Abstract
The impact of human milk oligosaccharides (HMO) on mucosal immunity, gut microbiota and response to rotavirus (RV) infection was investigated in the piglet model. Newborn piglets were fed with formula alone (FF) or formula supplemented with 4 g l−1 HMO (HMO) or a prebiotic mixture of 9:1 short-chain galactooligosaccharides (3.6 g l−1) and long-chain fructooligosaccharides (0.4 g l−1) (PRE) (n=19–21 per group) for 15 days. Piglets (n=7–8) in each dietary group were orally infected with porcine rotavirus (RV) OSU strain on d10, and stool consistency was assessed daily. Blood, small intestine and colonic contents were collected at day 15. Serum RV-specific antibody concentrations, intestinal histomorphology, RV non-structural protein-4 (NSP4) and cytokine mRNA expression were assessed. Colonic content pH, dry matter (DM) and short-chain fatty acid concentrations were measured. Ascending colonic microbiota was analyzed by 16S rRNA gene v1-3 region pyrosequencing. HMO- and PRE-fed groups had shorter duration of diarrhea than FF piglets. Infection changed intestinal histomorphology, increased serum RV-specific antibody response and intestinal RV NSP4 expression, and modulated ileal cytokine expression. HMO enhanced T helper type 1 (interferon-gamma) and anti-inflammatory (interleukin-10) cytokines in the ileum, while prebiotics promoted RV-specific immunoglobulin M response to the infection. RV infection and HMO supplementation altered intraluminal environment and gut microbiota. HMO increased pH and lowered DM of colonic contents and enhanced the abundance of unclassified Lachnospiraceae, which contains numerous butyrate-producing bacteria. In conclusion, HMO and prebiotics did not prevent the onset of RV infection but reduced the duration of RV-induced diarrhea in piglets, in part, by modulating colonic microbiota and immune response to RV infection.
Keywords: gut microbiota, human milk oligosaccharides, mucosal immunity, prebiotics, rotavirus
Introduction
Rotavirus (RV) infection is the leading cause of gastroenteritis and diarrhea in human infants and young children and accounts for 5% of all deaths in children aged <5 years (Parashar et al., 2006). Two commercially available RV vaccines effectively prevent RV infections in the developed countries (Giaquinto et al., 2011); however, these vaccines are not widely available and are less efficacious in the developing countries (Babji and Kang, 2012). Breastfed infants have a lower incidence of RV-induced acute gastroenteritis than formula-fed (FF) infants (Plenge-Bonig et al., 2010). A distinctive aspect of human milk is the high abundance (5–10 g l−1) and complexity (>200 forms) of oligosaccharides (HMO); whereas oligosaccharides are negligible in bovine milk and infant formula (Kunz et al., 2000). HMO are comprised of both neutral and anionic (acidic) components with a lactose core at the reducing end. Various structures can be formed by elongation of up to 15 N-acetyllactosamine units and by addition of fucose and/or sialic acid (SA) residues at the terminal positions (Kunz et al., 2000; Bode, 2006; Ninonuevo et al., 2006). The ratio of neutral-to-acidic HMO is 70:20, in which lacto-N-neotetraose (LNnT) and 2′-fucosyllactose (2′FL) are the predominant neutral HMO in human milk (Kunz et al., 2000; Tao et al., 2010).
HMO have prebiotic, immunoregulatory and anti-infective functions (Kunz et al., 2000; Bode, 2006). HMO are fermented by neonatal piglet microbiota to produce short-chain fatty acid (SCFA) in vitro (Li et al., 2012), and HMO may decrease inflammation by reducing neutrophil infiltration and activation (Bode et al., 2004a, 2004b). Finally, due to the structural similarities to mucus glycans, HMO serve to inhibit the adhesion of pathogens to epithelial surfaces (Newburg et al., 2005). SA-containing HMO inhibit RV binding to the host cells in vitro, and both neutral and acidic HMO reduced RV replication in an in situ acute RV infection piglet model (Hester et al., 2013). However, whether dietary HMO reduce RV infectivity and ameliorate clinical symptoms in vivo remains to be determined.
Herein, the impact of dietary HMO on intestinal immunity, gut microbiota and the response to RV infection was investigated using a clinically relevant animal model. Due to the challenge of isolating sufficient quantities of HMO from donor human milk for animal feeding, synthetic HMO composed of 75% neutral HMO and 25% acidic HMO were mixed according to their relative proportions in human milk (Kunz et al., 2000; Tao et al., 2010; Li et al., 2012). Although the mixture does not represent the full repertoire of HMO, it contains the most predominant HMO in human milk, several of which have been shown to have anti-microbial and immunomodulatory actions in vitro (Li et al., 2012; Comstock et al., 2013). The HMO diet was also compared with prebiotic blend comprised of 90% short-chain galactooligosaccharides (scGOS) and 10% long-chain fructooligosaccharides (lcFOS), as these have been proven to be beneficial to infant health (Knol et al., 2005; Boehm and Moro, 2008). We hypothesized that HMO would protect against RV infection by modulating the immune response and gut microbiota in piglets.
Materials and methods
Animal care and experimental diets
The study was approved by the Institutional Animal Care and Use Committee at the University of Illinois, Urbana, IL, USA. Piglets (n=60) were removed from the sow immediately following delivery to avoid ingestion of colostrum. To provide passive immunity, piglets received 5 ml of sow serum per kg body weight by oral gavage at birth and 8, 24 and 36 h postpartum. Piglets were individually housed in environmentally controlled rooms (25 °C) with a 12 h light/dark cycle and were fed a non-medicated milk replacer (183 g l−1) (Advance Liquiwean; Milk Specialties, Dundee, IL, USA) throughout the study, as previously described (Li et al., 2012). At birth, piglets were randomized to three dietary treatment groups: formula (FF, n=20), formula+4 g l−1 HMO consisting of 40% 2′FL (Glycom, Lyngby, Denmark), 35% LNnT (Glycom), 10% 6′-sialyllactose (6′SL) (Carbosynth, Compton, UK), 5% 3′-SL (Carbosynth) and 10% free SA (Glycom) (HMO, n=21) or formula+4 g l−1 prebiotics containing 90% scGOS (3.6 g l−1 Vivinal GOS; FrieslandCampina Domo, Zwolle, The Netherlands) and 10% lcFOS (0.4 g l−1 Orafti HP, BENEO-Orafti, Tienen, Belgium) (PRE, n=19). Piglet body weight, food consumption, stool consistency and rectal temperature were measured daily. The feeding system, cages and animal rooms were cleaned and disinfected by 10% bleach daily to minimize cross-contamination.
RV infection
Group A porcine RV strain OSU (P9[7], g5) (ATCC, Manassas, VA, USA) was propagated in neonatal piglets and purified by sucrose gradient centrifugation as previously described (Hester et al., 2013). Virus titer was determined by a focus-forming assay (Hester et al., 2013). Immediately before use, the RV suspensions were treated with trypsin at a final concentration of 10 μg ml−1 (Sigma Chemical Co., St Louis, MO, USA) for 30 min at 37 °C to activate the virus. On day 10 (d10), piglets in each diet group (FF, n=7; HMO, n=8; PRE, n=8) were infected with 5 × 106 focus-forming assay RV in 1 ml minimal essential medium (MEM) by oral gavage. Non-infected piglets (FF, n=13; HMO, n=13; PRE, n=11) were gavaged with 1 ml MEM. After infection, the infected and non-infected groups were separated into different rooms. Piglets were monitored three times daily after infection, and stool was scored based on consistency (1=hard pellets; 2=soft and formed feces; 3=flowing and unformed feces; 4=watery feces) (Zijlstra et al., 1999). Piglets with consistency scores of ⩾3 were considered diarrheic.
