Abstract
Recent coral optics studies have revealed the presence of steep light gradients and optical microniches in tissues of symbiont-bearing corals. Yet, it is unknown whether such resource stratification allows for physiological differences of Symbiodinium within coral tissues. Using a combination of stable isotope labelling and nanoscale secondary ion mass spectrometry, we investigated in hospite carbon fixation of individual Symbiodinium as a function of the local O2 and light microenvironment within the coral host determined with microsensors. We found that net carbon fixation rates of individual Symbiodinium cells differed on average about sixfold between upper and lower tissue layers of single coral polyps, whereas the light and O2 microenvironments differed ~15- and 2.5-fold, respectively, indicating differences in light utilisation efficiency along the light microgradient within the coral tissue. Our study suggests that the structure of coral tissues might be conceptually similar to photosynthetic biofilms, where steep physico-chemical gradients define form and function of the local microbial community.
The quantity and quality of solar radiation are arguably the most important environmental resources that affect the structure and function of photosynthetic communities in both terrestrial and aquatic environments. Sunlight is of key importance for symbiont-bearing corals, driving the symbiotic interaction between the coral animal and its photosynthetic microalgae of the genus Symbiodinium (Roth, 2014). Light attenuation through the water mass and over the reef matrix has a fundamental role in structuring morphology, function and distribution of corals and their symbiotic algae with depth (Falkowski et al., 1990). Recent studies on the optical properties of corals have shown that light is also a highly stratified resource at the level of individual coral polyps and tissue layers (Wangpraseurt et al., 2014). Steep light gradients exist within the polyp tissues of some corals and light can attenuate by more than an order of magnitude within tissues, that is, comparable to the attenuation that can occur in open oceanic waters between the surface and >25 m of water depth (Kirk, 1994; Wangpraseurt et al., 2012). In this study, we investigated whether such light gradients within coral tissues are correlated with a stratification of Symbiodinium physiology in hospite.
We used fibre-optic and electrochemical microsensors together with stable isotopic labelling and nanoscale secondary ion mass spectrometry (NanoSIMS) to estimate single-cell carbon fixation rates across light gradients within coral tissues. We collected several fragments of Favites sp. from the Heron Island reef flat (152°69' E, 20°299' S), Great Barrier Reef, Australia. Fragments were cultured under a downwelling photon irradiance (400–700 nm) of ~100 μmol photons per m2 per s (12/12 h cycle), in aerated seawater (25 °C, salinity 33). Photosynthesis-irradiance curves for the investigated corals were determined with an imaging pulse amplitude modulated fluorometer (I-PAM, Walz GmbH, Effeltrich, Germany; Ralph et al., 2005). Values for saturating irradiance, Emax, and irradiance at onset of saturation, Ek, were ~350 μmol photons per m2 per s and ~160 μmol photons per m2 per s, respectively (data not shown). These values are typical for healthy corals kept under moderate irradiance (Ralph et al., 2005). To ensure incubations at irradiance levels where photosynthesis and irradiance correlated linearly, that is, on the linearly increasing part of the P vs I curve, all experiments were performed at ~80 μmol photons per m2 per s (12/12 h cycle). Microsensor measurements of scalar irradiance (tip size ~60 μm; Lassen et al., 1992) and O2 concentration (OX-50, tip size 50 μm, Unisense A/S, Aarhus, Denmark) were performed within the polyp and coenosarc tissues of corals as described previously (Figures 1a and b; Wangpraseurt et al., 2012). After microsensor measurements, corals were incubated with 13C-bicarbonate (Supplementary Text S1). NanoSIMS imaging was then applied on coral tissue sections, as described by Pernice et al. (2014) to quantify the assimilation of dissolved inorganic carbon into individual Symbiodinium cells across polyp (oral and aboral) and coenosarc tissues of corals. Briefly, corals were incubated in small aquaria with 2 mm NaH13CO3 in artificial sea water (recipe adapted from Harrison et al., 1980). After 24 h of isotopic incubation, coral fragments were sampled, chemically fixed and processed for NanoSIMS analyses (see Kopp et al., 2013; Pernice et al., 2012, 2014; and Supplementary Text S1,Supplementary Figure S1).
