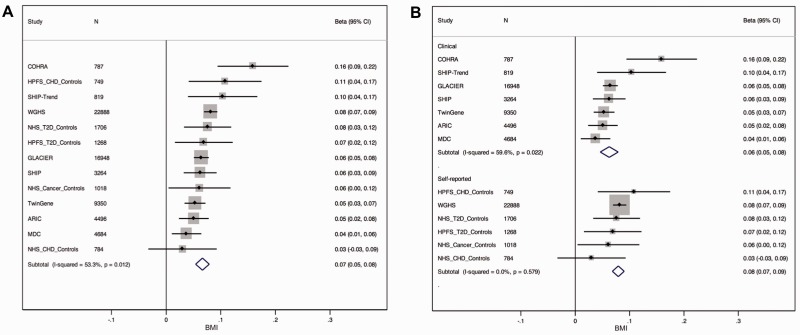
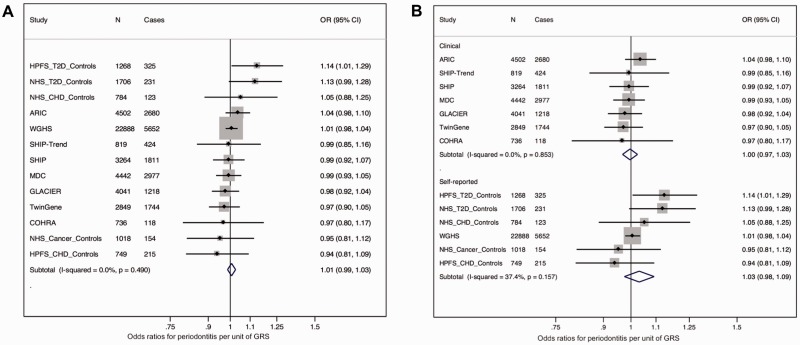
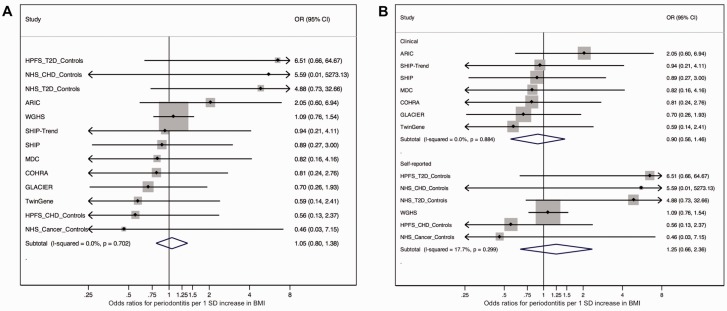
Dmitry Shungin
Dmitry Shungin
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
1-3,
Marilyn C Cornelis
Marilyn C Cornelis
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
4,
Kimon Divaris
Kimon Divaris
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
5,20,
Birte Holtfreter
Birte Holtfreter
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
6,
John R Shaffer
John R Shaffer
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
7,
Yau-Hua Yu
Yau-Hua Yu
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
8,
Silvana P Barros
Silvana P Barros
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
9,
James D Beck
James D Beck
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
10,
Reiner Biffar
Reiner Biffar
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
11,
Eric A Boerwinkle
Eric A Boerwinkle
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
12,13,
Richard J Crout
Richard J Crout
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
14,
Andrea Ganna
Andrea Ganna
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
15,
Goran Hallmans
Goran Hallmans
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
16,
George Hindy
George Hindy
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
17,
Frank B Hu
Frank B Hu
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
4,
Peter Kraft
Peter Kraft
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
33,
Daniel W McNeil
Daniel W McNeil
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
18,
Olle Melander
Olle Melander
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
19,
Kevin L Moss
Kevin L Moss
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
10,
Kari E North
Kari E North
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
20,21,
Marju Orho-Melander
Marju Orho-Melander
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
17,
Nancy L Pedersen
Nancy L Pedersen
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
15,
Paul M Ridker
Paul M Ridker
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
22,23,
Eric B Rimm
Eric B Rimm
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
4,
Lynda M Rose
Lynda M Rose
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
22,
Gull Rukh
Gull Rukh
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
17,
Alexander Teumer
Alexander Teumer
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
24,
Robert J Weyant
Robert J Weyant
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
25,
Daniel I Chasman
Daniel I Chasman
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
22,23,
Kaumudi Joshipura
Kaumudi Joshipura
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
26,
Thomas Kocher
Thomas Kocher
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
6,
Patrik KE Magnusson
Patrik KE Magnusson
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
15,
Mary L Marazita
Mary L Marazita
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
7,27,28,
Peter Nilsson
Peter Nilsson
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
29,
Steve Offenbacher
Steve Offenbacher
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
9,
George Davey Smith
George Davey Smith
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
30,31,
Pernilla Lundberg
Pernilla Lundberg
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
3,
Tom M Palmer
Tom M Palmer
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
32,
