Abstract
Northern and southern Ixodes scapularis Say populations differ greatly in density, host utilization, and especially questing behavior of the immatures. Haplotypes of I. scapularis in North America can be divided into two major clades—the All American Clade (haplotypes A through J) and the Southern Clade (M through O). This genetic variation may affect feeding success and vector competence. This study compared feeding success of larval I. scapularis measured by time-to-drop-off and subsequent transmissibility success of Borrelia burgdorferi to mice using ticks from Mississippi, Connecticut (both F haplotype), and Louisiana (haplotype O). Northern ticks (CT) fed to repletion much faster than MS and LA ticks: overall, 73.6% of CT ticks had dropped off mice at Day 3 compared to only 1.7% and 6.6% of ticks dropped off for MS and LA ticks at that same time point. As for vector competence, 4 of the 4 mice in each case (MS or CT) that had been fed on by infected nymphs tested positive for B. burgdorferi. In a second experiment, 5 of the 6 mice tested positive for B. burgdorferi after exposure to infected LA ticks as compared with 3 of the 4 mice exposed to infected CT ticks. These data demonstrate that there is no difference in northern and southern populations of I. scapularis in their ability to transmit B. burgdorferi, but the ability of the northern populations to feed rapidly on rodents exceeds that of southern populations.
Keywords: Ixodes scapularis, Immatures, Lyme disease, Feeding success, Vector competence
Introduction
Lyme borreliosis (LB), caused by one or more “genospecies” of Borrelia burgdorferi, is a systemic tick-borne illness displaying a variety of clinical manifestations that occurs over much of the world in temperate zones (Gray et al. 2002). In North America, Borrelia burgdorferi sensu stricto is the etiologic agent of LB, which is transmitted by Ixodes scapularis Say ticks in the northeastern and midwestern United States, while Ixodes pacificus Cooley and Kohls, is the vector along the Pacific Coast. Immature ticks acquire the infection in nature while feeding as larvae and nymphs on infected reservoir vertebrate hosts such as small mammals or birds. In the northeastern and midwestern United States, the cycle of tick transmission of B. burgdorferi sensu stricto is driven by a focus on Peromyscus mice, chipmunks, shrews, and to some extent birds, but in the southern United States, little is known about the reservoirs, other than that lizards may be involved (Apperson et al. 1993, Levin et al. 1996, Durden et al. 2002). Confounding the issue is controversy (often extreme) about whether and to what extent “true” Lyme borreliosis occurs in the southern states (Auwaerter et al. 2011, Goddard et al. 2012, Clark et al. 2013), thus highlighting the need for ecological and epidemiological research on LB in that region.
Tick–host surveys are important in determining host–vector–pathogen relationships. However, host surveys by themselves do not provide specific information about relative success of tick development. The host species’ influence on tick development and molting success is largely unexplored beyond a few studies found in the scientific literature (Bishopp and Hixson 1936, Trager 1939, Hixson 1940, Sonenshine and Atwood 1967, Amin 1969, Koch and Hair 1975, Moraru et al. 2012). Theoretically, when ticks take larger bloodmeals (and quicker) on one host as opposed to another, this means greater host–parasite synchronization, and that the particular host is well-suited for that tick species (Koch and Hair 1975).
I. scapularis populations from northern and southern parts of their range differ greatly in population density, host utilization, and, particularly, questing behavior of the immatures (Piesman 2002, Goddard and Goddard 2008, Goddard and Goddard 2010). The division between “All American” and “Southern” lineages was first established by Norris (Norris et al. 1996), but Qiu further refined the classification, stating that I. scapularis haplotypes in North America can be divided into two major clades—the All American Clade (haplotypes A through J), and the Southern Clade (M through O; Qiu et al. 2002). A recent analysis utilizing single-nucleotide polymorphisms supports this view, showing that I. scapularis ticks collected from Mississippi and Georgia display greater genetic variation than those from New Jersey or Virginia (Van Zee et al. 2013). This genetic variation may affect tick feeding success and vector competence among southern I. scapularis. Previous studies have compared the vector competence of I. scapularis collected from the northern and southern populations (Piesman and Sinksky 1988, Sanders and Oliver 1995), but these studies were performed before it was possible to characterize the genetic background of the populations used in the experiment. The present study compares feeding success of larval I. scapularis measured by time-to-drop-off and subsequent transmissibility success of B. burgdorferi to mice using ticks from Mississippi (F haplotype), Connecticut (F haplotype), and Louisiana (O haplotype).
