Abstract
Significance: Cutaneous wound healing is a serious problem worldwide that affects patients with various wound types, resulting from burns, traumatic injuries, and diabetes. Despite the wide range of clinically available skin substitutes and the different therapeutic alternatives, delayed healing and scarring are often observed.
Recent Advances: Stem cells have arisen as powerful tools to improve skin wound healing, due to features such as effective secretome, self-renewal, low immunogenicity, and differentiation capacity. They represent potentially readily available biological material that can particularly target distinct wound-healing phases. In this context, mesenchymal stem cells have been shown to promote cell migration, angiogenesis, and a possible regenerative rather than fibrotic microenvironment at the wound site, mainly through paracrine signaling with the surrounding cells/tissues.
Critical Issues: Despite the current insights, there are still major hurdles to be overcome to achieve effective therapeutic effects. Limited engraftment and survival at the wound site are still major concerns, and alternative approaches to maximize stem cell potential are a major demand.
Future Directions: This review emphasizes two main strategies that have been explored in this context. These comprise the exploration of hypoxic conditions to modulate stem cell secretome, and the use of adipose tissue stromal vascular fraction as a source of multiple cells, including stem cells and factors requiring minimal manipulation. Nonetheless, the attainment of these approaches to target successfully skin regeneration will be only evident after a significant number of in vivo works in relevant pre-clinical models.

Alexandra Pinto Marques, PhD
Scope and Significance
From a physiological perspective, effective skin wound healing still represents a major concern for global healthcare, as the currently available skin substitutes and alternative therapeutics lead to unsatisfactory results. This problematic affects a wide range of patients with various wound types resulting from burns, traumatic injuries, and diabetes, where delayed healing and scarring is a reality. In the past few years, new insights into the wound-healing process triggered the development of more sophisticated strategies that take advantage of specific performers such as artificial extracellular matrix (ECM)-like matrices, growth factors, and primarily stem cells.
Translational Relevance
Endogenous stem cells are vital players in the well-coordinated cell-signaling cascades of wound healing. From a therapeutic perspective, their mobilization to the wound site has been suggested; however, insights into their mechanism of action are particularly difficult to attain. This has been hampering clinically relevant outcomes, thus supporting the exploitation of the therapeutic action of exogenous stem cells. Whether these can act as building blocks and/or potent secretome units is deeply dependent on the cell source and on the administration strategies. Both effects have been shown to significantly impact wound healing, targeting wound re-epithelialization, hair follicle (HF) formation, and neovascularization.
Clinical Relevance
Despite all the pre-clinical studies using exogenous stem cells in a wound-healing context, translation into clinical trials is fairly recent. The majority of these focus on the use of bone marrow mesenchymal stem cells (BM-MSCs) mostly to treat chronic wounds. Alternatively, adipose stem cells (ASCs) potential to treat burn wounds and diabetic foot/venous ulcers is currently being evaluated, addressing variables such as cell number, administration mode, and wound area. The knowledge acquired from these trials is expected to lead the development of hybrid constructs as “engineered regenerative platforms” to actively encourage skin wound regeneration.
Stem cell involvement in wound-healing phases
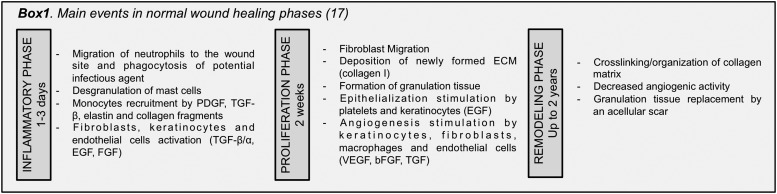
Wound healing is a complex dynamic response to a physical trauma that comprises three overlapping phases: inflammatory, proliferative, and remodeling, extensively reviewed elsewhere1,2 (Box 1 for a short overview of the main events). Their progression depends on a well-coordinated interplay of cell-signaling events at the wound site and surrounding tissues,2 in which endogenous stem cells are vital players. These include HF bulge3–5 and dermal sheath stem cells (DSCs),6 as well as mesenchymal stem cells (MSCs) such as BM-MSCs7–9 and speculatively ASCs,10 as very little is known in terms of its endogenous role during wound healing. These cells are located within distinct skin niches that are divided mainly in epidermal and dermal niches.11
During both embryonic development and adult homeostasis, epidermis and HF are distinct compartments formed by independent stem cell populations. HF, in particular, does not contribute to interfollicular epidermis maintenance.3 However, several authors revealed3–5 that after wounding, HF bulge stem cells rapidly migrate toward the interfollicular epidermis, generating short-lived transient-amplifying cells that promote re-epithelialization. Contrarily, DSCs surrounding HF units ensure dermal papilla cell maintenance and are involved in dermal repair by acquiring a myofibroblastic phenotype.6 Despite the compartmentalized structure of skin, paracrine signaling and cell–matrix communication are determinant to modulate an efficient wound-healing response.12 Details on the internal mechanisms of action within skin stem cell niches in a wound-healing scenario were recently reviewed elsewhere,11–14 and they are not the focus of this review.
In this context, the mechanism of action of endogenous stem cells outside the niche is particularly difficult to understand. The disclosure of their contribution in skin wound healing has been hampered by the use of rodent models, mechanistically different from humans, as well as by the lack of more sophisticated cell-tracking methodologies. Moreover, the role of BM is controversial due to its complex set of phenotypes and location, far from the cutaneous wound site.9 Nonetheless, it is known that BM-MSCs contribution to cutaneous wound healing is substantially greater than the previously recognized sub-population CD45+, which is composed by BM hematopoietic cells and antigen-presenting fibroblasts.15 Additional evidence showed that the contribution of BM-MSCs goes beyond early inflammation, contrary to what was initially believed.16 These cells are maintained in the newly formed dermis playing a substantial role in the formation of new connective tissue through the production of collagen I and III.7,15 More recently, further indications related to MSCs recruitment from bone marrow to participate in wound healing were demonstrated based on fetal microchimerism.9 However, the role of other endogenous MSCs in skin wound healing remains unknown. Therefore, despite the indications provided by exogenous application of ASCs on the regulation of blood vessels formation through a crosstalk with endothelial cells,10 studies that address its endogenous role for skin hemostasis/healing are still missing.
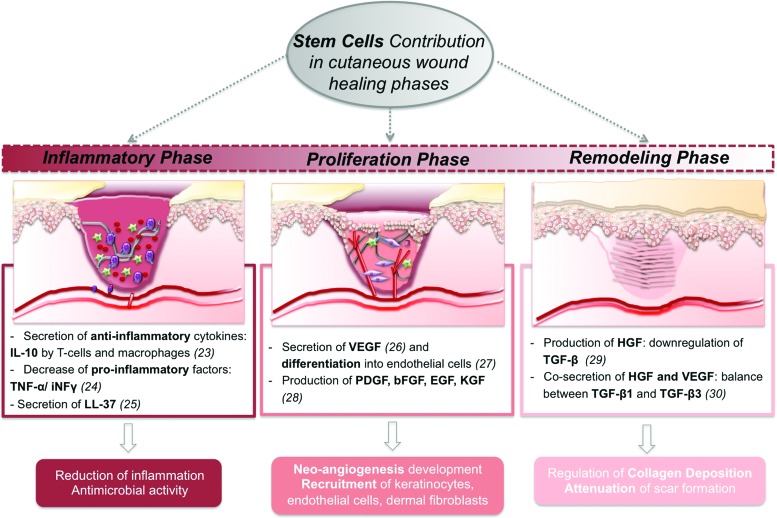
Despite this limited knowledge, local stem cell mobilization17,18 has been proposed as an answer to the low engraftment rate of transplanted exogenous stem cells.19 Nonetheless, it is important to highlight that these mechanisms have been studied in rodents and that the translational settings toward humans might represent a long leap for a clinical situation. Thus, the most followed approach for exploiting the therapeutic action of stem cells in the wound healing has been the application of exogenous MSCs at the wound site and their potential has been reviewed by several authors.14,20,21 They can regulate specific events that occur along the different phases of the wound-healing cascade, as reviewed by Maxson et al.22 and summarized in Fig. 1,23–30 opening distinct possibilities toward an effective healing.
