Abstract
Background. Noroviruses are a leading cause of acute gastroenteritis worldwide. Mucosal and cellular immune responses remain poorly understood, with most studies of noroviruses having focused on serological responses to infection.
Methods. We used saliva, feces, and peripheral blood mononuclear cells collected from persons who were administered Norwalk virus (NV) to characterize mucosal (salivary and fecal immunoglobulin A [IgA]) and cellular (NV-specific IgA and immunoglobulin G [IgG] antibody-secreting cells and total and NV-specific IgA and IgG memory B cells) immune responses following infection.
Results. Prechallenge levels of NV-specific salivary IgA and NV-specific memory IgG cells correlated with protection from gastroenteritis, whereas prechallenge levels of NV-specific fecal IgA correlated with a reduced viral load. Antibody-secreting cell responses were biased toward IgA, while memory B-cell responses were biased toward IgG. NV-specific memory B cells but not antibody-secreting cells persisted 180 days after infection.
Conclusions. NV-specific salivary IgA and NV-specific memory IgG cells were identified as new correlates of protection against NV gastroenteritis. Understanding the relative importance of mucosal, cellular, and humoral immunity is important in developing vaccine strategies for norovirus disease prevention.
Keywords: Norovirus, Norwalk virus, IgA, IgG, antibody-secreting cells, memory B cells, salivary IgA, fecal IgA, immune response, correlate of protection
Noroviruses (NoVs) are an important cause of epidemic and sporadic gastroenteritis and are associated with nearly one-fifth of all cases of acute gastroenteritis worldwide [1, 2]. In the United States, NoVs are the leading cause of gastroenteritis across all age groups [3]. The lack of a fully permissive cell culture system or small-animal model has hampered studies on NoV pathogenesis and immunity. Most knowledge of the immune response to NoVs comes from human challenge studies and clinical trials that used NoV virus-like particles (VLPs) as vaccines and have largely focused on serological responses [4, 5]. Histo-blood group antigens (HBGAs) serve as attachment factors for NoVs, and serum antibody that blocked the binding of NoV VLPs to HBGAs was the first identified correlate of protection from NoV gastroenteritis [4, 6]. Few studies have examined mucosal and cellular immunity following NoV infection [7–9].
We previously performed a randomized, double-blinded, placebo-controlled evaluation of different doses of Norwalk virus (NV), a prototype strain of human NoVs, to determine its median human infectious dose (HID50) [10]. Clinical data, virological characteristics, and serological response to infection have been published [5, 6, 10, 11]. We now report the mucosal and cellular immune responses to NV infection, using saliva specimens, fecal specimens, and peripheral blood mononuclear cells (PBMCs) collected from study participants before and at various time points after inoculation with NV.
METHODS
Study Design
The study design for the challenge of 57 healthy adult human volunteers with NV has been published previously [10]. Written informed consent was obtained from all participants. The study was approved by the institutional review boards at Baylor College of Medicine and the Houston Methodist Hospital. See the Supplementary Materials for details on study design and immunoassay methods.
Assessment of Disease Severity
An ordinal symptom scoring key was developed for calculation of severity of gastroenteritis, using data on diarrhea and vomiting recorded on days 1–4 after challenge. When a person experienced diarrhea, loose, unformed stool was assigned a score of 1, and liquid stools were given a score of 2. One or 2 episodes of vomiting per day were assigned a score of 1 and 3–6 episodes of vomiting were assigned a score of 2. The sum of scores provided the total severity score for gastroenteritis.
Measurement of Salivary and Fecal IgA
Total and NV-specific IgA was measured using antibody sandwich enzyme immunoassays. Briefly, 96-well microtiter plates were coated with a rabbit anti-human IgA antibody (Jackson Immuno) or a rabbit anti-NV antibody. NV VLPs were added at a concentration of 0.1 µg VLPs per well to the NV-specific IgA assay plates. Doubling dilutions of sample or standard human IgA (Sigma) were tested. IgA was detected using a mouse anti-human IgA (ABD Serotec), followed by a goat anti-mouse IgG conjugated to horseradish peroxidase (HRP; ABD Serotec) and 3,3′,5,5′-tetramethylbenzidine substrate (Thermo Scientific).
For fecal IgA measurement, fecal extracts (25% w/v) were prepared in a buffer containing soybean trypsin inhibitor (10% v/v), 0.5 M ethylenediaminetetraacetic, and 200 mM phenylmethanesulfonyl fluoride [12]. Total and NV-specific IgA levels were measured as described for saliva samples with minor modifications.
Measurement of IgA and IgG Antibody-Secreting Cells (ASCs) and Memory B Cells
Ninety-six–well polyvinylidene difluoride membrane plates (Millipore) were coated with 1 µg per well of NV VLP or phosphate-buffered saline (PBS) overnight at 4°C. PBMCs were thawed and doubling dilutions were prepared in complete AIM-V medium (Invitrogen) such that the first row contained an average of 2.56 × 105 PBMCs (range, 1.04 × 105–5.29 × 105 PBMCs). IgA or IgG ASCs were detected using a goat anti-human IgA or IgG antibody conjugated to HRP (Southern Biotech) and a stable DAB substrate (Invitrogen). The number of spots in PBS-coated wells was subtracted from the number of spots in the NV VLP–coated wells. Dilutions of PBMCs that gave 8–200 spots were used for calculation of ASCs.