Materials and Methods
Mice and Ticks
Mice used in these experiments were CD-1 females, 4–5 wk of age, purchased from Charles River Laboratories (Wilmington, MA). Mice were handled according to approved protocols on file with the Centers for Disease Control and Prevention, Division of Vector Borne Diseases Animal Care and Use Committee Protocol numbers 12-003 and 09-002. I. scapularis colonies were derived from three states: Connecticut (CT), Mississippi (MS), and Louisiana (LA). The CT colony originated from female I. scapularis collected in Bridgeport, CT, in 2009 and maintained as previously described (Piesman 1993). MS ticks were collected as adults from vegetation during March 2011 in Marshall County, MS, and fed on rabbits per previous protocols (Piesman 1991). LA ticks were derived from a colony maintained at the Tulane Primate Center, Covington, LA (but originally collected nearby).
Molecular Genetics
The genetic background of each tick colony was established by sequencing a 433 bp DNA fragment of the mitochondrial 16S rDNA gene (Van Zee et al. 2013). This fragment is enough to allow classification by haplotype as previously described (Qiu et al. 2002). Both the CT and MS ticks were classified as haplotype “F,” the most common haplotype of the “Northern Clade” or “All American Clade” (Norris et al. 1996), whereas the LA ticks were haplotype “O,” a haplotype restricted to southern states in its distribution.
Detection of B. burgdorferi
Nucleic acids were isolated from ticks using DNeasy Blood and Tissue kit (Qiagen, Valencia, CA) and a Mini-Beadbeater (Biospec, Bartlesville, OK; Hojgaard et al. 2014). To test for the presence of B. burgdorferi, a multiplex TaqMan PCR reaction was performed, targeting both B. burgdorferi and I. scapularis. As a control for both the DNA purification and the PCR reaction, a set of primers and probe against the actin gene of I. scapularis was used (Hojgaard et al. 2014). For detection of B. burgdorferi DNA, previously described primers and probes for the flagellar filament cap gene (fliD) were used (Dolan et al. 2011). The multiplex PCR reactions were performed using iQ Multiplex Powermix (BioRad), with primers in a final concentration of 300 nM, and probes in a final concentration of 200 nM. The PCR cycling conditions consisted of denature DNA at 95°C for 3 min followed by 40 cycles of 95°C for 10 s, and 60°C for 1 min on a C1000 Touch thermal cycler with a CFX96 real time system (BioRad).
Drop-off Study and Vector Competence
In order to infect mice, a total of five nymphal I. scapularis infected with the B31 strain of B. burgdorferi were allowed to feed on 4- to 5-wk-old mice ad libitum until repletion. At 3 weeks postnymphal exposure, an ear biopsy was obtained and cultured in Barbour-Stoenner-Kelly (BSK) to determine whether the animal was infected as previously described (Sinsky and Piesman 1989). All mice serving as hosts for larval ticks had positive ear biopsies on examination by darkfield microscopy. Mice were exposed to test larval ticks from different locations at 4 weeks postnymphal exposure. Logistically, we could not conduct this experiment all at once due to the number of mice involved. Therefore, three replicates (trials) for each location were performed; however, replicates were always run with northern (CT) ticks and one southern strain (MS or LA). In each of these trials, larval ticks were placed on mice and allowed to feed ad libitum. Larvae were not counted prior to being placed on hosts. Exact larval counts prior to infestation would have required prehandling and separating larvae into small batches for application. Prior experience in our lab has shown that prehandling larval batches notably reduces viability. At the time of these experiments, we only had small numbers of flat larvae available to us, especially the Louisiana larvae. Therefore, we made the decision not to precount larvae. Nonetheless, by a rough visual count, a minimum of 150 and a maximum of 350 larvae were placed on individual mice depending on the number of larvae available. Larval drop-off was assessed at least twice daily, and numbers of replete ticks found were counted and charted as to days postapplication to each mouse. Replete larval ticks were held in desiccators with saturated humidity at 22°C. At 10 days post-larval repletion, ≥5 larvae were tested for spirochetes by PCR.
