Abstract
Regulators of G protein signaling (RGS) proteins enhance the intrinsic GTPase activity of α subunits of the heterotrimeric G protein complex of G protein-coupled receptors (GPCRs) and thereby inactivate signal transduction initiated by GPCRs. The RGS family consists of nearly 37 members with a conserved RGS homology domain which is critical for their GTPase accelerating activity. RGS proteins are expressed in most tissues, including heart, lung, brain, kidney, and bone and play essential roles in many physiological and pathological processes. In skeletal development and bone homeostasis as well as in many bone disorders, RGS proteins control the functions of various GPCRs, including the parathyroid hormone receptor type 1 and calcium-sensing receptor and also regulate various critical signaling pathways, such as Wnt and calcium oscillations. This chapter will discuss the current findings on the roles of RGS proteins in regulating signaling of key GPCRs in skeletal development and bone homeostasis. We also will examine the current updates of RGS proteins’ regulation of calcium oscillations in bone physiology and highlight the roles of RGS proteins in selected bone pathological disorders. Despite the recent advances in bone and mineral research, RGS proteins remain understudied in the skeletal system. Further understanding of the roles of RGS proteins in bone should not only provide great insights into the molecular basis of various bone diseases but also generate great therapeutic drug targets for many bone diseases.
1. INTRODUCTION TO BONE
Bone is a connective tissue that plays crucial roles in mineral storage and homeostasis, organ support, and locomotion.1,2 Bone is composed of organic and inorganic materials. The inorganic component of bone is primarily constituted of hydroxyapatite, [Ca3(PO4)2]3 Ca(OH)2. The organic portion of bone consists mainly of type I collagen, a triple-helical molecule containing three polypeptide chains of amino acids that are each cross-linked by hydrogen bonds. The remaining organic constituent of bone contains various noncollagenous proteins, including hormones, growth factors, and cytokines.3–5 Bone formation occurs through two key mechanisms: intramembranous and endochondral ossification.4,6 Intramembranous mineralization gives rise to the cranial vault, some facial bones, parts of the mandible and clavicles, whereas endochondral ossification is responsible for the other bones of the skeleton. During intramembranous bone formation, undifferentiated mesenchymal cells give rise to osteoprogenitor cells which then differentiate into mature osteoblasts.3 During endochondral ossification, mesenchymal stem cells are first condensed to form a template of cartilage that is ultimately replaced by bone. Throughout adult life, bone is constantly remodeling through the synchronized and balanced activities of the osteoclasts, which derive from the hematopoietic stem cell lineage and are responsible for resorbing bone, and the osteoblasts, the bone-forming cells of the mesenchymal stem cell origin.7–9 Disruption which shifts the balance in the favor of osteoclasts can affect bone mass and causes many pathological bone disorders, including osteoporosis, periodontitis, endodontitis, and rheumatoid arthritis.10–14 Likewise, enhanced function of osteoblasts over osteoclasts can cause osteopetrosis.
2. THE GPCR–G PROTEIN–RGS SIGNALING PATHWAY
The activities of osteoclasts and osteoblasts are highly controlled by autocrine, paracrine, and endocrine factors from the external environment to ensure the systemic balance of calcium–phosphate metabolism while maintaining bone homeostasis.5 External stimuli can affect bone cells by binding to their receptors on the cell membranes and thereby trigger signals within the cells. G protein-coupled receptors (GPCRs) are an example of such receptors.15,16 GPCRs, also called seven-transmembrane domain receptors, are a large family of protein receptors. These receptors sense a variety of extracellular stimuli from growth factors, cytokines, hormones, neurotransmitters, light, to phospholipids to affect various cellular processes, such as cell proliferation, differentiation, activity, and apoptosis.17 Specifically, GPCR activation transduces intracellular signals through a hetero-trimeric G protein complex which can then direct the signals to downstream effectors for specific outcomes (Fig. 1). GPCRs regulate many physiological events and, consequently, have been exploited therapeutically in many disease states, including diabetes, various cancers as well as bone, neurological, diseases, blood, heart, and kidney diseases.18–21 Nearly 40% of the current drugs on the market target GPCRs.