Total and NV-specific IgG and IgA memory B cells were measured using modifications of published protocols as detailed in the Supplementary Materials [13].
Data Analysis
The amount of total IgA in test samples was calculated by interpolation from the linear portion of the standard curve, using purified IgA. To account for differences in total IgA between persons and time points, specific IgA was expressed as the amount per 100 µg of total IgA. For samples with undetectable NV-specific IgA but detectable total IgA, NV-specific IgA per 100 µg of total IgA could not be calculated. Such samples were assigned the minimum calculated value obtained when both NV-specific and total IgA levels could be determined [14]. IgA and IgG ASCs were expressed as number of cells per 106 PBMCs. The frequency of antigen-specific memory B cells was calculated as a percentage of total memory B cells. Samples with a total memory B cell count below the limit of detection of the assay were excluded from this analysis. The Pearson correlation coefficient was calculated to assess correlation between assays. Comparison between groups was performed using the Student t test or the Mann–Whitney U test. P values of <.05 were considered statistically significant.
RESULTS
Study Subjects
The study population demographic characteristics, clinical data, virological characteristics, and results of serological studies have been published previously [10]. In brief, of the 57 persons enrolled in the study, 8 received placebo, and an additional 8 were identified as lacking functional fucosyltransferase 2, a genetic factor required for susceptibility to NV infection. Further, 5 persons belonged to the B or AB blood type and were also not susceptible to NV infection [8, 15]. Of the remaining 36 susceptible persons challenged with NV, 21 became infected, of whom 20 experienced clinical symptoms during the 4 days after challenge. Nausea, abdominal cramps, and malaise were the most common symptoms. Fourteen persons developed viral gastroenteritis; all 14 vomited at least once, and 7 experienced watery diarrhea. None of the persons with gastroenteritis required intravenous rehydration. The duration of virus shedding ranged from 6 days to 55 days; the median duration of virus shedding was longer in persons with gastroenteritis (29 days), compared with those without gastroenteritis (19 days), but this difference was not statistically significant (P = .14). Peak fecal viral load was, however, significantly higher in persons with gastroenteritis, compared with those without gastroenteritis (geometric mean viral load, 160 billion vs 10 billion genomic equivalents per mL, respectively; P = .005) [10]. Serum IgA levels peaked at day 14 after inoculation and IgG levels peaked at day 28 [5]. The HBGA blocking antibody titer was significantly higher among infected persons who did not develop gastroenteritis than among those who did [6].
Mucosal Immune Response to NV Infection
Total and NV-specific salivary and fecal IgA levels were estimated in samples collected prior to challenge and at days 7, 14, and 28 after challenge wherever available. The geometric mean titers (GMTs) of IgA in samples collected from infected and uninfected subjects are given in Table 1. Uninfected subjects included persons challenged with NV who remained uninfected, nonsusceptible participants, and those who received placebo. There were no significant differences in total salivary and fecal IgA levels between infected and uninfected subjects at any time point. Similar to serum IgA levels, NV-specific IgA levels in saliva and feces peaked at day 14 after challenge in the infected participants. The geometric mean fold rise (GMFR) was higher for NV-specific fecal IgA than for NV-specific salivary IgA (Table 1). A ≥4-fold rise in the NV-specific salivary IgA level was observed in 70% of infected persons by day 28. None of the uninfected subjects showed a >4-fold change. For the NV-specific fecal IgA assay, all infected subjects (100%) showed a >4-fold change by day 28. A >4-fold rise was also observed in 5 other subjects, including 3 persons who were challenged with NV and were considered uninfected by the study definitions (study definitions are specified in the Supplementary Materials), 1 person who received placebo, and 1 nonsecretor (Table 1). Given that NV-specific IgA was expressed relative to the amount of total IgA, which varied between subjects and time points, at least some of these results were likely to be nonspecific.
Table 1.
