Abstract
The occurrence of nitrification in the oceanic water column has implications extending from local effects on the structure and activity of phytoplankton communities to broader impacts on the speciation of nitrogenous nutrients and production of nitrous oxide. The ammonia-oxidizing archaea, responsible for carrying out the majority of nitrification in the sea, are present in the marine water column as two taxonomically distinct groups. Water column group A (WCA) organisms are detected at all depths, whereas Water column group B (WCB) are present primarily below the photic zone. An open question in marine biogeochemistry is whether the taxonomic definition of WCA and WCB organisms and their observed distributions correspond to distinct ecological and biogeochemical niches. We used the natural gradients in physicochemical and biological properties that upwelling establishes in surface waters to study their roles in nitrification, and how their activity—ascertained from quantification of ecotype-specific ammonia monooxygenase (amoA) genes and transcripts—varies in response to environmental fluctuations. Our results indicate a role for both ecotypes in nitrification in Monterey Bay surface waters. However, their respective contributions vary, due to their different sensitivities to surface water conditions. WCA organisms exhibited a remarkably consistent level of activity and their contribution to nitrification appears to be related to community size. WCB activity was less consistent and primarily constrained to colder, high nutrient and low chlorophyll waters. Overall, the results of our characterization yielded a strong, potentially predictive, relationship between archaeal amoA gene abundance and the rate of nitrification.
Keywords: nitrification, upwelling, Thaumarchaeota, ecotype, amoA , euphotic zone
Introduction
Since their initial discovery in the coastal waters off California (DeLong 1992; Fuhrman et al., 1992), the debate about the metabolic lifestyle of planktonic marine Group I Archaea has persisted. The realization that mesophillic Crenarchaeota, now known as Thaumarchaeota, comprise as much as 20–30% of planktonic cells in the mesopelagic zone of the ocean—making them one of the most abundant groups of organisms on the planet (Karner et al., 2001)—drew widespread attention to the debate about their role in elemental cycles of the ocean. Significant advancements in our understanding came nearly a decade ago with the discovery of an ammonia monooxygenase subunit A (amoA) gene, putatively of archaeal origin, in the surface waters of the Sargasso Sea (Venter et al., 2004) and the cultivation of a marine ammonia-oxidizing archaeon (Könneke et al., 2005). Subsequent proof that these amoA genes are widespread throughout the marine environment (Francis et al., 2005) led many to focus their efforts on elucidating the role of these newly discovered ammonia-oxidizing archaea (AOA) in the nitrogen (N) cycle.
Nitrification, of which the first step is ammonia oxidation, can have a significant role in supplying nitrate (NO3−) for primary production and may be a source of N2O to the atmosphere (Yool et al., 2007; Clark et al., 2008; Santoro et al., 2011; Beman et al., 2012). There has been a widespread effort to determine the distribution of AOA in nearly all major oceanic regions (Lam et al., 2007; 2009; Beman et al., 2008; Church et al., 2010; Santoro et al., 2010; Christman et al., 2011; Alonso-Sáez et al., 2012; Baker et al., 2012; Sintes et al., 2013). Substantial evidence now exists that AOA have a primary role in determining the distribution and magnitude of nitrification in the sea (Mincer et al., 2007; Beman et al., 2008; Church et al., 2010; Hollibaugh et al., 2010; Newell et al., 2011; Santoro et al., 2013). Depending on depth in the water column, ammonia oxidation may be due to the activity of one or two dominant phylogenetic groups of AOA (Beman et al., 2008, 2010; Santoro et al., 2010). Water column groups A (WCA) and B (WCB) were identified as unique sequence types in the first survey of archaeal amoA diversity in the marine environment (Francis et al., 2005). In stratified marine water columns, the distribution of the two ecotypes is highly predictable: WCA are present throughout, whereas WCB are generally relegated to depths below the euphotic zone, the sunlit layer of the ocean (Beman et al., 2008, 2010; Santoro et al., 2010). Metagenomic studies later confirmed their phylogenetic separation and distinct depth distribution and suggested that the two groups represent ‘ecotypes' or closely related organisms with slight variations in physiological traits, leading to different niche specificities (Hallam et al., 2006; Mincer et al., 2007). This depth separation of WCA and WCB organisms has been supported by sequencing of archaeal 16S rRNA and intergenic spacer regions (Garcia-Martinez and Rodriguez-Valera, 2000), as well as in the phylogenies of archaeal carbon fixation and nitrogen metabolism genes (Yakimov et al., 2011; Lund et al., 2012), although identifying their role in ocean biogeochemical cycles has remained a challenge.
Evidence for widespread autotrophy, presumably linked to ammonia oxidation, suggests that mesopelagic Thaumarchaeota are a globally significant sink for inorganic carbon in the deep ocean (Hansman et al., 2009; Swan et al., 2011; Yakimov et al., 2011). However, several data sets support metabolic plasticity of the AOA, with a potential to live as heterotrophs or mixotrophs (Ouverney and Fuhrman, 2000; Herndl et al., 2005; Ingalls et al., 2006; Agogué et al., 2008). Understanding the metabolic lifestyles of AOA ecotypes is critical to determining their role in dark fixation of CO2 and may also explain the apparently different but overlapping niches they occupy in the ocean. Arriving at a holistic understanding of the role that WCA and WCB ecotypes of AOA play in oceanic nitrification and how their respective contributions may vary in response to changes in ocean conditions is paramount to our ability to forecast changes in elemental cycles under future ocean scenarios (Gruber, 2011). Projected declines in pH, for example, could alter their roles in NO3− and N2O production, depending on their susceptibility to such changes (Beman et al., 2011).
In order to advance our understanding of the roles of marine Thaumarchaeota in elemental cycles, efforts should now focus on identifying the ecophysiological differences among these organisms. Here, we sought to elucidate the biogeochemical roles of AOA ecotypes by examining the linkages between patterns of archaeal amoA gene abundance and expression from the two dominant ecotypes of marine Thaumarchaeota and measured rates of nitrification in a coastal upwelling ecosystem. In contrast to the deep (for example, mesopelagic) ocean, these surface waters experience dramatic physicochemical changes over short spatiotemporal scales that setup natural gradients in factors hypothesized to regulate the distributions and activity of dominant AOA ecotypes, including light, ammonia concentrations and phytoplankton abundance and activity (Horrigan et al., 1981; Olson, 1981b; Ward 1985; Kudela et al., 1997; Dugdale et al., 2006; Merbt et al., 2011; Sintes et al., 2013). Our approach has the distinct advantage of allowing the acquisition of both expressional and biogeochemical activity data from a wide array of water column samples with common origins, undergoing an evolution of community structure and activity. This data set yields new insights into the dynamics of AOA ecotypes in relation to nitrification in the ocean.