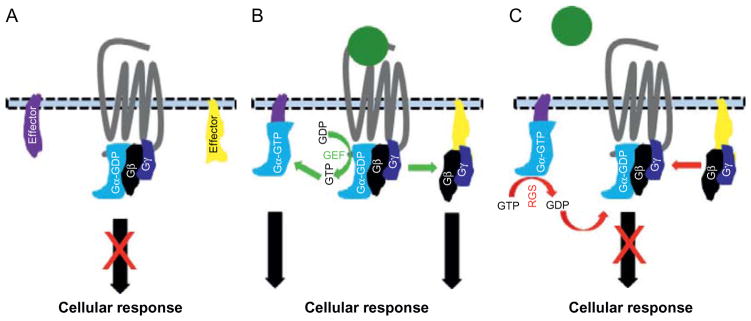
Figure 1.
Schematic of GPCR–G protein–RGS activation and inactivation cycle. (A) GPCR inactivation in the absence of ligands. In absence of ligands, Gα is linked to GDP (Gα-GDP) and forms a heterotrimeric complex with the Gγ and Gβ subunits, preventing their liberation to activate effectors to induce cellular response. (B) Ligand-induced GPCR activation. In the presence of ligands, GPCR undergoes a conformational change leading to the activation of Gα through the exchange of GDP for GTP by the receptor GEF activity. Gα-GTP and Gβγ subunits dissociate to modulate the activity of specific effectors to mediate cellular responses. (C) GPCR signaling inactivation by RGS proteins. RGS proteins enhance the GTPase activity of Gα to hydrolyze GTP to GDP leading to the inactivation of the Gα protein and its subsequent reunification with Gβγ subunits to reform the inactive G protein heterotrimeric complex. This prevents the G proteins from continuing activating effectors for cellular response and thus terminates the cellular response. GDP, guanosine diphosphate; GTP, guanosine triphosphate; GEF, guanine nucleotide exchange factor.
2.1 The G Protein Complex
GPCRs are linked to a heterotrimeric G protein complex at its cytoplasmic domain22 (Fig. 1). The G protein heterotrimeric complexes consist of three different subunits: Gα (33–55 kDa), Gβ (~35 kDa), and Gγ (~15 kDa).21,22 In humans, the Gα subunits are encoded by 16 genes and are divided into 4 subgroups (Gαs, Gαo/i, Gαq/11, and Gα12/13) which are further divided into other subgroups. The Gβ subunits are encoded by 5 genes and are divided into 6 subgroups. There are 12 different Gγ subunits. Given that signaling transduced by numerous combinations of βγ subunits is not significantly different from one another, the G protein heterotrimeric complexes are mostly defined according to their Gα constituents. Upon its activation, a GPCR undergoes a conformational change in its cytoplasmic domain which leads to the activation of the Gα submit through the exchange of its bound GDP for GTP by the guanine nucleotide exchange factor (GEF) activity of GPCRs16 (Fig. 1). The activated α subunit (Gα-GTP) then dissociates from the βγ submits to form two different G protein units which then transduce signaling through activation or inhibition of specific effectors to induce cellular responses.
Whereas Gαs can stimulate the adenylate cyclase (AC) pathway to activate the cyclic adenosine monophosphate (cAMP), activation of Gαi results in the inhibition of the cAMP pathway. Gαq activates the phospholipase C-β (PLCβ) pathway which cleaves phosphatidylinositol 4,5-biphosphate (PIP2) into inositol (1,4,5) triphosphate (IP3) and diacylgycerol (DAG), which can trigger an increase in intracellular Ca2+ levels. Gα12/13 activates the Rho pathway which is involved in cell cytoskeleton biology. The Gβγ submits can activate many ion channels. Notably, GPCR signaling is terminated when the Gα subunits hydrolyses their bound GTP to GDP, via their intrinsic GTPase activity, to reunite with the Gβγ subunits to reform an inactive G protein heterodimer complex (Fig. 1). The kinetics of G protein signaling are controlled by a family of proteins, named regulators of G protein signaling (RGS), which bind to activated Gα and accelerate their intrinsic GTPase activities.23,24 Therefore, RGS proteins are crucial for the rapid activation and inactivation of cellular responses that are initiated by GPCR stimulation.25