Figure 1.
Schematic overview of stem cell potential in the wound-healing scenario, along with their different mechanisms of action and potential outcome to target different stages, such as inflammation, proliferation, and remodeling. IL-10, interleukin 10; TNF-α, tumor necrosis factor alpha; INFγ, interferon gamma; VEGF, vascular endothelial growth factor; PDGF, platelet-derived growth factor; bFGF, basic fibroblast growth factor; EGF, epidermal growth factor; KGF, keratinocyte growth factor; HGF, hepatocyte growth factor; TGF-β, transforming growth factor beta. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Stem cells as building blocks and secretome units (771/8796)
Building blocks
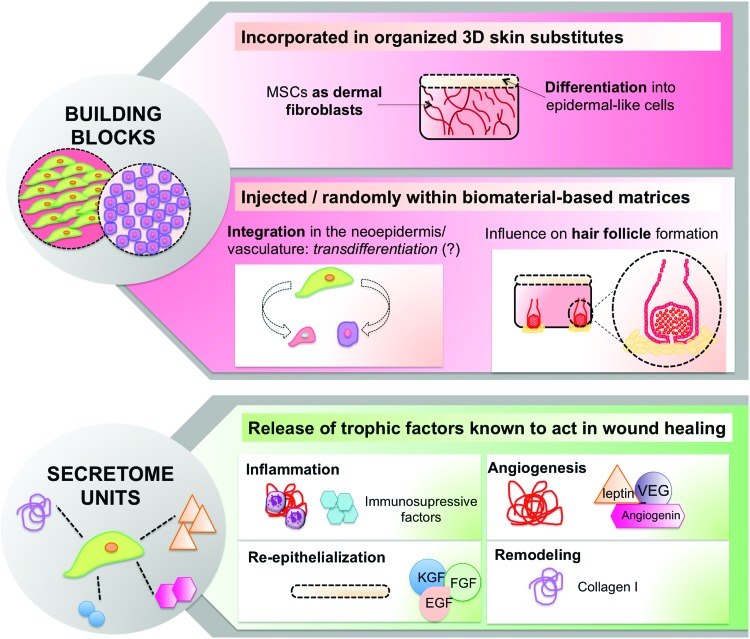
Autologous skin substitute application in large full-thickness wounds/burns is hampered by the scarcity of biological material.31 To counter this, different MSCs have started being used to replace dermal fibroblasts (DFbs).32–34 Tonsil-derived MSCs in a collagen-based dermal-epidermal construct promoted epidermal stratification, cornification, and pigmentation comparable to human dermal fibroblasts (hDFbs), after transplantation in immunocompromised rats.32 A similar outcome was found when the same cells were incorporated in a decellularized dermis and transplanted into a laser injury in humanized skin graft cultures.33 However, unlike ASCs,35 few studies detail the isolation procedures and expansion potential of tonsil-derived MSCs, hindering, for now, their positioning as a valid stem cell source. Epidermal-like cells and adipocytes generated from human adipose stem cells (hASCs) obtained from the debridement of burned patient's wounds34 were used to recreate the epidermal and hypodermal layers of a skin analogue where the hDFbs of the dermal-like layer are replaced by hASCs. Still, in vivo data are needed to validate such constructs for cutaneous wound healing.
The works described earlier32–34 represent the few stem cell-based alternatives to three-dimensional (3D) organized dermo-epidermal substitutes, highlighting the question whether it is necessary to recreate a 3D substitute with the classical organization of the skin tissue to improve wound healing. Considering the different number of works using stem cells either injected at the wound borders15,36 or randomly incorporated within biomaterial-based matrices,37–42 there is skepticism regarding the need for 3D organization. Nonetheless, this discrepancy can be correlated with the limitations of MSC differentiation toward epithelial and endothelial lineages,43 as well as to the deficient purity/stability of epidermal stem cells cultures.43 Stem cells from distinct sources, including MSCs, have shown a strong effect in HF formation.37,39,44,45 In our recently published work,37 the enhanced HF formation appears to be associated with keratinocyte growth factor (KGF) overexpression resulting from a direct interaction between resident keratinocytes (KCs) and transplanted hASCs cell sheet constructs. MSCs mechanisms that improve HF formation are, however, still elusive.45 The identification of transplanted MSCs in the neoepidermis27,42,46 has been supporting the hypothesis of transdifferentiation into epidermal cells. However, despite the set of in vitro studies,47–49 this hypothesis is still poorly explored. Transdifferentiation of MSCs into endothelial cells after transplantation into cutaneous wounds is also supported by few works showing mouse27,40 and human BM-MSCs-derived endothelial cell integration in the neo-vasculature. Interestingly, these findings were also associated to a pericytic phenotype of undifferentiated transplanted cells also described by others45 and in accordance to the reports that associate MSCs with perivascular niches and close/common origin with pericytes.50,51
Despite being able to act as building blocks, stem cells rich secretome (discussed in the next section “Secretome units”) appears to be the major tool to modulate functional regeneration (Fig. 2).
Figure 2.
Schematic perspective of stem cells as powerful tools in skin wound-healing improvement. Special highlight provided on their contribution as building blocks and as potent secretome units at different responses levels. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
Secretome units
MSCs secretome has been analyzed to assess stem cell mechanisms of action during the wound-healing process. BM-MSCs52 and hASCs53 secretome is immunosuppressant, modulating secretion of factors such as interleukin (IL)-6/10 and tumor necrosis factor alpha, as well as dendritic cell functions. Curiously, BM-MSCs-conditioned medium (CM) improved macrophage recruitment by potentiating secretion of macrophage inflammatory protein-1alpha/beta and erythropoietin.26 BM-MSCs,26,54 hASCs,55 and umbilical cord mesenchymal stem cells56 were also shown to release KGF, fibroblast growth factor 2, and epidermal growth factor. These promoted KCs migration in vivo, significantly influencing wound re-epithelialization.26,55,56 Likewise, the impact on wound angiogenesis/vascularization at the wound site was also associated to vascular endothelial growth factor (VEGF) secreted by mouse bone marrow mesenchymal stem cells26 and hASCs.55 Other important regulators of angiogenesis, namely angiogenin, VEGF-A, and leptin, were also identified in hASCs, human bone marrow mesenchymal stem cells, DSCs, and dermal papilla cells (DPCs) secretome.54 While angiogenin and VEGF-A secretion levels were comparable within MSCs populations, DSCs and DPCs released a significantly higher amount of leptin. These angiogenic features were confirmed in excisional26,57 and chronic wounds58 healing, and they can be maximized by playing with the oxygen concentration, as discussed in section “Hypoxia.” MSCs secretome can further act in the remodeling phase of cutaneous wound healing. hASCs-CM is shown to promote the synthesis of collagen I by DFbs,55,59,60 thus influencing wound closure. This effect on DFbs was also observed when human amniotic fluid MSCs-CM61,62 was administrated in a mouse excisional wound, suggesting their action via the transforming growth factor beta (TGF-β)/SMAD2 pathway.62
Overall, these data demonstrate that MSCs from different sources have a potent secretome that is capable of influencing different cells involved in the wound-healing process, modulating their activation, migration, and proliferation.
