Abstract
Background
CVD risk increases in women after menopause. Recent data suggest higher levels of complement protein C3 and cardiovascular fat (CF) in postmenopausal women. Whether complement proteins are associated with early markers of atherosclerosis in healthy midlife women has never been evaluated. Additionally, the potential impact of the local CF on these associations has never been assessed.
Methods
Participants (n=100, age (mean(SD)):50.48(2.63), 50% premenopausal) were from the Study of Women's Health Across the Nation (SWAN). Arterial calcification (aortic-AC and coronary-CAC) and CF volumes around the heart and aorta (total heart-TAT and aortic perivascular adipose tissue-PVAT) were quantified using EBCT scans. AC and CAC were each evaluated as presence (Agatston scores>0) and extent of calcification (log (Agatston scores +1)). Logistic and linear regression models were used for statistical analysis.
Results
Adjusting for age, race, menopausal status and lipids, C3 was significantly associated with both presence and extent of AC and CAC, all P values <0.05. Associations between C3 and presence and extent of AC and CAC were explained by additional adjustment for logTAT and logPVAT, respectively. Association between C3 and log(AC+1) was more pronounced at higher volumes of logTAT (interaction-P=0.013) adjusting for study variables. No associations were found with C4.
Conclusions
Higher C3 was significantly associated with presence and greater extent of arterial calcification in midlife women. These associations were explained by higher volumes of CF, suggesting CF as a potential source of C3. C3 could be a potential non-invasive biomarker of early diagnosis of atherosclerosis. These findings need to be replicated in larger studies.
Keywords: Subclinical atherosclerosis, complement proteins, cardiovascular fat, women, midlife
Introduction
The risk of cardiovascular diseases (CVD) in women increases significantly after the fifth decade of life (1), a time period coincident with the menopausal transition. During this period, women are subjected to several biological alterations including adverse changes in sex hormones, lipid/lipoprotein profile and body fat composition (2, 3). Interestingly, women over 50 years of age significantly had higher level of circulating complement protein C3 (4), which was found to be associated with postmenopausal status (5). Several lines of evidence suggest a potential role of complement proteins in the atherosclerotic process. Various studies have reported significant associations between complement proteins (e.g. C3, C4) and subclinical measures of atherosclerosis (6, 7) and demonstrated the activation of complement proteins within plaque in the arterial wall (8, 9). In light of the recent findings that complement protein C3 is higher in postmenopausal women (5), it is plausible to hypothesize that levels of complement proteins may play a potential role in explaining the higher risk of CVD after menopause (1). No previous study has assessed the association between complement proteins and subclinical measures of atherosclerosis in healthy midlife women.
Adipose tissue could be a potential source of complement proteins. C3 and C4 proteins are strongly associated with abdominal adiposity and visceral adipose tissue in human related studies (10). In mouse models, increased C3 and C4 deposition have been found connected to the adventitial and medial fibers including collagen and elastin at early time points prior to luminal lesion development (8). The detection of C3 and C4 depositions on the outside layers of the vasculature, which are usually surrounded by a local fat depot known as perivascular fat (11), suggested this fat depot as a potential local source of these proteins. Interestingly, postmenopausal women have higher volumes of cardiovascular fat (3). Therefore, it is possible that cardiovascular fat could be a potential local source of complement proteins (10, 12) and could contribute to any associations between complement proteins and subclinical atherosclerosis in midlife women. No previous study has assessed the role of cardiovascular fat in explaining or modifying associations between complement proteins and subclinical atherosclerosis in midlife women.
For the present study, we aim first to evaluate the association between circulating complement proteins (C3 and C4) and arterial calcification in the coronary arteries and aorta (measures of subclinical atherosclerosis, CAC and AC, respectively) in healthy midlife women, and then to evaluate whether this association could be explained or modified by the volume and location of the cardiovascular fat.
Methods
The Study of Women's Health Across the Nation (SWAN) is an ongoing, multisite, longitudinal study of women examining the physiological and psychological changes during their transition through the middle years. The study design has been previously published (13). Between 1996 and 1997, 3302 participants were recruited from 7 different sites across the US (Boston, MA; Oakland, CA; Los Angeles, CA; Detroit, MI; Chicago, IL; Pittsburgh, PA & Newark, NJ). The baseline eligibility criteria for the SWAN study were (1) An intact uterus and at least 1 ovary; (2) Not pregnant or breast feeding; (3) At least 1 menstrual period within the past 3 months; (4) No hormone therapy use within the past 3 months.
The current study was a pilot study conducted among participants from the SWAN Heart ancillary study at the Pittsburgh site. SWAN Heart ancillary study was a study of changes in subclinical measures of atherosclerosis during the menopausal transition (3). The sample size for this pilot study was determined based on several factors including availability of blood specimens, CT scans, subclinical atherosclerosis measures, and menopausal status at SWAN Heart baseline visit. Baseline blood specimens (stored locally at the Pittsburgh site) and CT scans were available for 100 SWAN Heart participants (50 pre/early peri-, and 50 late peri-/post-menopausal) who were all included in this pilot study.
The participants provided written informed consent prior to enrollment and research protocols were approved by the University of Pittsburgh institutional review board (IRB).