Figure 1.
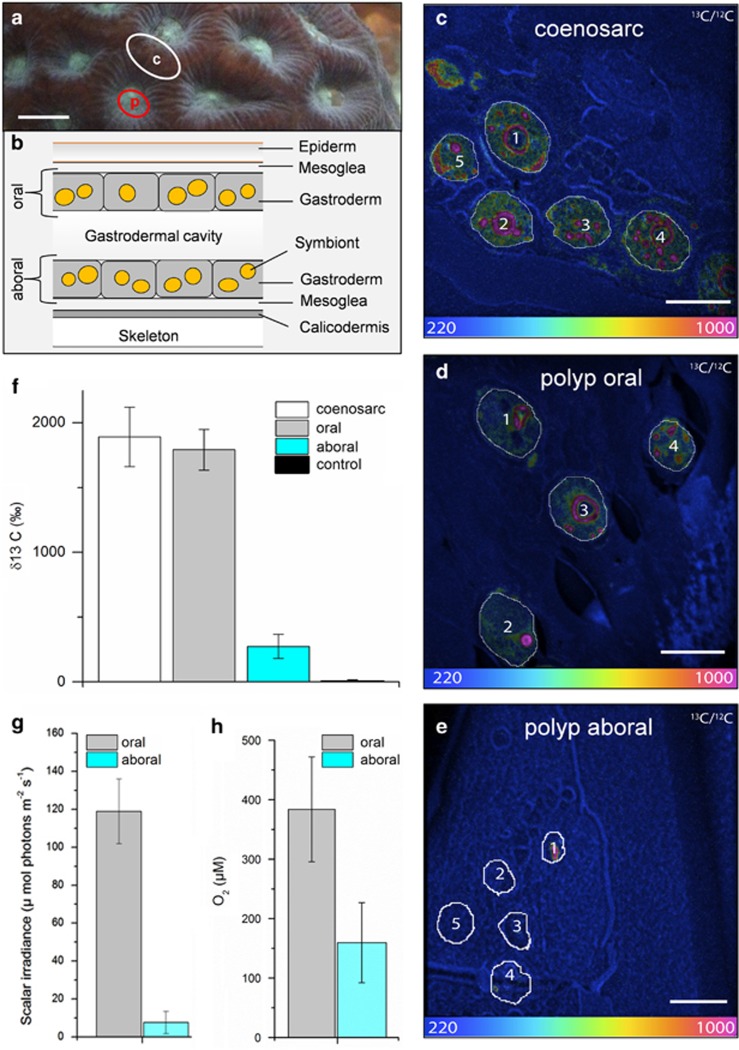
Internal microenvironment and single-cell 13C assimilation by Symbiodinium cells within Favites sp. (a) Representative measurement locations indicating connecting tissue (c, coenosarc; white circle) and polyp tissue (p; red circle). Scale bar is 0.5 cm. (b) Schematic diagram of the vertical arrangement of the polyp tissue structure (not drawn to scale). The coral tissue consists of oral and aboral gastrodermal tissues that contain photosymbiont cells (~10 μm in diameter). The two tissue layers are separated by a flexible gastrodermal cavity and the entire mean polyp tissue thickness was 1150 μm (±385 s.d., n=8) as determined by microsensor profiles. The NanoSIMS images (c–e) show the 13C/12C isotopic ratio for Symbiodinium cells in coenosarc tissue (c), the upper oral polyp tissue (d) and in the lowest layer of aboral polyp tissue (e). Scale bars are 10 μm. The colour scale of the NanoSIMS images is in hue saturation intensity ranging from 220 in blue (which corresponds to natural 13C/12C isotopic ratio of 0.0110) to 1000 in red (which corresponds to 13C/12C isotopic ratio of 0.05, ~4.5 times above the natural 13C/12C isotopic ratio). Quantification of 13C enrichment of individual Symbiodinium cells was obtained by selecting regions of interest that were defined in Open_MIMS (http://nrims.harvard.edu/software/openmims) by drawing the contours of the Symbiodinium cells directly on the NanoSIMS images. (f) Mean enrichment measured in Symbiodinium cells by NanoSIMS, in coenosarc tissue (in white, n=33), in upper oral polyp tissue (in grey, n=25), in the lowest layer of polyp tissue (in turquoise, n=17) and in the control treatment (n=20). Bars in the histograms indicate the s.e.m. enrichment quantified for the different whole Symbiodinium cells for each tissue category. Microsensor measurements of (g) scalar irradiance and (h) O2 performed along depth gradients within the polyp tissue (mean±s.d., n=4). Measurements were averaged for the first 100 μm from the tissue surface (oral) and the last 100 μm from the skeleton (aboral). The oral and aboral depth was defined through gentle touching of the microsensor tip at the surface of the coral tissue and skeleton, respectively.