Mucosal Response to Norwalk Virus (NV) Infection
| Assay | Uninfected |
Infected |
||||||
|---|---|---|---|---|---|---|---|---|
| Before Challenge | Day 7 | Day 14 | Day 28 | Before Challenge | Day 7 | Day 14 | Day 28 | |
| Total salivary IgA level, µg | ||||||||
| GMT (95% CI) | 260.9 (237.6–286.5) | 285.8 (259.7–314.6) | 246.8 (210.0–289.9) | 271.3 (243.5–302.1) | 262.9 (225.0–307.2) | 250.9 (215.1–292.6) | 275.7 (242.1–313.8) | 279.1 (249.2–312.7) |
| GMFR (95% CI) | … | 1.1 (1.0–1.2) | 0.9 (.8–1.1) | 1.0 (1.0–1.2) | … | 1.0 (.8–1.2) | 1.0 (.9–1.2) | 1.1 (.9–1.2) |
| NV-specific salivary IgA level, ng/100 µg of total IgA | ||||||||
| GMT (95% CI) | 69.7 (59.8–81.2) | 85.7 (76.2–96.3) | 87.8 (75.9–101.4) | 83.4 (74.0–93.9) | 76.8 (62.0–95.0) | 120.6 (93.7–155.3) | 340.3 (231.3–500.8) | 224.9 (175.3–288.7) |
| GMFR (95% CI) | … | 1.3 (1.1–1.4) | 1.2 (1.1–1.4) | 1.2 (1.1–1.4) | … | 1.6 (1.3–1.9) | 4.4 (3.2–6.1) | 2.9 (2.4–3.7) |
| Subjects with ≥4-fold rise, no. (%) | … | 0 (0) | 0 (0) | 0 (0) | … | 0 (0) | 13 (65) | 7 (35) |
| Total fecal IgA level, µg | ||||||||
| GMT (95% CI) | 123.5 (79.3–192.4) | 183.1 (123.9–270.7) | 148.5 (87.6–251.5) | 150.9 (93.5–243.6) | 122.9 (81.7–184.7) | 169.8 (101.7–283.2) | 138.1 (77.4–246.4) | 110.8 (63.7–192.8) |
| GMFR (95% CI) | … | 1.5 (1.0–2.1) | 1.2 (.8–1.8) | 1.1 (.7–1.7) | … | 1.4 (.9–2.0) | 1.1 (.8–1.7) | 0.9 (.6–1.3) |
| NV-specific fecal IgA level, ng/100 µg of total IgA | ||||||||
| GMT (95% CI) | 22.9 (16.0–32.8) | 24.6 (17.0–35.7) | 25.0 (16.5–37.9) | 23.9 (15.8–36.2) | 45.1 (19.5–104.5) | 69.6 (31.6–153.2) | 2715.0 (1083.0–6803.0) | 1544.0 (486.9–4897.0) |
| GMFR (95% CI) | … | 1.1 (.9–1.3) | 1.1 (.8–1.4) | 1.0 (.7–1.5) | … | 1.5 (.8–3.0) | 60.2 (24.4–148.5) | 34.2 (12.4–94.7) |
| Subjects with ≥4-fold rise, no. (%) | … | 1 (3) | 2 (6) | 3 (9) | … | 5 (25) | 19 (95) | 17 (85) |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; IgA, immunoglobulin A.
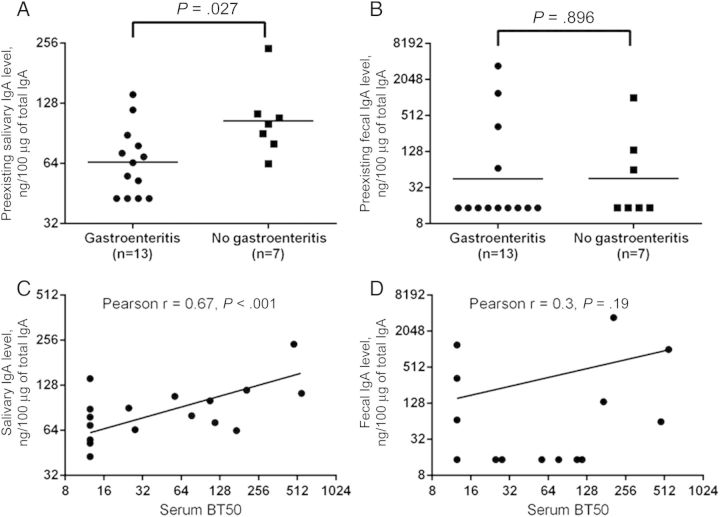
While preexisting NV-specific IgA levels in saliva were associated with protection from gastroenteritis, NV-specific fecal IgA levels were not. The geometric mean level of NV-specific preexisting salivary IgA was significantly greater in infected persons who did not develop gastroenteritis, compared with those with gastroenteritis (104.6 ng/100 µg of total IgA vs 65.0 ng/100 µg of total IgA, respectively; P < .05; Figure 1A). This difference was not seen in relation to preexisting fecal NV-specific IgA (45.6 ng/100 µg of total IgA in the nongastroenteritis group vs 44.9 ng/100 µg of total IgA in the gastroenteritis group; Figure 1B). Prechallenge serum HBGA blocking antibody (serum BT50) significantly correlated with prechallenge NV-specific salivary IgA but not with fecal IgA (Figure 1C and 1D, respectively).
Figure 1.
Salivary and fecal immunoglobulin A (IgA) responses to Norwalk virus (NV) infection. A significant difference in preexisting IgA levels between infected subjects with and those without gastroenteritis was seen with NV-specific salivary (A) but not fecal (B) IgA. Among the infected subjects, the preexisting histo-blood group antigen (HBGA) blocking antibody (BT50) in serum correlates with salivary (C) but not fecal (D) IgA.
Among the infected subjects, there was a significant inverse correlation between preexisting NV-specific fecal IgA levels and peak viral load (Pearson r, −0.47; P = .04). Similarly, there was an inverse correlation between preexisting NV-specific salivary IgA and severity score for diarrhea and vomiting (Pearson r, −0.48; P = .03). An inverse correlation was also present between the NV-specific fecal IgA level at day 7 after challenge and the duration of detectable virus shedding (Pearson r, −0.51; P = .02). Together, these findings are consistent with a role for the NV-specific mucosal immune response in reducing the viral load, the duration of virus shedding, and the severity of the disease.