Clinical placement
Clinical cases and trials
Despite all pre-clinical wound-healing studies, translation of stem cell-based strategies in the clinical settings is still reduced. Most of the clinical trials/case studies comprise the use of BM and adipose tissue (AT) MSCs, to treat various wound types, namely chronic wounds as recently revised by Maxson et al.22 BM-MSCs, still considered the gold standard for several applications, have a history of successful healing of chronic wound ulcerations,63,64 despite reported poor cellular engraftment. Therefore, new delivery strategies such as the use of a fibrin spray are currently under trial (NCT01751282). Alternatively, three different registered clinical trials (Table 1) using hASCs to treat burn wounds diabetic foot/venous ulcers are being undertaken. Variables such as cell number (NCT02092870), administration mode, including incorporation in platelet-rich plasma (2012-001596-36) or spraying (2009-016365-29) versus injection, and site of administration (NCT02092870) are being addressed.
Table 1.
Key registered clinical trials/case studies of stem cell-based strategies for skin wound healing
| Cell Type | Design | Wound Type | Time Frame | Status/Outcome | Reference |
|---|---|---|---|---|---|
| Allogeneic BM-MSCs | Injection: test of four different doses | Second-degree burns (<20% TBSA) | No less than 52 weeks | Not yet recruiting | NCT02104713a |
| Autologous BM-MSCs | Forty injections of CD34+ cells | Chronic critical limb ischemia, diabetic foot | ≈17 weeks | Therapy resulted in 79% limb salvage | NCT01232673a,36 |
| Autologous BM-MSCs | BM-MSCs in fibrin spray | Non-healing | 24 weeks | Ongoing | NCT01751282a |
| hASCs | Multiple injections within and periphery of the wound | Diabetic foot venous/pressure ulcer | ≈10 weeks | Recruiting | NCT02092870a |
| hASCs | Injection (3–8×106cells) and incorporation in PRP matrix | Burns | Not defined | Ongoing | 2012-001596-36b |
| hASCs | Injection and spraying (4.5×106 cells) | Burns | Not defined | Ongoing | 2009-016365-29c |
| SVF | Injection | Venous ulcers | Not defined | Not yet recruiting | d |
| Autologous lipoaspirate | Injection | Diabetic/venous stasis wounds | 52 weeks | Unknown | NCT00815217a |
| Autologous hair-follicles | 20 hair grafts/2×2 cm2 ulcers | Chronic leg ulcers | 18 weeks | Ulcer area reduction, enhanced epithelialization, neovascularization, dermal reorganization | 66 |
| Follicular epidermal stem cells | Fully differentiated autologous epidermal equivalent | Recalcitrant vascular leg ulcers | — | Healing and complete closure | 67 |
Identifier on US clinical trials website: http://clinicaltrials.gov/results for: stem+cells+wounds.
Identifier on EU clinical trials website: http://clinicaltrialsregister.eu/results for: stem+cells+wounds.
Identifier on EU clinical trials website: http://clinicaltrialsregister.eu/results for: burn+mesenchymal stem cells.
Australian Clinical trials website: http://australianclinicaltrials.gov.au/.
BM-MSCs, bone marrow mesenchymal stem cells; TBSA, total body surface area; hASCs, human adipose stem cells; PRP, platelet-rich plasma; SVF, stromal vascular fraction.
In a different perspective, other tissues/fractions such as lipoaspirate and stromal vascular fraction (SVF) that fit “minimal manipulation” criteria, possibly with the rationale of particularly tackling the lack of vasculature of venous ulcers, are being tested (Box 1). In the same context, autologous scalp HF grafts were directly transplanted into chronic leg ulcers65 as healing units. Although yielding promising results, limitations such as sample size, absence of stratification in the whole ulcer area, and difficult follow-up due to fibrotic healing in some patients, among others, were also identified.65
Interestingly, with the exception of BM-MSCs delivered in fibrin, none of the most recent trials has tested the combination of stem cells with biomaterial-based matrices. Apart from an earlier trial that confirmed that autologous epidermal equivalents engineered from outer root sheath KCs (EpiDex™) were safe and as effective as a standard mesh graft in the treatment of recalcitrant vascular leg ulcers,66 new skin analogues have not reached clinical trials/case studies. However, artificial dermis with non-cultured autologous hASCs and exogenous basic fibroblast growth factor (bFGF) showed a high degree of success in the healing of patients who suffered chronic radiation injuries.67
Overview of regulatory aspects for clinical application
Stem cell-based products (apart some exceptions) are advanced therapy medicinal products (ATMPs). These products imply “substantial manipulation” of tissues/cells, resulting in biological/physiological function alteration.68 Cell products with substantial or minimal manipulation are distinguished by their regulatory systems. In vitro cultivation of isolated cells represents substantial manipulation, while the simple isolation of BM mononuclear cells or SVF for an autologous approach enters the minimal manipulation category. Regulatory requirements for these minimally manipulated products are similar to the ones for blood banking products. This allows their preparation in suitable transfusion or blood banking facilities.69
The ATMP regulation has been differently managed worldwide; the EU and the United States have implemented new systems for the regulation of ATMPs that intend to merge, as much as possible, the regulatory requirements worldwide.70 In the EU, the European Medicines Agency (EMA) established a Committee for Advanced Therapies (CAT) (in accordance to 1394/2007). The CAT has the responsibility to analyze each ATMP application submitted to EMA. The approval process also requires the Committee for Medicinal Products for Human Use feedback, whose decision is subsequently formalized by the European Commission that binds all EU member states. In the United States, applications are directed to the Food and Drug Administration (FDA), the Office of Cellular, Tissue, and Gene Therapies, and the Center for Biologics Evaluation and Research; the review process is conducted. Nonetheless, FDA has very much adopted a case-by-case analysis of ATMPs with little product “class” guidance to instruct compliance.
Maximization of stem cell potential in wound healing
Despite all the hype on stem cells' therapeutic application for cutaneous wound healing, it is clear that there is still significant room for improvements. A new trend of the field has been following strategies that can target mechanistic features of a skin wound-healing microenvironment to maximize the performance of stem cell-based approaches.
Hypoxia
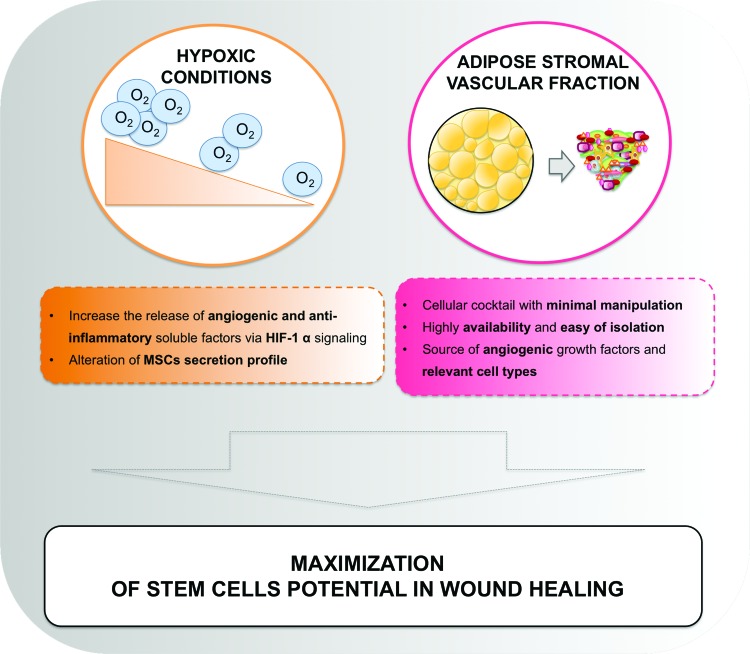
In most of the human tissues, the oxygen (O2) concentration that cells sense is significantly lower than the atmospheric concentration, or normoxia (20–21%, v/v), and it varies from 14% in highly perfused tissues to 1% or less in the bone marrow.71 Moreover, it is widely known that a wounded tissue is hypoxic due to the disruption of the blood vessels and increased O2 consumption by the local cells.72 Because “hypoxia” denotes a state where the O2 concentration to which a cell is subjected is lower than that at the cell native site or niche, it is a relative term. Under the scope of this review, hypoxia refers to O2 concentration lower than the atmospheric one.