Our combined approach of using NanoSIMS and microsensors within the tissue of corals provides, to the best of our knowledge, the first evidence for physiological differences of individual Symbiodinium cells in hospite in relation to the local microenvironmental conditions across different coral tissue layers, that is, oral vs aboral parts of polyp and coenosarc. Quantitative analysis based on tissue sections from different coral tissue layers showed that mean incorporation of 13C-bicarbonate by individual Symbiodinium cells was up to 6.5-fold higher in the upper oral polyp and coenosarc tissues compared with the lowermost layer of polyp tissues (δ13C: 1609±147‰, n=25 for Symbiodinium cells in upper oral polyp tissue; 1696±205‰, n=33 for Symbiodinium cells in coenosarc tissue and 246±82‰, n=17 for Symbiodinium cells in the lowest aboral layer of polyp tissue). Although the sample sizes in this study are small and the 13C signal is heterogeneous within individual Symbiodinium cells (because of carbon fixation hotspots in specific compartments; Supplementary Figure S2; Kopp et al., 2015), the magnitude of the difference in mean 13C incorporation between the aboral part of the polyp and the two other parts of coral tissue was clear and statistically significant (one-way analysis of variance (ANOVA) F2,75=15.91; P<0.0001; 6.5-fold increase in polyp oral vs aboral polyp tissue, Fischer's least significant difference (LSD) P<0.0001; 6.9-fold increase in coenosarc vs aboral polyp tissue Fischer's LSD P<0.0001; and no significant difference between oral polyp vs coenosarc tissue, Fischer's LSD P=0.718; Figure 1c–f; Supplementary Table S1). The internal microenvironment within the corresponding polyp tissues was highly stratified with respect to light and O2 (Figures 1g and h). Scalar irradiance decreased about 15-fold from the surface to the bottom of the polyp tissues. Gradients of O2 were less steep but still significant, with an approximate reduction in O2 concentration by about 2.5 times (Figure 1; Supplementary Table S2; ANOVA F1,6= 16.4; P=0.006).
These results suggest that coral tissues are vertically stratified systems that affect the physiological activity of their symbionts along a fine-scale microenvironmental gradient. The presence and role of microscale heterogeneity has hitherto largely been ignored in the field of coral symbiosis research, while much is known for other photosynthetic tissues. For instance, for terrestrial plant leaves and for aquatic photosynthetic biofilms, it is known that the photosynthetic unit can adapt to microenvironmental light gradients, where chloroplasts/phototrophs harboured in low-light niches show increased photosynthetic quantum efficiencies at low light levels (Terashima and Hikosaka, 1995; Al-Najjar et al., 2012). Although the steady-state O2 concentration values reported here are a function of the different metabolic processes of the coral holobiont (that is, Symbiodinium photosynthesis and the combined respiration by the coral host, Symbiodinium and microbes), the NanoSIMS approach allowed us to separate 13C fixation of Symbiodinium from the host metabolic activity. Our study provides the first experimental evidence from carbon fixation measurements that Symbiodinium cells can adapt to optical microniches in coral tissues. The 15-fold reduction in irradiance with depth in the coral tissue led only to an ~6.5-fold reduction in net carbon fixation suggesting enhanced light-harvesting efficiency or a reduced P/R ratio for Symbiodinium harboured in aboral tissues. Although such enhanced efficiency under low light often reflects the adaptation of the photosynthetic apparatus (for example, an increase in light-harvesting complexes (Walters, 2005) and reduced cell respiration (Givnish, 1988), it might additionally be the result of physiologically distinct populations or clades of Symbiodinium. Several studies have revealed remarkable genetic and physiological diversities among different Symbiodinium clades (Loram et al., 2007; Stat et al., 2008; Baker et al., 2013; Pernice et al., 2014). Although Favites sp. corals from Southern Great Barrier Reef are generally reported in association with one specific Symbiodinium type (clade C3; Tonk et al., 2013), Symbiodinium diversity within the microenvironment of these common corals could have been overlooked and such physiological diversity could further provide selective advantage to different genotypes in microenvironments within coral tissue. Coral tissues might thus exhibit similar characteristics to photosynthetic biofilms where steep physico-chemical microgradients give rise to different pheno- and ecotypes of phototrophs along those gradients (Musat et al., 2008; Ward et al., 1998).