Materials and methods
Sample collection
Water samples were collected in April and June of 2011 aboard the R/V John Martin with a rosette consisting of 12 × 2.5 l Niskin bottles, a Seabird SBE 19plus conductivity–temperature–depth (CTD) sensor package equipped with SBE 43 oxygen sensor (Seabird Electronics, Bellevue, WA, USA), a Wetlabs C-Star transmissometer and a Wetlabs WETstar fluorometer (Wetlabs, Philomath, OR, USA). Seawater density was determined from CTD data, based on measured temperature and pressure and the calculated salinity. Resultant densities are expressed as σt=density−1000 referenced to surface pressure. Determination of macronutrient concentrations, chlorophyll and rates of primary production on whole seawater samples were conducted as described previously (Pennington and Chavez, 2000). Ammonium concentrations were measured by ortho-phthalaldehyde fluorescence (Holmes et al., 1999). Seawater samples collected from the same rosette bottles as the whole water used in rate incubations were collected into graduated 1-L polycarbonate bottles and immediately placed into a dark cooler. Cells were harvested by pressure filtration through 25-mm filters housed in Swinnex filter holders (Millipore, Billerica, MA, USA); each sample was first passed through a 10-μm pore size polyester prefilter (GE Osmonics, Trevose, PA, USA) and then a 0.2-μm filter (Supor, Pall Inc., Port Washington, NY, USA). For each co-extraction, 1-L sample volumes were collected (at each station and depth for which an incubation was conducted) and the filters were flash frozen in liquid nitrogen in gasketed 2-ml bead tubes containing a mixture of 0.1 and 0.5 mm glass beads. The shallow sampling depths for this study allowed fast deployment and recovery of the CTD-Rosette sampler and thus fast sample acquisition. Filtration and cell harvesting were initiated within 10 min of sample collection from the rosette and within 30 min after deployment of the CTD-Rosette system. Samples from April (N=41) were from depths of 2 m (N=7), 5 m (N=7), 10 m (N=20) and 20 m (N=7). June samples (N=32) were from depths of 5 m (N=15) and 10 m (N=18).
Co-extraction of DNA and RNA
Total nucleic acids were extracted from a single filter sample using a two-step co-extraction protocol. First, the samples were removed from storage at −80 °C and immediately placed on ice. Then, 750 μl of lysis buffer (mirVana miRNA isolation kit, Life Technologies, Carlsbad, CA, USA) was added to each tube, which was then sealed with parafilm and vortexed briefly to disperse the lysis buffer containing RNAse inhibitors. Following this, cell lysis was executed by mechanical agitation in a FastPrep bead beater (MP Biomedicals, Santa Ana, CA, USA) for two cycles of 45 s at setting 5.5. The tubes were then spun down to reduce foam. The supernatant was passed through a DNeasy DNA capture column (Qiagen, Valenica, CA, USA). Columns were then stored at 4 °C until RNA extraction was completed (ca. 2 h). Column-bound DNA was purified and eluted using the DNeasy kit according to the manufacturer's protocol, yielding an average (±s.d.) of 1.6±0.6 μg DNA l−1 of seawater.
Following passage through the DNeasy column, the eluent was immediately processed for total RNA purification using the mirVana miRNA isolation kit (Life Technlogies) following the manufacturer's protocol. RNA was eluted from the capture column with 75 μl of 95 °C nuclease-free water. RNA yields averaged (±s.d.) 2.1±0.6 μg RNA l−1 seawater. An aliquot of the purified RNA was immediately subjected to removal of contaminating DNA using the Turbo DNA-free kit following the manufacturer's protocol. cDNA was synthesized with random hexamers using the SuperScript III First-Strand Synthesis System for reverse transcriptase–PCR (Invitrogen, Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol, except for increasing the reverse transcription incubation step to 5 h at 50 °C (Lund et al., 2012). Negative control reactions were performed for each sample by replacing the reverse transcriptase enzyme with water.
Estimation of amoA gene and transcript abundances
Thaumarchaeal amoA genes and mRNA transcripts were estimated by quantitative PCR (qPCR) in all samples from Monterey Bay (MB) taken in April and June of 2011. Total thaumarchaeal amoA was estimated by summation of two independent, non-overlapping qPCR assays targeting the two most common phylogenetic groups of pelagic marine AOA (Mosier and Francis, 2011). The assays were run with identical qPCR reaction chemistries as follows: 12.5 μl Taqman Environmental Master Mix 2.0 (Applied Biosystems, Life Technologies), 200 nM of each primer, 300 nM of each probe and either 1 μl of DNA or 2 μl of cDNA template per reaction, to a final volume of 25 μl. Cycling conditions were: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, and 56 °C for 1 min, followed by detection.
All qPCR assays were run in triplicate using a StepOnePlus Real-Time PCR System (Applied Biosystems, Life Technologies). Standard curves ranging from 1 to 1 × 106 copies per reaction were generated from purified, linearized plasmids obtained from clone libraries constructed with each primer set. The limit of detection for both qPCR assays was 102 copies l−1 of seawater (1 copy per reaction). In the event that the coefficient of variation for a set of triplicate reactions exceeded 10%, one of the replicates was omitted or the sample was reanalyzed. Both assays had efficiencies of 94–99% across all samples; all results were consistent and reproducible.
15NH+4 ammonia oxidation
Nitrification rate measurements were performed at four depths at station M0 (0, 5, 10, 20 m) and at 10-m depth at all other stations. For each set of incubations, 800 ml of water was spiked with 15N-labelled NH4Cl to a nominal final concentration of 750 nmol l−1. The spiked whole water was then split into duplicate 280-ml polycarbonate incubation bottles, which were capped tightly and incubated on board the ship using a flow-through incubator cooled with local surface waters. Seawater samples were incubated at estimated in situ levels of light using stainless steel tubes pre-drilled with evenly spaced and sized holes, which were submerged in the deck incubator (Pennington and Chavez, 2000). Samples from depths 0, 5, 10 and 20 m were incubated in light tubes transmitting 50, 15, 1 and 0% light, respectively. Replicate 50-ml samples were removed from each incubation bottle at 0 and 24 h after the start of the incubation, as well as two intermediate time points (between 6–18 h), filtered through a 0.2-micron syringe filter (Sterivex, Millipore) and stored at −20 °C until analysis.
Stable isotope analysis and rate calculations
Analyses of the δ15N in NO3−+NO2− were performed using the denitrifier method (Sigman et al., 2001; Casciotti et al., 2002) either at the University of California Davis Stable Isotope Facility using a ThermoFinnigan GasBench and PreCon trace gas concentration system interfaced to Delta VPLUS or at the Stanford University, Stanford, CA, USA using a Finnigan Delta PLUS isotope ratio mass spectrometer. δ15N values were calibrated against nitrate isotope reference materials USGS32, USGS34 and USGS35 analyzed in parallel (Casciotti et al., 2008). Nitrification rates were calculated according to the method developed by Santoro et al. (2010), based on modeling the 15N and 14N contents of the NO3−+NO2− pool over time, with inputs from both the 15N-labeled NH4 pool and losses through uptake of NO3−+NO2. We used a mass balance approach to estimate the 15NH4+ content of the ammonium pool using the ambient concentrations of NH4+ and those measured following tracer addition.
Statistical analysis
All statistical analyses were performed on untransformed data in SPSS version 20 (IBM, Armonk, NY, USA). Fit analysis for the predictive relationships was done with Igor Pro version 6.04 (Wavemetrics, Portland, OR, USA).
Results and Discussion
Upwelling-driven biogeochemical variability
During spring and summer months, variations in the strength and direction of coastal winds drive frequent, intense changes in the chemical and biological properties of surface waters of MB (Pennington and Chavez, 2000), a deep, non-estuarine embayment on the central coast of California (Supplementary Figure S1). When northwesterly winds increase, cold, nutrient-rich waters are brought to the surface at the upwelling center just north of the bay (Breaker and Broenkow, 1994). As these waters mature and mix with inshore waters, warming increases stratification and rates of primary productivity, which drives the transformation (maturation) of newly upwelled waters into those representing ‘typical' bay surface waters, with high quantities of chlorophyll and low macronutrient concentrations (Kudela et al., 1997; Pennington and Chavez, 2000). We measured the abundance of AOA amoA genes and transcripts, as well as 15N-based nitrification rates, in 73 locations (stations/depths) collected during two different months of the 2011 upwelling season in MB.