Study Measures
Arterial Calcification
Coronary artery calcification (CAC) and aortic calcification (AC) were measured using electron beam computed tomography (EBCT) scans in 3 passes. The first pass marked the anatomical landmarks for the coronary and aortic scans. The second pass showed the coronary arteries and was captured at maximal breath hold using electrocardiographic triggering to obtain the 100-ms exposure in the same phase of cardiac cycle of R-R interval (60%). The third pass captured the aortic artery from the aortic arch to the aortic bifurcation. The scans were saved on optical discs. To assess CAC, 30-40 contiguous scans of 3-mm were obtained from the level of root of aorta to the apex of the heart at maximal breath holding. To assess aortic calcification, 6-mm images were obtained picturing the arch of the aorta to bifurcation of the iliac vessels with a 300-ms exposure. AC and CAC were assessed using a DICOM workstation equipped with AcuImage, Inc software (South San Francisco, CA) at the University of Pittsburgh using the Agatston scoring technique (14). Calcification was determined as present if three contiguous pixels showed > 130 Hounsfield units (HU). CAC was determined as the sum of Agatston scores of the 4 major coronary arteries (15).
Blood assay
Blood samples were collected in the morning after fasting overnight (8-12 hours). The blood collection was scheduled on days 2-5 of a regular menstrual cycle. In cases when a scheduled sample could not be obtained (due to less regular menstrual cycles), a random fasting sample was collected within 3 months of the scheduled annual visit. After sample collection, the blood was maintained at 4° C and separated and frozen at -80° C. The sample was then transported to the medical research laboratories on dry ice for analysis. High density lipoprotein cholesterol (HDL-C) was measured using heparin-2M manganese chloride while triglycerides and total cholesterol were measured by enzymatic methods using a Hitachi 747 analyzer (16-18). Low density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation (19), after excluding triglyceride value >=400 mg/dl. Insulin resistance was measured as homeostasis model assessment insulin resistance index (HOMA-IR) using fasting insulin and glucose levels (20). C3 and C4 were assessed from frozen serum samples using commercial immunoturbidimetric assay kits (Tina-quant C3 cassette and a Tina-quant C4 cassette) that were run on the Integra 800.
Cardiovascular fat (CF) volumes
Volumes of total heart adipose tissue (TAT) and perivascular adipose tissue (PVAT) of the descending thoracic aorta were quantified using existing EBCT scans previously obtained to measure arterial calcification. TAT was defined as the fat around the heart and PVAT was defined as the fat around the descending thoracic aorta. TAT volume was quantified at the Biomedical lab at the Harbor UCLA Medical center. For TAT volume (cm3), slices within 15 mm above and 30 mm below the superior extent of the left main coronary artery were included. The anterior border was defined by the chest wall and the posterior border by aorta and bronchus. This region was chosen as it also consists of the epicardial fat around the proximal coronary arteries including left anterior descending, left main coronary artery, right coronary artery and circumflex arteries. The protocol to quantify TAT showed excellent reproducibility with a between-readers Spearman correlation coefficient of 0.99 and within-reader Spearman coefficients of at least 0.97 (21). PVAT volume was quantified as previously described (22) using the existing scans originally obtained to quantify AC. The scans were re-read at the University of Pittsburgh using the software Slice-O-matic (Tomovision, Montreal, Canada) by one local reader. The posterior border for PVAT was defined as the anterior portion of the spinal foramen while the anterior and lateral borders were defined by the left bronchus, esophagus and crus of diaphragm. The pulmonary bifurcation was used as the proximal boundary of the descending aorta while the distal boundary was marked by the initial image of the first lumbar vertebrae. The adipose tissue volumes for both TAT and PVAT were quantified by using Hounsfield Unit (HU) attenuation values for fat (-190HU to -30 HU) (23). Similar to TAT, the PVAT measure has shown excellent reproducibility statistics with a Spearman correlation coefficient for both between reader and within reader of 0.99 (22).
Study covariates
Age was calculated using the birth date reported upon screening. Race/ethnicity was self-reported. The body mass index (BMI) was calculated as weight/height2 (kg/m2). Heart medications and smoking (current smoker or non-smoker) were self-reported. Menopausal status was determined based on reports of the frequency, and regularity of menstrual bleeding and use of hormone therapy and were classified into: Premenopausal - women experienced no changes in cycle intervals; Early peri-menopausal - women had at least 1 bleeding cycle in the last 3 months with a perceived change in the cycle interval; Late peri-menopausal - had 3 consecutive months without a menstrual cycles; Postmenopausal - had 12 consecutive months without a menstrual cycle. Due to small sample size, the premenopausal and early peri-menopausal groups were combined together as premenopausal, while the late-peri and post-menopausal groups were combined together as postmenopausal.