Sample collection
On day 15, that is, 5 days post infection (PI), all piglets were euthanized, and blood and tissue samples were collected as previously described (Li et al., 2012). Jejunum and ileum sections (5 cm) were frozen in liquid nitrogen or were preserved in formalin solution. Another section (25 cm) of jejunum and ileum was opened and was gently scraped with a microscope slide to collect the mucosa, which was snap-frozen in liquid nitrogen. Ascending (AC) and descending (DC) colonic contents were collected in HCl or were snap-frozen in liquid nitrogen. During the sample collection, infected and non-infected animals were processed separately to avoid cross-contamination. The surgery tools were soaked in 10% bleach and disinfected by ethanol between animals.
Intestinal histomorphology
Formalin-fixed sections were embedded in paraffin, sliced (∼5 μm) with a microtome and mounted on glass microscope slides. The slides were stained with hematoxylin and eosin by the Veterinary Diagnostic Laboratory (University of Illinois). Intestinal images were captured by NanoZoomer Digital Pathology System (Hamamatsu Corporation, Bridgewater, NJ, USA). The villus height and crypt depth (∼20 per tissue per animal) were measured using the AxioVision 4.8 Digital Image Processing Software (Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA).
Serum RV-specific antibody response
RV-specific immunoglobulin G (IgG) and IgM in serum at day 15 were detected by an enzyme-linked immunosorbent assay developed in our laboratory.
RV-IgG
Ninety-six-well flat-bottomed plates were incubated with 100 μl per well of crude porcine OSU RV (diluted 1:400 in coating buffer (0.5 M carbonate–bicarbonate buffer, pH 9.6)) overnight at 4 °C. The plates were then blocked with 300 μl per well of 3% bovine serum albumin/phosphate-buffered saline (PBS; Sigma Chemical Co.) for 1 h at room temperature (RT), followed by washing three times with 0.1% Tween 20/PBS. Serum samples were twofold serially diluted from 1:25 to 1:1600 in 0.05% fish gelatin/PBS and added to the wells (100 μl per well) in triplicate. RV-infected piglet serum (positive control) was run on each plate in dilutions ranging from 1:200 to 1:8000 and was used as the standard curve. The plates were incubated at RT for 1 h and then washed three times with 0.1% Tween 20/PBS. Goat anti-pig IgG conjugated to horseradish peroxidase (100 μl per well) (HRP, Bethyl Labs, Montgomery, TX, USA) was then added at 1:20 000 dilution and incubated for 1 h, followed by washing four times. Then, 100 μl of tetramethylbenzidine substrate reagent (BD Biosciences, San José, CA, USA) was added and incubated in the dark at RT for 25 min. Finally, 100 μl of 2 N sulfuric acid was added. The plates were read at 450 nm on a spectrophotometer (SpectraMax M2e, Molecular Devices, Sunnyvale, CA, USA) with 570 nm plate correction wavelength. OSU RV for coating and RV-positive piglet serum for standard curve were each from single lots stored in aliquots at −80 °C.
RV-IgM
Ninety-six-well enzyme-linked immunosorbent assay plates were coated with 100 μl of 10 μg ml−1 goat anti-pig IgM (Bethyl Labs) in coating buffer overnight at 4 °C. The plates were then blocked and washed. Serially diluted samples and RV-positive piglet serum were added in triplicates in wells and incubated for 1 h. RV-positive serum was diluted from 1:200 to 1:8000, and samples were diluted from 1:50 to 1:3200. After washing, 100 μl of biotinylated porcine OSU strain RV at a dilution of 1:5000 in 0.5% fish gelatin/PBS was added and incubated for 1 h, followed by washing three times with 0.1% Tween 20/PBS. Then, 100 μl of streptavidin–horseradish peroxidase (1:200, R&D Systems, Minneapolis, MN, USA) was added to each well and incubated for 1 h. After washing, 100 μl tetramethylbenzidine was added to each well, and the plates were incubated for 35 min. Sulfuric acid was added. The plates were read at 450 and 570 nm. RV-specific IgG and IgM are expressed in arbitrary units calculated based on the standard curve.
Cytokine and RV non-structural protein-4 (NSP4) mRNA expression
RNA isolation and quantification were performed as previously described (Hester et al., 2013). Jejunal and ileal mucosal RNA were used for RV NSP4 mRNA expression. RNA isolated from ileal tissue was used for cytokine (interleukin (IL)-4, -6, -8, -10, -12 and interferon (IFN)-γ) gene expression. Reverse transcription was performed by a reverse transcription kit (Invitrogen Life Technologies Corporation, Carlsbad, CA, USA). Reference complementary DNA ribosomal protein L19 (RPL19) was used as an endogenous control (Invitrogen). Taqman real-time quantitative PCR was performed in triplicate using the TaqMan ABI 7900 PCR system (Invitrogen). Taqman primer/probe sequences and assay IDs are provided in Supplementary Table 1. Standard curves consisted of fivefold serial dilutions of pooled cDNA from all the samples of each tissue. Normalized values for each target were calculated by dividing the target quantity mean by the RPL19 quantity mean. The average of normalized values from FF non-infected animals was used as the calibrator. Fold changes were calculated for each measurement by dividing the normalized target values by the normalized calibrator sample.
Colonic content analyses
Dry matter (DM) and pH of AC and DC contents were measured as previously described (Wang et al., 2013). The SCFA and branched-chain fatty acid (BCFA) concentrations in AC contents were analyzed by gas chromatography (Li et al., 2012). Bacterial DNA from AC contents was extracted by using a bead beating method followed by purification with a QIAamp Stool Mini Kit (Qiagen, Valencia, CA, USA) (Li et al., 2012). Bacterial tag-encoded FLX amplicon pyrosequencing of 16S rRNA gene v1-3 region was performed at the Research and Testing Laboratory (Lubbock, TX, USA) using primers 28F (5′-GAGTTTGATCNTGGCTCAG-3′) and 519R (5′-GTNTTACNGCGGCKGCTG-3′). The tagging and sequencing protocol were performed as previously described (Handl et al., 2011). Raw sequence data were denoised and chimera checked by UCHIME (Edgar et al., 2011) and trimmed with the QIIME pipeline (Caporaso et al., 2010) with the following parameters: minimal read length >200 bp; no ambiguous base; mean quality score >25; maximal homopolymer ⩽6; no mismatches in primer; and no uncorrected barcodes. High-quality sequences were grouped into operational taxonomic units (OTUs) at the cutoff of 97% similarity. The most abundant sequence of each OTU was selected as the representative sequence and submitted to RDP classifier (Ribosomal Database Project, RDP version 10.31) for taxonomical assignment with a bootstrap threshold of 80% (Wang et al., 2007). The representative sequences from each OTU are available in the GenBank database under accession numbers KF517438—KF52179.
Statistical analysis
Univariate analysis
Data were analyzed as a randomized complete block design using the Proc Mixed procedure of SAS (SAS, Cary, NC, USA) with a Tukey adjustment. Fixed effects included diet, infection and the interaction of diet and infection. Litter and replicate were included as random effects. Normality was checked by the Shapiro–Wilk test, and outliers were identified by the Proc Robustreg procedure. Data were log-transformed if not normally distributed. When a main effect was significant, a post hoc least significant difference test was used to compare the differences among the treatments. All data are reported as means and s.e.ms. A probability of P⩽0.05 was considered as statistically significant.
Multivariate analysis
The Shannon diversity index and Chao 1 estimates of microbiota community were calculated in QIIME. The relative abundance of genus from each sample was log-transformed for redundancy analysis (RDA). RDA was performed in CANOCO for Windows 4.5 (Microcomputer Power, Ithaca, NY, USA). The statistical significance was assessed by the Monte Carlo test with 500 random permutations under the full model.
Results
RV infection and dietary supplementation did not change weight gain or formula intake
Neither diet nor infection affected body weight (Supplementary Figure 1). Piglets did not reduce their voluntary food intake (data not shown) or lose weight after RV infection. No piglets were pyrexic, defined as rectal temperature >104 °F (normal piglet body temperature is 101.6–104 °F°) or dehydration during the infection (data not shown). No clinical symptoms of RV infection (for example, diarrhea, fever) were observed in the non-infected piglets.