Cellular Immune Response to NV Infection
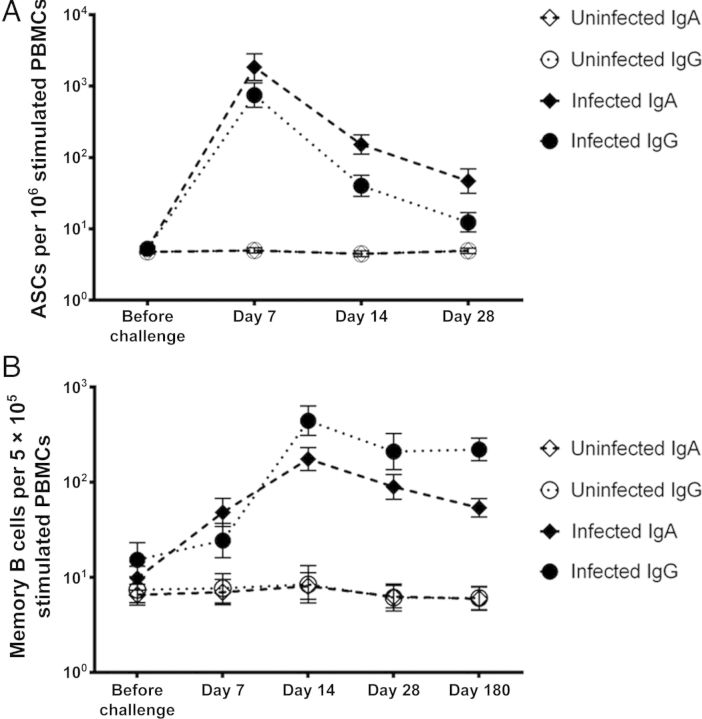
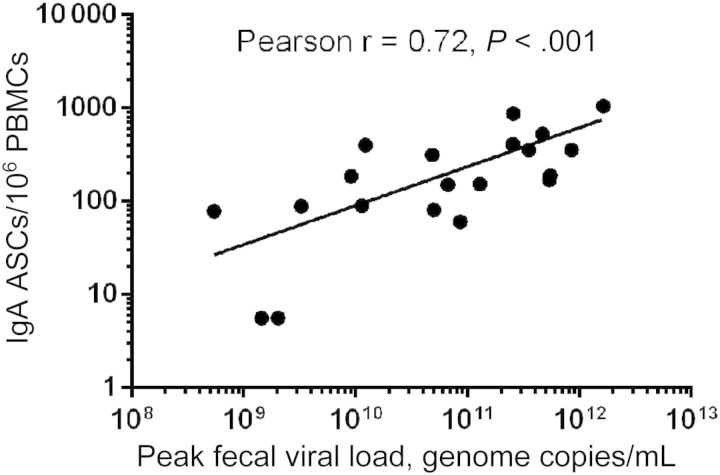
ASCs and memory B cells were analyzed to characterize cellular immune responses. ASC studies were performed on samples collected from 20 infected and 11 uninfected persons, including 6 who were challenged with NV, 4 placebo recipients, and 1 nonsusceptible individual. Initial studies for assessment of ASCs were performed using PBMCs collected prior to challenge and at days 2, 7, 14, 28, and 180 after challenge. However, since the number of ASCs at days 2 and 180 were not above background levels (data not shown), these time points were excluded from the remainder of the studies. In NV-infected subjects, IgA and IgG ASCs peaked at day 7 after challenge and reduced dramatically by day 14 (Table 2 and Figure 2A). No change in ASC levels occurred in uninfected subjects. All infected subjects (100%) showed a >4-fold rise in IgA and IgG ASC levels from baseline. The increase in GMFR from prechallenge levels was greater at all time points after infection for IgA ASCs, compared with IgG ASCs. The peak IgA ASC level precedes the IgA antibody level peak in serum, saliva, and feces. A strong correlation was present between peak cellular immune response and antibody levels, as observed between IgA ASC levels at day 7 and NV-specific antibody levels in serum, salivary, and fecal IgA levels at day 14 (Pearson r, 0.86, 0.78, and 0.70, respectively; P < .0001). Among infected subjects, levels of IgA ASC correlated with peak viral load in feces at day 7 (Pearson r, 0.5; P = .01). A greater correlation was seen at day 14 (Pearson r, 0.72; P < .0001; Figure 3), indicating a possible role for higher viral load in persistence of IgA ASC response beyond the peak levels at day 7.
Table 2.
Antibody-Secreting Cell (ASC) Response to Norwalk Virus (NV) Infection
| Assay | Uninfected |
Infected |
||||||
|---|---|---|---|---|---|---|---|---|
| Before Challenge | Day 7 | Day 14 | Day 28 | Before Challenge | Day 7 | Day 14 | Day 28 | |
| NV-specific IgA ASCs/106 PBMCs | ||||||||
| GMT (95% CI) | 4.9 (4.2–5.8) | 4.8 (3.9–5.8) | 4.7 (3.8–5.9) | 4.9 (4.1–5.9) | 5.3 (4.5–6.3) | 1845.0 (751.8–4530.0) | 151.8 (79.4–290.1) | 46.7 (20.4–107.1) |
| GMFR (95% CI) | … | 1.0 (.7–1.3) | 1.0 (.7–1.3) | 1.0 (.8–1.3) | … | 339.5 (117.3–982.2) | 29.4 (13.4–64.2) | 8.2 (3.4–19.8) |
| Subjects with ≥4-fold rise, no. (%) | … | 0 (0) | 0 (0) | 0 (0) | … | 18 (95) | 17 (90) | 12 (67) |
| NV-specific IgG ASCs/106 PBMCs | ||||||||
| GMT (95% CI) | 4.9 (4.2–5.8) | 4.8 (3.9–5.8) | 4.7 (3.8–5.9) | 4.9 (4.1–5.9) | 5.3 (4.5–6.3) | 751.2 (329.1–1715.0) | 40.2 (19.8–81.9) | 12.4 (6.4–23.9) |
| GMFR (95% CI) | … | 1.0 (.7–1.3) | 1.0 (.7–1.3) | 1.0 (.8–1.3) | 132.5 (50.0–351.5) | 8.7 (4.1–18.1) | 2.6 (1.4–5.1) | |
| Subjects with ≥4-fold rise, no. (%) | … | 0 (0) | 0 (0) | 0 (0) | 18 (95) | 14 (74) | 7 (39) | |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; IgA, immunoglobulin A; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cell.