2.2 Introduction to RGS Proteins
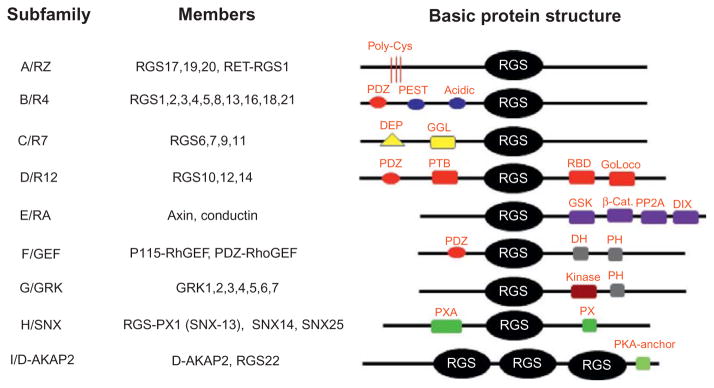
The RGS protein family consists of at least 37 members in mammals and is divided into nine subfamilies, including RZ/A, R4/B, R7/C, R12/E, GEF/F, GRK/G, SNX/H, and D-AKAP2/I based on structural analysis18 (Fig. 2). Nevertheless, all RGS proteins contain a highly conserved 120-amino acid RGS domain, which can enhance the GTPase activity of the Gα proteins.26 The GTPase accelerating protein (GAP) of RGS proteins is mainly limited to Gαq, Gαi/o, and Gα12/13 subunits. The functions of RGS proteins are essential for coordinating signaling output elicited by GPCRs. Indeed, RGS proteins can regulate G protein-mediated receptors, ion channels, and other signaling pathways and can also serve as key modulators in many physiological processes, including cardiovascular, respiratory, nervous and immune functions as well as many disease states, such as diabetes, cardiovascular diseases, cancers, inflammatory diseases, and neurological diseases.27–31 RGS proteins are also critical for the skeletal system by regulating various signaling pathways, including Wnt, parathyroid hormone (PTH), and calcium-sensing receptor (CaSR) pathways and Ca2+ signaling.32,33 Consistent with their critical roles in bone, it was shown that the expression of RGS proteins can be regulated by many bone factors, including proinflammatory cytokines, lipopolysaccharide (LPS), PTH and PTH-related peptide (PTHrP), and thyroid-stimulating hormone (TSH).33–35 LPS can inhibit the expression of RGS2 in macrophages but increases the expression of RGS1 in dendritic cells and macrophages.36–38 Likewise, interferon β can upregulate the expression of RGS1 in monocytes, B cells, and T cells and induce the expression of RGS2 and RGS16 in mononuclear leukocytes.38,39 Moreover, PTH and PTHrP can induce RGS2 in osteoblasts. Injection of PTH in femoral metaphysical spongiosa of young male rats can increase the expression of RGS2 in a rapid but transient fashion. Moreover, the expression of RGS2 can also be induced by TSH stimulation.35 As such, targeting RGS protein functions can attenuate the symptoms associated with many bone pathological disorders. Here, we summarized the current knowledge on the roles of RGS proteins in the bone-forming osteoblasts and bone-resorbing osteoclasts. We also reviewed the roles of RGS proteins in some key-skeletal signaling pathways under normal physiological and pathological states.
Figure 2.
Subclassification of RGS family members. All members of the RGS family possess a common RGS domain but are subdivided into different subfamilies based on protein structures. β-Cat, β-catenin-binding; D-AKAP, dual-specificity A-kinase anchoring protein; DEP, dishevelled/EGL-10/pleckstrin; DH, double homology; DIX, dishevelled homology domain; GAIP, Gα interacting protein; GEF, guanine nucleotide exchange factor; GGL, Gγ-like; Goloco, Gαi/o-Loco; GRK, G protein-coupled receptor kinase; GSK, glycogen synthase kinase 3β-binding; PDZ, PSD95/dlg/Z0-1/2; PEST, proline, glutamine, serine, threonine-rich; PH, pleckstrin homology; PP2A, protein phosphatase 2A; PTB, phosphotyrosine-binding; PX, phosphatidylinositol-binding; PXA, PX-associated; RBD, Ras-binding domain; SNX, sorting nexin.