The O2 concentration is an integral part of the stem cells' niche71; BM-MSC, in particular, are subjected to physiologic O2 concentrations between 1% and 3%.73 hASCs can face from 5% to 9%, but some controversy regarding these values can be found.74 Cells respond to O2 variations mainly through the action of the hypoxia-inducible factor (HIF)-1α.75,76 This highly conserved protein is degraded at atmospheric O2 concentrations77 but stable in hypoxic conditions, promoting the activation of target genes76,77 such as the angiogenesis- and the wound healing-related genes VEGF, platelet-derived growth factor, TGF-β3, TGF-β1, stromal cell-derived factor 1, and bFGF.75,78–83
Despite the limited attention given to hypoxia culture, an increasing number of works demonstrate that MSCs cultured under low O2 concentrations have improved proliferation, clonogenicity, survival, and, notably, an enhanced secretome.
Hypoxia and wound healing
As referred in section “Stem cells as building blocks and secretome units (771/8796),”, the major effect of stem cells on skin healing has been associated to paracrine signaling either directly or through their CM. Thus, considering the impact of hypoxic conditions in the nature of their secretome, it is likely that their healing potential can be modulated under those conditions. Chen et al.84 demonstrated that hypoxic culture (2% O2) increases the expression and secretion of bFGF, VEGF-A, and IL-6/8 in mouse BM-MSCs, subsequently inducing proliferation and migration of KCs, fibroblasts, endothelial cells, and monocytes. This was also confirmed in vivo after topical application of CM from hypoxia-cultured BM-MSC in full-thickness excisional wounds. In comparison to the use of CM from BM-MSC cultured in normoxia, increased cell proliferation, neovascularization, as well as recruitment of inflammatory macrophages were observed. Increased amounts of VEGF and bFGF in the CM of BM-MSC cultured in hypoxia were suggested to stimulate those processes that will, ultimately, lead to enhanced skin wound healing, although increased skin contraction was also observed. While the involvement of VEGF and bFGF was similarly reported by Lee et al.85 using CM of hASC cultured in hypoxic conditions (2% O2), contrarily to what is stated by the authors, the effect of neutralizing antibodies to VEGF and bFGF was not reflected in the reduced wound area, and it was most likely directly correlated with rodent skin contraction. Interestingly, Frazier et al.86 analyzed the secretome of hASCs cultured in hypoxia (5% O2) and observed that low oxygen conditions may favor a fibrosis-inhibiting, regeneration-promoting immunoregulatory role of hASCs in wound healing. In addition, the use of a splinted wound imprinting control region mouse model, where the effect of wound contraction in wound closure is eliminated,87 confirmed enhanced wound closure in animals treated with CM of amniotic fluid-derived MSC (AF-MSCs) cultured in hypoxia (1% O2) versus normoxia. CM of hypoxic AF-MSCs CM contained elevated levels of VEGF and TGF-β1 that induced hDFb migration in vitro. This effect was reversed by the inhibition of TGF-β/SMAD2 and PI3K/AKT pathways, suggesting that wound-healing enhancement by AF-MSC hypoxia CM happens, at least partially, via increased fibroblast recruitment and migration.
Overall, these works show how culture in hypoxia can alter the MSC secretion patterns, likely to have a paracrine effect on other cells and tissues and impact the wound-healing process at different stages. Hypoxia culture seems to be able to modulate inflammatory cell recruitment due to strong secretion of immunomodulatory molecules such as programed death ligand-1 and indoleamine 2,3-dioxygenase,74 which will positively impact the inflammatory phase of wound healing. Furthermore, increased secretion of VEGF and bFGF in the hypoxic CM strongly suggests that the proliferative wound-healing stage is affected by the consequent increase in neo-angiogenesis by paracrine signaling75,83 independently of the MSC source. In opposition, the fibrosis-inhibiting signaling might be dependent on both the O2 concentration and the cell type, as a balance between TGF-β1 and TGF-β3 is needed to achieve skin regeneration rather than fibrosis and scarring (Fig. 3). However, to effectively harness this potential of hypoxic-cultured stem cells, appropriate ways to deliver cells to the wound site will have to be developed. As shown in Table 1, there is a multitude of strategies to deliver cells to the wound site, ranging from injection to spraying or incorporation in a matrix. An incorrect strategy may not translate the full potential of the cells. Therefore, for each particular clinical setting, specific approaches must be defined to allow hypoxic-cultured cells to maintain a favorable secretome that results in improved healing or regeneration.
Figure 3.
Overview of strategies to maximize stem cell application/potential in the wound-healing context. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/wound
AT stromal vascular fraction
As described in section “Overview of regulatory aspects,” despite the attractiveness of hASCs for cutaneous healing,88 their use is associated with complex regulatory issues.68 In opposition, the use of SVF, a heterogeneous mixture of 30–40% of ASCs and endothelial cells, smooth muscle cells, pericytes, fibroblasts, mast cells, and preadipocytes,89 enters the “minimal manipulation” category, facilitating clinical applications. The SVF unique composition has been posed as beneficial to enhance the ASCs regenerative potential, offering unique benefits in cutaneous wound-healing applications.55,90 However, few clinical studies directly compare the administration of cultured hASCs versus SVF and additional data are still needed to reinforce the current pre-clinical findings.
Current data show not only an effect of SVF on collagen synthesis91 but also its strong angiogenic potential.92,93 Very recently, Klar et al. suggested that SVF possesses similar percentages of stromal and endothelial cells, demonstrating that when incorporated in 3D fibrin or collagen, SVF cells were capable of de novo formation of microvascular networks, causing a rapid anastomosis with the host vasculature and a sustained epidermal coverage.92 Moreover, when applied to burn wounds, increased VEGF production and reduced inflammation was observed.93 Likewise, in a scenario of diabetic wounds, VEGF and bFGF expression was also increased,94 accelerating the wound-healing process.
The several clinical studies using SVF to treat several conditions of very distinct diseases reviewed elsewhere95,96 suggest that this stromal cell fraction is safe and can be efficiently transplanted in either an autologous or allogeneic manner. However, as mentioned earlier, to our knowledge only one trial is addressing the effectiveness of autologous SVF under the scope of skin wound healing in the treatment of venous ulcers.
Future Trends
A new era in skin wound healing and regeneration has risen in the past few years having stem cells as central players. MSCs from bone marrow, AT, AF, and umbilical cord matrix are among the most explored. Their role is being discussed from two intimately connected viewpoints: as building blocks of the newly formed tissue due to their (trans)differentiation ability and as potent secretory units that can modulate the wound-healing cell-signaling pathways. However, while transdifferentiation mechanisms are poorly dissected, the interest in MSCs secretome has been increasingly rising. The spatiotemporal regulation of MSCs secretome in the context of the different wound types is, nevertheless, not achieved.