Statistical Analysis
Independent (C3, C4) and dependent variables (CAC and AC) as well as covariates were examined for distribution and outliers. The distributions of TAT and PVAT were skewed and therefore log transformation was applied to these measures. Each of the CAC and AC scores was analyzed as both categorical (presence of calcification) and continuous (extent of calcification) outcomes. Presence of calcification was a two levels variable: none; if Agatston score =0 and any; if Agatston score >0. Extent of calcification was the log-transformation of Agatston scores+1. Logistic regression was used for presence of calcification outcomes and linear regression was used for extent of calcification outcomes. Models were adjusted for potential covariates based on results from univariate analysis. Only the covariates found to be significantly associated with arterial calcification with a p-value of <0.05 were considered in multivariable analyses. To avoid multicollinearity and since the negative effects of multicollinearity are magnified at smaller sample sizes (24, 25), only covariates with weak correlations (<0.30) with each others and with complement proteins were considered. BMI, HDL-C and triglyceride levels were moderatly to highly correlated with complement proteins (C3) (>0.30), and therefore were not included in multivariable analyses. Additionally, BMI could not be included in the same models with CF depots due to the high correlations between these measures (>0.6). Including BMI with CF volumes in the same models in exploratory analyses resulted in reversing the direction of the detected association, which is a sign of model instability. The effect sizes were calculated per 1SD of the complement protein levels. Statistical analyses were performed using STATA 13 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP) with two sided test and significance level of 0.05.
Results
The characteristics of the study population are summarized in Table 1. The mean age of the women in the current study was 50.48 years (SD=2.63 years) with 73% of them being Whites. The population tended to be overweight with a mean BMI of 28.66 kg/m2 (SD=5.92 kg/m2) and the majority being nonsmokers (84%). The study population had high LDL-C values with a median of 120 mg/dl (SD= 34.3 mg/dl). The average complement protein C3 (162.3 mg/dl) and C4 levels (31.93 mg/dl) were within the normal ranges (26). AC and CAC were present among 67% and 48% of the current study population, respectively.
Table 1. Characteristics of the study population.
| Characteristic | Total (N=100) |
|---|---|
| Age (years), Mean (SD) | 50.48(2.63) |
| Race, N(%) | |
| Black | 27(27) |
| White | 73(73) |
| Menopausal Status, N(%) | |
| Premenopausal | 50(50) |
| Postmenopausal | 50(50) |
| Body mass index (kg/m2), Mean (SD) | 28.66(5.92) |
| Current smoking, N(%) | 15(15.15) |
| Use of Heart medication, N(%) | 03(03) |
| HDL-C(mg/dl), Median(Q1, Q3) | 57(48, 68) |
| LDL-C(mg/dl), Median(Q1, Q3) | 120(105, 145) |
| Triglycerides(mg/dl), Median(Q1, Q3) | 101(79, 144) |
| HOMA-IR, Median(Q1, Q3) | 1.83(1.48, 2.75) |
| TAT (cm3), Median(Q1, Q3) | 43.47(35.12, 66.59) |
| PVAT (cm3), Median(Q1, Q3) | 27.31(22.73, 36.39) |
| C3 (mg/dl), Mean (SD) | 162.3(33.28) |
| C4 (mg/dl), Mean (SD) | 31.93(8.78) |
| Aortic calcification (AC) Agatston score, Median(Q1, Q3) | 2.80(0, 4.67) |
| Presence of AC (AC>0), N(%) | 67(67) |
| Coronary calcification (CAC) Agatston score, Median(Q1, Q3) | 0(0, 2.29) |
| Presence of CAC (CAC>0), N(%) | 48(48) |
In univariate analysis of extent of calcification with study covariates (Table 2), higher levels of log(CAC+1) were significantly associated with higher BMI, LDL-C, log triglycerides, HOMA-IR, log TAT, log PVAT and C3 (all P<0.05). Similar associations were seen with log(AC+1). Both log(CAC+1) and log(AC+1) were inversely and significantly associated with HDL-C levels. Univariate analyses of presence of calcification with study covariates yielded similar results with the exception that Black women were significantly more likely to have CAC and AC than White women, data not shown.
Table 2. Univariate analyses between study variables and extent of arterial calcification.