Figure 2.
Kinetics of cellular immune response in Norwalk virus (NV) infection. Geometric mean number of NV-specific antibody-secreting cells (ASCs; A) and memory B cells (B) were plotted for each time point. Error bars represent the standard error of mean. Abbreviations: IgA, immunoglobulin A; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cell.
Figure 3.
Correlation of day 14 Norwalk virus–specific immunoglobulin A (IgA) antibody-secreting cell (ASC) levels with peak viral load in 20 infected persons. Abbreviation: PBMC, peripheral blood mononuclear cell.
Memory B-cell assays were performed using PBMCs from 35 study participants, including 29 persons challenged with NV (19 infected and 10 uninfected) and 6 who received placebo. PBMCs collected prior to challenge and at days 7, 14, 28, and 180 after challenge were tested. NV-specific memory B-cell response peaked at day 14 and persisted at day 180 after challenge (Table 3 and Figure 2B). Taking data from all time points, NV-specific IgA memory B-cell levels correlated well with serum, salivary, and fecal IgA titers (Pearson r, 0.86, 0.72, and 0.61, respectively).
Table 3.
Memory B-Cell Response to Norwalk Virus (NV) Infection
| Assay | Uninfected |
Infected |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before Challenge | Day 7 | Day 14 | Day 28 | Day 180 | Before Challenge | Day 7 | Day 14 | Day 28 | Day 180 | |
| NV-specific IgA memory B cells, %/5 × 105 PBMCs | ||||||||||
| GMT (95% CI) | 0.1 (.0–.1) | 0.1 (.0–.2) | 0.1 (.0–.3) | 0.1 (.0–.2) | 0.1 (.0–.1) | 0.2 (.1–.3) | 0.8 (.4–1.7) | 2.5 (1.6–4.0) | 1.3 (.7–2.3) | 0.7 (.4–1.2) |
| GMFR (95% CI) | … | 1.1 (.8–1.5) | 1.4 (1.0–2.0) | 1.1 (.8–1.4) | 0.9 (.6–1.2) | … | 7.2 (3.2–16.3) | 15.7 (8.3–29.5) | 7.3 (3.4–15.7) | 4.5 (2.3–8.9) |
| Subjects with ≥4-fold rise, no. (%) | … | 1 (7) | 1 (7) | 0 (0) | 0 (0) | … | 10 (67) | 16 (84) | 12 (63) | 9 (50) |
| NV-specific IgG memory B cells, %/5 × 105 PBMCs | ||||||||||
| GMT (95% CI) | 0.1 (.0–.2) | 0.1 (.0–.2) | 0.1 (.0–.3) | 0.1 (.0–.1) | 0.1 (.0–.2) | 0.1 (.1–.3) | 0.3 (.1–.7) | 4.7 (2.5–8.8) | 2.2 (.9–5.5) | 2.2 (1.4–3.4) |
| GMFR (95% CI) | … | 1.2 (.8–1.6) | 1.2 (.7–2.2) | 0.9 (.5–1.6) | 1.1 (.8–1.6) | … | 1.5 (.5–5.0) | 30.5 (15.1–61.4) | 13.0 (5.1–33.1) | 12.0 (4.8–30.2) |
| Subjects with ≥4-fold rise, no. (%) | … | 0 (0) | 0 (0) | 1 (11) | 0 (0) | … | 2 (20) | 11 (92) | 9 (75) | 9 (75) |
Abbreviations: CI, confidence interval; GMFR, geometric mean fold rise; GMT, geometric mean titer; IgA, immunoglobulin A; IgG, immunoglobulin G; PBMC, peripheral blood mononuclear cell.
GMFR was calculated from prechallenge levels or, when prechallenge PBMC samples were not available, from day 7 values. At day 7 after infection, the GMFR from baseline levels was higher for IgA memory B cells than IgG memory B cells. However, the peak GMFR from baseline levels (at day 14) was higher for IgG memory B cells, compared with the IgA memory B cells. Eighteen of 19 infected subjects (94.7%) showed a >4-fold change in the percentage of NV-specific IgA memory B cells from baseline levels. Prechallenge PBMCs were not available for the one nonresponder, and therefore, fold change was calculated from day 7 values. A 5-fold change from the prechallenge NV-specific IgA memory B-cell level was seen in 1 uninfected subject at day 7. One person receiving placebo also showed a 6-fold change from baseline at day 14. Total IgG memory B cell levels were below the limit of detection at all time points for 11 persons, including 6 infected, 1 uninfected, and 4 persons who received placebo; therefore the percentage of NV-specific IgG memory B cells could not be calculated for these subjects. Twelve of 13 infected subjects (92.3%) showed a >4-fold change in the percentage of NV-specific IgG memory B cells from prechallenge levels. The uninfected, NV-challenged subject who showed a 5-fold change in NV-specific IgA memory B-cell level at day 7 also showed a 6-fold change in NV-specific IgG memory B cells at day 14 after challenge.