HMO and PRE supplementation shortened the duration of RV-induced diarrhea
The onset of the RV-induced diarrhea occurred at 36.3±1.8 h PI in all infected piglets, with no diet effect (Table 1). A biphasic diarrhea response was observed (Supplementary Figure 2). The time to recovery from the diarrhea was considered the time of the first observation of solid feces (stool consistency ⩽2). All RV-infected piglets recovered from the initial diarrhea on average 76.6±5.0 h PI, with no differences among the diet groups (Table 1). The duration of the initial diarrhea, which was calculated as the time of the onset of the diarrhea subtracted from the time of initial recovery, did not differ among the diet groups either. However, some of the RV-infected piglets had a second wave of diarrhea after the initial recovery. In the FF group, 57% (4/7) of the infected piglets had secondary diarrhea, whereas only 25% (2/8) of the infected piglets in either the HMO or PRE groups experienced a second incidence of diarrhea (Supplementary Figure 2). With the exception of one RV-infected piglet from the HMO group that had normal stool consistency on the day of sample collection, no piglets with secondary diarrhea fully recovered by the end of the study; thus the recovery time of the secondary diarrhea of those piglets was set at 120 h PI. The total duration of diarrhea, which was calculated as the time of the onset of the initial diarrhea subtracted from the time to recovery of diarrhea, was shorter in the HMO and PRE groups than in the FF group (48.8±9.8 h in the HMO group and 53.1±11.1 h in the PRE group vs 80.6±4.5 h in the FF group, P=0.0377; Table 1).
Table 1. Time (h) to recovery and duration of rotavirus (RV)-induced diarrhea is reduced in the RV-infected piglets fed formula with 4 g l−1 human milk oligosaccharides (HMO) or 4 g l−1 short-chain galactooligosaccharides and long-chain fructooligosaccharides (9:1) (PRE) compared with unsupplemented formula (FF).
| Onset of diarrhea (h PI) | Recovery of initial diarrheaa (h PI) | Duration of initial diarrheab (h) | Total duration of diarrheab (h) | |
|---|---|---|---|---|
| FF | 35.1±3.8 | 81.4±10.5 | 46.3±9.4 | 80.6±4.5a |
| HMO | 38.3±2.3 | 76.5±9.0 | 38.3±7.6 | 48.8±9.8b |
| PRE | 35.1±3.8 | 72.0±7.2 | 36.9±6.6 | 53.1±11.1b |
Abbreviations: FF, formula fed; PI, post infection.
The time of the first observation of solid feces (when stool consistency reaches 1–2) post-RV infection.
The duration of diarrhea is calculated as the time (h) to recovery of diarrhea minus the time (h) of onset of diarrhea. Values are means±s.e.m. (n=7–8 per group). Different superscript numbers indicate significant difference among the treatment groups (P⩽0.05).
Infection changed small intestinal morphology, with no diet effect
Villus height in the jejunum was decreased (P<0.0001) by 50%, whereas the crypt depth was increased by 20% in the RV-infected piglets compared with the non-infected groups, with no differences among the diet groups (Supplementary Figure 3a). Similarly, in the ileum, villus height was decreased by 40%, and crypt depth was increased by 25% in the RV-infected pigs, with no diet effect (Supplementary Figure 3b).
Infection resulted in RV replication in small intestine, with no diet effect
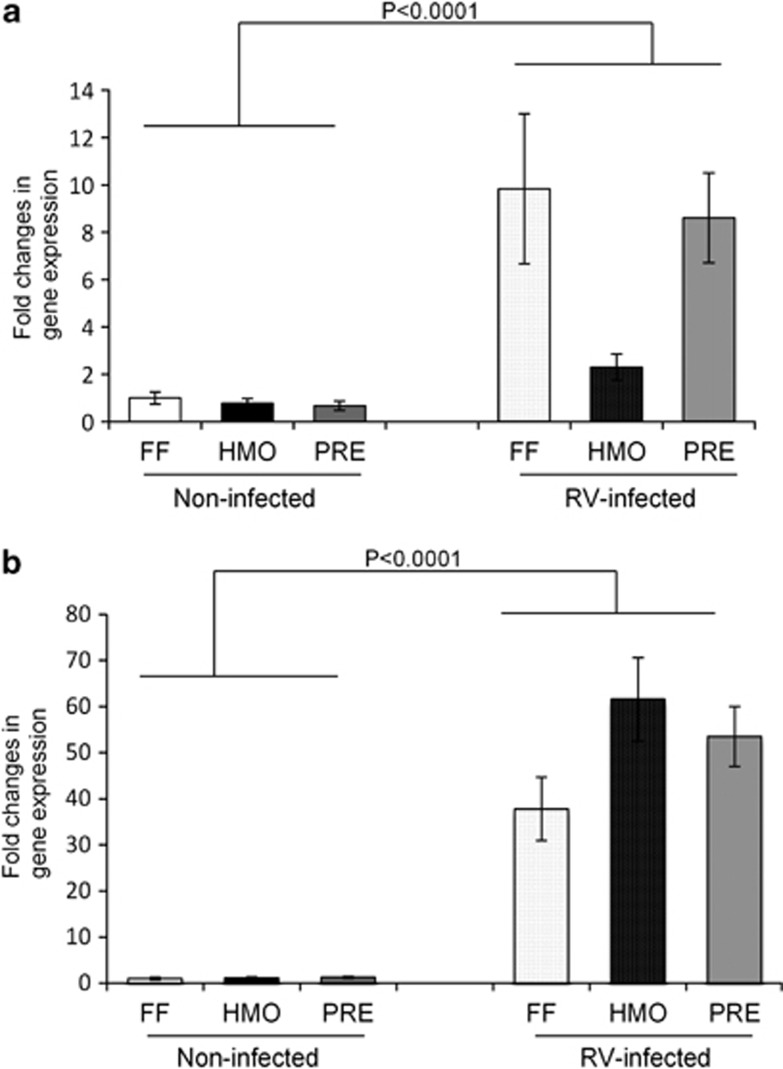
RV NSP4 mRNA abundance, which was assessed as a marker of viral replication, was increased (P<0.0001) in jejunal and ileal mucosa of the infected piglets compared with the non-infected, with no diet effect (Figure 1).
Figure 1.
RV NSP4 mRNA expression was increased in the jejunal (a) and ileal (b) mucosa of RV-infected piglets at 5 days PI with no effect of diet. The expression levels of NSP4 were standardized to ribosomal protein L19 mRNA and expressed as fold changes. Values are means±s.e.m.
Infection and diet modulated systemic and intestinal immunity responses to RV infection
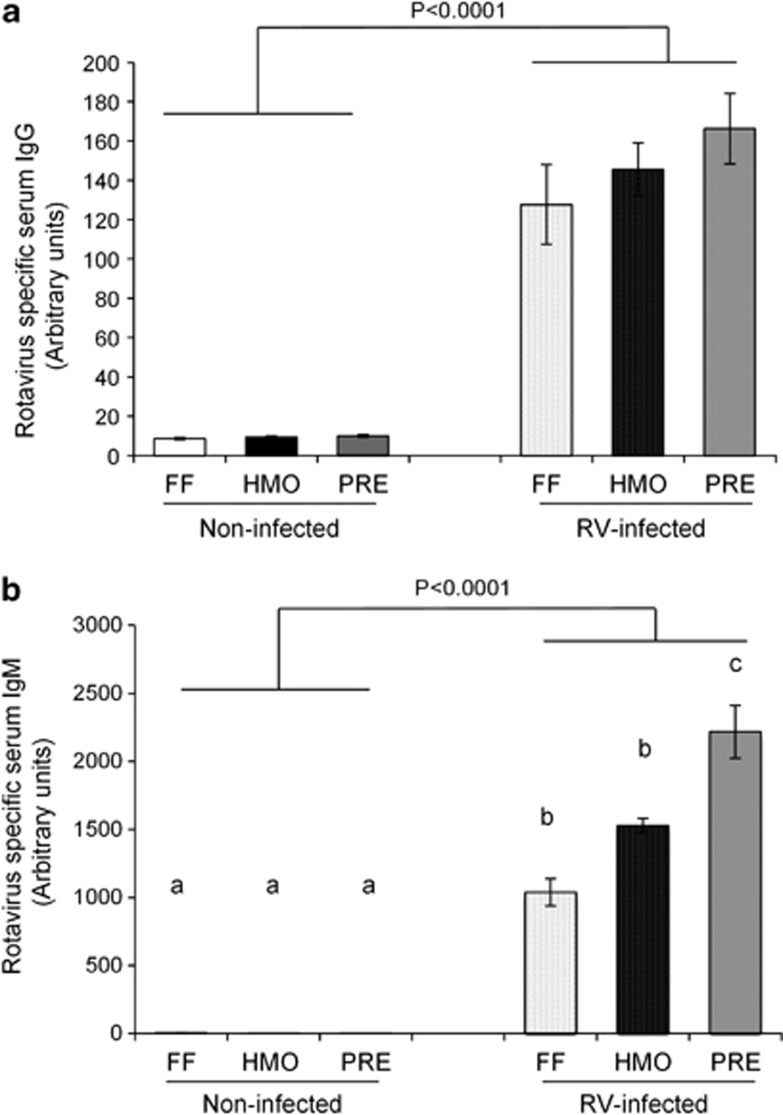
The concentrations of RV-specific IgG and IgM in serum were increased in the infected piglets at 5 days PI (Figure 2). Diet had no effect on RV-IgG (Figure 2a). In contrast, infected PRE piglets had higher circulating RV-IgM than the FF- and HMO-fed groups (P=0.0021) (Figure 2b).