3. RGS PROTEINS IN OSTEOBLASTS
Osteoblasts are mononucleated cells that derive from the mesenchymal stem cell lineage in the bone marrow and are responsible for bone formation.6 Osteoblasts induce bone mineralization by producing new bone called “osteoid.” Bone formation by osteoblasts are influenced by many GPCRs, including PTH1R, frizzled (Fz), and CaSR,21 which are critical for osteoblast differentiation and function. Transgenic mice bearing a constitutively active form of CaSR in mature osteoblasts via the osteocalcin promoter cause a decrease in bone volume and density in cancellous bone.21,40 These effects result from enhanced osteoclast formation and activity triggered by an increase in the expression of receptor activator of nuclear factor κ β ligand (RANKL). Furthermore, CaSR can also promote osteoblast proliferation in part through the activation of the Gαq-PLCβ pathway leading to increased Ca2+ oscillations.16 Collectively, these studies demonstrate a role for GPCRs in osteoblast differentiation and suggest a role for RGS proteins in osteoblast biology. Further studies reported that overexpression of RGS2 or RGS4 and pretreatment with pertussis toxin can inhibit the activation of GPRC6A by extracellular cations.41 However, RGS proteins have not been highly studied in osteoblasts. Here, we discussed the current understanding of the known roles of RGS2, RGS5, and axin in osteoblasts.
3.1 RGS2 in Osteoblasts
RGS2 regulates G protein-mediated functions in various tissues, including lung, brain, heart, and bone.18 RGS2 is expressed in the rat metaphyseal and diaphyseal, mouse calvarial culture, and cultured osteoblasts and plays a critical role in the skeletal system. Nevertheless, RGS2 function in osteoblasts is a bit complex. RGS2 expression is upregulated by forskolin, PTH, and PTHrP in osteoblasts through the stimulation of the AC-cAMP pathway by Gαs activation,42–45 which is critical for osteoblast differentiation. It was reported that a high level of RGS2 is required to attenuate signaling by GPCRs that promote Ca2+ mobilization via the activation of the Gαq-PLCβ pathway β.16,18 RGS2 can also be induced by ATP-induced activation of Gαq to attenuate PTH-induced activation of cAMP. Overall, the finding indicates that, at basal levels RGS2 does not regulate Gαq or Gαs signaling, but at higher levels, RGS2 may cross-desensitize both Gαs and Gαq in osteoblasts.
3.2 RGS5 in Osteoblasts
Recent findings have suggested that PTH-induced RGS5 expression may play a crucial role in cellular responses to extracellular calcium level through activation of CaSR.46 RGS5 was reported to be markedly elevated in parathyroid adenomas, and mice deficient in RGS5 showed a reduced level of PTH plasma levels.46–48 Notably, forced expression of RGS5 in cells expressing CaSR abrogates the Ca2+-induced IP3 induction by CaSR. These results indicate that RGS5 can act as a physiological negative regulator of CaSR in the parathyroid gland. Unfortunately, our understanding of the role of RGS5 in organ physiology is mostly unknown because of a lack of RGS5-targeted mouse models.18 Nonetheless, RGS5 is also expressed in smooth muscle and heart. A novel splice variant of RGS5, RGS5S, which lacks 104 amino acids has recently been cloned and reported to be expressed in the human brain, skeletal system, eye, and small intestine.49
3.3 Axin in Osteoblasts
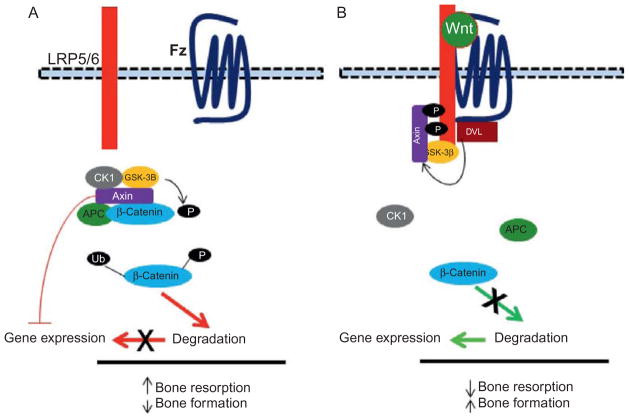
Axin, a member of E/RA subfamily of the RGS protein family, is an important regulator of skeletal homeostasis (Fig. 3).50–53 Axin serves as molecular scaffold for the β-catenin destruction complex by interacting with other proteins, such as protein phosphatase 2A (PP2A), adenomatosis polyposis coli (APC), glycogen synthase kinase 3 (GSK3), and casein kinase 1 (CK1), thus functioning as a negative regulator of the canonical Wnt–β-catenin signaling pathway.32 Mice deficient in the axin gene display a significant increase in bone density stemming from an increase in osteoblast differentiation and a decrease in osteoclast differentiation.50 Axin can also negatively regulate bone mass through the β-catenin-bone morphogenetic proteins 2 and 4 (BMP2/4)–osterix (Osx) signaling pathway in osteoblasts.16 Axin has also been shown to interact with N-cadherin to mediate β-catenin degradation leading to decrease osteoblast differentiation.54 Consistent with that posture, transgenic mice expressing N-cadherin under the control of the Col1 2.3 promoter display an increase in β-catenin degradation leading to a decrease in osteoblasts and bone mass.54,55 Notably, axin may also control the Wnt–β-catenin signaling pathway through actions on Gα proteins. Whereas Gαs proteins bearing activating mutations can interact with axin to enhance β-catenin activation, a lack of Gαs causes an increase in β-catenin degradation which affects bone mass. All together, these results demonstrate a crucial role for axin as an important negative regulator of bone formation through inhibition of the Wnt–β-catenin pathway.