This uncertainty, as well as the reduced control of the artificial hybrid microenvironments that are being proposed, is greatly hampering the translation of stem cell-based strategies. Moreover, pre-clinical validation using closer to human models comprises a crucial step to support this translation. The mouse skin full-thickness wound model is the most used animal model, which, regardless of its contribution and convenience, presents limitations resulting from healing dissimilarities between humans and rodents.97 While in rodents the wound closure occurs mainly via contraction, human skin is tethered to subcutaneous tissues and wounds heal by granulation tissue formation and re-epithelialization. Due to the anatomic similarities with the human skin as well as to the identical mechanisms of healing, the porcine model has been described as the most suitable pre-clinical model in the cutaneous wound context.98
Advances in material science opened up the possibility of specifically tailoring a microenvironment to modulate cell behavior,99 namely by incorporating cell-adhesion motifs,100 cell-signaling elements,101–104 and genetic encoding sequences.105–107 However, the sophistication of the designed matrices is still not satisfactory. Likewise, the understanding/control of biomechanical mechanisms at the wound site through mechanically tailored materials, minimizing scar tissue formation, has been underexplored.108 Thus, the sustained knowledge acquired from the studies on the wound-healing processes/events is expected to lead the development of hybrid constructs as “engineered regenerative platforms” to actively encourage skin wound regeneration. Sophisticated techniques that allow combining stem cells with a maximized potential, within a matrix capable of mimicking different aspects of the natural ECM, will certainly have a significant impact. In this context, 3D biofabrication appears as a breakthrough technology in the field of tissue engineering that offers advantages such as the automated creation of hybrid structures that differently combine living and non-living materials in a pre-defined 3D micro-organization.108–110 Biofabrication strategies for skin regeneration/repair are limited to the creation of skin grafts consisting of layered fibroblasts and KCs embedded in collagen,111,112 and more recently AF-MSCs within fibrin-collagen.113 So far, major limitations have been associated to the materials' features that have to comply not only with the fabrication requirements but also with cellular phenotypic demands. But ultimately, the design of engineered regenerative platforms in which the placement of different cellular units is within a specific microenvironment, potentially biofunctional and mechanically adjusted, will lead the path to regenerate skin with compartments, such as HF and other appendages.
Take-Home Messages.
• Endogenous stem cells are recruited to the wound site, triggering specific responses in the different healing phases but approaches that are capable of precisely controlling cell recruitment are yet to be defined.
• Exogenous stem cells from distinct sources have demonstrated potential therapeutic action, often limited due to poor cellular engraftment and low transdifferentiation ability.
• Despite all the studies conducted at a research level with exogenous stem cells, this trend has just recently started being translated into clinical trials.
• Alternative approaches to maximize stem cell potential that goes beyond a single cell's injection are a major demand to tackle reduced engraftment and to tailor their secretome.
• The use of hypoxia and the exploration of SVF have been proposed to maximize stem cell potential.
• Cultured MSCs in hypoxia have shown increased proliferation, survival, and, more importantly, a healing-driven secretome.
• Minimal cell manipulation to reduce regulatory issues has been posed as an advantage of SVF, whose angiogenic potential and enhanced effect on collagen production are being reported.
• Sophisticated techniques that allow combining maximized stem cells within a matrix that is capable of mimicking different aspects of the natural ECM will lead the path to regenerate skin.
Abbreviations and Acronyms
- 3D
three-dimensional
- AF-MSC
amniotic fluid-derived MSC
- ASC
adipose stem cell
- AT
adipose tissue
- ATMP
advanced therapy medicinal product
- bFGF
basic fibroblast growth factor
- BM-MSC
bone marrow mesenchymal stem cell
- CAT
Committee for Advanced Therapies
- CM
conditioned medium
- DFb
dermal fibroblast
- DPC
dermal papilla cell
- DSC
dermal sheath stem cell
- EGF
epidermal growth factor
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
- hASC
human adipose stem cell
- HF
hair follicle
- HGF
hepatocyte growth factor
- HIF
hypoxia inducible factor
- IL
interleukin
- INFγ
interferon gamma
- KC
keratinocyte
- KGF
keratinocyte growth factor
- MSC
mesenchymal stem cell
- PDGF
platelet-derived growth factor
- PRP
platelet-rich plasma
- SVF
stromal vascular fraction
- TBSA
total body surface area
- TGF-β
transforming growth factor beta
- TNF-α
tumor necrosis factor alpha
- VEGF
vascular endothelial growth factor
Acknowledgments and Funding Sources
The authors would like to acknowledge “RL3–TECT—NORTE-01-0124-FEDER-000020” cofinanced by North Portugal Regional Operational Program (ON.2—O Novo Norte), under the National Strategic Reference Framework (NSRF), through the European Regional Development Fund (ERDF), and Portuguese Foundation for Science and Technology (FCT) for Mariana Cerqueira Post-doctoral grant (SFRH/BPD/96611/2013).
Author Disclosure and Ghostwriting
No competing financial interests exist. The content of this article was expressly written by the authors listed. No ghostwriters were used to write this article.
About the Authors
Mariana Teixeira Cerqueira, PhD, is a post-doc researcher at the 3B's Research Group and her main scientific interests reside in the study of stem cell-based strategies for skin regeneration. She has been comparing different elements in the tissue engineering ground to target especially neovascularization and re-epithelialization in the cutaneous wound-healing context, namely by the interaction of cellular elements and native or artificial extracellular matrix interaction. Rogério Pedro Pirraco, PhD, is an invited assistant researcher at the 3B's Research Group. His work focuses on developing scaffold-free approaches for Tissue Engineering. His research interests have been centered in proposing new strategies to promote the vascularization of engineered tissues and in maximizing the angiogenic potential of different stem cells. Alexandra Pinto Marques, PhD, is Principal Investigator at 3B's Research Group. She has been integrating knowledge in the field of biomaterials and tissue engineering as a way to define innovative strategies to improve the functionality of skin tissue-engineered constructs. She has also paid particular attention to the vascularization hurdle by exploring the potential of endothelial cells obtained from different sources.