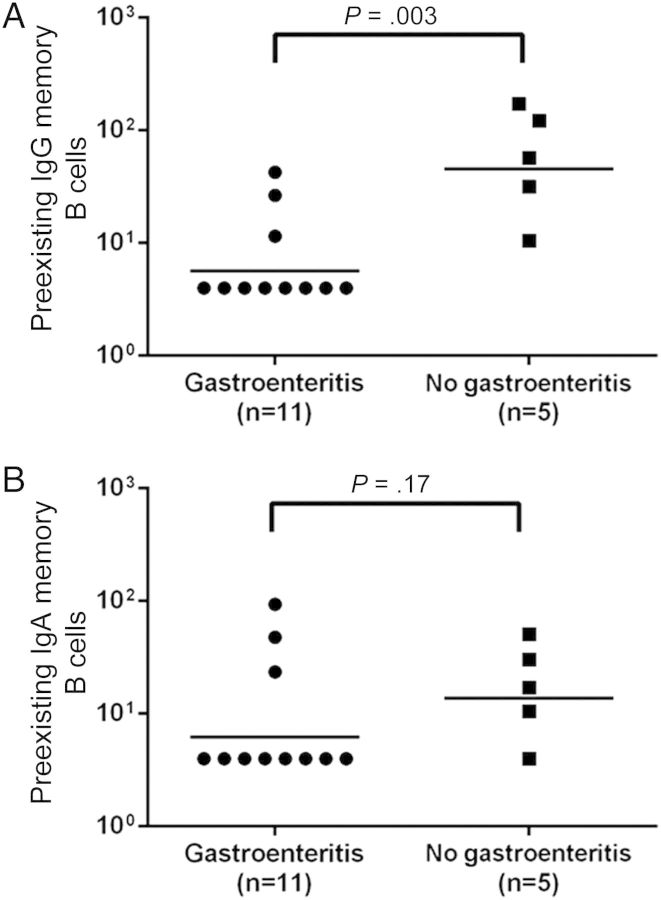
The level of preexisting NV-specific IgG memory B cells was significantly associated with protection from gastroenteritis (Figure 4A) and correlated with preexisting serum HBGA blocking antibody (Pearson r, 0.61; P < .05). The level of NV-specific IgA memory B cells was not associated with protection against gastroenteritis (Figure 4B) and showed a poor correlation with HBGA blocking antibody (Pearson r, 0.37; P = .15). However, total IgA memory B cell levels correlated with protection from NV infection and gastroenteritis. The GMT of total IgA memory B cells was significantly lower across all time points in susceptible persons who became infected and developed gastroenteritis, compared with those who became infected but did not develop gastroenteritis or who remained uninfected (Supplementary Figure 1). There were no significant correlations between memory B-cell response and viral load, duration of virus shedding, or disease severity.
Figure 4.
Comparison of prechallenge memory B cells levels among Norwalk virus (NV)-infected persons with and those without gastroenteritis. There were significant differences in preexisting NV-specific immunoglobulin G (IgG) memory B cells (A) but not NV-specific immunoglobulin A (IgA) memory B cells (B).
DISCUSSION
This study addresses a current gap in knowledge on mucosal and cellular immunity to NoV and includes characterization of salivary IgA, fecal IgA, ASC, and memory B-cell responses following experimental NV infection. Two new potential correlates of protection against NV gastroenteritis, specifically, levels of prechallenge NV-specific salivary IgA and NV-specific memory B cells, were identified. NV-specific salivary IgA levels before challenge correlated with reduced severity of gastroenteritis. NV-specific fecal IgA levels before challenge were associated with a reduction in peak viral load, whereas fecal IgA measured day 7 after infection correlated with a shorter duration of virus shedding. A higher viral load also correlated with persistence of the IgA ASC response.
A key finding of this study was that preexisting NV-specific salivary IgA was associated with a lower risk of gastroenteritis and reduced severity of gastroenteritis. IgA is abundantly present in the gastrointestinal tract and, in the secreted form, is a key immune effector against enteric pathogens [16]. Secretory IgA is a dimeric molecule; monomers of IgA are bound by the J-chain and released with the secretory component attached to them. Since exudation of monomeric serum IgA in saliva is known to occur, the correlation of salivary IgA with serum IgA raises the possibility that IgA detected in saliva may be serum-derived monomeric IgA, rather than secretory IgA. However, serum IgA by itself did not correlate with protection from illness. The detected salivary IgA was therefore possibly reflective of mucosal immunity. However, we were unable to confirm a mucosal source by using an antibody to secretory component to detect virus-specific dimeric IgA (data not shown).