The inherent displacement of water associated with upwelling means that the chemical and biological parameters of a water parcel, determined at the time of sampling, are reflective of events that occurred before and subsequent to the water being upwelled. Thus the chemical and biological characteristics of a given water sample may not be entirely reflective of the depth they were acquired but also of the one from which they came. In consideration of this, and the overlapping ranges in the chemical and biological data from different stations and sampling depths (Supplementary Figure S2), we analyzed data acquired during April and June of 2011, two different periods of upwelling intensity (Supplementary Figure S3), as a composite for each period of study using density as the master variable (Figure 1). Samples from April were composited (N=41) from depths of 2 m (N=7), 5 m (N=7), 10 m (N=20) and 20 m (N=7). June samples were composited (N=32) from depths of 5 m (N=15) and 10 m (N=18). Correlation coefficients for depth with all variables were statistically supported (P<0.05) but lower (R<0.7) than that of density (or other factors co-varying with it) (Supplementary Table S1).
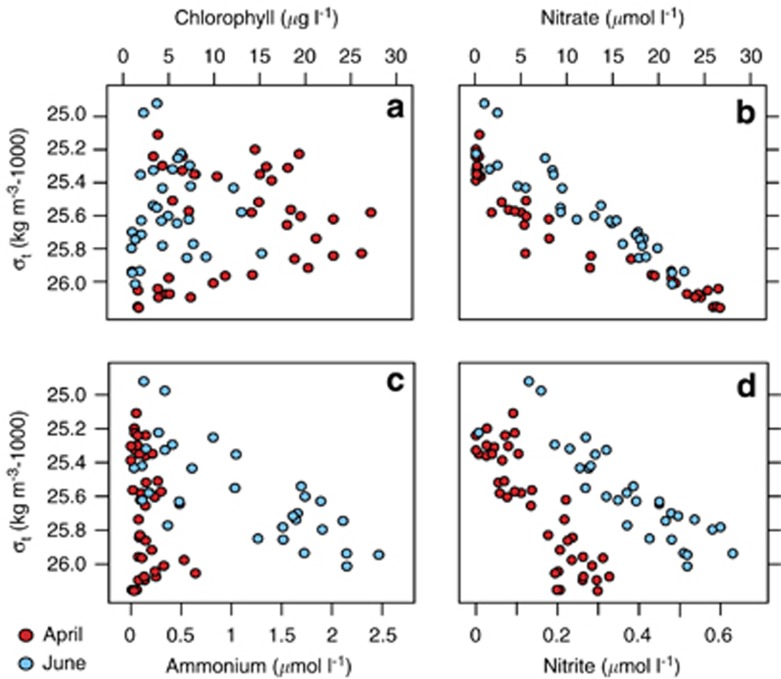
Figure 1.
Distribution and concentrations of (a) chlorophyll, (b) nitrate, (c) ammonium and (d) nitrite in the surface waters of Monterey Bay. Samples from April are composited (N=41) from depths of 2 m (N=7), 5 m (N=7), 10 m (N=20) and 20 m (N=7). June samples are composited (N=32) from depths of 5 m (N=15) and 10 m (N=18).
April sampling expeditions took place following the first major upwelling event of the year, during a period of wind relaxation. At this time, waters in the bay contained higher chlorophyll and lower macronutrient concentrations (Table 1 and Figures 1a and b). As upwelled waters warm and stratify, a large, diverse assemblage of phytoplankton reliant on nitrate for growth establishes itself, driving macronutrient depletion in the near surface and a deepening of the nitracline (Figures 1a and b). The relatively high chlorophyll and longer apparent history of nitrate drawdown are characteristics of ‘aged upwelled' waters typical of MB during periods of wind relaxation (Kudela and Dugdale, 2000; Pennington and Chavez, 2000).
Table 1. Average physicochemical conditions in samples taken from Monterey Bay surface waters during April and June 2011.
| Temperature† (oC) | Salinity | Chl a (μg l−1) | NH4+ (μM) | NO2− (μM) | NO3− (μM) | SiO44− (μM) | PO43− (μM) | O2 (ml l−1) | 15NH4+oxidation (nmol l−1 day−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| April | ||||||||||
| Mean | 11.0a | 33.6a | 12.6a | 0.15a | 0.15a | 10.0 | 10.8 | 1.2 | 5.9 | 23.5 |
| s.d. | 1.1 | 0.2 | 8.2 | 0.13 | 0.09 | 10.3 | 11.2 | 0.7 | 2.0 | 21.9 |
| N | 41 | 41 | 41 | 41 | 41 | 41 | 41 | 41 | 41 | 27 |
| June | ||||||||||
| June | ||||||||||
| Mean | 11.9b | 33.7b | 4.8b | 1.0b | 0.37b | 12.6 | 14.6 | 1.4 | 5.4 | 14.8 |
| s.d. | 1.2 | 0.1 | 3.4 | 0.79 | 0.15 | 6.8 | 7.4 | 0.5 | 1.5 | 15.1 |
| N | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 32 | 14 |
Different letters between April and June denote significantly different means (α=0.05), within a variable; when letters are absent, no statistical difference between means was found.
The effects of nitrate uptake by phytoplankton are less evident in June samples, which were obtained during a period of active upwelling. The low apparent rates of nitrate uptake in June can be attributed to the lower phytoplankton abundances (Figure 1a) (F1,72=25.39, P<0.001) and the higher availability of ammonium (Figure 1c) (F1,72=56.57, P<0.001) (Table 1). Interaction of waters with the continental shelf during upwelling leads to pronounced enrichment of ammonium in surface waters (Dugdale et al., 2006). This pool can persist for several days following an upwelling event, where it serves to restrict rates of nitrate drawdown until it is depleted below a certain threshold (Dortch 1990; Kudela et al., 1997; Fawcett and Ward, 2011). Concentrations of nitrite were also elevated in June compared with April (Figure 1d), although the reason for this is less clear. Unlike ammonium, nitrite does not accumulate to appreciable levels in sediments, suggestive of a water column source (for example, ammonia oxidation or nitrite excretion by light-stressed phytoplankton) (Olson 1981a; Santoro et al., 2013)
The influence of upwelling on the rate of nitrification was assessed by measurement of 15NH4 oxidation rates at all locations sampled during the course of our study (N=73). However, rates were below detection at 31 sites (stations/depths), mostly in waters shallower than 10 m (Supplementary Figure S2F). The inability to measure rates of 15NH4 oxidation in some highly productive surface waters is most likely related to the intense competition for ammonia and nitrate by phytoplankton during periods following upwelling (Kudela et al., 1997; Kudela and Dugdale, 2000). Measured nitrification rates in MB surface waters ranged from 0.03 to 69 nmol l−1 day−1 (Figure 2), consistent with those reported previously (Ward, 2005). No statistical differences were evident in average rates for each sampling period (F1,40=1.76, P=0.19; Table 1).
