
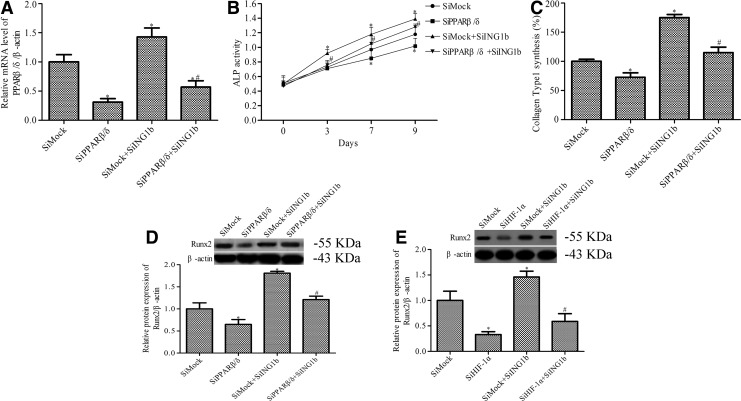
To further study the mechanism of ING1b regulation of osteoblast differentiation, we determined the effect of PPAR-β/δ silencing on the ALP activity, Runx2 expression, and collagen type 1 synthesis induced by ING1b silencing. We first silenced the expression of PPAR-β/δ and HIF1α by PPAR-β/δ siRNA and HIF1α siRNA, respectively (Figs. 3D and 4A). PPAR-β/δ silencing blocked the effect of ING1b silencing on the promotion of ALP activity (Fig. 4B). ING1b silencing-induced collagen type 1 (Fig. 4C) and Runx2 (Fig. 4D) expression were also inhibited by PPAR-β/δ silencing. To further demonstrate this effects is HIF1α dependence, the expression of Runx2 marker were also determined in HIF1α siRNA treated cell. As shown in Figure 4E, ING1b silencing induced Runx2 expression was also suppressed by HIF1α silencing.
FIG. 4.
The effects of PPAR-β/δ siRNA on ING1b-mediated osteoblast differentiation. (A) The PPAR-β/δ level in MC3T3-E1 cells was detected by RT-PCR. (B) ALP activity was measured using an ALP activity kit. (C) The synthesis of collagen type 1 was measured using the Prolagen-C kit. (D, E) Runx2 expression was detected by Western blotting in MC3T3-E1 preosteoblasts following osteoblast differentiation. *p < 0.05 versus SiMock group, #p < 0.05 versus SiMock+SiING1b group.
Discussion
The main findings in this study are as follows: (1) ING1b is decreased during osteoblast differentiation; (2) ING1b overexpression markedly decreased osteoblast differentiation, whereas ING1b silencing significantly promoted osteoblast differentiation; (3) ING1b can regulate the expression of PPAR-β/δ in a HIF1α-dependent manner. Overexpression of ING1b markedly decreased the HIF1α level, which resulted in a low level of PPAR-β/δ expression, while ING1b silencing significantly increased the expression of PPAR-β/δ and HIF1α; (4) silencing the expression of PPAR-β/δ and/or HIF1α significantly inhibited osteoblast differentiation marker ALP activity and Runx2 and collagen type 1 expression. Bone formation involves osteoblast differentiation, which is controlled by different important transcription factors. Our study was aimed to show the effects and mechanism of ING1b in osteoblast differentiation; thus, our study was choosing the preosteoblast cell line MC3T3-E1 and, before the study, cells were transferred to the differentiation medium to induce MC3T3-E1 cell differentiation. Our data demonstrate that ING1b is an important regulator of osteoblast differentiation.