Figure 3.
Role of axin in Wnt signaling in bone. (A) In the absence of Wnt ligands, β-catenin is constructively targeted for degradation via a multiprotein destruction complex. β-Catenin is phosphorylated by GSK-3β and then targeted for ubiquitination (Ub) and proteosomal degradation. Axin plays a key role in this process of β-catenin degradation and thus functions as an inhibitor of the canonical Wnt-β pathway. (B) Binding of canonical Wnt ligands to their co-receptors Fz and LRP5/6 prevent β-catenin phosphorylation by GSK-3β, which in turn prevent its degradation and allows it to move to the nucleus to mediate gene expression.
4. RGS PROTEINS IN OSTEOCLASTS
Osteoclasts play essential roles in skeletal development and bone homeostasis.7 Enhanced osteoclast formation and/or activity are responsible for many disorders of skeletal deficiency.10 Osteoclastogenesis requires two key cytokines: RANKL and macrophage colony-stimulation factor (M-CSF). Whereas M-CSF maintains the proliferation of osteoclast precursors, RANKL is responsible for the differentiation of these precursors into mature osteoclasts. RANKL functions by activating its receptor, RANK, to induce the expression of osteoclast marker genes, including the nuclear factor of activated T cells, c1 (NFATc1), a master regulator of osteoclast differentiation.56,57 Osteoclastogenesis also requires the activation of costimulatory signals from immunoreceptor tyrosine-based activation motif-containing (ITAM-containing) receptors, mainly the DNAX-activating protein 12 (DAP12) and Fc receptor common γ (FcRγ) subunits, in osteoclast precursors.58 Activation of DAP12 and FcRγ triggers the activation of PLC which can maintain intracellular calcium [Ca2+]i oscillations, which is critical for the activation of NFATc1.58–60 Consequently, recombinant recognition sequence binding protein at the Jκ site (RBP-J), one of the key inhibitors of osteoclastogenesis, functions by blocking PLC-induced [Ca2+]i oscillations in osteoclast precursors.61,62
In line with the requirement for Ca2+, osteoclasts have an active Ca2+ regulatory system, including a membrane Ca2+-ATPase and endoplasmic Ca2+-ATPase as well as receptors, such as ovarian cancer G protein-coupled receptor (ORG1) and N-type Ca2+ channel, to regulate [Ca2+]i levels.16 Also, the basomembrane of osteoclasts is very sensitive to elevated Ca2+ levels.16,21,63 Despite the essential requirement for calcium, which can be activated by the GPCR–Gαq–PLC signaling and the findings that osteoclasts expressed many GPCRs, such as calcitonin receptor, ORG1, and CaSR, the roles of GPCR–G proteins–RGS proteins in regulating osteoclast formation and/or activity remain understudied.3,21,64,65 Below, we reviewed the reported roles of RGS10, RGS12, and RGS18 in osteoclasts.
4.1 RGS10 in Osteoclasts
RGS10 is expressed by many tissues and cells, including brain, testis, atrial monocytes, B lymphocytes, dendritic cells, and osteoclasts.16 RGS10 has two key isoforms: RGS10A and RGS10B. We found that, through a differential screening of a human osteosarcoma cDNA library, RGS10A, but not RGS10B, is specifically expressed by human osteoclasts.66 Consequently, we demonstrated that RGS10A is upregulated by RANKL in osteoclast precursors and highly expressed in murine osteoclast-like cells. RGS10A silencing by RNA interference blocks [Ca2+]i oscillations, the expression of NFATc1, and osteoclast differentiation in bone marrow cells and the osteoclast cell lines. Most importantly, we also showed that mice deficient in the RGS10 gene (RGS10−/−mice) display a severe osteopetrotic phenotype as a result of defective osteoclast formation.67 RGS10 overexpression markedly enhances the sensitivity of osteoclast differentiation to RANKL stimulation, restores [Ca2+]i oscillations, and induces NFATc1 expression. Interestingly, forced expression of NFATc1 could also rescue osteoclastogenesis in RGS10−/−bone marrow cells, indicating that RGS10 functions upstream of NFATc1. Hence, we revealed that RGS10 competitively interacts with Ca2+/calmodulin (CaM) and IP3 in a calcium-dependent manner to mediate PLC activation and [Ca2+]i oscillations for the ultimate activation of NFATc1.3 Hence, our studies have established RGS10 as a crucial regulator of osteoclast differentiation and bone homeostasis.