Figure 2.
RV-specific IgG (a) and IgM (b) concentrations were increased in the serum of RV-infected piglets at 5 days PI. Prebiotic supplementation promoted RV-IgM response. Values are means±s.e.m. Different letters indicate significant differences among the treatment groups (P⩽0.05).
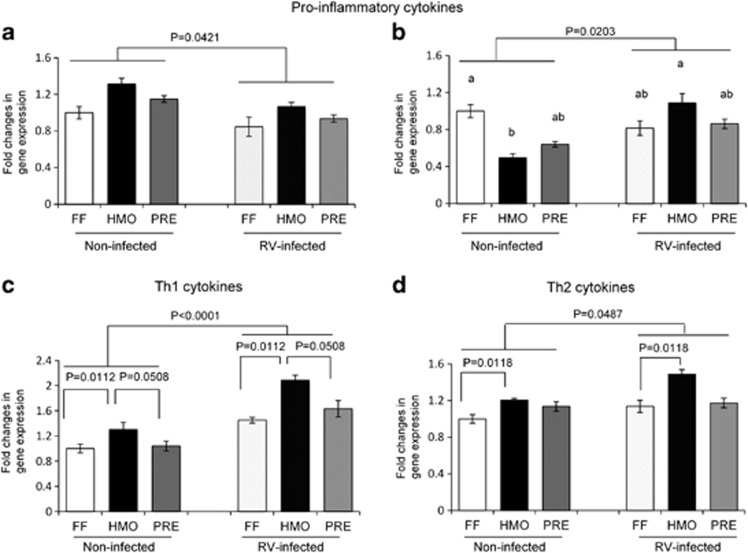
To determine the effects of RV infection and diet on the intestinal immune response, pro-inflammatory (IL-6, IL-8), T helper type 1 (Th1; IL-12, IFN-γ) and Th2 (IL-4, IL-10) cytokine gene expression in ileal tissue was measured by reverse transcriptase–PCR (Figure 3). IL-6 expression was decreased in the RV-infected piglets (P=0.0421), with no diet effect (Figure 3a). IL-8 expression was affected by both diet and infection (Figure 3b). IL-8 expression was higher (P=0.0077) in FF non-infected than in HMO non-infected piglets and was higher in RV-infected than in the non-infected piglets in the HMO group (P=0.0039). Ileal IFN-γ (Figure 3c) and IL-10 (Figure 3d) expression levels were also greater in the RV-infected piglets, with both diet and infection being significant but no interaction. HMO-fed piglets had greater expression of IFN-γ and IL-10 than the FF group regardless of infection status. IFN-γ tended (P=0.0508) to be higher in the HMO groups compared with the PRE groups. IL-4 and IL-12 expression levels did not differ among the treatment groups.
Figure 3.
RV infection and HMO supplementation modulated IL-6 (a), IL-8 (b), IFN-γ (c) and IL10 (d) mRNA expression in the ileum as measured by reverse transcriptase–PCR. Values are means±s.e.m. Different letters indicate significant difference among the treatment groups (P⩽0.05).
Infection and HMO supplementation influenced gut intraluminal environment
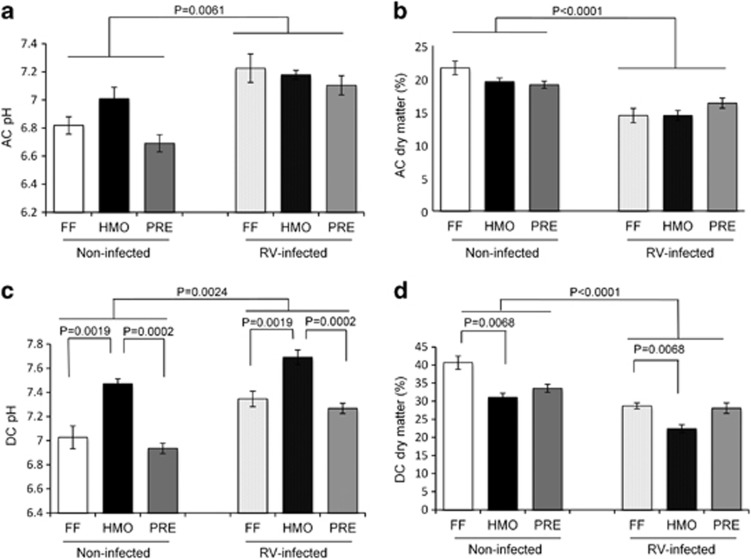
AC and DC content pH was increased, and DM was decreased in the RV-infected piglets at 5 days PI (Figure 4). In DC contents, the HMO group had higher pH than the FF and PRE groups and lower DM than the FF group (Figures 4c and d). There were no significant differences in most of the SCFA concentrations (mg g−1 wet content) in AC among the treatment groups, with the exception of propionate, which was higher in the AC of RV-infected piglets (P=0.0045; Table 2). The concentrations of isobutyrate (P=0.06) and valerate (P=0.07) and total branched BCFA (the sum of isobutyrate and isovalerate) (P=0.06) tended to be higher in the RV-infected piglets.
Figure 4.
pH of AC (a) and DC (c) colonic contents increased and DM (AC: b, DC: d) decreased in the RV-infected piglets at 5 days PI. HMO had higher pH and lower DM of DC content. Values are means±s.e.m.
Table 2. Short-chain fatty acid (SCFA) concentration (mg g−1 wet content) of the ascending colon contents of rotavirus (RV)-infected and non-infected piglets at day 15.
| SCFA (mg g−1 wet content) |
Non-infected |
RV-infected |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FF | HMO | PRE | FF | HMO | PRE | Diet | Infection | Diet × infection | |
| Acetate | 1.71±0.37 | 1.84±0.38 | 2.2±0.36 | 1.74±0.3 | 1.94±0.41 | 1.94±0.23 | 0.91 | 0.39 | 0.83 |
| Propionate | 0.26±0.05 | 0.36±0.09 | 0.45±0.08 | 0.44±0.06 | 0.57±0.13 | 0.53±0.07 | 0.39 | 0.0045 | 0.64 |
| Butyrate | 0.29±0.07 | 0.26±0.07 | 0.45±0.12 | 0.27±0.04 | 0.28±0.06 | 0.33±0.04 | 0.30 | 0.88 | 0.56 |
| Total SCFAa | 2.26±0.48 | 2.46±0.52 | 3.24±0.6 | 2.46±0.38 | 2.79±0.57 | 2.8±0.29 | 0.73 | 0.37 | 0.63 |
| Isobutyrate | 0.04±0.01 | 0.03±0.01 | 0.04±0.01 | 0.04±0.01 | 0.05±0.02 | 0.04±0.01 | 0.51 | 0.06 | 0.45 |
| Isovalerate | 0.06±0.01 | 0.05±0.01 | 0.08±0.02 | 0.05±0.01 | 0.08±0.02 | 0.07±0.01 | 0.39 | 0.10 | 0.51 |
| Valerate | 0.05±0.01 | 0.05±0.01 | 0.06±0.01 | 0.05±0.02 | 0.07±0.02 | 0.06±0.01 | 0.63 | 0.07 | 0.46 |
| Total BCFAb | 0.09±0.01 | 0.08±0.01 | 0.12±0.03 | 0.09±0.01 | 0.13±0.04 | 0.11±0.02 | 0.49 | 0.07 | 0.48 |
Abbreviations: BCFA, branch-chained fatty acid; FF, formula fed; HMO, formula supplemented with 4 g l−1 human milk oligosaccharides; PRE, formula supplemented with 4 g l−1 short-chain galactooligosaccharides and long-chain fructooligosaccharides (9:1).