Based on selection of gene fragments that can cause transformation and block the activity of tumor suppressors, ING1 was identified as a candidate tumor suppressor (Garkavtsev et al., 1996). ING1 is well conserved and encodes three protein isoforms: ING1a, ING1b, and ING1c (Saito et al., 2000; Chen et al., 2013). ING1b is the major isoform expressed in human tissues and cells and plays an important role in most functions of ING1 (Chen et al., 2013). ING1b can stimulate cell cycle arrest, apoptosis, senescence, and repair. ING1b overexpression results in cell cycle arrest at the G1 phase with ensuing cell apoptosis, whereas ING1b silencing increases cell growth in vitro and promotes tumorigenesis in vivo (Garkavtsev et al., 1996). More recently, Bigot et al. (2015) showed that hypoxia-induced downregulation of adipocyte differentiation was highly dependent on ING1b expression during the early days of this process. The authors indicated that ING1 silencing increased BGLAP, RUNX2, and SP7 levels, as well as calcium deposits at 7 or 14 days (Bigot et al., 2015). In accordance with these results, our data indicate that ING1b overexpression markedly decreased osteoblast differentiation marker ALP, Runx2, and collagen type 1 activity and expression, whereas ING1b silencing significantly increased osteoblast differentiation. ING1b level and the effects of ING1b on ALP activity in C3H10T1/2 mesenchymal stem cells and MC3T3-E1 preosteoblasts were similar; thus, we choose MC3T3-E1 preosteoblasts for further study. Our study showed that overexpression of ING1b is partly inhibited osteoblast differentiation and silencing its expression accelerates osteoblast differentiation. There needs to be further study to show overexpression of ING1b will stop the osteoblast differentiation or just slow it down, and we will do some experiments about this issue in our further studies.
Our data show that the function of ING1b in osteoblast differentiation is associated with the level of PPAR-β/δ. PPARs play an important role in bone metabolism and may be involved in the regulation of bone formation (Still et al., 2008). PPAR has three isotypes: PPAR-α, PPAR-β/δ, and PPAR-γ. Agonists of PPAR-α increase bone mass and promote osteoblast differentiation, whereas PPAR-γ agonists induce bone loss and inhibit osteoblast differentiation (Mosti et al., 2014). PPAR-β/δ agonists are known to influence skeletal muscle metabolism, and our study indicated that ING1b silencing increased the level of PPAR and promoted osteoblast differentiation.
Ample evidence indicates that ING1 can negatively regulate the level of HIF1 and that HIF1 can activate expression of a broad range of genes, including PPAR (Zhao et al., 2014; Bigot et al., 2015). Therefore, our study also measured the expression of HIF1α in MC3T3-E1 preosteoblast cells after osteoblast differentiation. ING1 overexpression significantly inhibited the level of HIF1α, and its silence markedly increased HIF1α expression. In addition, ING1b silence cannot rescue the expression of PPAR-β/δ after suppression of HIF1α. Our study also showed that ING1b regulates osteoblast differentiation by targeting PPAR-β/δ or HIF1α. These results demonstrated that ING1 affected osteoblast differentiation through suppression of PPAR expression in a HIF1-dependent manner.
Conclusion
In summary, our data suggest that suppressing PPAR-β/δ expression with ING1b in a HIF1-dependent manner inhibits osteoblast differentiation, while suppressing ING1b expression accelerates osteoblast differentiation, thus suggesting that ING1b may be a potential new therapeutic target for improving bone homeostasis.
Acknowledgment
Financial support was provided by the Projects of Science and Technology Department of Sichuan Province (2015SZ0167).
Disclosure Statement
No competing financial interests exist.