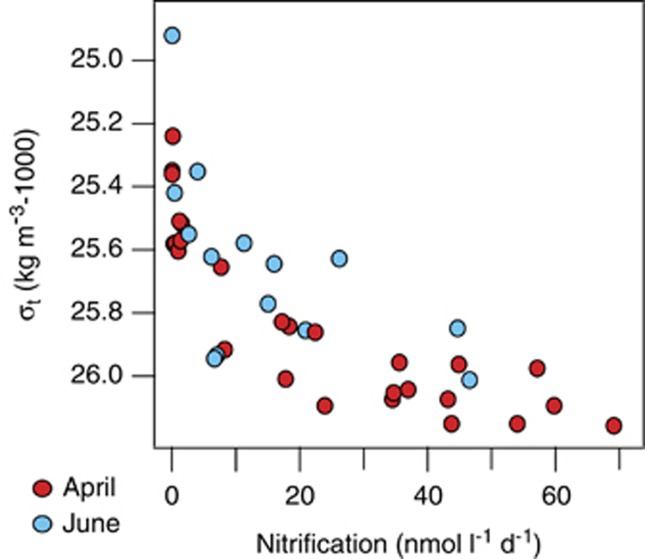
Figure 2.
Nitrification rates in Monterey Bay surface waters in April (N=27) and June (N=15) of 2011. Data are plotted against density rather than with depth.
Correlation analysis of rate data from both study periods with other physicochemical and biological variables revealed strong (R>0.7), significant (P<0.05) positive correlations with density, salinity and macronutrient concentrations and negative correlations with temperature and oxygen (Supplementary Table S1). All of these point toward nitrification rates being maximal deeper in the euphotic zone, where phytoplankton growth and light intensities are lower (Ward, 2005). No relationship between nitrification rates and ammonium concentrations was observed (Supplementary Table S1). Overall, rates were widely variable at a given depth (Supplementary Figure S2F). However, when analyzed by density, there was a clear and consistent pattern in the distribution and magnitude of nitrification rates; they were relatively low throughout the less dense surface waters, then increased dramatically below σt=25.5 kg m−3, continuing to a maximum in the densest waters sampled, σt=26.2 kg m−3 (10–20 m depth) (Figure 2).
Abundance of ecotype-specific amoA genes in relation to upwelling dynamics
Archaeal amoA genes from WCA and WCB AOA ecotypes were present in 100% of samples collected (N=73). WCA amoA gene abundances spanned four orders of magnitude, from 103 to 107 genes l−1 (Figure 3a). WCB amoA genes were considerably more variable, ranging over six orders of magnitude from 101 to 107 genes l−1 (Figure 3b). The abundance of amoA genes related to both ecotypes co-varied strongly with each other during both months of study (R=0.67, P<0.05) (Supplementary Figure S4A) and were correlated positively with density, macronutrient concentrations and nitrification rates and negatively with temperature and oxygen (Supplementary Table S1), all of which suggest that AOA community sizes are larger in waters where a history of phytoplankton growth is less apparent. Overall, the sum of WCA and WCB amoA genes closely matches the abundances of ‘total' AOA amoA genes observed in MB surface waters (Mincer et al. 2007). However, there is still some disagreement with cell abundances determined by fluorescence in situ hybridization, possibly due to material loss during DNA extraction (Mincer et al. 2007).
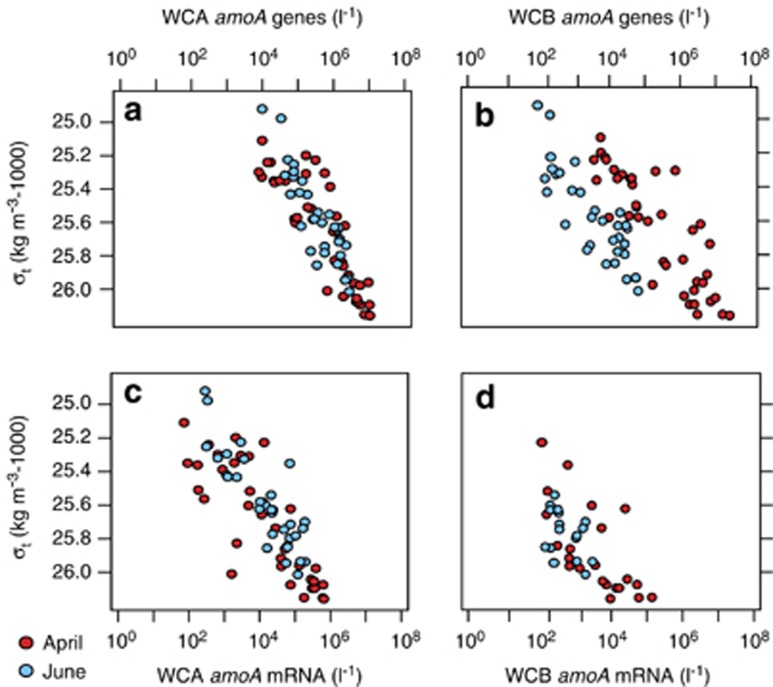
Figure 3.
The distribution and abundance of archaeal amoA genes and transcripts along density surface in Monterey Bay surface waters. (a) WCA and (b) WCB amoA gene abundances plotted along density surface in April (N=41 for both genes) and June (N=32 for both genes). Transcript abundances for (c) WCA amoA (N=37/31 for April/June) and (d) WCB amoA (N=22/17). All data are plotted on the same axis for comparison purposes.
Based on our current understanding of the central California Current system (CCS), the maximum depth of origin for upwelled waters during a wind-driven event varies from 30 to 60 m (Messié et al., 2009). AOA typically increase in abundance between these depths in MB and the CCS (Mincer et al. 2007; Santoro et al. 2010). A decline in WCA (F1,72=6.95, P=0.01) and WCB (F1,72=8.96, P=0.004) community sizes between April and June when upwelling was more intense (Figure 1), was surprising (Supplementary Table S2). These findings suggest that physical processes alone do not influence the size and structure of AOA communities in MB surface waters.
WCA amoA transcriptional activity
WCA amoA mRNA transcripts, detected in 95% of samples (N=69), ranged in abundance from 101 to 105 l−1 (Figure 3c). Genes and transcripts of WCA were highly correlated with one another (R=0.80, P<0.05; Supplementary Figure S4B). There was no significant variation in the ratio of WCA transcripts to genes between April (mean±s.e.=3.4 × 10−2±1.5 × 10−2) and June (6.2 × 10−2±1.2 × 10−2) (t-test, P>0.05), despite the pronounced changes in hydrographic conditions (Figures 1a–d, Supplementary Figure S4B). These data suggest that an average of 1 in every 22 (±14) WCA cells was expressing amoA at the time of sampling. The relatively consistent relationship between gene and transcript abundances suggests that the role of WCA in nitrification may be determined largely by the factors that regulate their abundance rather than those influencing their cellular activity. If the latter were more important, less coherence between gene and transcript abundances should have been apparent (Figures 3a and c).