Values are mean±s.e.m. (n=7–13 per group).
Total SCFA=acetate+propionate+butyrate.
Total BCFA=isobutyrate+isovalerate.
Infection and HMO supplement influenced the AC microbiota
Piglet AC microbiota was analyzed by 16S rRNA gene v1-3 region pyrosequencing. After trimming, a total of 335 564 high-quality reads were obtained from 60 samples, with an average length of 400 bp, and grouped into 4631 OTUs at 97% similarity. The numbers of observed and estimated OTUs (Chao 1), which represent the richness of the microbial community, were lower in the RV-infected piglets regardless of diet (Table 3). The Shannon diversity index was also lower in the infected piglets (P=0.0024), with no diet effect.
Table 3. Overall diversity of gut microbiota was reduced in the rotavirus (RV)-infected piglets at 5 days post infection.
| Diversity index |
Non-infected |
RV-infected |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FF | HMO | PRE | FF | HMO | PRE | Diet | Infection | Diet × infection | |
| No. of sequences | 6102±1180 | 5407±656 | 6565±773 | 4072±562 | 6032±800 | 4621±584 | 0.10 | 0.13 | 0.16 |
| No. of OTUs | 339±63 | 278±49 | 356±47 | 222±46 | 265±61 | 314±103 | 0.44 | 0.01 | 0.47 |
| Shannon H | 3.70±0.15 | 3.54±1.05 | 3.74±0.23 | 3.17±0.31 | 3.02±0.36 | 3.38±0.33 | 0.17 | 0.0024 | 0.11 |
| Chao 1 | 501.3±104.9 | 385.3±79.9 | 502.7±70.6 | 322.9±77.6 | 374.1±94.8 | 487.5±195.5 | 0.12 | 0.03 | 0.48 |
Abbreviations: FF, formula fed; HMO, formula supplemented with human milk oligosaccharides (HMO) (4 g l−1); OTU, operational taxonomic unit; PRE, formula supplemented with short-chain galactooligosaccharides and long-chain fructooligosaccharides (4 g l−1).
16S rRNA gene v1-3 pyrosequencing reads were assigned to OTUs at the cutoff of 97% similarity. Shannon H diversity index and Chao 1 estimator were calculated in QIIME according to the OTU numbers of each group (Caporaso et al., 2010). Values are means±s.e.m. (n=7–13 per group).
The taxonomic analysis of the OTUs showed that Firmicutes and Bacteroidetes are the most abundant phyla in piglet colonic microbiota, and the proportion of these two phyla was significantly affected by RV infection, regardless of diet. RV-infected piglets had higher relative abundances of Bacteroidetes (53.15±3.80 vs 39.46±2.81, P=0.02) and lower Firmicutes (42.67±3.25 vs 56.70±2.76, P=0.05) than the non-infected piglets (Supplementary Figure 3). At class/order level, the proportions of certain bacterial groups were also impacted by RV infection, with no diet effect. Class Bacilli (P<0.0001), Erysipelotrichia (P=0.0183) and a group of unclassified Firmicutes (P=0.0511) were decreased, whereas class Bacteroidia (P=0.086) tended to increase in the infected piglets. The family abundance of the piglet microbiota was significantly changed by both RV infection and diet. Infection resulted in the significant increase of Bacteroidaceae and Helicobacteraceae and reduction of Porphyromonadaceae, Lactobacillaceae, Erysipelotrichaceae, unclassified Bacteroidales and unclassified Lactobacillales in the infected piglets, while diet mainly affected on the abundance of Lachnospiraceae, which was exclusively increased in the HMO group regardless of infection (P=0.0003) and Ruminococcaceae, which was lower in the HMO than in the FF and PRE groups (P=0.0133) (Supplementary Table 2).
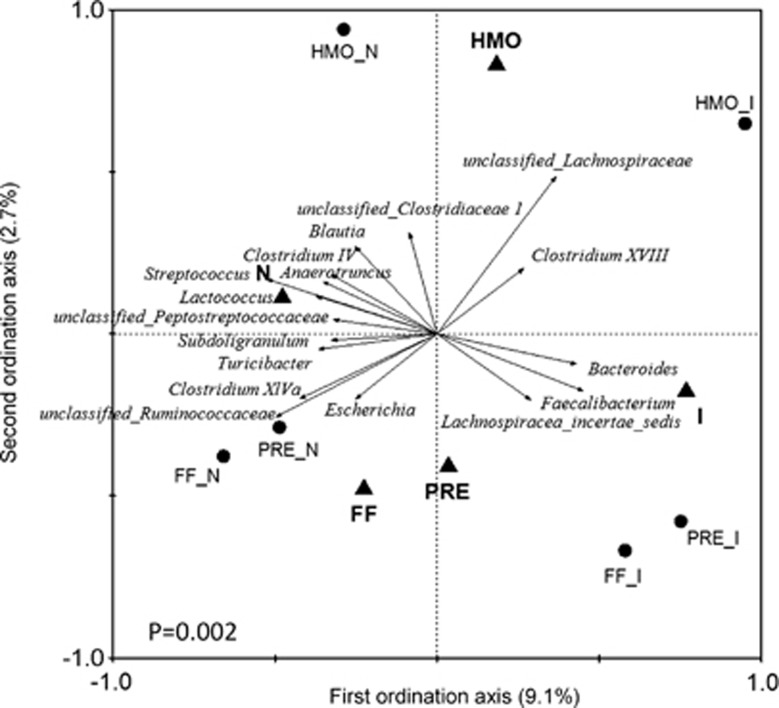
The impact of RV infection and diet on gut microbiota was further evaluated by RDA of the bacterial genus abundance. The triplot of RDA showed that the RV-infected and non-infected piglets were separated at the first constrained axis (explaining 9.1% of the total variability), while the HMO-fed groups differed from the FF- and PRE-fed groups at the second constrained axis (explaining 2.7% of the total variability) (Figure 5), indicating that both RV infection and HMO supplementation changed the gut microbiota. The significance of the data classification was validated by the Monte Carlo permutation test (P=0.002). The genera that contributed at least 10% of the total variability of the samples were identified as key bacterial groups responding to RV infection and dietary treatment, which was confirmed further by univariate statistics (PROC MIXED) (Table 4). Among the 15 key genera, 6 corresponded to the infection effect alone, including Bacteroides, which was increased in the RV-infected piglets, and Parabacteroides, Blautia, Clostridium IV, Anaerotruncus and unclassified Peptostreptococcaceae, which were decreased in the RV-infected piglets. Two genera were changed by the diet effect alone: Clostridium XVIII and unclassified Clostridiaceae 1, which were both higher in the HMO-fed groups compared with the PRE groups. Unclassified Lachnospiraceae, Clostridium XlVa, Faecalibacterium, unclassified Ruminococcaceae, Lactococcus, Streptococcus and Turicibacter were affected by both infection and diet treatment. The major genus that distinguished the HMO from the FF and PRE groups was unclassified Lachnospiraceae.
Figure 5.