Nicholas J Timpson
Nicholas J Timpson
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
30,31,
Ingegerd Johansson
Ingegerd Johansson
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
3,16,
Paul W Franks
Paul W Franks
1Department of Public Health and Clinical Medicine, Section for Medicine, Umeå University, Umeå, Sweden, 2Genetic and Molecular Epidemiology Unit, Department of Clinical Sciences, Skåne University Hospital, Lund University, Malmö, Sweden, 3Department of Odontology, Umeå University, Umeå, Sweden, 4Department of Nutrition, Harvard School of Public Health, Boston, MA, USA, 5Department of Pediatric Dentistry, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 6Department of Restorative Dentistry, Periodontology and Endodontology, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 7Department of Human Genetics, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA, USA, 8Department of Oral Medicine, Infection and Immunity, Harvard School of Dental Medicine, Boston, MA, USA, 9Department of Periodontology, and 10Department of Dental Ecology, School of Dentistry, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 11Department of Prosthodontics, Gerodontology and Biomaterials, University Medicine, Ernst-Moritz-Arndt University Greifswald, Greifswald, Germany, 12Human Genetics Center, and 13Brown Foundation Institute of Molecular Medicine for the Prevention of Human Diseases, University of Texas Health Science Center at Houston, Houston, TX, USA, 14Emeritus, Department of Periodontics, West Virginia University School of Dentistry, Morgantown, WV, USA, 15Department of Medical Epidemiology and Biostatistics, Karolinska Institutet, Stockholm, Sweden, 16Department of Biobank Research, Umeå University, Umeå, Sweden, 17Department of Clinical Sciences, Diabetes and Cardiovascular Disease Genetic Epidemiology, Lund University Diabetes Center, Skåne University Hosptial, Malmö, Sweden, 18Dental Practice and Rural Health, Morgantown, WV, USA, 19Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Lund University Diabetes Center, Skåne University Hospital, Malmö, Sweden, 20Department of Epidemiology, Gillings School of Global Public Health, and 21Carolina Center for Genome Sciences, University of North Carolina-Chapel Hill, Chapel Hill, NC, USA, 22Division of Preventive Medicine, Brigham and Women's Hospital, Boston, MA, USA, 23Harvard Medical School, Boston, MA, USA, 24Institute for Community Medicine, University Medicine Greifswald, Greifswald, Germany, 25Department of Dental Public Health and Information Management, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 26Center for Clinical Research and Health, Promotion, School of Dental Medicine, University of Puerto Rico, San Juan, Puerto Rico, 27Center for Craniofacial and Dental Genetics, Department of Oral Biology, School of Dental Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 28Clinical and Translational Science Institute, and Department of Psychiatry, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA, 29Department of Clinical Sciences, Internal Medicine, Lund University, Malmö, Sweden, 30MRC Integrative EpidemiologyUnit (IEU) at the University of Bristol, Bristol, UK, 31School of Social and Community Medicine, University of Bristol, Bristol, UK, 32Division of Health Sciences, Warwick Medical School, University of Warwick, Coventry, UK and 33Program in Genetic Epidemiology and Statistical Genetics, Harvard School of Public Health, Boston, MA, USA
1,2,4,*