References
- 1.Yamaguchi Y, Yoshikawa K. Cutaneous wound healing: an update. J Dermatol 2001;28:521–534 [DOI] [PubMed] [Google Scholar]
- 2.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature 2008;453:314–321 [DOI] [PubMed] [Google Scholar]
- 3.Ito M, Liu Y, Yang Z, Nguyen J, Liang F, Morris RJ, et al. . Stem cells in the hair follicle bulge contribute to wound repair but not to homeostasis of the epidermis. Nat Med 2005;11:1351–1354 [DOI] [PubMed] [Google Scholar]
- 4.Levy V, Lindon C, Zheng Y, Harfe BD, Morgan BA. Epidermal stem cells arise from the hair follicle after wounding. FASEB J 2007;21:1358–1366 [DOI] [PubMed] [Google Scholar]
- 5.Nowak JA, Polak L, Pasolli HA, Fuchs E. Hair follicle stem cells are specified and function in early skin morphogenesis. Cell Stem Cell 2008;3:33–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jahoda CAB, Reynolds AJ. Hair follicle dermal sheath cells: unsung participants in wound healing. Lancet 2001;358:1445–1448 [DOI] [PubMed] [Google Scholar]
- 7.Opalenik SR, Davidson JM. Fibroblast differentiation of bone marrow-derived cells during wound repair. FASEB J 2005;19:1561–1563 [DOI] [PubMed] [Google Scholar]
- 8.Wang Y, Sun Y, Yang XY, Ji SZ, Han S, Xia ZF. Mobilised bone marrow-derived cells accelerate wound healing. Int Wound J 2013;10:473–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seppanen E, Roy E, Ellis R, Bou-Gharios G, Fisk NM, Khosrotehrani K. Distant mesenchymal progenitors contribute to skin wound healing and produce collagen: evidence from a murine fetal microchimerism model. PLoS One 2013;8:e62662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Traktuev DO, Prater DN, Merfeld-Clauss S, Sanjeevaiah AR, Saadatzadeh MR, Murphy M, et al. . Robust functional vascular network formation in vivo by cooperation of adipose progenitor and endothelial cells. Circ Res 2009;104:1410–1420 [DOI] [PubMed] [Google Scholar]
- 11.Wong VW, Levi B, Rajadas J, Longaker MT, Gurtner GC. Stem cell niches for skin regeneration. Int J Biomater 2012;2012:926059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerqueira MC, Reis RL, Marques AP. Wound healing microenvironmental cues: from tissue analogs to skin regeneration. Curr Tissue Eng 2013;2:145–153 [Google Scholar]
- 13.Wong VW, Gurtner GC, Longaker MT. Wound healing: a paradigm for regeneration. Mayo Clin Proc 2013;88:1022–1031 [DOI] [PubMed] [Google Scholar]
- 14.Cha J, Falanga V. Stem cells in cutaneous wound healing. Clin Dermatol 2007;25:73–78 [DOI] [PubMed] [Google Scholar]
- 15.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, et al. . Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 17.Lin Q, Wesson R, Maeda H, Wang Y, Cui Z, Jo L, et al. . Pharmacological mobilization of endogenous stem cells significantly promotes skin regeneration after full-thickness excision: the synergistic activity of AMD3100 and tacrolimus. J Invest Dermatol 2014;134:2458–2468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whyte JL, Smith AA, Liu B, Manzano WR, Evans ND, Dhamdhere GR, et al. . Augmenting endogenous Wnt signaling improves skin wound healing. PLoS One 2013;8:e76883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin L, Peterson DA. Human mesenchymal stem cell grafts enhance normal and impaired wound healing by recruiting existing endogenous tissue stem/progenitor cells. Stem Cells Transl Med 2013;2:33–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millard SM, Fisk NM. Mesenchymal stem cells for systemic therapy: shotgun approach or magic bullets? Bioessays 2014;35:173–182 [DOI] [PubMed] [Google Scholar]
- 21.Jackson WM, Nesti LJ, Tuan RS. Concise review: clinical translation of wound healing therapies based on mesenchymal stem cells. Stem Cells Transl Med 2012;1:44–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maxson S, Lopez EA, Yoo D, Danilkovitch-Miagkova A, Leroux MA. Concise review: role of mesenchymal stem cells in wound repair. Stem Cells Transl Med 2012;1:142–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Németh K, Leelahavanichkul A, Yuen PST, Mayer B, Parmelee A, Doi K, et al. . Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med 2009;15:42–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005;105:1815–1822 [DOI] [PubMed] [Google Scholar]
- 25.Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, et al. . Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 2010;28:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–2587 [DOI] [PubMed] [Google Scholar]
- 28.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res 2008;103:1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono I, Yamashita T, Hida T, Jin HY, Ito Y, Hamada H, et al. . Combined administration of basic fibroblast growth factor protein and the hepatocyte growth factor gene enhances the regeneration of dermis in acute incisional wounds. Wound Repair Regen 2004;12:67–79 [DOI] [PubMed] [Google Scholar]
- 30.Colwell AS, Beanes SR, Soo C, Dang C, Ting K, Longaker MT, et al. . Increased angiogenesis and expression of vascular endothelial growth factor during scarless repair. Plast Reconstr Surg 2005;115:204–212 [PubMed] [Google Scholar]
- 31.Shevchenko RV, James SL, James SE. A review of tissue-engineered skin bioconstructs available for skin reconstruction. J R Soc Interface 2010;7:229–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Böttcher-Haberzeth S, Biedermann T, Klar AS, Pontiggia L, Rac J, Nadal D, et al. . Tissue engineering of skin: human tonsil-derived mesenchymal cells can function as dermal fibroblasts. Pediat Surg Int 2014;30:213–222 [DOI] [PubMed] [Google Scholar]
- 33.Collawn SS, Banerjee NS, de la Torre J, Vasconez L, Chow LT. Adipose-derived stromal cells accelerate wound healing in an organotypic raft culture model. Ann Plast Surg 2012;68:501–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan RK, Zamora DO, Wrice NL, Baer DG, Renz EM, Christy RJ, et al. . Development of a vascularized skin construct using adipose-derived stem cells from debrided burned skin. Stem Cells Int 2012;2012:841203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puissant B, Barreau C, Bourin P, Clavel C, Corre J, Bousquet C, et al. . Immunomodulatory effect of human adipose tissue-derived adult stem cells: comparison with bone marrow mesenchymal stem cells. Br J Haematol 2005;129:118–129 [DOI] [PubMed] [Google Scholar]
- 36.Procházka V, Gumulec J, Jalůvka F, Salounová D, Jonszta T, Czerný D, et al. . Cell therapy, a new standard in management of chronic critical limb ischemia and foot ulcer. Cell Transplant 2010;19:1413–1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cerqueira MT, Pirraco RP, Santos TC, Rodrigues DB, Frias AM, Martins AR, et al. . Human adipose stem cells cell sheet constructs impact epidermal morphogenesis in full-thickness excisional wounds. Biomacromolecules 2013;14:3997–4008 [DOI] [PubMed] [Google Scholar]
- 38.Shen Y, Dai L, Li X, Liang R, Guan G, Zhang Z, et al. . Epidermal stem cells cultured on collagen-modified chitin membrane induce in situ tissue regeneration of full-thickness skin defects in mice. PLoS One 2014;9:e87557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tam K, Cheyyatraviendran S, Venugopal J, Biswas A, Choolani M, Ramakrishna S, et al. . A nanoscaffold impregnated with human Wharton's jelly stem cells or its secretions improves healing of wounds. J Cell Biochem 2014;115:794–803 [DOI] [PubMed] [Google Scholar]
- 40.Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, et al. . Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2011;33:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zamora DO, Natesan S, Becerra S, Wrice N, Chung E, Suggs LJ, et al. . Enhanced wound vascularization using a dsASCs seeded FPEG scaffold. Angiogenesis 2013;16:745–757 [DOI] [PubMed] [Google Scholar]