Triplot of RDA based on genus abundance of gut microbiota relative to RV infection and diet. Nominal environmental variables (infection: I vs N, diet: FF, HMO, PRE) were indicated by triangles. The center of the samples from each group (n=7–13 per group) was indicated by circles. The genera with at least 10% of the variability in their values explained by the first axis are indicated by arrows. P-value was assessed by a Monte Carlo test. FF_N, formula-fed non-RV infected; HMO_N, formula supplemented with human milk oligosaccharides (4 g l−1) non-infected; PRE_N, formula supplemented with short-chain galactooligosaccharides and long-chain fructooligosaccharides (4 g l−1; 9:1) non-infected, FF_I, formula-fed and RV infected; HMO_I, formula supplemented with HMO and RV-infected; PRE_I, formula supplemented with prebiotics and RV-infected.
Table 4. Relative abundances of key genera responding to rotavirus (RV) infection and dietary treatment identified by redundancy analysis and univariate statistics (PROC MIXED).
| Genus |
Non-infected |
RV-infected |
P-value |
||||||
|---|---|---|---|---|---|---|---|---|---|
| FF | HMO | PRE | FF | HMO | PRE | Diet | Infection | Diet × infection | |
| Anaerotruncus | 0.26±0.07 | 0.26±0.03 | 0.15±0.03 | 0.07±0.02 | 0.09±0.04 | 0.15±0.05 | 0.73 | 0.02 | 0.04 |
| Bacteroides | 26.19±4.12 | 21.60±3.81 | 20.65±4.97 | 47.95±8.76 | 45.7±8.61 | 42.15±7.33 | 0.69 | <0.0001 | 0.85 |
| Blautia | 5.47±1.14 | 11.2±1.95 | 5.71±1.46 | 4.6±1.58 | 4.25±1.72 | 3.69±0.96 | 0.56 | 0.01 | 0.44 |
| Clostridium IV | 0.35±0.1 | 0.51±0.2 | 0.14±0.05 | 0.14±0.05 | 0.05±0.03 | 0.07±0.03 | 0.38 | <0.0001 | 0.53 |
| Clostridium XlVa | 3.69±1.22 | 1.78±0.16 | 5.06±1.13 | 1.91±0.75 | 1.21±0.52 | 2.38±1.05 | 0.03 | 0.0033 | 0.64 |
| Clostridium XVIII | 0.03±0.02 | 0.12±0.05 | 0.01±0.01 | 0.19±0.18 | 0.64±0.5 | 0.06±0.03 | 0.05 | 0.07 | 0.55 |
| Faecalibacterium | 0.14±0.04 | 0.32±0.12 | 0.56±0.31 | 1.46±0.67 | 1.62±0.89 | 8.47±5.41 | 0.0045 | 0.0002 | 0.32 |
| Lactococcus | 0.2±0.07 | 0.16±0.05 | 0.04±0.03 | 0.04±0.02 | 0.01±0.01 | 0.01±0.01 | 0.05 | 0.00 | 0.25 |
| Parabacteroides | 9.33±2.93 | 11.02±3.86 | 10.00±3.86 | 2.84±0.98 | 3.54±1.55 | 3.73±1.69 | 0.88 | 0.0014 | 0.66 |
| Streptococcus | 2.99±0.93 | 2.22±0.53 | 1.06±0.41 | 0.35±0.07 | 0.32±0.06 | 0.5±0.26 | 0.05 | <0.0001 | 0.03 |
| Turicibacter | 0.56±0.22 | 0.54±0.22 | 2.68±1.19 | 0.14±0.08 | 0.02±0.01 | 0.13±0.06 | 0.05 | 0.0004 | 0.20 |
| Unclassified Clostridiaceae 1 | 0.03±0.01 | 0.19±0.1 | 0.02±0.01 | 0.01±0.01 | 0.04±0.03 | 0.02±0.01 | 0.05 | 0.28 | 0.19 |
| Unclassified Lachnospiraceae | 6.93±0.97 | 15.69±2.39 | 7.28±1.18 | 10.67±2.95 | 19.89±2.91 | 9.68±1.21 | <0.0001 | 0.01 | 1.00 |
| unclassified Peptostreptococcaceae | 0.08±0.02 | 0.11±0.04 | 0.14±0.03 | 0.05±0.02 | 0.02±0.01 | 0.04±0.02 | 0.82 | 0.01 | 0.66 |
| Unclassified Ruminococcaceae | 13.41±2.93 | 4.66±0.77 | 7.16±1.53 | 5.59±1.25 | 2.92±0.68 | 3.62±0.86 | 0.0002 | 0.003 | 0.68 |
Abbreviations: FF, formula fed; HMO, formula supplemented with human milk oligosaccharides (HMO) (4 g l−1); PRE, formula supplemented with short-chain galactooligosaccharides and long-chain fructooligosaccharides (4 g l−1).
Values are means±s.e.m. (n=7–13 per group).
Discussion
Herein, we have shown for the first time that feeding HMO modulates the gut microbiota, intestinal immunity and shortens the duration of diarrhea following RV infection. We found that neither HMO nor scGOS/lcFOS mixtures prevented the initial RV infection. However, HMO and PRE supplementation shortened the duration of diarrhea by 31 and 27.5 h, respectively, compared with FF-infected piglets. We observed a biphasic diarrheal response. HMO and PRE decreased the overall duration of diarrhea by reducing the incidence of the second wave of diarrhea from 57% in FF to 25%. Previous work in our lab characterized the time course of RV replication in the RV-infected piglet intestine and demonstrated a biphasic response in RV NSP4 mRNA expression; one at 2 days PI and the second at 4 days PI (unpublished data), corroborating the course of diarrhea in the current study. The secondary peak of diarrhea is due to the release of newly synthesized RV particles by infected enterocytes. Furthermore, in an in situ acute RV infection piglet model, we showed that both neutral (LNnT) and acidic HMO decreased NSP4 expression in the loops (Hester et al., 2013). Taken together, it appears that HMO and prebiotics inhibit RV binding and/or replication predominantly during the second phase of the infection. However, a significant difference in NSP4 mRNA abundance among the diet groups was not observed. As RV replication occurs in the cells at the villi tips of the small intestine, the villus blunting caused by the initial infection may have precluded detection of the differences in mucosal NSP4 expression between the diet groups. Thus multiple time point sampling may be needed to determine whether HMO is in fact inhibiting RV replication at earlier time points in infection. The prebiotic effect is consistent with results of similar studies showing that feeding prebiotics or probiotics reduced the duration, severity and incidence of diarrhea in human infants or animals (Shu et al., 2001; de Vrese and Marteau, 2007), suggesting that HMO or prebiotics confers a degree of protection against RV infection in piglets.
RV infection stimulated a vigorous systemic antibody response, in agreement with studies showing increased serum and intestinal RV-specific IgM, IgG and IgA in RV-infected children (O'Ryan et al., 1994; Velazquez et al., 2000) and animals (Desselberger and Huppertz, 2011). Secretory IgA is associated with protection from infection (Velazquez et al., 2000; Azevedo et al., 2004); however, we did not detect RV-IgA in serum or colonic contents at 5 days PI. Grimwood et al. (1988) found that RV-IgA was frequently undetectable in duodenal fluid or feces in the first week of acute infection in children. Thus, 5 days PI may not have been sufficient time to detect an IgA response to RV.
Interestingly, the prebiotic mixture of scGOS and lcFOS enhanced RV-IgM response in the infected piglets. This prebiotic blend induced a beneficial Ig profile in infants at high risk of allergy (van Hoffen et al., 2009). Benyacoub et al. (2008) also demonstrated that feeding FOS enhanced serum IgG and fecal IgA following Salmonella vaccination in mice. In contrast, an effect of prebiotics on antibody response to vaccination in infants was not detected (Stam et al., 2011). The systemic immune-enhancing effects of prebiotics on RV infection may be relevant to the reduction of diarrhea duration; however, the mechanism warrants further investigation.