4.2 RGS12 in Osteoclasts
RGS12 is expressed in many tissues and cells, including lung, spleen, ovary, kidney, prostate, brain, testis, and osteoclasts.16 RGS12 is the largest member of the RGS protein family (Fig. 2). It contains many domains, including the RGS domain, a GoLoco motif which bears guanine nucleotide dissociation inhibitor (GDI) activity toward Gαi subunits, and a Ras-binding domain which provides additional functions to RGS12. Also, RGS12 possesses a PDZ and PTB domains which bind to the C terminus of GPCRs and interacts with GPCR chemokine receptors, respectively.16 Given its multiple domains, RGS12 may regulate numerous signaling pathways. In fact, it has been shown that RGS12 can interact with certain types of calcium channels to control calcium oscillations in many cell types. In line with this finding, we found that RGS12 is highly induced by RANKL in osteoclast precursors and plays a crucial role in osteoclastogenesis.68 RGS12 knockdown by RNA interference impairs osteoclastogenesis by affecting PLC activation which in turn impacts Ca2+ oscillations and the subsequent NFATc1 activation. In addressing this issue, we reported that RANKL can activate the N-type calcium channels to increase [Ca2+]i levels.69–71 Notably, we revealed that RGS12 can interact with N-type calcium channel to regulate its activity. Consistently, targeted deletion of the RGS12 in the hematopoietic cell lineage results in a severe osteopetrotic phenotype from impaired osteoclastogenesis stemming from defective Ca2+ signaling. Whereas the defect in osteoclastogenesis from RGS12−/−osteoclast precursor cells could be rescued by overexpressing either RGS10 or RGS12, those in RGS10−/−bone marrow cells can only be rescued by RGS10. Given that RGS10 can activate calcium oscillations through induction of the IP3 pathway while RGS12 can promote calcium influxes through a specific calcium channel, these findings suggest that [Ca2+]i oscillations may be the most effective way to maintain [Ca2+]i levels in osteoclasts. Hence, our studies reveal critical roles for RGS10 and RGS12 in regulating calcium levels for osteoclast differentiation.
4.3 RGS18 in Osteoclasts
RGS18 is predominantly expressed by selected bone marrow cells, such as granulocytes, monocytes, and platelets but not by lymphocytes and erythrocytes.18 Investigation of the role of RGS18 in osteoclasts revealed that RANKL attenuates RGS18 expression in the osteoclast precursor cell line and in the primary bone marrow-derived osteoclast precursor monocytes during osteoclastogenesis.72 And, RGS18 depletion by RNA interference can promote osteoclastogenesis by RANKL stimulation. However, deletion of RGS18 is unable to promote osteoclast differentiation in the absence of RANKL stimulation, indicating that the RGS18 deficiency requires RANKL to stimulate osteoclastognesis. Previous reports indicated a role for OGR1 in osteoclastogenesis which stimulates the Gαq–PLC pathway leading to the activation of NFATc1.73–76 Interestingly, RGS18 inhibits osteoclastogenesis by acidosis-induced ORGR1 activation by affecting PLC-induced NFATc1 activation because the enhanced osteoclastogenesis from RGS18−/−cells can be revered by treatment with anti-ORG1 blocking antibody. Moreover, whereas overexpression of RGS18 can inhibit the NFATc1 activation triggered by ORG1 activation, RGS18 deletion can enhance NFATc1 activation in these conditions. These findings indicate that RGS18 can negatively regulate osteoclastogenesis induced by extracellular acidosis via activation of ORG1.