For vector competence studies, in two separate experiments, groups of five nymphs (at least 2 mo postmolt) resulting from the above feedings were placed on test mice and allowed to feed to repletion, comparing the ability of MS and LA ticks to transmit B. burgdorferi to mice as compared with CT ticks (Note: in the case of LA ticks, three mice received less than five nymphs each due to low numbers available; Table 1). No effort was made to compare nymphal drop-off times of ticks from different locations due to the small numbers of nymphs used. Replete nymphs were subsequently tested for the presence of spirochetes by PCR. Exposed mice were then tested for transmission of spirochetes by culturing ear, bladder, and heart at 1-mo postnymphal exposure (Piesman and Happ 1997). Each organ cultured in BSK was examined by darkfield microscopy weekly for 1 mo to detect live spirochetes.
Table 1.
Experiments assessing ability of nymphal I. scapularis from different locations to transmit B. burgdorferi to mice
| Mousea | No. placed on mouse | No. fed on mouse | No. ticks PCR ++ | Mouse culture for Bbb |
|---|---|---|---|---|
| MS-1 | 5 | 4 | 4 | Pos |
| MS-2 | 5 | 2 | 2 | Pos |
| MS-3 | 5 | 1 | 1 | Pos |
| MS-4 | 5 | 4 | 4 | Pos |
| CT-1 | 5 | 4 | 2 | Pos |
| CT-2 | 5 | 5 | 3 | Pos |
| CT-3 | 5 | 5 | 1 | Pos |
| CT-4 | 5 | 5 | 4 | Pos |
| Second experiment | ||||
| LA-1 | 5 | 2 | 2 | Pos |
| LA-2 | 5 | 2 | 2 | Pos |
| LA-3 | 4 | 4 | 4 | Pos |
| LA-4 | 3 | 1 | 1 | Pos |
| LA-5 | 3 | 1 | 1 | Pos |
| LA-6 | 3 | 1 | 1 | Neg |
| CT-1 | 5 | 1 | 1 | Pos |
| CT-2 | 5 | 4 | 3 | Neg |
| CT-3 | 5 | 1 | 1 | Pos |
| CT-4 | 5 | 4 | 3 | Pos |
a Mouse number with ticks from three locations—either Mississippi, Connecticut, or Louisiana.
b Cultured for B. burgdorferi.
Statistical Analysis
Pearson’s Chi-squared test was used to evaluate statistical significance of differences in tick drop-off rates in northern versus southern ticks (Table 2). Specifically, this test was used to evaluate whether location and tick drop-off times were independent or related.
Table 2.