Intestinal cytokine profiles were also changed by RV infection. A significant increase in IL-8, IFN-γ and IL-10 expression in the ileum of infected piglets was observed, consistent with previous reports of upregulated pro-inflammatory Th1 and Th2 cytokine response in RV-infected gnotobiotic piglets (Azevedo et al., 2006). Additionally, HMO significantly increased IFN-γ and IL-10 expression in the intestine of both non-infected and infected piglets. IFN-γ inhibits RV entry into human intestinal epithelial cells (Bass, 1997), while IL-10 is known as an anti-inflammatory cytokine that inhibits the synthesis of the major pro-inflammatory cytokines and chemokines (Opal et al., 1998). Further, acidic HMO were shown to stimulate IFN-γ and IL-10 production in cord blood-derived mononuclear cells in vitro, whereas scGOS/lcFOS had no effect on cytokine production (Eiwegger et al., 2010). Neutral HMO, such as LNnT, also increased IL-10 production in mice (Terrazas et al., 2001). A similar study in which the RV-infected piglets were fed lactic acid bacteria resulted in an increase in both Th1 and Th2 cytokines in piglets (Azevedo et al., 2012). Taken together, we hypothesize that HMO influenced host protective immunity by stimulating a balanced Th1 and Th2 cytokine response, consequently, enhancing recovery from diarrhea, that is, shorten the duration of diarrhea.
RV infection altered both the intraluminal environment and the composition of the colonic microbial community. The increase in pH and decrease in DM in the lumen were likely the result of an acid–base and electrolyte disturbance and increased intestinal secretion in response to RV infection (Gennari and Weise, 2008). The infection also reduced gut microbial diversity and increased Bacteroides relative abundance, which is consistent with the greater abundance of Bacteroides fragilis reported in RV-infected children compared with healthy controls (Zhang et al., 2009). B. fragilis is a commensal gut bacteria but is also recognized as an opportunistic pathogen commonly associated with diarrheal disease and clinical infections (Kato et al., 1999). The increase in opportunistic pathogenic bacteria might be caused by the perturbation of the entire microbial community during diarrhea.
Evidence has shown that probiotics or prebiotics may be promising preventive and curative treatments for diarrheal diseases (deVrese and Marteau, 2007). Due to the prebiotic effects of HMO observed in vitro (Li et al., 2012), we anticipated that HMO would exert a protective effect against RV infection via modulation of the gut microbiota. The sequencing results confirmed that the microbiota of HMO-fed piglets differed from that of the FF and PRE groups. Most interestingly, HMO specifically increased the amount of unclassified Lachnospiraceae. A recent study found that a Lachnospiraceae isolate suppressed Clostridium difficile colonization in the gut of germ-free mice (Reeves et al., 2012). Likewise, clinical studies have linked reduced Lachnospiraceae abundance with chronic intestinal disorders, such as inflammatory bowel disease (Frank et al., 2007). The Lachnospiraceae family contains numerous butyrate-producing bacteria (Cotta and Forster, 2006), which could ferment HMO to SCFA that are beneficial for intestinal morphology and barrier function (Scheppach, 1994) and, hence, protect against RV infection. However, we did not observe a diet effect on SCFA production in the current study; thus, other protective mechanisms also may be considered. There was a consistent increase in IFN-γ mRNA expression and the abundance of unclassified Lachnospiraceae in the HMO-fed groups, and we also observed a positive correlation between IFN-γ and the abundance of unclassified Lachnospiraceae (Pearson's correlation, r=0.32, P=0.027). Therefore, an interaction between the gut bacteria and mucosal immune system mediated by HMO may contribute to the protection conferred against RV infection by HMO feeding. However, this postulate requires further investigation.
Previous in vitro studies showed that HMO promoted the growth of Bifidobacterium isolated from human infants' feces (Ward et al., 2007; Marcobal et al., 2010). In this study, the level of Bifidobacterium measured by quantitative PCR was reduced in the infected piglets but was not affected by diet (data not shown). This is likely due to the phenotypic differences in bifidobacteria in humans and pigs (Gavini et al., 1991) and the bifidobacterial species/strain specificity of HMO metabolism (LoCascio et al., 2007; Ward et al., 2007).
In summary, this study describes for the first time in a clinically relevant, a large mammalian, non-rodent animal model the in vivo effects of HMO on mucosal immunity, composition of the gut microbiota and response to RV infection. Combined with our previous in vitro study, we concluded that HMO supplementation could protect neonates against RV infection, as evidenced by the shorter duration of diarrhea, by inhibiting RV binding and/or replication, enhancing mucosal Th1/Th2 cytokine response and modulating the composition and, thus, metabolic potential of the gut microbiota. In contrast, the prebiotic scGOS/lcFOS mixture only promoted a systemic antibody response to infection. Therefore, supplementing formula with HMO may represent a novel nutritional approach to protect against RV infection in human infants and animals.
Acknowledgments
We thank Laura Bauer for technical assistance with the SCFA measurement. We also thank Glycom (Lyngby, Denmark) for providing 2′FL, LNnT and SA through their donation program. This research was funded by an NIH Grant R01 HD061929 to SMD.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Azevedo MS, Zhang W, Wen K, Gonzalez AM, Saif LJ, Yousef AE et al. (2012). Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef Microbes 3: 33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MS, Yuan L, Pouly S, Gonzales AM, Jeong KI, Nguyen TV et al. (2006). Cytokine responses in gnotobiotic pigs after infection with virulent or attenuated human rotavirus. J Virol 80: 372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo MS, Yuan L, Iosef C, Chang KO, Kim Y, Nguyen TV et al. (2004). Magnitude of serum and intestinal antibody responses induced by sequential replicating and nonreplicating rotavirus vaccines in gnotobiotic pigs and correlation with protection. Clin Diagn Lab Immunol 11: 12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babji S, Kang G. (2012). Rotavirus vaccination in developing countries. Curr Opin Virol 2: 443–448. [DOI] [PubMed] [Google Scholar]
- Bass DM. (1997). Interferon gamma and interleukin 1, but not interferon alfa, inhibit rotavirus entry into human intestinal cell lines. Gastroenterology 113: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benyacoub J, Rochat F, Saudan KY, Rochat I, Antille N, Cherbut C et al. (2008). Feeding a diet containing a fructooligosaccharide mix can enhance Salmonella vaccine efficacy in mice. J Nutr 138: 123–129. [DOI] [PubMed] [Google Scholar]
- Bode L. (2006). Recent advances on structure, metabolism, and function of human milk oligosaccharides. J Nutr 136: 2127–2130. [DOI] [PubMed] [Google Scholar]
- Bode L, Kunz C, Muhly-Reinholz M, Mayer K, Seeger W, Rudloff S. (2004. a). Inhibition of monocyte, lymphocyte, and neutrophil adhesion to endothelial cells by human milk oligosaccharides. Thromb Haemost 92: 1402–1410. [DOI] [PubMed] [Google Scholar]
- Bode L, Rudloff S, Kunz C, Strobel S, Klein N. (2004. b). Human milk oligosaccharides reduce platelet-neutrophil complex formation leading to a decrease in neutrophil beta 2 integrin expression. J Leukoc Biol 76: 820–826. [DOI] [PubMed] [Google Scholar]
- Boehm G, Moro G. (2008). Structural and functional aspects of prebiotics used in infant nutrition. J Nutr 138: 1818S–1828S. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7: 335–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comstock SS, Wang M, Hester SN, Li M, Donovan SM. (2013). Select human milk oligosaccharides directly modulate peripheral blood mononuclear cells isolated from 10-d-old pigs. Br J Nutr 17: 1–10. [DOI] [PubMed] [Google Scholar]