Most striking is the unresponsiveness of WCA expressional activity (Figure 3c), or nitrification rates (Figure 2), to a nearly three-order of magnitude variation in ammonium concentrations during the course of our study (Figure 1c). No apparent effect of increased ammonium availability on nitrification rates is contrary to recent findings, which showed a positive relationship between oxidation rates and substrate concentrations in archaeal-dominated assemblages of ammonia-oxidizing microorganisms (Horak et al., 2013; Newell et al., 2013). From our data, it seems unlikely that ammonium addition stimulated WCA activity. If WCA were substrate limited, there should have been a more pronounced response at the level of activity (transcripts or rates of nitrification) in association with the wide swings in ammonium concentrations during our study, leading to more variability in gene-normalized transcript abundances or increased nitrification rates. In particular, this response should have been quite clear between sampling months. During April, ammonium concentrations were near limiting levels for archaeal ammonia oxidation(Martens-Habbena et al., 2009; Horak et al., 2013; Newell et al., 2013), but increased an order of magnitude to concentrations well above typical oceanic levels in June (Figure 1c and Table 1)(Raimbault et al., 2008).
Overall, the nearly ‘fixed' level of WCA activity in relation to their abundance defines these organisms as being relatively insensitive to large variations in environmental conditions. Within a given community, the specific-activity level remains relatively constant irrespective of changes in the physicochemical environment or size of the community. Future work to identify the factors that most strongly influence the size of the WCA community in the photic zone will greatly further efforts to model their role in ocean biogeochemical cycles.
WCB amoA transcriptional activity
WCB transcripts were only detected in 55% of samples. Their abundances ranged from 102 to 105 l−1 across the sample set (Figure 3d and Supplementary Table S2). As with their amoA genes, average WCB transcript abundances declined from 104 to 102 l−1 between April and June (F1,38=4.37, P=0.04; Supplementary Table S2). Unlike WCA, the abundance of WCB amoA genes and transcripts were not well correlated with each other (R=0.49, P<0.05; Figures 3b and d). There were 1.2 × 10−2 (±6.2 × 10−3) and 4 × 10−2 (±8.2 × 10−3) WCB amoA transcripts per gene in April and June, respectively. This means that, when transcripts were detectable, 1 of every 41 (±30) WCB cells was expressing amoA at the time of sampling. No significant variation between these ratios was observed between months or between those observed for the WCA (t-test, P>0.05), indicative of a relatively consistent level of specific activity between WCA and WCB communities.
The strong correlation between WCA and WCB amoA genes (Supplementary Figure S4A) indicates that the abundance of both ecotypes is influenced by the same set of depth-related factors. For the WCA community, increased transcript abundances are reflective of increased community size (Figures 3a and c). WCB amoA transcripts did not demonstrate this level of consistency; transcripts were sometimes absent when gene abundances were high (Figures 3b and d). The less consistent expressional patterns of WCB suggest an additional level of control (or greater sensitivity to one set of factors), beyond that which controls abundance. Although a difference in transcript stabilities, or lifetimes, is a plausible explanation, we hypothesize that WCB amoA transcriptional activity differs from that of the WCA, because they are more responsive to changes in environmental conditions. Two sets of factors influence their activity, namely those that influence community size and those that exert regulatory control over activity.
When present, WCB transcripts comprised ∼10% (±20%) of the combined pool, which suggests that the role of WCB in nitrification cannot be entirely overlooked. Further insights into the reason for the narrower range of ‘optimal' conditions for WCB amoA expression may prove most useful to our ability to predict when they might be actively oxidizing ammonia. Partial correlations of environmental data with transcript abundances did not yield any clear insight into potential regulatory factors (R<0.5 for all variables, Supplementary Table S1). However, simplifying the data by comparison of samples binned based on the presence or absence of WCB transcripts provided more insight (Table 2). Their preferred niche within the euphotic zone appears to be colder, lower chlorophyll waters with higher macronutrient and ammonium concentrations (Table 2)(Ward et al., 1989). In stratified water columns, these conditions are characteristic of the lower euphotic zone, or the nitracline, where phytoplankton growth rates and light intensities are much lower than in shallower layers (Ward et al., 1989). However, transcripts were observed in a number of samples from 5 m depth (Supplementary Figure S2H), in the presence of 8 μg l−1 of chlorophyll (range, 1–14 μg l−1) and 0.8 μM ammonium (Table 2), which suggests that WCB activity is not confined soley to conditions typical of the lower euphotic or upper mesopelagic zones.
Table 2. Comparison of average conditions when WCB amoA transcripts were present or absent in Monterey Bay surface waters.
| Temperature† (oC) | Salinity | Chl a (μg l−1) | NH 4 + (μM) | NO 2 − (μM) | NO 3 − (μM) | SiO 4 4− (μM) | PO 4 3− (μM) | O2 (ml l−1) | 15NH4+oxidation (nmol l−1 day−1) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Absent | ||||||||||
| Mean | 12.3a | 33.5a | 10.2 | 0.24a | 0.16a | 4.4a | 5.6a | 0.8a | 7.0a | 5.2a |
| s.d. | 0.9 | 0.2 | 6.9 | 0.27 | 0.12 | 5.1 | 5.3 | 0.4 | 1.1 | 6.7 |
| N | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 34 | 15 |
| Present | ||||||||||
| Mean | 10.6b | 33.7b | 8.4 | 0.81b | 0.33b | 17.1b | 18.5b | 1.7b | 4.6b | 29.4b |
| s.d. | 0.9 | 0.1 | 8.0 | 0.83 | 0.16 | 7.3 | 8.9 | 0.4 | 1.4 | 20.0 |
| N | 39 | 39 | 39 | 39 | 39 | 39 | 39 | 39 | 39 | 26 |
Different letters between April and June denote significantly different means (α=0.05), within a variable; when letters are absent, no statistical difference between means was found.
The roles of WCA and WCB in nitrification
Although there have been quantitative assessments of AOA amoA transcript abundances in the ocean, those published have focused on whole community activity, rather than ecotypes (Lam et al., 2007, 2009; Church et al., 2010; Labrenz et al., 2010). Considering the sum of WCA and WCB genes and transcripts to represent the total community of AOA in the water column (Beman et al., 2008), a consistent level of specific activity (transcript to gene ratio) was observed. On average (±s.e.), there were 4 × 10−2 (±8 × 10−3) transcripts per amoA gene (median, 2 × 10−2; N=73) for the sum of both ecotypes. Assuming one amoA gene per genome (Walker et al., 2010; Blainey et al., 2011) and that one transcript is present per active cell, only 4% (median, 3% range, 0.05–18%) of cells (1 in 25) were expressing the gene when sampled. Quantitatively, this percentage of ‘active' cells seems low but is remarkably consistent with transcript data from the suboxic zone of the Baltic Sea (Labrenz et al., 2010) and the meso- and bathypelagic zones of the oligotrophic Pacific Ocean (Church et al., 2010).
However, if only the amoA-expressing cells were active, these ‘active' members of the AOA community would have to be oxidizing ammonia at a rate of 886 fmol cell−1 d−1, several orders of magnitude higher than other published estimates, which range from 0.2–13 fmol cell−1 d−1 (Wuchter et al., 2006; Martens-Habbena et al., 2009; Santoro et al., 2010; 2011). AOA greatly outnumber ammonia-oxidizing bacteria (AOB) in surface waters of MB and the CCS (Mincer et al., 2007; Santoro et al., 2010). Thus, factoring the contributions of β- and γ-AOB into our calculations is unlikely to decrease the cell-specific ammonia oxidation rates by the two orders of magnitude necessary to align them with other published estimates. Potential underestimates of mRNA abundances due to inefficiency of our nucleic acid extraction or at the stages of cDNA synthesis and quantification of transcripts should be considered when making these calculations. However, we assert that the disparity in our transcript-based calculations of per cell rates is reflective of the fact that, while a transcript may represent an active cell, the lifetime of a transcript dictates the fraction of the community able to be deduced as ‘active' in a given sample (Moran et al., 2012). The half-life of AOA mRNA transcripts, in culture or whole seawater, has not been published. However, our results suggest that they are relatively short-lived, most likely falling within the range of those of the slow-growing marine prochlorophytes and the crenarchaeote Sulfolobus, both of which have half-lives of the order of minutes (Andersson et al., 2006; Steglich et al., 2010). Post-transcriptional and -translational regulation has an important and often overlooked role in determining the abundance of gene products in transcript or protein pools, such that mRNA abundances may not be related to the abundance of the proteins they encode at all (Moran et al., 2012 and references therein), as has been observed for oceanic prochlorophytes (Waldbauer et al., 2012).