Statistical analysis of tick drop-off data
| A | ||||
| Day 2 | Day 3 | Day 4 | Day 5 | |
| MS–LA ticks | 0 | 11 | 267 | 136 |
| CT ticks | 158 | 225 | 100 | 33 |
| χ2 = 485.4705, df = 3, P < 2.2e-16 | ||||
| B | ||||
| Day 3 | Day 4 | |||
| MS–LA ticks | 11 | 267 | ||
| CT ticks | 225 | 100 | ||
| χ2 = 265.2742, df = 1, P < 2.2e-16 | ||||
| C | ||||
| Day 4 | Day 5 | |||
| MS–LA ticks | 267 | 136 | ||
| CT ticks | 100 | 33 | ||
| χ2 = 3.2955, df = 1, P < 0.06947 | ||||
Results and Discussion
Drop-off Study
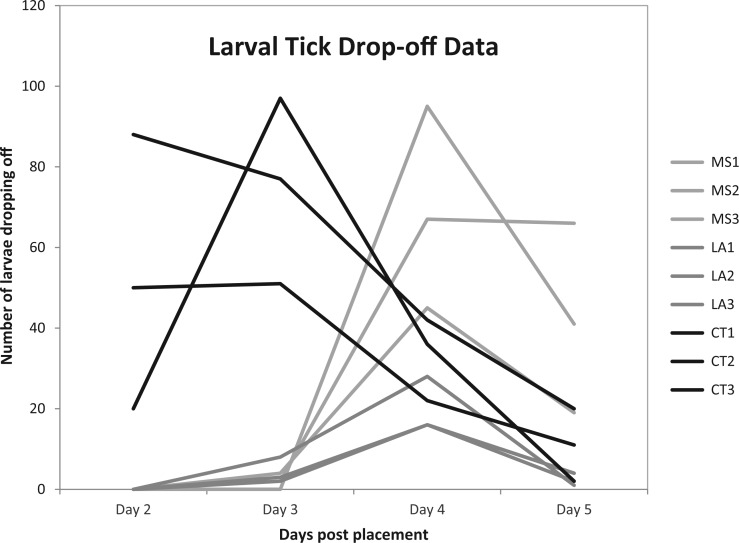
Despite our efforts to roughly place equal numbers of tick larvae on each mouse, the numbers feeding to repletion on experimental mice were widely disparate—339 MS ticks; 75 LA ticks; and 516 CT ticks (Fig. 1). However, because actual larval numbers were not counted, no conclusions about the relative numbers of ticks recovered per location will be made here. Future studies are warranted to more accurately determine feeding success on mice of immature I. scapularis ticks derived from different locations. As for drop-off times, CT ticks fed to repletion much faster than MS and LA ticks: overall, 73.6% of CT ticks had dropped off mice at Day 3 compared with only 1.7% and 6.6% of ticks dropped off for MS and LA ticks at that same time point (Fig. 1). Statistical analysis of the data showed that location (tick origin) and time until tick drop-off were related (P < 0.001, Table 2). Further analysis showed that the timeframe of days 3–4 for tick drop-off was significantly related to location (P < 0.001, Table 2B), while the timeframe of days 4–5 for tick drop-off was not significantly related to location (P > 0.05, Table 2C). It is important to note that our study included only two (F and O) of several genetic haplotypes of northern and southern populations of I. scapularis, thereby limiting the conclusions we can make to these two particular haplotypes. Despite this limitation, there were marked differences in feeding success (as measured by drop-off times) between northern and southern larval ticks. Interestingly, even southern haplotype F (from Mississippi) did not have the same drop-off rate as northern haplotype F (from Connecticut). Perhaps there is selection pressure on southern populations of I. scapularis immatures to feed on lizards and thus they are not adapted to feeding rapidly on rodents. In fact, previous studies of small rodents in Mississippi have found few, if any of them infested with immature I. scapularis (Norment et al. 1985, Clark and Durden 2002, Moraru et al. 2012, 2013).
Fig. 1.
Larval I. scapularis drop-off times from three different locations, repeated three times (three trials of each).
Vector competence
All I. scapularis ticks, regardless of geographic origin, easily transmitted B. burgdorferi to mice (Table 1). In the first experiment, 4 of the 4 mice in each case (MS or CT) that had been fed on by infected nymphs tested positive for B. burgdorferi. In the second experiment, 5 of the 6 mice tested positive for B. burgdorferi after exposure to infected LA ticks as compared with 3 of the 4 mice exposed to infected CT ticks. These data demonstrate that there is no difference in northern and southern populations of I. scapularis in their ability to serve as vectors of B. burgdorferi and points to lack of anthropophily of local immature ticks as a reason for scarcity of LB in southern states. Certainly, there may be many ecological or host-preference differences in tick populations that indirectly affect Lyme disease epidemiology in the southern United States, but southern populations of I. scapularis are indeed able to acquire and transmit the agent of Lyme disease.