These first experiments were performed under sub-saturating irradiance of ~80 μmol photons per m2 per s. Earlier studies showed that the local scalar irradiance in upper vs deeper tissue layers relates to the incident photon irradiance in a linear fashion such that at stressful incident irradiance levels of, for example, 2000 μmol photons per m2 per s, light levels in the lowermost polyp tissue layers are ~200 μmol photons per m2 per s (Wangpraseurt et al., 2012), still representing optimal conditions for photosynthesis. We thus consider it likely that excess irradiance triggering photoinhibition in oral tissues is unlikely to cause photoinhibition of Symbiodinium in aboral polyp tissues. The internal light field is species specific and in some thin-tissued, branching corals such as Pocillopora damicornis, intra-tissue light attenuation is not very pronounced (Wangpraseurt et al., 2012; Szabó et al., 2014). The ability to harbour Symbiodinium cells in low-light niches might be an important resilience factor for thick-tissued corals, such as massive faviids, during and after coral bleaching. Our study gives first insights to the functional diversity of Symbiodinium along microscale gradients in coral tissue and underscores the importance of considering such heterogeneity in studies linking symbiont diversity and coral physiology responses to environmental stress factors.
Acknowledgments
We thank Peter J. Ralph for logistical and administrative support. This research was funded by the Danish Council for Independent Research | Natural Sciences (MK), the Plant Functional Biology and Climate Change Cluster (MK, MP and DW) and a postgraduate stipend from the University of Technology, Sydney (DW). We acknowledge access to the Australian Microscopy and Microanalysis Research Facility at the Centre for Microscopy, Characterisation and Analysis, UWA, a facility funded by the University, State and Commonwealth Governments. The NanoSIMS facility at Utrecht University is funded by the large infrastructure subsidy awarded to Jack M. B. Middelburg by the Netherlands Foundation for Science and Research (NWO). Isabelle Domart-Coulon, Anders Meibom and Christophe Kopp are thanked for discussions.
Author contributions
DW, MP and MK conceived and designed the experiments; DW and MP performed the experiments; DW, MP, PG, MRK, PLC, LP and MK analysed and interpreted the data; PG, MRK and MK contributed reagents, materials and analysis tools; DW, MP and MK wrote the paper with contributions from all co-authors.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Al-Najjar MA, de Beer D, Kühl M, Polerecky L. (2012). Light utilization efficiency in photosynthetic microbial mats. Environ Microbiol 14: 982–992. [DOI] [PubMed] [Google Scholar]
- Baker DM, Andras JP, Jordan-Garza AG, Fogel ML. (2013). Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J 7: 1248–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkowski PG, Jokiel PL, Kinzie R (1990). Irradiance and corals. In: Dubinsky Z (ed). Ecosystems of the World 25: Coral Reefs. Elsevier: Amsterdam, The Netherlands, pp 59-107.