In a previous human challenge study with NV, susceptible persons who produced salivary IgA within 5 days of challenge were less likely to be infected than those who produced salivary IgA later, suggesting a role for memory salivary response in protection from infection [8]. Although the percentage of subjects showing a >4-fold change was similar between both studies, salivary IgA response in the present study did not correlate with protection from infection. However, the first time point evaluated after challenge was 7 days, and it is possible that early differences were missed. Also, there were fewer subjects who were uninfected in the current study who received a dose of NV higher than the HID50 [10]. The other marker of mucosal immunity we assessed was fecal IgA. NV-specific fecal IgA levels did not correlate with protection from infection or disease, as reported previously [9]. However, prechallenge levels of fecal IgA correlated with reduced viral load, and the induction of a fecal IgA response appeared to be important in controlling the duration of virus shedding.
Cellular immune response to NoVs in humans has been examined mainly in the context of VLP vaccines [7, 17, 18]. As observed in vaccine trials, ASC responses following infection peaked at day 7 and correlated with serum and mucosal IgA responses [17]. Memory B-cell responses to both infection and vaccination persist at day 180 [18]. An interesting difference between the ASC and memory B-cell responses in this study was that ASCs were more biased toward IgA, while memory B-cell responses were more biased toward IgG. It remains to be seen whether this bias also occurs following vaccination. We also observed that IgA ASCs persisted beyond peak levels at day 7 in persons with a higher viral load and that prechallenge NV-specific IgG memory B cells correlated with protection from clinical disease. This raises the question whether the IgA cellular response is critical for the clearance of infection, while IgG is important for long-term immunity. Lipopolysaccharide-specific IgG memory B cells were found to be important in protection against infection with another enteric pathogen, Vibrio cholerae O1 [19]. Long-term protection against NoV was not seen in early volunteer studies, although a recent modeling study suggests that immunity lasts 4–9 years following infection [20]. Analysis of memory B cells at later time points could be important for understanding long-term protection against NoV infection.
Another important consideration in the comparison of cellular immunity after infection and vaccination is the role of live virus versus VLPs in the induction of robust immunity. Three weeks after intranasal immunization with NoV VLPs, only 20% of vaccinated subjects showed >4-fold levels of IgA ASCs, compared with 67% following infection [17]. While 100% of subjects showed >4-fold change from prevaccination levels after a second dose, the number of ASCs produced were lower than observed after the first dose. Overall, the magnitude of ASC and memory B-cell responses were lower following intranasal vaccination, compared with infection [18]. Trials of parenteral immunization with NoV vaccines are being conducted, and it remains to be seen whether this approach results in better cellular immunity [21].
Although not NV-specific, an interesting observation was that total IgA memory B cells correlated with protection from NV infection and gastroenteritis. Whether these were cells with mucosal homing potential and would therefore be reflective of higher levels of IgA in the gut of uninfected subjects and those who did not develop gastroenteritis can be evaluated in future studies. The identification of new correlates of protection in this study raises questions on whether multiple mechanisms contribute to protection from NV gastroenteritis and whether 1 or more protective effector molecules can compensate for the lack of other responses. A recently introduced concept in the nomenclature of correlates of protection is the distinction into mechanistic correlates (mCoP) and nonmechanistic correlates (nCoP) [22]. The first identified correlate of protection was serum antibodies that block the binding of virus to HBGA. More recently, NV-specific serum IgA also was found to correlate with protection in vaccine studies [23]. In the absence of an efficient replication system, which of the effectors are mCoP or nCoP remains to be confirmed. It is possible that non-HBGA blocking mechanisms also contribute to protection. The relatively small number of subjects enrolled and the likelihood that some of the immune responses covary make it difficult to assess whether one correlate is better than another and which is the most important predictor of protection.
As studies with VLP-based NoV vaccines progress, dissection of the repertoire of immune responses will be important to aid the development of more-effective vaccines. Assays to test the new correlates of protection can be incorporated in the study design of the next phase of NoV VLP vaccine trials. The isotype of HBGA blocking antibodies remains to be identified. Characterization of the isotype of these protective antibodies will be important to inform decisions on the use of specific adjuvants and routes of immunization as NoV-VLP vaccine trials proceed. The present study was performed in adults, and future studies will determine whether similar patterns of responses will be seen in an unprimed population, such as children. In the case of influenza virus, a mucosal pathogen for which parental immunization is used for vaccination, the percentage of IgA memory B cells does not change with age, while the percentage of IgG memory B cells increases [24]. How these differences might affect protection from NoV in children in comparison to adults needs to be studied. The role of additional factors, such as cytokine production in disease manifestation or driving cellular immunity, remains unknown. This study reiterates the importance of developing an efficient replication system for human NoVs to facilitate more progress in understanding immune responses and pathogenesis.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Financial support. The work was supported by the National Institutes of Health) (NIH (M01-RR00188, N01-AI25465, P01-AI057788, P30-DK56338, and HHSN272200800002C) and the John S. Dunn Research Foundation (to R. L. A.).