5. GPCR–RGS PROTEINS SIGNALING IN SKELETAL PHYSIOLOGY
5.1 RGS Proteins and PTH/PTHrP Signaling in Bone
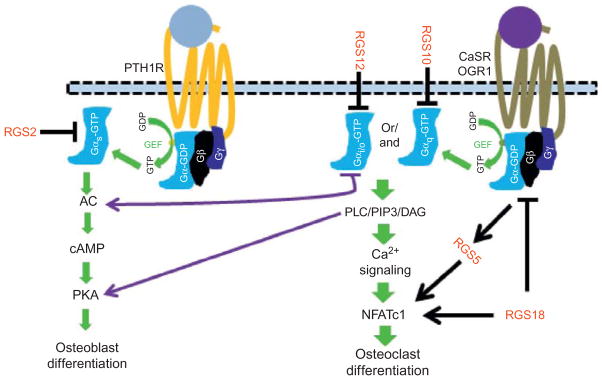
PTH and PTHrP play crucial roles in bone homeostasis through their actions on osteoblasts via the PTH1R, a member of the GPCR family (Fig. 4).77–80 Ligand binding on PTHR1 triggers the activation of the Gαq–PLC and Gαs–AC signaling pathways leading to [Ca2+]i mobilizations from IP3 and increases in cAMP levels, respectively.32 Increased [Ca2+] and cAMP levels are critical for osteoblast differentiation and function. Notably, the inactivation of PTHR1 signaling, mediated by specific RGS proteins, is vital to maintain the rapid turnover of cellular responses. Many studies have demonstrated a role for RGS2 in inactivating PTH1R signaling through the evidence that forced expression of RGS2 can attenuate PTH-induced cAMP production in osteoblasts.16,81 Consistent with the role of RGS2 in inactivating PTHR1 signaling, PTH has been shown to be able to upregulate RGS2 expression both in vivo and in vitro.81,82 Notably, PTH-induced RGS2 expression can also be inhibited by 1,25-(OH)2-D3 in a dose-dependent manner.81 Moreover, while pretreatment with vitamin D can promote PTH-induced RGS2 expression, pretreatment with gluco-corticoids such as dexamethasone can inhibit upregulation of RGS2 induced by PTH stimulation.82 Hence, PTH-induced RGS2 upregulation may play an important mechanism by which PTH controls its activation.
Figure 4.
Role of RGS proteins in selected GPCR signaling in bone. In osteoblasts, PTH1R activation upon binding of PTH or PTHrP triggers the activation of the Gαs–AC–cAMP signaling pathway which ultimately promotes osteoblast differentiation. Activation of PTH1R can induce the expression of many RGS proteins, including RGS2 and RGS5. RGS2 can target the cAMP signaling to regulate PTH1R signaling, and RGS5 is likely to regulate osteoblast differentiation by controlling PTH levels. In osteoclasts, activation of GPCRs, such as CasR and OGR1, can activate Gαq or Gαi/o to activate the PLC–Ca2+ signaling pathway for NFATc1 activation or Gαs to activate the Ac–cAMP pathway to promote osteoblast differentiation and function. RGS10, RGS12, and RGS18 can regulate osteoclast differentiation.
5.2 RGS Proteins and Wnt Signaling in Bone
The canonical Wnt–β-catenin pathway is critical for skeletal development and bone homeostasis by its central function in osteoblast differentiation.32 Wnt signaling is induced by the binding of Wnt ligands to the dual receptor complex consisted of Fz, a member of the GPCR family, and either lipoprotein receptor-related protein 5 (LRP5) or LRP6 (Fig. 3). In the absence of Wnt signaling, β-catenin is found in complex in the cytoplasm with many proteins, including axin, PP2A, APC, GSK3, and CK1, which results in the phosphorylation of β-catenin by GSK-3β. Phosphorylated β-catenin is subsequently targeted for ubiquitination and proteosomal degradation. Axin plays a key role in assembling the destruction complex to reduce the β-catenin levels in the cytoplasm32 as demonstrated by many studies.
Wnt signaling is initiated upon the binding of canonical Wnt ligands to their coreceptors Fz and LRP5/6 to stimulate the Wnt–β-catenin signaling pathway by destroying the β-catenin destruction complex (Fig. 3). Axin moves to the plasma membrane at the tail of LRP5/6 via interaction with dishevelled, which is bound on the tail of Fz. They dissociate the β-catenin destruction complex to form a complex which also includes CSk-3β and FRAT1 (not shown) that prevents the phosphorylation of β-catenin by Csk-3β.32,50 This leads to the accumulation of β-catenin in the cytoplasm and then its migration in the nucleus to activate osteoblast genes and thus promote bone formation.83,84 Finally, Wnt can also regulate bone mass through a noncanonical pathway, which does not involve the utilization of LRP5/6 and activation of β-catenin.85 Furthermore, Wnt can induce [Ca2+]i oscillations independently of the canonical and noncanonical pathways.86 Hence, axin is an important regulator of Wnt signaling for osteoclast differentiation and bone formation.