In contrast to transcript-based assessments, per cell rates calculated based on amoA gene abundances were 14 and 454 fmol cell−1 d−1 for WCA and WCB, respectively. Estimates for the WCA AOA are directly in line with those previously reported from culture studies and natural assemblages (Martens-Habbena et al., 2009; Santoro et al., 2010). Inclusion of WCB abundances further decreases per cell estimates for the combined community, to 10 fmol cell−1 d−1. However, WCB AOA alone cannot explain the observed rates of nitrification, which remained relatively constant between April and June, despite a pronounced decrease in WCB abundance (Figure 3b).
Molecular markers for estimating nitrification
Our extensive survey of AOA communities and how they relate to physicochemical parameters and rates of nitrification in surface waters yielded a potentially valuable relationship for prediction of nitrification rates from amoA gene abundances (Figure 4). In our data sets of amoA genes and nitrification, there is good representation over the range of typical values observed in coastal surface waters (Ward, 2005; Mincer et al., 2007; Beman et al., 2008; Santoro et al., 2010). Consistent with our finding that their abundance alone can explain observed rates of nitrification in surface waters of MB, a direct relationship between WCA amoA genes and nitrification rates was observed (Figure 4).
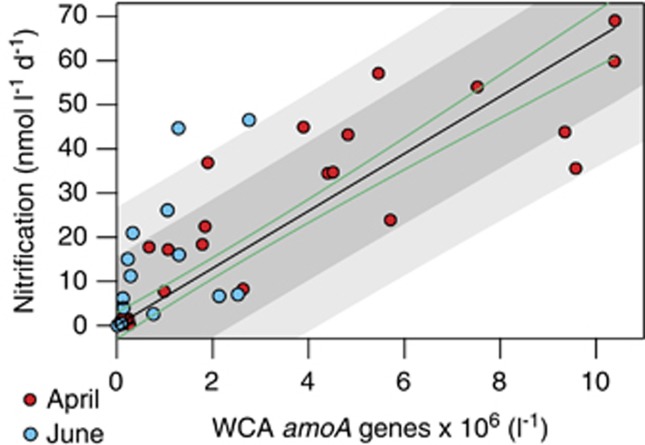
Figure 4.
Results of fit analysis between WCA amoA gene abundances and nitrification rates (N=42). The relationship was fit with a linear regression model with an intercept of zero and slope (m) of 6.48 × 10−6±1 × 10−6. The line of best fit is shown in black. Green lines represent the 95% confidence interval of the model fit. Shaded regions show the prediction envelope at 75% (α=0.25, light gray) and 95% (α=0.05, dark gray) confidence intervals.
The fit of the linear regression model (R2=0.59, P=0.01) had a slope (±95% confidence interval) of 6.5 × 10−6 (±1.0 × 10−6). This relationship indicates that WCA AOA oxidize ammonia at a consistent per cell rate of ∼6 fmol cell−1 day−1across three orders of magnitude shifts in community size. The fit estimates for the linear model are weaker at lower rates (Figure 4), which is likely due to the paucity of nitrification rate data below ∼1 nmol l−1 day−1 (Figure 2). It should also be noted that this relationship was assessed with WCB amoA gene abundances (Supplementary Figure S5A), as well as the sum of amoA genes from both ecotypes (Supplementary Figure S5B). In both cases, statistical support and strength of the linear fit decreased substantially.
Data presented here clearly establish the WCA as the most promising of the two AOA ecotypes for further development of quantitative relationships between amoA gene abundances and nitrification rates in the upper ocean. Their abundances were more strongly correlated to nitrification rates (Figure 4), their transcripts were almost always present across a wide range of surface waters conditions and the abundance of their genes and transcripts remained relatively constant (at a given density) over the course of the study (Figures 3a and c) in agreement with the consistency in nitrification rates between months (Figure 2). Transcript data suggest that the WCB may, at times, have a role in determining rates of nitrification (Figure 3d). However, their lack of consistent transcriptional activity, especially at high gene abundances, makes their role more difficult to predict when developing operational metrics using gene abundances to estimate rates of nitrification.
The measurement of nitrification rates is labor intensive and requires specialized equipment, however, the quantification of amoA genes and transcripts is becoming more streamlined and automated. In light of the significant relationship (Figure 4) between the abundance of WCA amoA genes and nitrification rate, a number of exciting applications could be pursued. For example, the Environmental Sample Processor (Preston et al., 2011) has been implemented to study the distribution of WCA and WCB amoA genes in situ (Mosier and Francis, 2011; Robidart et al., 2011), opening up the opportunity to infer rates of nitrification remotely and without incubation. The Environmental Sample Processor also offers automated sample collection and processing at the higher spatial and temporal resolutions required to fully understand the role this process will have in upper ocean biogeochemistry in the future. Strengthening the predictive nature of this relationship in the lower range of rates would allow extension of this approach to estimate rates of nitrification in oligotrophic surface waters; current estimates are not well constrained, due in part to analytical limitations stemming from the extremely low concentrations of ammonium and nitrate (Raimbault et al., 2008). As with nitrogen fixation, and other key biogeochemical process in the surface ocean, quantitatively small, but widespread, rates of nitrification in the surface ocean could significantly alter our understanding of elemental cycling on a global scale (Yool et al., 2007).