- 42.Jiang D, Qi Y, Walker NG, Sindrilaru A, Hainzl A, Wlaschek M, et al. . The effect of adipose tissue derived MSCs delivered by a chemically defined carrier on full-thickness cutaneous wound healing. Biomaterials 2013;34:2501–2515 [DOI] [PubMed] [Google Scholar]
- 43.Cerqueira MT, Marques AP, Reis RL. Using stem cells in skin regeneration: possibilities and reality. Stem Cells Dev 2012;21:1201–1214 [DOI] [PubMed] [Google Scholar]
- 44.Lam MT, Nauta A, Meyer NP, Wu JC, Longaker M. Effective delivery of stem cells using an extracellular matrix patch results in increased cell survival and proliferation and reduced scarring in skin wound healing. Tissue Eng Part A 2013;19:738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rustad KC, Wong VW, Sorkin M, Glotzbach JP, Major MR, Rajadas J, et al. . Enhancement of mesenchymal stem cell angiogenic capacity and stemness by a biomimetic hydrogel scaffold. Biomaterials 2012;33:80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altman AM, Matthias N, Yan Y, Song YH, Bai X, Chiu ES, et al. . Dermal matrix as a carrier for in vivo delivery of human adipose-derived stem cells. Biomaterials 2008;29:1431–1442 [DOI] [PubMed] [Google Scholar]
- 47.Chun-Mao H, Su-Yi W, Ping-Ping L, Hang-Hui C. Human bone marrow-derived mesenchymal stem cells differentiate into epidermal-like cells in vitro. Differentiation 2007;75:292–298 [DOI] [PubMed] [Google Scholar]
- 48.Brzoska M, Geiger H, Gauer S, Baer P. Epithelial differentiation of human adipose tissue-derived adult stem cells. Biochem Biophys Res Commun 2005;330:142–150 [DOI] [PubMed] [Google Scholar]
- 49.Kamolz L-P, Kolbus A, Wick N, Mazal PR, Eisenbock B, Burjak S, et al. . Cultured human epithelium: human umbilical cord blood stem cells differentiate into keratinocytes under in vitro conditions. Burns 2006;32:16–19 [DOI] [PubMed] [Google Scholar]
- 50.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. . A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008;3:301–313 [DOI] [PubMed] [Google Scholar]
- 51.Da Silva Meirelles L, Caplan AI, Nardi NB. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008;26:2287–2299 [DOI] [PubMed] [Google Scholar]
- 52.Aksu AE, Horibe E, Sacks J, Ikeguchi R, Breitinger J, Scozio M, et al. . Co-infusion of donor bone marrow with host mesenchymal stem cells treats GVHD and promotes vascularized skin allograft survival in rats. Clin Immunol 2008;127:348–358 [DOI] [PubMed] [Google Scholar]
- 53.Lee SM, Lee SC, Kim SJ. Contribution of human adipose tissue-derived stem cells and the secretome to the skin allograft survival in mice. J Surg Res 2014;188:280–289 [DOI] [PubMed] [Google Scholar]
- 54.Hsiao ST-F, Asgari A, Lokmic Z, Sinclair R, Dusting GJ, Lim SY, et al. . Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 2012;21:2189–2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park BS, Jang KA, Sung JH, Park JS, Kwon YH, Kim KJ, et al. . Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol Surg 2008;34:1323–1326 [DOI] [PubMed] [Google Scholar]
- 56.Miranda JP, Filipe E, Fernandes AS, Almeida JM, Martins JP, De La Fuente A, et al. . The human umbilical cord tissue-derived MSC population UCX® promotes early motogenic effects on keratinocytes and fibroblasts and G-CSF-mediated mobilization of BM-MSCs when transplanted in vivo. Cell Transplant 2013. [Epub ahead of print]; DOI: 10.3727/096368913X676231] [DOI] [PubMed] [Google Scholar]
- 57.Yew T-L, Hung Y-T, Li H-Y, Chen H-W, Chen L-L, Tsai K-S, et al. . Enhancement of wound healing by human multipotent stromal cell conditioned medium: the paracrine factors and p38 MAPK activation. Cell Transplant 2011;20:693–706 [DOI] [PubMed] [Google Scholar]
- 58.Maharlooei MK, Bagheri M, Solhjou Z, Jahromi BM, Akrami M, Rohani L, et al. . Adipose tissue derived mesenchymal stem cell (AD-MSC) promotes skin wound healing in diabetic rats. Diabetes Res Clin Pract 2011;93:228–234 [DOI] [PubMed] [Google Scholar]
- 59.Smith AN, Willis E, Chan VT, Muffley LA, Isik FF, Gibran NS, et al. . Mesenchymal stem cells induce dermal fibroblast responses to injury. Exp Cell Res 2009;316:48–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SH, Jin SY, Song JS, Seo KK, Cho KH. Paracrine effects of adipose-derived stem cells on keratinocytes and dermal fibroblasts. Ann Dermatol 2012;24:136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim SW, Zhang HZ, Guo L, Kim JM, Kim MH. Amniotic mesenchymal stem cells enhance wound healing in diabetic NOD/SCID mice through high angiogenic and engraftment capabilities. PLoS One 2012;7:e41105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yoon BS, Moon J-H, Jun EK, Kim J, Maeng I, Kim JS, et al. . Secretory profiles and wound healing effects of human amniotic fluid-derived mesenchymal stem cells. Stem Cells Dev 2010;19:887–902 [DOI] [PubMed] [Google Scholar]
- 63.Badiavas EV, Falanga V. Treatment of chronic wounds with bone marrow-derived cells. Arch Dermatol 2003;139:510–516 [DOI] [PubMed] [Google Scholar]
- 64.Falanga V, Iwamoto S, Chartier M, Yufit T, Butmarc J, Kouttab N, et al. . Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 65.Jiménez F, Garde C, Poblet E, Jimeno B, Ortiz J, Martínez ML, et al. . A pilot clinical study of hair grafting in chronic leg ulcers. Wound Repair Regen 2012;20:806–814 [DOI] [PubMed] [Google Scholar]
- 66.Tausche AK, Skaria M, Bohlen L, Liebold K, Hafner J, Friedlein H, et al. . An autologous epidermal equivalent tissue-engineered from follicular outer root sheath keratinocytes is as effective as split-thickness skin autograft in recalcitrant vascular leg ulcers. Wound Repair Regen 2003;11:248–252 [DOI] [PubMed] [Google Scholar]
- 67.Akita S, Akino K, Hirano A, Ohtsuru A, Yamashita S. Noncultured autologous adipose-derived stem cells therapy for chronic radiation injury. Stem Cells Int 2010;2010:532704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schneider CK, Salmikangas P, Jilma B, Flamion B, Todorova LR, Paphitou A, et al. . Challenges with advanced therapy medicinal products and how to meet them. Nat Rev Drug Discov 2010;9:195–201 [DOI] [PubMed] [Google Scholar]
- 69.Pytlik, Slanar, Stehlik, Matejkova. Production of clinical grade mesenchymal stromal cells. In: Eberli D, ed. Regenerative Medicine and Tissue Engineering-Cells and Biomaterials. Croatia: InTech, 2011:145–178 [Google Scholar]
- 70.European Medicines Agency, CAT Secretariat and US Food and Drug Administration. Regen Med 2011;6:90–96 [DOI] [PubMed] [Google Scholar]
- 71.Chung H, Won C-H, Sung J. Responses of adipose-derived stem cells during hypoxia: enhanced skin-regenerative potential. Expert Opin Biol 2009;9:1499–1508 [DOI] [PubMed] [Google Scholar]
- 72.Gordillo GM, Sen CK. Revisiting the essential role of oxygen in wound healing. Am J Surg 2003;186:259–263 [DOI] [PubMed] [Google Scholar]
- 73.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. . Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014;508:269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Roemeling-Van Rhijn M, Mensah FKF, Korevaar SS, Leijs MJ, Van Osch GJVM, Ijzermans JNM, et al. . Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol 2013;4:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rezvani HR, Ali N, Nissen LJ, Harfouche G, de Verneuil H, Taïeb A, et al. . HIF-1α in epidermis: oxygen sensing, cutaneous angiogenesis, cancer, and non-cancer disorders. J Invest Dermatol 2011;131:1793–1805 [DOI] [PubMed] [Google Scholar]
- 76.Semenza GL. Signal transduction to hypoxia-inducible factor 1. Biochem Pharmacol 2002;64:993–998 [DOI] [PubMed] [Google Scholar]
- 77.Lee J-W, Bae S-H, Jeong J-W, Kim S-H, Kim K-W. Hypoxia-inducible factor (HIF-1)alpha: its protein stability and biological functions. Exp Mol Med 2004;36:1–12 [DOI] [PubMed] [Google Scholar]