References
- Bialek P., Kern B., Yang X., Schrock M., Sosic D., Hong N., et al. (2004). A twist code determines the onset of osteoblast differentiation. Dev Cell 6, 423–435 [DOI] [PubMed] [Google Scholar]
- Bigot N., Guérillon C., Loisel S., Bertheuil N., Sensebé L., Tarte K., et al. (2015). ING1b negatively regulates HIF1α protein levels in adipose-derived stromal cells by a SUMOylation-dependent mechanism. Cell Death Disease 6, e1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G., Deng C., and Li Y.-P. (2012). TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci 8, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Tran U., Rajarajacholan U., Thalappilly S., and Riabowol K. (2013). ING1b-inducible microRNA203 inhibits cell proliferation. Br J Cancer 108, 1143–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Zhang R., Geoffroy V., Ridall A.L., and Karsenty G. (1997). Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89, 747–754 [DOI] [PubMed] [Google Scholar]
- Eriksen E., Langdahl B., and Kassem M. (1994). The cellular basis of osteoporosis. Spine 8, 23–62 [Google Scholar]
- Feng X., Hara Y., and Riabowol K. (2002). Different HATS of the ING1 gene family. Trends Cell Biol 12, 532–538 [DOI] [PubMed] [Google Scholar]
- Fung C.-H., Cheung W.-H., Pounder N.M., Harrison A., and Leung K.-S. (2014). Osteocytes exposed to far field of therapeutic ultrasound promotes osteogenic cellular activities in pre-osteoblasts through soluble factors. Ultrasonics 54, 1358–1365 [DOI] [PubMed] [Google Scholar]
- Galzio R., Cristiano L., Fidoamore A., Cifone M.G., Benedetti E., Cinque B., et al. (2012). Hypoxia modulation of peroxisome proliferator-activated receptors (PPARs) in human glioblastoma stem cells. Implications for therapy. J Cell Biochem 113, 3342–3352 [DOI] [PubMed] [Google Scholar]
- Garkavtsev I. (1999). Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat Genet 23, 373. [DOI] [PubMed] [Google Scholar]
- Garkavtsev I., Demetrick D., and Riabowol K. (1997). Cellular localization and chromosome mapping of a novel candidate tumor suppressor gene (ING1). Cytogenet Genome Res 76, 176–178 [DOI] [PubMed] [Google Scholar]
- Garkavtsev I., Grigorian I.A., Ossovskaya V.S., Chernov M.V., Chumakov P.M., and Gudkov A.V. (1998). The candidate tumour suppressor p33ING1cooperates with p53 in cell growth control. Nature 391, 295–298 [DOI] [PubMed] [Google Scholar]
- Garkavtsev I., Kazarov A., Gudkov A., and Riabowol K. (1996). Suppression of the novel growth inhibitor p33ING1 promotes neoplastic transformation. Nat Genet 14, 415–420 [DOI] [PubMed] [Google Scholar]
- Guérillon C., Larrieu D., and Pedeux R. (2013). ING1 and ING2: multifaceted tumor suppressor genes. Cell Mol Life Sci 70, 3753–3772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbing C.C., Veillette C., Riabowol K., Johnston R.N., and Garkavtsev I. (1997). A novel candidate tumor suppressor, ING1, is involved in the regulation of apoptosis. Cancer Res 57, 1255–1258 [PubMed] [Google Scholar]
- Hiraga T., Kizaka-Kondoh S., Hirota K., Hiraoka M., and Yoneda T. (2007). Hypoxia and hypoxia-inducible factor-1 expression enhance osteolytic bone metastases of breast cancer. Cancer Res 67, 4157–4163 [DOI] [PubMed] [Google Scholar]
- Kim S., Koga T., Isobe M., Kern B.E., Yokochi T., Chin Y.E., et al. (2003). Stat1 functions as a cytoplasmic attenuator of Runx2 in the transcriptional program of osteoblast differentiation. Genes Dev 17, 1979–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T., Yagi H., Nomura S., Yamaguchi A., Sasaki K., Deguchi K., et al. (1997). Targeted disruption of Cbfa1results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 [DOI] [PubMed] [Google Scholar]