Acknowledgments
Tim Pennington, Marguerite Blum, Marie Lund and Julian Damashek provided invaluable logistical assistance with expedition planning and sample acquisition. This work was supported in part by the National Science Foundation Grant OCE-0825363 to CAF. Salary support for JMS came from a graduate research fellowship from the Northern California chapter of the ARCS Foundation. FPC was supported by the David and Lucile Packard Foundation.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Agogué H, Brink M, Dinasquet J, Herndl GJ. (2008). Major gradients in putatively nitrifying and non-nitrifying Archaea in the deep North Atlantic. Nature 456: 788–791. [DOI] [PubMed] [Google Scholar]
- Alonso-Sáez L, Waller AS, Mende DR, Bakker K, Farnelid H, Yager PL et al. (2012). Role for urea in nitrification by polar marine Archaea. Proc Natl Acad Sci USA 109: 17989–17994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson AF, Lundgren M, Eriksson S, Rosenlund M, Bernander R, Nilsson P. (2006). Global analysis of mRNA stability in the archaeon. Sulfolobus Genome Biol 7: R99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker BJ, Lesniewski RA, Dick GJ. (2012). Genome-enabled transcriptomics reveals archaeal populations that drive nitrification in a deep-sea hydrothermal plume. ISME J 6: 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beman JM, Chow C-E, King AL, Feng Y, Fuhrman JA, Andersson A et al. (2011). Global declines in oceanic nitrification rates as a consequence of ocean acidification. Proc Natl Acad Sci USA 108: 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beman JM, Popp BN, Alford SE. (2012). Quantification of ammonia oxidation rates and ammonia-oxidizing archaea and bacteria at high resolution in the Gulf of California and eastern tropical North Pacific Ocean. Limnol Oceanogr 57: 711–726. [Google Scholar]
- Beman JM, Popp BN, Francis CA. (2008). Molecular and biogeochemical evidence for ammonia oxidation by marine Crenarchaeota in the Gulf of California. ISME J 2: 429–441. [DOI] [PubMed] [Google Scholar]
- Beman JM, Sachdeva R, Fuhrman JA. (2010). Population ecology of nitrifying Archaea and Bacteria in the Southern California Bight. Environ Microbiol 12: 1282–1292. [DOI] [PubMed] [Google Scholar]
- Blainey PC, Mosier AC, Potanina A, Francis CA, Quake SR. (2011). Genome of a low-salinity ammonia oxidizing archaeon determined by single-cell and metagenomic analysis. PLoS One 6: e16626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breaker LC, Broenkow WW. (1994). The circulation of Monterey Bay and related processes. Oceanogr Mar Biol 32: 1–64. [Google Scholar]
- Casciotti KL, Sigman DM, Galanter Hastings M, Böhlke JK, Hilkert A. (2002). Measurement of the oxygen isotopic composition of nitrate in seawater and freshwater using the denitrifier method. Anal Chem 74: 4905–4912. [DOI] [PubMed] [Google Scholar]
- Casciotti KL, Trull TW, Glover DM, Davies D. (2008). Constraints on nitrogen cycling at the subtropical North Pacific Station ALOHA from isotopic measurements of nitrate and particulate nitrogen. Deep-Sea Res Pt II 55: 1661–1672. [Google Scholar]
- Christman GD, Cottrell MT, Popp BN, Gier E, Kirchman DL. (2011). Abundance, diversity, and activity of ammonia-oxidizing prokaryotes in the coastal Arctic Ocean in summer and winter. Appl Environ Microbiol 77: 2026–2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church MJ, Wai B, Karl DM, DeLong EF. (2010). Abundances of crenarchaeal amoA genes and transcripts in the Pacific Ocean. Environ Microbiol 12: 679–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DR, Rees AP, Joint I. (2008). Ammonium regeneration and nitrification rates in the oligotrophic Atlantic Ocean: implications for new production estimates. Limnol Oceanogr 53: 52. [Google Scholar]
- DeLong EF. (1992). Archaea in coastal marine environments. Proc Natl Acad Sci USA 89: 5685–5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dortch Q. (1990). The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol Prog Ser 61: 183–201. [Google Scholar]
- Dugdale RC, Wilkerson FP, Hogue VE, Marchi A. (2006). Nutrient controls on new production in the Bodega Bay, California, coastal upwelling plume. Deep-Sea Res Pt II 53: 3049–3062. [Google Scholar]
- Fawcett SE, Ward BB. (2011). Phytoplankton succession and nitrogen utilization during the development of an upwelling bloom. Mar Ecol Prog Ser 428: 13–31. [Google Scholar]
- Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB. (2005). Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proc Natl Acad Sci USA 102: 14683–14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman JA, McCallum K, Davis AA. (1992). Novel major archaebacterial group from marine plankton. Nature 356: 148–149. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez J, Rodriguez-Valera F. (2000). Microdiversity of uncultured marine prokaryotes: the SAR11 cluster and the marine Archaea of Group I. Mol Ecol 9: 935–948. [DOI] [PubMed] [Google Scholar]
- Gruber N. (2011). Warming up, turning sour, losing breath: ocean biogeochemistry under global change. Philos Trans R Soc A Math Phys Eng Sci 369: 1980–1996. [DOI] [PubMed] [Google Scholar]
- Hallam SJ, Mincer TJ, Schleper C, Preston CM, Roberts K, Richardson PM et al. (2006). Pathways of carbon assimilation and ammonia oxidation suggesed by environmental genomic analyses of marine Crenarchaeota. PLoS Biol 4: e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansman RL, Griffin S, Watson JT, Druffel ERM, Ingalls AE, Pearson A et al. (2009). The radiocarbon signature of microorganisms in the mesopelagic ocean. Proc Natl Acad Sci USA 106: 6513–6518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herndl GJ, Reinthaler T, Teira E, van Aken H, Veth C, Pernthaler A et al. (2005). Contribution of Archaea to total prokaryotic production in the deep Atlantic Ocean. Appl Environ Microbiol 71: 2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollibaugh JT, Gifford S, Sharma S, Bano N, Moran MA. (2010). Metatranscriptomic analysis of ammonia-oxidizing organisms in an estuarine bacterioplankton assemblage. ISME J 5: 866–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes RM, Aminot A, Kérouel R, Hooker BA, Peterson BJ. (1999). A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can J Fish Aquat Sci 56: 1801–1808. [Google Scholar]
- Horak REA, Qin W, Schauer AJ, Armbrust EV, Ingalls AE, Moffett JW et al. (2013). Ammonia oxidation kinetics and temperature sensitivity of a natural marine community dominated by Archaea. ISME J doi:10.1038.ismej2013.75. [DOI] [PMC free article] [PubMed]
- Horrigan SG, Carlucci AF, Williams PM. (1981). Light inhibition of nitrification in sea-surface films. J Mar Res 39: 557–565. [Google Scholar]
- Ingalls AE, Shah SR, Hansman RL, Aluwihare LI, Santos GM, Druffel ERM et al. (2006). Quantifying archaeal community autotrophy in the mesopelagic ocean using natural radiocarbon. Proc Natl Acad Sci USA 103: 6442–6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karner MB, DeLong EF, Karl DM. (2001). Archaeal dominance in the mesopelagic zone of the Pacific Ocean. Nature 409: 507–510. [DOI] [PubMed] [Google Scholar]
- Könneke M, Bernhard AE, la Torre de JR, Walker CB, Waterbury JB, Stahl DA. (2005). Isolation of an autotrophic ammonia-oxidizing marine archaeon. Nature 437: 543–546. [DOI] [PubMed] [Google Scholar]
- Kudela RM, Cochlan WP, Dugdale RC. (1997). Carbon and nitrogen uptake response to light by phytoplankton during an upwelling event. J Plankton Res 19: 609–630. [Google Scholar]