- 78.Calvani M, Rapisarda A, Uranchimeg B, Shoemaker RH, Melillo G. Hypoxic induction of an HIF-1α-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood 2006;107:2705–2712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Black SM, Devol JM, Wedgwood S. Regulation of fibroblast growth factor-2 expression in pulmonary arterial smooth muscle cells involves increased reactive oxygen species generation. Am J Physiol Cell Physiol 2008;294:C345–C354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang SXL, Gozal D, Sachleben LR, Rane M, Klein JB, Gozal E. Hypoxia induces an autocrine-paracrine survival pathway via platelet-derived growth factor (PDGF)-B/PDGF-β receptor/phosphatidylinositol 3-kinase/Akt signaling in RN46A neuronal cells. FASEB J 2003;17:1709–1711 [DOI] [PubMed] [Google Scholar]
- 81.Nishi H, Nakada T, Hokamura M, Osakabe Y, Itokazu O, Huang LE, et al. . Hypoxia-inducible factor-1 transactivates transforming growth factor-beta3 in trophoblast. Endocrinology 2004;145:4113–4118 [DOI] [PubMed] [Google Scholar]
- 82.Carmeliet P, Dor Y, Herbert J-M, Fukumura D, Brusselmans K, Dewerchin M, et al. . Role of HIF-1[alpha] in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998;394:485–490 [DOI] [PubMed] [Google Scholar]
- 83.Moon KM, Park Y-H, Lee JS, Chae Y-B, Kim M-M, Kim D-S, et al. . The effect of secretory factors of adipose-derived stem cells on human keratinocytes. Int J Mol Sci 2012;13:1239–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chen L, Xu Y, Zhao J, Zhang Z, Yang R, Xie J, et al. . Conditioned medium from hypoxic bone marrow-derived mesenchymal stem cells enhances wound healing in mice. PLoS One 2014;9:e96161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee EY, Xia Y, Kim WS, Kim MH, Kim TH, Kim KJ, et al. . Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 2009;17:540–547 [DOI] [PubMed] [Google Scholar]
- 86.Frazier TP, Gimble JM, Kheterpal I, Rowan BG. Impact of low oxygen on the secretome of human adipose-derived stromal/stem cell primary cultures. Biochimie 2013;95:2286–2296 [DOI] [PubMed] [Google Scholar]
- 87.Jun EK, Zhang Q, Yoon BS, Moon J-H, Lee G, Park G, et al. . Hypoxic conditioned medium from human amniotic fluid-derived mesenchymal stem cells accelerates skin wound healing through TGF-β/SMAD2 and PI3K/Akt pathways. Int J Mol Sci 2013;15:605–628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res 2007;100:1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gentile P, Orlandi A, Scioli MG, Di Pasquali C, Bocchini I, Cervelli V. Concise review: adipose-derived stromal vascular fraction cells and platelet-rich plasma: basic and clinical implications for tissue engineering therapies in regenerative surgery. Stem Cells Transl Med 2012;1:230–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoshimura K, Shigeura T, Matsumoto D, Sato T, Takaki Y, Aiba-Kojima E, et al. . Characterization of freshly isolated and cultured cells derived from the fatty and fluid portions of liposuction aspirates. J Cell Physiol 2006;208:64–76 [DOI] [PubMed] [Google Scholar]
- 91.Han SK, Kim HR, Kim WK. The treatment of diabetic foot ulcers with uncultured, processed lipoaspirate cells: a pilot study. Wound Repair Regen 2010;18:342–348 [DOI] [PubMed] [Google Scholar]
- 92.Klar AS, Güven S, Biedermann T, Luginbühl J, Böttcher-Haberzeth S, Meuli-Simmen C, et al. . Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014;35:5065–5078 [DOI] [PubMed] [Google Scholar]
- 93.Atalay S, Coruh A, Deniz K. Stromal vascular fraction improves deep partial thickness burn wound healing. Burns 2014;40:1375–1383 [DOI] [PubMed] [Google Scholar]
- 94.Jiang Y, Chen B, Liu Y, Zhufu Z, Yan X, Hou X, et al. . Effect of collagen scaffold with adipose-derived stromal vascular fraction cells on diabetic wound healing: a study in a diabetic porcine model. Tissue Eng Regen Med 2013;10:192–199 [Google Scholar]
- 95.Schäffler A, Büchler C. Concise review: adipose tissue-derived stromal cells—basic and clinical implications for novel cell-based therapies. Stem Cells 2007;25:818–827 [DOI] [PubMed] [Google Scholar]
- 96.Casteilla L, Planat-Benard V, Laharrague P, Cousin B. Adipose-derived stromal cells: their identity and uses in clinical trials, an update. World J Stem Cells 2011;3:25–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong VW, Sorkin M, Glotzbach JP, Longaker MT, Gurtner GC. Surgical approaches to create murine models of human wound healing. J Biomed Biotechnol 2011;2011:969618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sullivan TP, Eaglstein WH, Davis SC, Mertz P. The pig as a model for human wound healing. Wound Repair Regen 2001;9:66–76 [DOI] [PubMed] [Google Scholar]
- 99.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol 2005;23:47–55 [DOI] [PubMed] [Google Scholar]
- 100.Jeong SI, Jeon O, Krebs MD, Hill MC, Alsberg E. Biodegradable photo-crosslinked alginate nanofibre scaffolds with tuneable physical properties, cell adhesivity and growth factor release. Eur Cell Mater 2012;24:331–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi JS, Leong KW, Yoo HS. In vivo wound healing of diabetic ulcers using electrospun nanofibers immobilized with human epidermal growth factor (EGF). Biomaterials 2008;29:587–596 [DOI] [PubMed] [Google Scholar]
- 102.Gil ES, Panilaitis B, Bellas E, Kaplan DL. Functionalized silk biomaterials for wound healing. Adv Healthc Mater 2013;2:206–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sun X, Cheng L, Zhao J, Jin R, Sun B, Shi Y, et al. . bFGF-grafted electrospun fibrous scaffolds via poly(dopamine) for skin wound healing. J Mater Chem B 2014;2:3636–3645 [DOI] [PubMed] [Google Scholar]
- 104.Xie Z, Paras CB, Weng H, Punnakitikashem P, Su LC, Vu K, et al. . Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013;9:9351–9359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kobsa S, Kristofik NJ, Sawyer AJ, Bothwell ALM, Kyriakides TR, Saltzman WM. An electrospun scaffold integrating nucleic acid delivery for treatment of full-thickness wounds. Biomaterials 2013;34:3891–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Guo R, Xu S, Ma L, Huang A, Gao C. The healing of full-thickness burns treated by using plasmid DNA encoding VEGF-165 activated collagen-chitosan dermal equivalents. Biomaterials 2011;32:1019–1031 [DOI] [PubMed] [Google Scholar]
- 107.Liu X, Ma L, Liang J, Zhang B, Teng J, Gao C. RNAi functionalized collagen-chitosan/silicone membrane bilayer dermal equivalent for full-thickness skin regeneration with inhibited scarring. Biomaterials 2013;34:2038–2048 [DOI] [PubMed] [Google Scholar]
- 108.Gurtner GC, Dauskardt RH, Wong VW, Bhatt KA, Wu K, Vial IN, et al. . Improving cutaneous scar formation by controlling the mechanical environment: large animal and phase I studies. Ann Surg 2011;254:217–225 [DOI] [PubMed] [Google Scholar]
- 109.Norotte C, Marga FS, Niklason LE, Forgacs G. Scaffold-free vascular tissue engineering using bioprinting. Biomaterials 2009;30:5910–5917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kolesky DB, Truby RL, Gladman AS, Busbee TA, Homan KA, Lewis JA. 3D Bioprinting of vascularized, heterogeneous cell-laden tissue constructs. Adv Mater 2014;21:3124–3130 [DOI] [PubMed] [Google Scholar]
- 111.Koch L, Deiwick A, Schlie S, Michael S, Gruene M, Coger V, et al. . Skin tissue generation by laser cell printing. Biotechnol Bioeng 2012;109:1855–1863 [DOI] [PubMed] [Google Scholar]
- 112.Lee VK, Singh G, Trasatti JP, Bjornsson C, Tran TN, Xu G, et al. . Design and fabrication of human skin by 3D bioprinting. Tissue Eng Part C Methods 2014;20:473–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, et al. . Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 2012;1:792–802 [DOI] [PMC free article] [PubMed] [Google Scholar]