- Cotta M, Forster R. (2006). The Family Lachnospiraceae, including the Genera Butyrivibrio, Lachnospira and Roseburia. Prokaryotes 4: 1002–1021. [Google Scholar]
- de Vrese M, Marteau PR. (2007). Probiotics and prebiotics: effects on diarrhea. J Nutr 137: 803S–811SS. [DOI] [PubMed] [Google Scholar]
- Desselberger U, Huppertz HI. (2011). Immune responses to rotavirus infection and vaccination and associated correlates of protection. J Infect Dis 203: 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 27: 2194–2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiwegger T, Stahl B, Haidl P, Schmitt J, Boehm G, Dehlink E et al. (2010). Prebiotic oligosaccharides: in vitro evidence for gastrointestinal epithelial transfer and immunomodulatory properties. Pediatr Allergy Immunol 21: 1179–1188. [DOI] [PubMed] [Google Scholar]
- Frank DN St, Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci USA 104: 13780–13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini F, Pourcher AM, Neut C, Monget D, Romond C, Oger C et al. (1991). Phenotypic differentiation of bifidobacteria of human and animal origins. Int J Syst Bacteriol 41: 548–557. [DOI] [PubMed] [Google Scholar]
- Gennari FJ, Weise WJ. (2008). Acid-base disturbances in gastrointestinal disease. Clin J Am Soc Nephrol 3: 1861–1868. [DOI] [PubMed] [Google Scholar]
- Giaquinto C, Dominiak-Felden G, Van Damme P, Myint TT, Maldonado YA, Spoulou V et al. (2011). Summary of effectiveness and impact of rotavirus vaccination with the oral pentavalent rotavirus vaccine: a systematic review of the experience in industrialized countries. Hum Vaccin 7: 734–748. [DOI] [PubMed] [Google Scholar]
- Grimwood K, Lund JC, Coulson BS, Hudson IL, Bishop RF, Barnes GL. (1988). Comparison of serum and mucosal antibody responses following severe acute rotavirus gastroenteritis in young children. J Clin Microbiol 26: 732–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. (2011). Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol 76: 301–310. [DOI] [PubMed] [Google Scholar]
- Hester SN, Chen X, Li M, Monaco MH, Comstock SS, Kuhlenschmidt TB et al. (2013). Human milk oligosaccharides inhibit rotavirus infectivity in vitro and in acutely infected piglets. Br J Nutr 26: 1–10. [DOI] [PubMed] [Google Scholar]
- Kato N, Liu C, Kato H, Watanabe K, Nakamura H, Iwai N et al. (1999). Prevalence of enterotoxigenic Bacteroides fragilis in children with diarrhea in Japan. J Clin Microbiol 37: 801–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knol J, Boehm G, Lidestri M, Negretti F, Jelinek J, Agosti M et al. (2005). Increase of faecal bifidobacteria due to dietary oligosaccharides induces a reduction of clinically relevant pathogen germs in the faeces of formula-fed preterm infants. Acta Paediatr 94(Suppl): 31–33. [DOI] [PubMed] [Google Scholar]
- Kunz C, Rudloff S, Baier W, Klein N, Strobel S. (2000). Oligosaccharides in human milk: structural, functional, and metabolic aspects. Annu Rev Nutr 20: 699–722. [DOI] [PubMed] [Google Scholar]
- Li M, Bauer LL, Chen X, Wang M, Kuhlenschmidt TB, Kuhlenschmidt MS et al. (2012). Microbial composition and in vitro fermentation patterns of human milk oligosaccharides and prebiotics differ between formula-fed and sow-reared piglets. J Nutr 142: 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LoCascio RG, Ninonuevo MR, Freeman SL, Sela DA, Grimm R, Lebrilla CB et al. (2007). Glycoprofiling of bifidobacterial consumption of human milk oligosaccharides demonstrates strain specific, preferential consumption of small chain glycans secreted in early human lactation. J Agric Food Chem 55: 8914–8919. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Barboza M, Froehlich JW, Block DE, German JB, Lebrilla CB et al. (2010). Consumption of human milk oligosaccharides by gut-related microbes. J Agric Food Chem 58: 5334–5340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newburg DS, Ruiz-Palacios GM, Morrow AL. (2005). Human milk glycans protect infants against enteric pathogens. Annu Rev Nutr 25: 37–58. [DOI] [PubMed] [Google Scholar]
- Ninonuevo MR, Park Y, Yin H, Zhang J, Ward RE, Clowers BH et al. (2006). A strategy for annotating the human milk glycome. J Agric Food Chem 54: 7471–7480. [DOI] [PubMed] [Google Scholar]
- Opal SM, Wherry JC, Grint P. (1998). Interleukin-10: potential benefits and possible risks in clinical infectious diseases. Clin Infect Dis 27: 1497–1507. [DOI] [PubMed] [Google Scholar]
- O'Ryan ML, Matson DO, Estes MK, Pickering LK. (1994). Anti-rotavirus G type-specific and isotype-specific antibodies in children with natural rotavirus infections. J Infect Dis 169: 504–511. [DOI] [PubMed] [Google Scholar]
- Parashar UD, Gibson CJ, Bresee JS, Glass RI. (2006). Rotavirus and severe childhood diarrhea. Emerg Infect Dis 12: 304–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenge-Bonig A, Soto-Ramirez N, Karmaus W, Petersen G, Davis S, Forster J. (2010). Breastfeeding protects against acute gastroenteritis due to rotavirus in infants. Eur J Pediatr 169: 1471–1476. [DOI] [PubMed] [Google Scholar]
- Reeves AE, Koenigsknecht MJ, Bergin IL, Young VB. (2012). Suppression of Clostridium difficile in the gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun 80: 3786–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W. (1994). Effects of short chain fatty acids on gut morphology and function. Gut 35: S35–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Q, Qu F, Gill HS. (2001). Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhea associated with rotavirus and Escherichia coli infection in a piglet model. J Pediatr Gastroenterol Nutr 33: 171–177. [DOI] [PubMed] [Google Scholar]
- Stam J, van Stuijvenberg M, Garssen J, Knipping K, Sauer PJ. (2011). A mixture of three prebiotics does not affect vaccine specific antibody responses in healthy term infants in the first year of life. Vaccine 29: 7766–7772. [DOI] [PubMed] [Google Scholar]
- Tao N, Ochonicky KL, German JB, Donovan SM, Lebrilla CB. (2010). Structural determination and daily variations of porcine milk oligosaccharides. J Agric Food Chem 58: 4653–4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrazas LI, Walsh KL, Piskorska D, McGuire E, Harn DA Jr. (2001). The schistosome oligosaccharide lacto-N-neotetraose expands Gr1(+) cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4(+) cells: a potential mechanism for immune polarization in helminth infections. J Immunol 167: 5294–5303. [DOI] [PubMed] [Google Scholar]
- van Hoffen E, Ruiter B, Faber J, M'Rabet L, Knol EF, Stahl B et al. (2009). A specific mixture of short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides induces a beneficial immunoglobulin profile in infants at high risk for allergy. Allergy 64: 484–487. [DOI] [PubMed] [Google Scholar]
- Velazquez FR, Matson DO, Guerrero ML, Shults J, Calva JJ, Morrow AL et al. (2000). Serum antibody as a marker of protection against natural rotavirus infection and disease. J Infect Dis 182: 1602–1609. [DOI] [PubMed] [Google Scholar]
- Wang M, Radlowski EC, Monaco MH, Fahey GC Jr, Gaskins HR, Donovan SM. (2013). Mode of delivery and early nutrition modulate microbial colonization and fermentation products in neonatal piglets. J Nutr 143: 795–803. [DOI] [PubMed] [Google Scholar]
- Wang Q, Garrity GM, Tiedje JM, Cole JR. (2007). Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73: 5261–5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward RE, Ninonuevo M, Mills DA, Lebrilla CB, German JB. (2007). In vitro fermentability of human milk oligosaccharides by several strains of bifidobacteria. Mol Nutr Food Res 51: 1398–1405. [DOI] [PubMed] [Google Scholar]
- Zhang M, Zhang M, Zhang C, Du H, Wei G, Pang X et al. (2009). Pattern extraction of structural responses of gut microbiota to rotavirus infection via multivariate statistical analysis of clone library data. FEMS Microbiol Ecol 70: 21–29. [DOI] [PubMed] [Google Scholar]
- Zijlstra RT, McCracken BA, Odle J, Donovan SM, Gelberg HB, Petschow BW et al. (1999). Malnutrition modifies pig small intestinal inflammatory responses to rotavirus. J Nutr 129: 838–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.