- Kudela RM, Dugdale RC. (2000). Nutrient regulation of phytoplankton productivity in Monterey Bay, California. Deep-Sea Res Pt II 47: 1023–1053. [Google Scholar]
- Labrenz M, Sintes E, Toetzke F, Zumsteg A, Herndl GJ, Seidler M et al. (2010). Relevance of a crenarchaeotal subcluster related to Candidatus Nitrosopumilus maritimus to ammonia oxidation in the suboxic zone of the central Baltic Sea. ISME J 4: 1496–1508. [DOI] [PubMed] [Google Scholar]
- Lam P, Jensen MM, Lavik G, McGinnis DF, Müller B, Schubert CJ et al. (2007). Linking crenarchaeal and bacterial nitrification to anammox in the Black Sea. Proc Natl Acad Sci USA 104: 7104–7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam P, Lavik G, Jensen MM, van de Vossenberg J, Schmid M, Woebken D et al. (2009). Revising the nitrogen cycle in the Peruvian oxygen minimum zone. Proc Natl Acad Sci USA 106: 4752–4757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund MB, Smith JM, Francis CA. (2012). Diversity, abundance and expression of nitrite reductase (nirK)-like genes in marine thaumarchaea. ISME J 6: 1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens-Habbena W, Berube PM, Urakawa H, la Torre de JR, Stahl DA. (2009). Ammonia oxidation kinetics determine niche separation of nitrifying Archaea and Bacteria. Nature 461: 976–979. [DOI] [PubMed] [Google Scholar]
- Merbt SN, Stahl DA, Casamayor EO, Martí E, Nicol GW, Prosser JI. (2011). Differential photoinhibition of bacterial and archaeal ammonia oxidation. FEMS Microbiol Lett 327: 41–46. [DOI] [PubMed] [Google Scholar]
- Messié M, Ledesma J, Kolber DD, Michisaki RP, Foley DG, Chavez FP. (2009). Potential new production estimates in four eastern boundary upwelling ecosystems. Prog Oceanogr 83: 151–158. [Google Scholar]
- Mincer TJ, Church MJ, Taylor LT, Preston C, Karl DM, DeLong EF. (2007). Quantitative distribution of presumptive archaeal and bacterial nitrifiers in Monterey Bay and the North Pacific Subtropical Gyre. Environ Microbiol 9: 1162–1175. [DOI] [PubMed] [Google Scholar]
- Moran MA, Satinsky B, Gifford SM, Luo H, Rivers A, Chan L-K et al. (2012). Sizing up metatranscriptomics. ISME J 7: 237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier AC, Francis CA. (2011). Determining the distribution of marine and coastal ammonia-oxidizing archaea and bacteria using a quantitative approach. Method Enzymol 486: 206–221. [DOI] [PubMed] [Google Scholar]
- Newell SE, Babbin AR, Jayakumar A, Ward BB. (2011). Ammonia oxidation rates and nitrification in the Arabian Sea. Global Biogeochem Cy 25: GB4016. [Google Scholar]
- Newell SE, Facwett SE, Ward BB. (2013). Depth distribution of ammonia oxidation rates and ammonia-oxidizer community composition in the Sargasso Sea. Limnol Oceanogr 58: 1491–1500. [Google Scholar]
- Olson RJ. (1981. a). 15N tracer studies of the primary nitrite maximum. J Mar Res 39: 203–226. [Google Scholar]
- Olson RJ. (1981. b). Differential photoinhibition of marine nitrifying bacteria: a possible mechanism for the formation of the primary nitrite maximum. J Mar Res 39: 227–238. [Google Scholar]
- Ouverney CC, Fuhrman JA. (2000). Marine planktonic archaea take up amino acids. Appl Environ Microbiol 66: 4829–4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington JT, Chavez FP. (2000). Seasonal fluctuations of temperature, salinity, nitrate, chlorophyll and primary production at station H3/M1 over 1989–1996 in Monterey Bay, California. Deep-Sea Res Pt II 47: 947–973. [Google Scholar]
- Preston CM, Harris A, Ryan JP, Roman B, Marin R, Jensen S et al. (2011). Underwater application of quantitative PCR on an ocean mooring. PLoS One 6: e22522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raimbault P, Garcia N, Cerutti F. (2008). Distribution of inorganic and organic nutrients in the south pacific ocean—evidence for long-term accumulation of organic matter in nitrogen-depleted waters. Biogeosci Discuss 5: 281–298. [Google Scholar]
- Robidart JC, Preston CM, Paerl RW, Turk KA, Mosier AC, Francis CA et al. (2011). Seasonal Synechococcus and Thaumarchaeal population dynamics examined with high resolution with remote in situ instrumentation. ISME J 6: 513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro AE, Buchwald C, McIlvin MR, Casciotti KL. (2011). Isotopic signature of N2O produced by marine ammonia-oxidizing archaea. Science 333: 1282–1285. [DOI] [PubMed] [Google Scholar]
- Santoro AE, Casciotti KL, Francis CA. (2010). Activity, abundance and diversity of nitrifying archaea and bacteria in the central California Current. Environ Microbiol 12: 1989–2006. [DOI] [PubMed] [Google Scholar]
- Santoro AE, Sakamoto CM, Smith JM, Plant JN, Gehman AL, Worden AZ et al. (2013). Measurements of nitrite production and nitrite-producing organisms in and around the primary nitrite maximum in the central California Current. Biogeosci Discuss 10: 5803–5840. [Google Scholar]
- Sigman DM, Casciotti KL, Andreani M, Barford C, Galanter M, Böhlke JK. (2001). A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater. Anal Chem 73: 4145–4153. [DOI] [PubMed] [Google Scholar]
- Sintes E, Bergauer K, De Corte D, Yokokawa T, Herndl GJ. (2013). Archaeal amoA gene diversity points to distinct biogeography of ammonia—oxidizing Crenarchaeota in the ocean. Environ Microbiol 15: 1647–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steglich C, Lindell D, Futschik M, Rector T, Steen R, Chisholm SW. (2010). Research Short RNA half-lives in the slow-growing marine cyanobacterium Prochlorococcus. Genome Biol 11: R54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan BK, Martinez-Garcia M, Preston CM, Sczyrba A, Woyke T, Lamy D et al. (2011). Potential for chemolithoautotrophy among ubiquitous bacteria lineages in the dark ocean. Science 333: 1296–1300. [DOI] [PubMed] [Google Scholar]
- Venter JC, Remington K, Heidelberg JF, Halpern AL, Rusch D, Eisen JA et al. (2004). Environmental genome shotgun sequencing of the Sargasso Sea. Science 304: 66–74. [DOI] [PubMed] [Google Scholar]
- Waldbauer JR, Rodrigue S, Coleman ML, Chisholm SW. (2012). Transciptome and proteome dynamics of a light-dark synchronized bacterial cell cycle. PLoS One 7: e43432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CB, la Torre de JR, Klotz MG, Urakawa H, Pinel N, Arp DJ et al. (2010). Nitrosopumilus maritimus genome reveals unique mechanisms for nitrification and autotrophy in globally distributed marine crenarchaea. Proc Natl Acad Sci USA 107: 8818–8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BB. (1985). Light and substrate concentration relationships with marine ammonium assimilation and oxidation rates. Mar Chem 16: 301–316. [Google Scholar]
- Ward BB. (2005). Temporal variability in nitrification rates and related biogeochemical factors in Monterey Bay, California, USA. MarEcol Prog Ser 292: 97–109. [Google Scholar]
- Ward BB, Kilpatrick KA, Renger EH, Eppley RW. (1989). Biological nitrogen cycling in the nitracline. Limnol Oceanogr 34: 493–513. [Google Scholar]
- Wuchter C, Abbas B, Coolen MJL, Herfort L, van Bleijswijk J, Timmers P et al. (2006). Archaeal nitrification in the ocean. Proc Natl Acad Sci USA 103: 12317–12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakimov MM, La Cono V, Smedile F, DeLuca TH, Juárez S, Ciordia S et al. (2011). Contribution of crenarchaeal autotrophic ammonia oxidizers to the dark primary production in Tyrrhenian deep waters (Central Mediterranean Sea). ISME J 5: 945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yool A, Martin AP, Fernández C, Clark DR. (2007). The significance of nitrification for oceanic new production. Nature 447: 999–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.