| CACa | ACa | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| β(SE) | 95% CI | P-value | β(SE) | 95% CI | P-value | |
| Age (years) | 0.09(0.06) | (-0.02, 0.21) | 0.111 | 0.07(0.09) | (-0.11, 0.25) | 0.418 |
| Race | ||||||
| Black | 0.33(0.35) | (-0.37, 1.02) | 0.353 | 0.10(0.53) | (-0.96, 1.16) | 0.851 |
| White | -- | -- | ||||
| BMI (kg/m2) | 0.16(0.02) | (0.11, 0.19) | <0.001 | 0.18(0.04) | (0.11, 0.25) | <0.001 |
| Smoking | ||||||
| Current smoker | -0.39(0.43) | (-1.25, 0.47) | 0.372 | 0.57(0.66) | (-0.74, 1.88) | 0.388 |
| Non smoker | -- | -- | ||||
| Menopausal status | ||||||
| Postmenopausal | 0.39(0.31) | (-0.23, 0.99) | 0.214 | 0.28(0.47) | (-0.66, 1.22) | 0.557 |
| Premenopausal | -- | -- | ||||
| HDL-C(mg/dl) | -0.04(0.01) | (-0.06, 0.02) | <0.001 | -0.05(0.02) | (-0.08, -0.02) | 0.002 |
| LDL-C(mg/dl) | 0.01(0.004) | (0.001, 0.02) | 0.028 | 0.01(0.007) | (-0.001,0.03) | 0.074 |
| Triglycerides(mg/dl)b | 1.28(0.29) | (0.69, 1.86) | <0.001 | 2.11(0.44) | (1.24, 2.98) | <0.001 |
| HOMA-IRb | 0.11(0.04) | (0.04, 0.18) | 0.002 | 0.16(0.06) | (0.05, 0.27) | 0.005 |
| TAT(cm3)b | 1.66(0.32) | (1.03, 2.29) | <0.001 | 1.67(0.53) | (0.63, 2.72) | 0.002 |
| PVAT (cm3)b | 2.25(0.40) | (1.46, 3.05) | <0.001 | 2.44(0.66) | (1.13, 3.75) | <0.001 |
| C3 (mg/dl)c | 0.57(0.15) | (0.28, 0.86) | <0.001 | 0.63(0.23) | (0.17, 1.09) | 0.007 |
| C4 (mg/dl)c | 0.16(0.16) | (-0.15, 0.46) | 0.320 | 0.04(0.34) | (-0.43, 0.52) | 0.854 |
log(CAC+1), log(AC+1);
log transformed;
β per standard deviation (SD) ; SD of C3 = 33.28 mg/dl ; SD of C4= 8.77 mg/dl
In minimally adjusted models for age, race and menopausal status (Model 1), C3 was significantly associated with greater extent of calcification, Table 3. Additional adjustment for LDL-C (Model 2) did not explain the association with log(CAC+1) but attenuated the association with log(AC+1). Adjusting further for either log TAT or log PVAT largely explained the association between C3 and arterial calcification. No significant associations were found between C4 and extent of calcification, Table 3. Similar results were seen between complement proteins and presence of calcification, Table 4.
Table 3. Multivariable analyses between complement proteins and extent of calcification.
| CACa | ACa | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| C3 | ||||||
|
| ||||||
| β(SE)b | 95% CI | P-value | β(SE)b | 95% CI | P-value | |
| Unadjusted | 0.57(0.15) | (0.28, 0.86) | <0.001 | 0.63(0.23) | (0.17, 1.09) | 0.007 |
| Model 1c | 0.55(0.17) | (0.22, 0.88) | <0.001 | 0.70(0.26) | (0.18, 1.23) | 0.009 |
| Model 2c | 0.47(0.18) | (0.11, 0.84) | 0.011 | 0.56(0.28) | (-0.001,1.13) | 0.051 |
| Model 2 + TATd | 0.30(0.18) | (-0.06, 0.67) | 0.103 | 0.14(0.31) | (-0.48, 0.76) | 0.654 |
| Model 2 + PVATd | 0.21(0.18) | (-0.14, 0.56) | 0.238 | 0.27(0.29) | (-0.31, 0.85) | 0.356 |
|
| ||||||
| C4 | ||||||
|
| ||||||
| β(SE)b | 95% CI | P-value | β(SE)b | 95% CI | P-value | |
|
| ||||||
| Unadjusted | 0.16(0.16) | (-0.15, 0.46) | 0.320 | 0.04(0.24) | (-0.43, 0.52) | 0.854 |
| Model 1c | 0.14(0.16) | (-0.18, 0.46) | 0.378 | 0.05(0.25) | (-0.45, 0.55) | 0.853 |
| Model 2c | 0.13(0.17) | (-0.21, 0.46) | 0.445 | -0.001(0.26) | (-0.51, 0.51) | 0.997 |
| Model 2 + TATd | 0.08(0.16) | (-0.23, 0.39) | 0.595 | -0.27(0.26) | (-0.79, 0.25) | 0.309 |
| Model 2 + PVATd | 0.02(0.15) | (-0.28, 0.32) | 0.878 | -0.12(0.25) | (-0.61, 0.37) | 0.618 |
log(CAC+1), log(AC+1)
β per standard deviation (SD) ; SD of C3= 33.28 mg/dl, SD of C4= 8.77 mg/dl
Model 1: Adjusted for age, race and menopausal status; Model 2: Model 1+ LDL-C
log transformed
Table 4. Multivariable analyses of complement proteins with presence of calcification.