Potential conflicts of interest. S. R. received support from the National Institute of Allergy and Infectious Disease (NIAID), NIH, to conduct the study. F. H. N. received support from the NIAID, NIH, to conduct the study. A. R. O. received support from the NIAID, NIH, to conduct the study. M. A. G. received support from the NIAID, NIH, to conduct the study. D. Y. G. received support from the NIAID, NIH, to conduct the study, is named as an inventor on patents related to cloning of the Norwalk virus genome, and has received research grant funding from Takeda Vaccines (Montana). M. K. E. received support from NIAID, NIH, to conduct the study, is named as an inventor on patents related to cloning of the Norwalk virus genome, and has served as a consultant to Takeda Vaccines (Montana). R. L. A. received support from NIAID, NIH, to conduct the study and has received research grant funding from and is a consultant to Takeda Vaccines (Montana).
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Ahmed SM, Hall AJ, Robinson AE, et al. Global prevalence of norovirus in cases of gastroenteritis: a systematic review and meta-analysis. Lancet Infect Dis 2014; 14:725–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ramani S, Atmar RL, Estes MK. Epidemiology of human noroviruses and updates on vaccine development. Curr Opin Gastroenterol 2014; 30:25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall AJ, Lopman BA, Payne DC, et al. Norovirus disease in the United States. Emerg Infect Dis 2013; 19:1198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atmar RL, Bernstein DI, Harro CD, et al. Norovirus vaccine against experimental human Norwalk Virus illness. New Engl J Med 2011; 365:2178–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kavanagh O, Estes MK, Reeck A, et al. Serological responses to experimental Norwalk virus infection measured using a quantitative duplex time-resolved fluorescence immunoassay. Clin Vaccine Immunol 2011; 18:1187–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reeck A, Kavanagh O, Estes MK, et al. Serological correlate of protection against norovirus-induced gastroenteritis. J Infect Dis 2010; 202:1212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lindesmith L, Moe C, Lependu J, Frelinger JA, Treanor J, Baric RS. Cellular and humoral immunity following Snow Mountain virus challenge. J Virol 2005; 79:2900–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lindesmith L, Moe C, Marionneau S, et al. Human susceptibility and resistance to Norwalk virus infection. Nat Med 2003; 9:548–53. [DOI] [PubMed] [Google Scholar]
- 9.Okhuysen PC, Jiang X, Ye L, Johnson PC, Estes MK. Viral shedding and fecal IgA response after Norwalk virus infection. J Infect Dis 1995; 171:566–9. [DOI] [PubMed] [Google Scholar]
- 10.Atmar RL, Opekun AR, Gilger MA, et al. Determination of the 50% human infectious dose for Norwalk virus. J Infect Dis 2014; 209:1016–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ajami NJ, Barry MA, Carrillo B, et al. Antibody responses to norovirus genogroup GI.1 and GII.4 proteases in volunteers administered Norwalk virus. Clin Vaccine Immunol 2012; 19:1980–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gaspari MM, Brennan PT, Solomon SM, Elson CO. A method of obtaining, processing, and analyzing human intestinal secretions for antibody content. J Immunol Methods 1988; 110:85–91. [DOI] [PubMed] [Google Scholar]
- 13.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods 2004; 286:111–22. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Choo S, Everard J, Jennings R, Finn A. Mucosal immune responses to meningococcal group C conjugate and group A and C polysaccharide vaccines in adolescents. Infect Immun 2000; 68:2692–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutson AM, Airaud F, LePendu J, Estes MK, Atmar RL. Norwalk virus infection associates with secretor status genotyped from sera. J Med Virol 2005; 77:116–20. [DOI] [PubMed] [Google Scholar]
- 16.Janeway CA, Travers P, Walport M, Shlomchik M. The mucosal immune system. Immunobiology: The Immune System in Health and Disease. 5th ed New York: Garland Science, 2001. [Google Scholar]
- 17.El-Kamary SS, Pasetti MF, Mendelman PM, et al. Adjuvanted intranasal Norwalk virus-like particle vaccine elicits antibodies and antibody-secreting cells that express homing receptors for mucosal and peripheral lymphoid tissues. J Infect Dis 2010; 202:1649–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ramirez K, Wahid R, Richardson C, et al. Intranasal vaccination with an adjuvanted Norwalk virus-like particle vaccine elicits antigen-specific B memory responses in human adult volunteers. Clin Immunol 2012; 144:98–108. [DOI] [PubMed] [Google Scholar]
- 19.Patel SM, Rahman MA, Mohasin M, et al. Memory B cell responses to Vibrio cholerae O1 lipopolysaccharide are associated with protection against infection from household contacts of patients with cholera in Bangladesh. Clin Vaccine Immunol 2012; 19:842–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simmons K, Gambhir M, Leon J, Lopman B. Duration of immunity to norovirus gastroenteritis. Emerg Infect Dis 2013; 19:1260–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Treanor JJ, Atmar RL, Frey SE, et al. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate-reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J Infect Dis 2014; 210:1763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plotkin SA, Gilbert PB. Nomenclature for immune correlates of protection after vaccination. Clin Infect Dis 2012; 54:1615–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atmar RL, Bernstein DI, Lyon GM, et al. Serological correlates of protection against a GII.4 norovirus. Presented at: 32nd Annual Meeting of the European Society for Paediatric Infectious Diseases, Dublin, Ireland, 2014. [Google Scholar]
- 24.Sasaki S, Jaimes MC, Holmes TH, et al. Comparison of the influenza virus-specific effector and memory B-cell responses to immunization of children and adults with live attenuated or inactivated influenza virus vaccines. J Virol 2007; 81:215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.