Acknowledgments
Dr. Janice Van Zee provided valuable assistance with the molecular genetics used during this study. Dr. Kristine Edwards (Mississippi State University) provided technical assistance and John Caskey (Tulane National Primate Research Center) performed the statistical analysis. This article has been approved for publication as Journal Article No. J-12521 of the Mississippi Agriculture and Forestry Experiment Station, Mississippi State University.
References Cited
- Amin O. M. 1969. Growth of the dog tick, Dermacentor variabilis Say. II. The effect of starvation and host species on its growth and fecundity. J. Med. Entomol. 6: 321–326. [DOI] [PubMed] [Google Scholar]
- Apperson C. S., Levine J. F., Evans T. L., Braswell A., Heller J. 1993. Relative utilization of reptiles and rodents as hosts by immature Ixodes scapularis in the coastal plain of North Carolina. Exp. Appl. Acarol. 17: 719–731. [DOI] [PubMed] [Google Scholar]
- Auwaerter P. G., Bakken J. S., Dattwyler R. J., Dumler J. S., Halperin J. J., McSweegan E., Nadelman R. B., O'Connell S., Shapiro E. D., Sood S. K., et al. 2011. Antiscience and ethical concerns associated with advocacy of Lyme disease. Lancet Infect. Dis. 11: 713–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishopp F. C., Hixson H. 1936. Biology and economic importance of the Gulf Coast tick. J. Econ. Entomol. 20: 1068–1076. [Google Scholar]
- Clark K. L., Durden L. A. 2002. Parasitic Arthropods of small mammals in Mississippi. J. Mammol. 83: 1039–1048. [Google Scholar]
- Clark K. L., Brian L., Hartman S. 2013. Lyme borreliosis in human patients in Florida and Georgia, USA. Intl. J. Med. Sci. 10: 915–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M. C., Schulze T. L., Jordan R. A., Dietrich G., Schulze C. J., Hojgaard A., Ullmann A. J., Sackal C., Zeidner N. S., Piesman J. 2011. Elimination of Borrelia burgdorferi and Anaplasma phagocytophilum in rodent reservoirs and Ixodes scapularis ticks using a doxycycline hyclate-laden bait. Am. J. Trop. Med. Hyg. 85: 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durden L. A., Oliver J. H., Jr., Banks C. W., Vogel G. N. 2002. Parasitism of lizards by immature stages of the blacklegged tick, Ixodes scapularis (Acari, Ixodidae). Exp. Appl. Acarol. 26: 257–66. [DOI] [PubMed] [Google Scholar]
- Goddard J., Goddard J., II 2008. Estimating populations of adult Ixodes scapularis in Mississippi using a sequential Bayesian algorithm. J. Med. Entomol. 45: 556–562. [DOI] [PubMed] [Google Scholar]
- Goddard J., Goddard J., II 2010. Relative risk of acquiring black-legged ticks, Ixodes scapularis, in central Mississippi. Midsouth Entomol. 3: 97–100. [Google Scholar]
- Goddard J., Varela-Stokes A., Finley R. W. 2012. Lyme-disease-like illnesses in the South. J. Miss. State Med. Assoc. 53: 68–72. [PubMed] [Google Scholar]
- Gray J., Kahl O., Lane R. S., Stanek G. 2002. Lyme Borreliosis: Biology, Epidemiology, and Control. CABI Publishing, New York, NY. [Google Scholar]
- Hixson H. 1940. Field biology and environmental relationships of the Gulf Coast tick in southern Georgia. J. Econ. Entomol. 33: 179–189. [Google Scholar]
- Hojgaard A., Lukacik G., Piesman J. 2014. Detection of Borrelia burgdorferi, Anaplasma phagocytophilum, and Babesia microti, with two different multiplex PCR assays. Ticks Tick Borne Dis. 5: 349–351. [DOI] [PubMed] [Google Scholar]
- Koch H. G., Hair J. A. 1975. The effect of host species on engorgement, molting success, and molted weight of the Gulf Coast tick, Amblyomma maculatum. J. Med. Entomol. 12: 213–219. [DOI] [PubMed] [Google Scholar]