| Presence of CACa | Presence of ACa | |||
|---|---|---|---|---|
|
| ||||
| C3 | ||||
| OR (95%CI)b | P-value | OR (95%CI)b | P-value | |
| Unadjusted | 2.64 (1.58-4.42) | <0.001 | 2.19 (1.32-3.64) | 0.003 |
| Model 1c | 2.32 (1.33-4.03) | 0.003 | 1.97 (1.13-3.43) | 0.016 |
| Model 2c | 1.90 (1.07-3.39) | 0.029 | 1.76 (0.98-3.16) | 0.056 |
| Model 2 + TATd | 1.47 (0.744-2.93) | 0.265 | 1.06 (0.52-2.15) | 0.876 |
| Model 2 + PVATd | 1.31 (0.71-2.43) | 0.385 | 1.48 (0.81-2.74) | 0.206 |
|
| ||||
| C4 | ||||
|
| ||||
| OR (95%CI)b | P-value | OR (95%CI)b | P-value | |
| Unadjusted | 1.42 (0.94-2.14) | 0.093 | 1.32 (0.86-2.04) | 0.203 |
| Model 1c | 1.36 (0.87-2.11) | 0.180 | 1.20 (0.76-1.91) | 0.434 |
| Model 2c | 1.32 (0.83-2.11) | 0.244 | 1.15 (0.71-1.85) | 0.572 |
| Model 2 + TATd | 1.25 (0.72-2.17) | 0.435 | 0.83 (0.47-1.45) | 0.511 |
| Model 2 + PVATd | 1.16 (0.69-1.93) | 0.572 | 1.04 (0.65-1.70) | 0.848 |
CAC>0, AC>0
OR per standard deviation (SD); SD of C3= 33.28 mg/dl, SD of C4= 8.77 mg/dl
Model 1: Adjusted for age, race and menopausal status; Model 2: Model 1+ LDL-C
log transformed
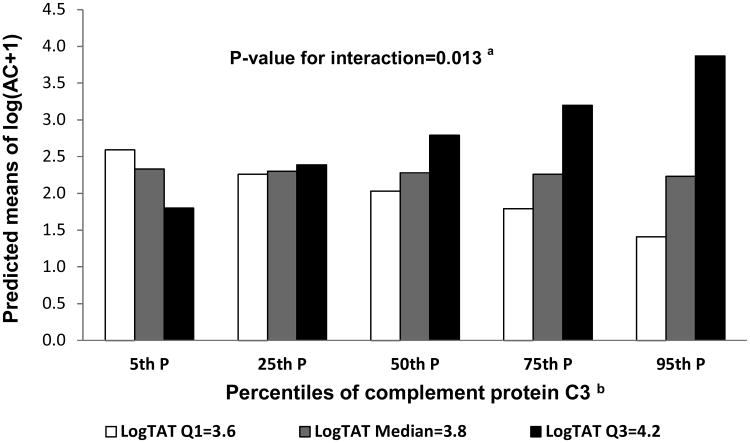
Next, we assessed the interactions between complement proteins and CF volumes in relation to the arterial calcification measures (extent and presence of calcification). A significant interaction was found between C3 and logTAT (p=0.005) in relation to extent but not presence of aortic calcification in unadjusted analyses. This interaction remained significant even after adjusting for age, race, menopausal status and LDL-C (p=0.013). Figure 1 provides the predicted mean of aortic calcification score by complement protein C3 percentiles at selected values of log TAT (at Q1=3.6, median=3.8 and Q3=4.2). Higher levels of complement protein C3 were associated with higher predicted means of log(AC+1) mainly at greater volumes of log TAT.
Figure 1.
Predicted values of aortic calcification by C3 percentiles at selected values of log TAT
aInteraction between C3 and log TAT relative to log(AC+1) adjusted for age, race, menopausal status and LDL-C
bC3 percentiles: 5th P=110 mg/dl, 25th P=141 mg/dl, 50th P=162 mg/dl, 75th P=183 mg/dl, 95th P= 218 mg/dl
Discussion
Our study showed that higher levels of circulating complement proteins were significantly associated with presence and greater extent of CAC and AC in healthy midlife women. These associations were explained by the volumes of CF fat depots. Furthermore, the association between greater extent of AC and C3 was more pronounced at greater volumes of TAT. These findings suggest a potential link between complement proteins and CF in relation to subclinical atherosclerosis levels in women at midlife.
Complement proteins – especially C3, have been vastly studied in relation to CVD and subclinical atherosclerosis. Higher levels of circulating C3 have been shown to be associated with arterial calcification in different at-CVD risk populations including patients suffering from various autoimmune diseases like systemic lupus erythematosus (SLE) and psoriasis, and elderly patients(>70 years) (6, 7, 10, 12). In addition, follow-up studies in women with pre-existing CAD have shown that higher levels of circulating C3 could predict the complications of atherosclerosis (27). The results of our study are consistent with these findings and support the evaluation of circulating complement protein levels in association with CVD risk in a broader population of healthy women at midlife.
Although most of the human studies that evaluated the associations between complement protein C3 and CVD risk suggested a pro-atherogenic role of C3 (6,7,10,12,27), the evidence from animal models were not consistent (8, 28-30) emphasizing the complexity of this association. C3 deficient mice showed greater extent of aortic atherosclerosis as compared with controls. Additionally, in the absence of C3, lesions maturation is impaired supporting a protective role of C3 on plaque stability (29). On the other hand, in mice deficient of decay-accelerating factor, a membrane protein that regulates complement pathway activity at the level of C3, greater deposition of C3d (a cleavage product of complement protein C3) were found in aortic root lesion, an indication of increased activation of C3 which suggests a potential pro-atherogenic role (30).
Complement proteins are plasma proteins that play a central role in the innate immunity (31). The exact role of complement system activation in the development of atherosclerosis is not fully understood. Complement system can be activated by different pathways including the classical, lectin, alternative and coagulation pathways (9). It has been suggested that the potential role of complement proteins in the atherosclerotic process is dependent on which pathway is activated. Complement activation by the classic and lectin pathways has been suggested to be protective through eliminating cell debris and apoptotic cells from atherosclerotic plaques. However, activation of the complement cascade by the alternative pathway has been proposed to accelerate atherogenesis via proinflammatory effects (9). Complement activation is responsible for recruitment of other inflammatory mediators at the targeted site resulting in the formation of the membrane attack complex (MAC) (32), which leads to microvasculature damage through the production of reactive oxygen species (ROS) (33). Interestingly, complement protein C3 and MAC have been found within the atherosclerotic plaques (34, 35). Clearly, the mechanism by which complement proteins impact the atherosclerotic process warranted more investigation.