- Mosti M.P., Stunes A.K., Ericsson M., Pullisaar H., Reseland J.E., Shabestari M., et al. (2014). Effects of the peroxisome proliferator-activated receptor (PPAR)-delta agonist GW501516 on bone and muscle in ovariectomized rats. Endocrinology 155, 2178–2189 [DOI] [PubMed] [Google Scholar]
- Mullender M., Van der Meer D., Huiskes R., and Lips P. (1996). Osteocyte density changes in aging and osteoporosis. Bone 18, 109–113 [DOI] [PubMed] [Google Scholar]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. (2001). Osteoporosis prevention, diagnosis, and therapy. JAMA 285, 785–79511176917 [Google Scholar]
- Park S.J., Jung S.-H., Jogeswar G., Ryoo H.-M., Yook J.I., Choi H.S., et al. (2010). The transcription factor snail regulates osteogenic differentiation by repressing Runx2 expression. Bone 46, 1498–1507 [DOI] [PubMed] [Google Scholar]
- Reffitt D., Ogston N., Jugdaohsingh R., Cheung H., Evans B., Thompson R., et al. (2003). Orthosilicic acid stimulates collagen type 1 synthesis and osteoblastic differentiation in human osteoblast-like cells in vitro. Bone 32, 127–135 [DOI] [PubMed] [Google Scholar]
- Saito A., Furukawa T., Fukushige S., Koyama S., Hoshi M., Hayashi Y., et al. (2000). p24/ING1-ALT1 and p47/ING1-ALT2, distinct alternative transcripts of p33/ING1. J Hum Genet 45, 177–181 [DOI] [PubMed] [Google Scholar]
- Semenza G.L. (2003). Targeting HIF-1 for cancer therapy. Nat Rev Cancer 3, 721–732 [DOI] [PubMed] [Google Scholar]
- Still K., Grabowski P., Mackie I., Perry M., and Bishop N. (2008). The peroxisome proliferator activator receptor alpha/delta agonists linoleic acid and bezafibrate upregulate osteoblast differentiation and induce periosteal bone formation in vivo. Calcif Tissue Int 83, 285–292 [DOI] [PubMed] [Google Scholar]
- Takano M., Otsuka F., Matsumoto Y., Inagaki K., Takeda M., Nakamura E., et al. (2012). Peroxisome proliferator-activated receptor activity is involved in the osteoblastic differentiation regulated by bone morphogenetic proteins and tumor necrosis factor-α. Mol Cell Endocrinol 348, 224–232 [DOI] [PubMed] [Google Scholar]
- Utting J., Robins S., Brandao-Burch A., Orriss I., Behar J., and Arnett T. (2006). Hypoxia inhibits the growth, differentiation and bone-forming capacity of rat osteoblasts. Exp Cell Res 312, 1693–1702 [DOI] [PubMed] [Google Scholar]
- Vieyra D., Toyama T., Hara Y., Boland D., Johnston R., and Riabowol K. (2002). ING1 isoforms differentially affect apoptosis in a cell age-dependent manner. Cancer Res 62, 4445–4452 [PubMed] [Google Scholar]
- Wagegg M., Gaber T., Lohanatha F.L., Hahne M., Strehl C., Fangradt M., et al. (2012). Hypoxia promotes osteogenesis but suppresses adipogenesis of human mesenchymal stromal cells in a hypoxia-inducible factor-1 dependent manner. PLoS One 7, e46483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Wan C., Deng L., Liu X., Cao X., Gilbert S.R., et al. (2007). The hypoxia-inducible factor α pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117, 1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J., Shi Y., Zheng L., Zhou B., Inose H., Wang J., et al. (2012). miR-34s inhibit osteoblast proliferation and differentiation in the mouse by targeting SATB2. J Cell Biol 197, 509–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y.Z., Liu X.L., Shen G.M., Ma Y.N., Zhang F.L., Chen M.T., et al. (2014). Hypoxia induces peroxisome proliferator-activated receptor gamma expression via HIF-1-dependent mechanisms in HepG2 cell line. Arch Biochem Biophys 543, 40–47 [DOI] [PubMed] [Google Scholar]