- Levin M., Levine J. F., Yang S., Howard P., Apperson C. S. 1996. Reservoir competence of the southeastern five-lined skink (Eumeces inexpectatus) and the green anole (Anolis carolinensis) for Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 54: 92–97. [DOI] [PubMed] [Google Scholar]
- Moraru G. M., Goddard J., Varela-Stokes A. S. 2012. Observations on host preference and feeding success of immature Amblyomma maculatum. J. Entomol. Sci. 47: 221–226. [Google Scholar]
- Moraru G. M., Goddard J., Murphy A., Link D., Belant J. L., Varela-Stokes A. 2013. Evidence of antibodies to spotted fever group rickettsiae in small mammals and quail from Mississippi. Vector Borne Zoonotic Dis. 13: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norment B. R., Stricklin L. S., Burgdorfer W. 1985. Rickettsia-like organisms in ticks and antibodies to spotted fever-group rickettsiae in mammals from Northern Mississippi. J. Wildl. Dis. 21: 125–131. [DOI] [PubMed] [Google Scholar]
- Norris D. E., Klompen J. S. H., Keirans J. E., Black W. C. 1996. Population genetics of Ixodes scapularis based on mitochondrial 16S and 12S genes. J. Med. Entomol. 33: 78–89. [DOI] [PubMed] [Google Scholar]
- Piesman J. 1991. Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method. J. Infect. Dis. 163: 895–897. [DOI] [PubMed] [Google Scholar]
- Piesman J. 1993. Standard system for infecting ticks with the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 30: 199–203. [DOI] [PubMed] [Google Scholar]
- Piesman J. 2002. Ecology of Borrelia burgdorferi sensu lato in North America, pp. 223–249. In Gray J. S., Kahl O., Lane R. S., Stanek G. (eds.), Lyme Borreliosis – Biology, Epidemiology, and Control. CABI Publishing, New York, NY. [Google Scholar]
- Piesman J., Sinksky R. J. 1988. Ability of Ixodes scapularis, Dermacentor variabilis, and Amblyomma americanum to acquire, maintain, and transmit Lyme disease spirochetes. J. Med. Entomol. 25: 336–339. [DOI] [PubMed] [Google Scholar]
- Piesman J., Happ C. M. 1997. Ability of the Lyme disease spirochete Borrelia burgdorferi to infect rodents and three species of human biting ticks (black-legged tick, American dog tick, lone star tick). J. Med. Entomol. 34: 451–456. [DOI] [PubMed] [Google Scholar]
- Qiu W. G., Dykhuizen D. E., Acosta M. S., Luft B. J. 2002. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics 160: 833–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders F. H., Jr., Oliver J. H., Jr 1995. Evaluation of Ixodes scapularis, Amblyomma americanum, and Dermacentor variabilis from Georgia as vectors of a Florida strain of the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 32: 402–406. [DOI] [PubMed] [Google Scholar]
- Sinsky R. J., Piesman J. 1989. Ear punch biopsy method for detection and isolation of Borrelia burgdorferi from rodents. J. Clin. Microbiol. 27: 1723–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonenshine D. E., Atwood E. L. 1967. Dynamics of feeding of the American dog tick, Dermacentor variabilis . Ann. Entomol. Soc. Am. 60: 362–373. [DOI] [PubMed] [Google Scholar]
- Trager W. 1939. Acquired immunity to ticks. J. Parasitol. 25: 57–81. [Google Scholar]
- Van Zee J., Black W. C., Levin M., Goddard J., Smith J., Piesman J. 2013. High SNP density in the blacklegged tick, Ixodes scapularis, the principal vector of Lyme disease spirochetes. Ticks Tick Borne Dis. 4: 63–71. [DOI] [PubMed] [Google Scholar]