Similar to complement proteins, adiposity is a well-established risk factor for CVD and subclinical atherosclerosis (36). In particular, visceral adiposity has been known to be associated with various cardiometabolic risk factors and higher risk of CVD (37). Additionally, local visceral adipose tissues such as those around the coronary blood vessels (38) and the aorta (39) have been shown to be positively associated with greater risk of atherosclerosis. This suggests a plausible role of CF in the atherosclerotic process. The “outside-in” theory of atherosclerosis proposes that the atherosclerotic process is initiated within the surrounding PVAT and the adventitial layers and then progressed towards the inner layers of the blood vessel (22). This theory has been supported by the presence of various inflammatory cells including complement proteins C3 and C4 within the outside layers of the vasculature (39, 8). Previous studies provide evidence for a potential link between adipose tissue and complement proteins (10). Complement protein expression has been detected in visceral (omental) adipose tissue in obese individuals (40). The results from our study showing a potential role of CF in explaining the association between levels of complement protein C3 and arterial calcification are consistent with the “outside-in” theory of atherosclerosis and suggest TAT as a potential local source of complement proteins, specifically at higher volumes of this fat depot. To the best of our knowledge, such an association between complement proteins, CF volumes and arterial calcification in healthy women at midlife transitioning through menopause has not been assessed before.
The current findings should be viewed in the context of several limitations. We were largely limited by the small sample size of this pilot study. This may limit the power to detect significant associations between complement protein C4 and arterial calcification. Additionally, the small sample size limited our ability to adjust for multiple important covariates in the same model. Many factors were involved in the determination of the sample size for this study, and this limited the generalizability of our findings. The cross-sectional design of our study prevented us from assessing temporality and could potentially introduce reverse causality issue. For example, it is unknown whether higher levels of complement proteins might be a source or a byproduct of the inflammatory process of atherosclerosis. Finally, our study population was predominantly Whites, and thus our findings cannot be generalized to other racial/ethnic groups.
Despite these limitations, our study is the first to evaluate associations between complement proteins and calcification in healthy women at midlife and to assess the potential role of CF in explaining this association. Additionally, our findings would help in enhancing our understanding of the possible mechanisms of atherosclerosis. Future studies should replicate our study, however in a larger multi-racial/ethnic population and followed up over time to look for risk and occurrence of CVD. In addition, studies exploring the other circulating and deposited complement protein levels within the plaques and adipose tissue volumes shall help explain specific complement activation pathways and mechanisms.
Conclusions
In conclusion, circulating complement protein C3 levels were significantly associated with arterial calcification measures in women at midlife. These associations were explained by volumes of fat around the heart and thoracic aorta. Higher volumes of TAT significantly amplify the association between C3 and AC, suggesting this fat depot as a potential source of complement proteins when expanded and extend support to the “outside-in” theory. By extending the findings of our study to the general population, future studies should test in a larger setting whether C3 could be used as a non-invasive biomarker for the early detection of subclinical atherosclerosis in women at midlife. Considering that women are at a higher risk of CVD after the 5th decade of life, early detection of arterial calcification without the use of EBCT scans in this population would not only be helpful in developing appropriate preventive strategies but prove to be cost effective. It is important to replicate our study in a larger study of multi/ethnic women at midlife over time.
Highlights as per journal instructions.
Higher levels of C3 were associated with greater CAC and AC scores.
Association between C3 and arterial calcification was explained by cardiovascular fat volume.
C3 was associated with greater arterial calcification at greater volumes of heart fat.
Acknowledgments
University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994-2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda H. Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012 - present; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Maria Mori Brooks, PI 2012 - present; Kim Sutton-Tyrrell, PI 2001 – 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair
Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of Funding:
The Study of Women's Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this presentation is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
SWAN Heart was supported by grants from the NHLBI (HL065581, HL065591).
The SWAN Cardiovascular Fat Ancillary Study was supported by an award from the American Heart Association Great River Affiliation Clinical Research Program: 12CRP11900031.
C3 and C4 assays were funded through the small Department of Epidemiology grant program at the University of Pittsburgh.
Footnotes
Disclosures: Dr. Matthew Budoff is a consultant to GE. Other authors have no disclosures.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nayana Nagaraj, Email: nayana.nagaraj@pitt.edu.
Kelly J Shields, Email: kshield1@wpahs.org.
Emma Barinas-Mitchel, Email: barinas@edc.pitt.edu.
Matthew Budoff, Email: mbudoff@labiomed.org, matthewska@upmc.edu.