- Givnish TJ. (1988). Adaptation to sun and shade: a whole-plant perspective. Funct Plant Biol 15: 63–92. [Google Scholar]
- Harrison PJ, Waters RE, Taylor FJR. (1980). A broad spectrum artificial seawater medium for coastal and open ocean phytoplankton. J Phycol 16: 28–35. [Google Scholar]
- Kirk J. (1994) Light and Photosynthesis in Aqatic Ecosystems Cambridge University Press: New York, NY, USA.
- Kopp C, Pernice M, Domart-Coulon I, Djediat C, Spangenberg J, Alexander D et al. (2013). Highly dynamic cellular-level response of symbiotic coral to a sudden increase in environmental nitrogen. mBio 4: e00052–00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp C, Domart-Coulon I, Escrig S, Humbel BM, Hignette M, Meibom A. (2015). Subcellular investigation of photosynthesis-driven carbon assimilation in the symbiotic reef coral Pocillopora damicornis. mBio 6: e02299–02214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen C, Ploug H, Jørgensen BB. (1992). A fiberoptic scalar irradiance microsensor—application for spectral light measurements in sediments. Fems Microbiol Ecol 86: 247–254. [Google Scholar]
- Loram JE, Trapido-Rosenthal HG, Douglas AE. (2007). Functional significance of genetically different symbiotic algae Symbiodinium in a coral reef symbiosis. Mol Ecol 16: 4849–4857. [DOI] [PubMed] [Google Scholar]
- Musat N, Halm H, Winterholler B, Hoppe P, Peduzzi S, Hillion F et al. (2008). A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc Natl Acad Sci USA 105: 17861–17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernice M, Meibom A, Van Den Heuvel A, Kopp C, Domart-Coulon I, Hoegh-Guldberg O et al. (2012). A single-cell view of ammonium assimilation in coral-dinoflagellate symbiosis. ISME J 6: 1314–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pernice M, Dunn SR, Tonk L, Dove S, Domart-Coulon I, Hoppe P et al. (2014). A nanoscale secondary ion mass spectrometry study of dinoflagellate functional diversity in reef-building corals. Env Microbiol e-pub ahead of print 30 June 2014; doi:10.1111/1462-2920.12518. [DOI] [PubMed]
- Ralph PJ, Schreiber U, Gademann R, Kühl M, Larkum AW. (2005). Coral photobiology studied with a new imaging pulse amplitude modulated fluorometer. J Phycol 41: 335–342. [Google Scholar]
- Roth MS. (2014). The engine of the reef: photobiology of the coral–algal symbiosis. Front Microbiol 5: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stat M, Morris E, Gates RD. (2008). Functional diversity in coral -dinoflagellate symbiosis. Proc Natl Acad Sci USA 105: 9256–9261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó M, Wangpraseurt D, Tamburic B, Larkum AW, Schreiber U, Suggett DJ et al. (2014). Effective light absorption and absolute electron transport rates in the coral Pocillopora damicornis. Plant Physiol Bioch 83: 159–167. [DOI] [PubMed] [Google Scholar]
- Terashima I, Hikosaka K. (1995). Comparative ecophysiology of leaf and canopy photosynthesis. Plant Cell Environ 18: 1111–1128. [Google Scholar]
- Tonk L, Bongaerts P, Sampayo EM, Hoegh-Guldberg O. (2013). SymbioGBR: a web-based database of Symbiodinium associated with cnidarian hosts on the Great Barrier Reef. BMC ecology 13: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RG. (2005). Towards an understanding of photosynthetic acclimation. J Exp Bot 56: 435–447. [DOI] [PubMed] [Google Scholar]
- Wangpraseurt D, Larkum AW, Ralph PJ, Kühl M. (2012). Light gradients and optical microniches in coral tissues. Front Microbiol 3: 316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangpraseurt D, Polerecky L, Larkum AW, Ralph PJ, Nielsen DA, Pernice M et al. (2014). The in situ light microenvironment of corals. Limnol Oceanogr 59: 917–926. [Google Scholar]
- Ward DM, Ferris MJ, Nold SC, Bateson MM. (1998). A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiol Mol Biol Rev 62: 1353–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.