Samar R. El Khoudary, Email: elkhoudarys@edc.pitt.edu.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee and Stroke Statistics Subcommittee; Heart disease and stroke statistics--2015 update: a report from the American Heart Association. Circulation. 2015;131:29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Freeman EW, Sammel MD, Hui Lin, Gracia CR. Obesity and Reproductive Hormone Levels in the Transition to Menopause. Menopause. 2010;17:718–26. doi: 10.1097/gme.0b013e3181cec85d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Khoudary SR, Shields KJ, Janssen I, Hanley C, Budoff MJ, Barinas-Mitchell E, Everson-Rose SA, Powell L, Matthews KA. Cardiovascular Fat, Menopause and Sex Hormones in Women: The SWAN Cardiovascular Fat Ancillary Study. J Clin Endocrinol Metab. 2015;100:3304–12. doi: 10.1210/JC.2015-2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muscari A, Massarelli G, Bastagli L, Poggiopollini G, Tomassetti V, Volta U, Puddu GM, Puddu P. Relationship between serum C3 levels and traditional risk factors for myocardial infarction. Acta Cardiol. 1998;53:345–54. [PubMed] [Google Scholar]
- 5.El Khoudary SR, Shields KJ, Chen HY, Matthews KA. Menopause, complement, and hemostatic markers in women at midlife: The Study of Women's Health Across the Nation. Atherosclerosis. 2013;231:54–8. doi: 10.1016/j.atherosclerosis.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parra S, Vives G, Ferré R, González M, Guardiola M, Ribalta J, Castro A. Complement system and small HDL particles are associated with subclinical atherosclerosis in SLE patients. Atherosclerosis. 2012;225:224–30. doi: 10.1016/j.atherosclerosis.2012.08.029. [DOI] [PubMed] [Google Scholar]
- 7.Alpsoy S, Akyuz A, Erfan G, Akkoyun DC, Topcu B, Guzel S, Kaya S, Kulac M. Atherosclerosis, some serum inflammatory markers in psoriasis. G Ital Dermatol Venereol. 2014;149:167–75. [PubMed] [Google Scholar]
- 8.Shields KJ, Stolz D, Watkins SC, Ahearn JM. Complement proteins C3 and C4 bind to collagen and elastin in the vascular wall: a potential role in vascular stiffness and atherosclerosis. Clin Transl Sci. 2011;4:146–52. doi: 10.1111/j.1752-8062.2011.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Speidl WS, Kastl SP, Huber K, Wojta J. Complement in atherosclerosis: friend or foe? J Thromb Haemost. 2011;9:428–40. doi: 10.1111/j.1538-7836.2010.04172.x. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson B, Hamad OA, Ahlström H, Kullberg J, Johansson L, Lindhagen L, Haenni A, Ekdahl KN, Lind L. C3 and C4 are strongly related to adipose tissue variables and cardiovascular risk factors. Eur J Clin Invest. 2014;44:587–96. doi: 10.1111/eci.12275. [DOI] [PubMed] [Google Scholar]
- 11.Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernández-Alfonso MS. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab. 2015;26:367–75. doi: 10.1016/j.tem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Hertle E, Stehouwer CD, Van Greevenbroek MM. The complement system in human cardiometabolic disease. J Mol Immunol. 2014;61:135–48. doi: 10.1016/j.molimm.2014.06.031. [DOI] [PubMed] [Google Scholar]
- 13.Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, Lo A, Weiss G, Kelsey J. SWAN: A multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–88. [Google Scholar]
- 14.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 15.Rumberger JA, Brundage BH, Rader DJ, Kondos G. Electron beam computed tomographic coronary calcium scanning: A review and guidelines for use in asymptomatic persons. Mayo Clin Proc. 1999;74:243–52. doi: 10.4065/74.3.243. [DOI] [PubMed] [Google Scholar]
- 16.Myers GL, Cooper GR, Winn CL, Smith SJ. The centers for disease control-national heart, lung and blood institute lipid standardization program. An approach to accurate and precise lipid measurements. Clin Lab Med. 1989;9:105–35. [PubMed] [Google Scholar]
- 17.Steiner P, Freidel J, Bremner W, Stein E. Standardization of micromethods for plasma cholesterol, triglyceride, and HDL-cholesterol with the lipid clinics' methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 18.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–02. [PubMed] [Google Scholar]
- 20.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–19. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 21.El Khoudary SR, Shin C, Masaki K, Miura K, Budoff M, Edmundowicz D, Kadowaki S, Barinas-Mitchell E, El-Saed A, Fujiyoshi A, Evans RW, Hisamatsu T, Ohkubo T, Willcox BJ, Kuller LH, Ueshima H, Sekikawa A. Ectopic cardiovascular fat in middle-aged men: effects of race/ethnicity, overall and central adiposity. The ERA JUMP study. Int J Obes. 2015;39:488–94. doi: 10.1038/ijo.2014.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shields KJ, Barinas-Mitchell E, Gingo MR, Tepper P, Goodpaster BH, Kao AH, Manzi S, Sutton-Tyrrell K. Perivascular adipose tissue of the descending thoracic aorta is associated with systemic lupus erythematosus and vascular calcification in women. Atherosclerosis. 2013;231:129–35. doi: 10.1016/j.atherosclerosis.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy RA, Register TC, Shively CA, Carr JJ, Ge Y, Heilbrun ME, Cummings SR, Koster A, Nevitt MC, Satterfield S, Tylvasky FA, Strotmeyer ES, Newman AB, Simonsick EM, Scherzinger A, Goodpaster BH, Launer LJ, Eiriksdottir G, Sigurdsson S, Sigurdsson G, Gudnason V, Lang TF, Kritchevsky SB, Harris TB. Adipose tissue density, a novel biomarker predicting mortality risk in older adults. The Journals of Gerontology, Series A, Biological Sciences and Medical Sciences. 2013;69:109–17. doi: 10.1093/gerona/glt070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kiers HAL, Smilde AK. A comparison of various methods for multivariate regression with highly collinear variables. Statistical Methods and Applications. 2007;16:193–228. [Google Scholar]
- 25.Mason CH, Perreault WD. Collinearity, Power, and Interpretation of Multiple Regression Analysis. J Market Res. 1991;28:268–80. [Google Scholar]
- 26.Hussain N, Jaffery G, Hasnain S. Serum Complement C3 and C4 Levels in Relation to Diagnosis of Lupus Nephritis. Tropl J of Pharm Res. 2008;7:1117–21. [Google Scholar]
- 27.Széplaki G, Prohászka Z, Duba J, Rugonfalvi-Kiss S, Karádi I, Kókai M, Varga L. Association of high serum concentration of the third component of complement (C3) with pre-existing severe coronary artery disease and new vascular events in women. Atherosclerosis. 2004;177:383–89. doi: 10.1016/j.atherosclerosis.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 28.Oksjoki R, Kovanen PT, Pentikäinen MO. Role of complement activation in atherosclerosis. Current Opinion in Lipidology. 2003;14:477–82. doi: 10.1097/00041433-200310000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 Deficiency on Atherosclerosis. Circulation. 2002;105:3025–31. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- 30.Leung VWY, Yun S, Botto M, Mason JC, Malik TH, Song W, Paixao-Cavalcante D, Pickering MC, Boyle JJ, Haskard DO. Decay-accelerating factor suppresses complement C3 activation and retards atherosclerosis in low-density lipoprotein receptor-deficient mice. The American Journal of Pathology. 2009;175:1757–67. doi: 10.2353/ajpath.2009.090183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Janeway CA, Jr, Travers P, Walport M, Shlomchik MJ. Immunobiology: The Immune System in Health and Disease. 5th. New York, NY: Garland Science; 2001. [Google Scholar]
- 32.Yasojima K, Schwab C, McGeer EG, McGeer PL. Complement components, but not complement inhibitors, are upregulated in atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2001;21:1214–19. doi: 10.1161/hq0701.092160. [DOI] [PubMed] [Google Scholar]
- 33.Gross ML, Meyer HP, Ziebart H, Rieger P, Wenzel U, Amann K, Ritz E. Calcification of coronary intima and media: immunohistochemistry, backscatter imaging, and x-ray analysis in renal and nonrenal patients. Clinical Journal of the American Society of Nephrology. 2007;2:121–34. doi: 10.2215/CJN.01760506. [DOI] [PubMed] [Google Scholar]
- 34.Hansson GK, Holm J, Kral JG. Accumulation of IgG and complement factor C3 in human arterial endothelium and atherosclerotic lesions. Acta Pathol Microbiol Immunol Scand. 1984;92:429–35. doi: 10.1111/j.1699-0463.1984.tb04424.x. [DOI] [PubMed] [Google Scholar]
- 35.Vlaicu R, Rus HG, Niculescu F, Cristea A. Immunoglobulins and complement components in human aortic atherosclerotic intima. Atherosclerosis. 1985;55:35–50. doi: 10.1016/0021-9150(85)90164-9. [DOI] [PubMed] [Google Scholar]
- 36.Hegazi RAF, Sutton-Tyrrell K, Evans RW, Kuller LH, Belle S, Yamamoto M, Kelley DE. Relationship of adiposity to subclinical atherosclerosis in obese patients with type 2 diabetes. Obesity Research. 2003;11:1597–05. doi: 10.1038/oby.2003.212. [DOI] [PubMed] [Google Scholar]
- 37.Mathieu P, Lemieux I, Després JP. Obesity, inflammation, and cardiovascular risk. Clin Pharm and Therap. 2010;87:407–16. doi: 10.1038/clpt.2009.311. [DOI] [PubMed] [Google Scholar]
- 38.Verhagen SN, Vink A, Van der Graaf Y, Visseren FLJ. Coronary perivascular adipose tissue characteristics are related to atherosclerotic plaque size and composition. A post-mortem study Atherosclerosis. 2012;225:99–04. doi: 10.1016/j.atherosclerosis.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 39.Britton KA, Fox CS. Perivascular adipose tissue and vascular disease. Clinical Lipidology. 2011;6:79–91. doi: 10.2217/clp.10.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabrielsson BG, Johansson JM, Lönn M, Jernås M, Olbers T, Peltonen M, Larsson I, Lönn L, Sjöström L, Carlsson B, Carlsson LM. High expression of complement components in omental adipose tissue in obese men. Obes Res. 2003;11:699–08. doi: 10.1038/oby.2003.100. [DOI] [PubMed] [Google Scholar]