5.3 RGS Proteins and Ca2+ Oscillations
Ca2+ is an important intracellular messenger that is critical for many cellular processes including cell differentiation.40 Calcium levels can be maintained or regulated via cellular Ca2+ influxes and/or the stimulation-specific calcium channel. CaSR, a member of the GPCR family, acts as a Ca2+ sensor in the parathyroid and kidney to detect extracellular Ca2+ concentration and thus, helps control systemic calcium homeostasis by activating the Gαq/11 pathway.40 Activation of PLC then induces the formation of DAG and IP3, which then boost [Ca2+]i levels through activation of store-operated Ca2+ channel in the plasma membrane and Ca2+ release from the endoplasmic reticulum (ER). The effects of Ca2+ on cellular functions are mainly mediated through the Ca2+-binding protein, CaM, which activates the phosphatase calcineurin,87–89 which in turn dephosphorylates target genes for activation.90,91 Conversely, an increase in intracellular Ca2+ levels can activate the plasma membrane Ca2+ATPase and sarco/endoplasmic reticulum Ca2+ ATPase pumps to remove Ca2+ ions from the cytosol.63
While RGS proteins can regulate the duration of high Ca2+ states and control Ca2+ oscillations in different cell types by desensitizing the associated GPCRs through inactivation of Gαq, the influences of Ca2+ oscillations on the regulation of many tissue functions and cell differentiation have not been reported.92 RGS proteins, such as RGS1, RGS2, and RGS4, can bind to Gαq to enhance its GAP activity, leading to the inactivation of GPCR signaling and a decrease in [Ca2+]i in a cell-specific manner.23 Consistent with that notion, deletion of RGS2 can reduce cell sensitivity to enhance Ca2+ oscillations from ER Ca2+ release into the cytosol.63 Indeed, the amplitude and frequency of [Ca2+]i changes can influence cellular responses.93,94 This is underscored by the findings that components of the Ca2+ signaling, including IP3 production and Ca2+ influxes across the ER and plasma membranes, can regulate the frequency and amplitude of Ca2+ oscillations.16 Importantly, Ca2+ oscillation frequency may target cells along specific developmental pathways by differentially controlling the activation of distinct sets of genes.95,96 Whereas rapid [Ca2+]i oscillations can stimulate the activation of NFAT, Oct/OAP, and NF-κB, infrequent [Ca2+]i oscillations can activate only NF-κB.
Similar to many cell types, Ca2+ is also critical for osteoblast and osteoclast differentiation.40,67 CaSR is expressed in osteoblast precursors and can promote osteoblast differentiation in response to the elevated extracellular Ca2+ levels.16 Also, recent reports have demonstrated that CaM kinase (CaMK) is expressed in osteoblasts, and pharmacological inhibition of CaMK can inhibit osteoblast differentiation both in vivo and in vitro.40 Notably, PTH can influence Ca2+ oscillations and promote the expression of several genes in osteoblasts, including the matrix metallopeptidase 13 (Mmp13). Interestingly, CaMKII inhibition significantly decreases Mmp13 expression in osteoblasts in response to PTH stimulation, suggesting that some of the osteoblast responses to PTH are mediated by CaMKII. Moreover, another key osteoblastogenic agent, vitamin D3, can also activate CaMKII in response to Ca2+ influxes.40 Collectively, these studies demonstrate that Ca2+ is a critical factor for osteoblast differentiation.
In osteoclast differentiation, RANKL can induce Ca2+ oscillations via the activation of PLC in osteoclast precursors through a crosstalk between RANK and the ITAM-based receptors Dap12 and FcRy, leading to the activation of calcineurin which dephosphorylates and activates NFATc1.58,61 Hence, a threshold level of calcium is required to mediate a sustained level of NFATc1 for osteoclast differentiation.61 Notably, the role of Ca2+ oscillations in osteoclast differentiation is buttressed by the reports showing that mice deficient in both Dap12 and FcRy display a severe osteopetrosis phenotype due to impaired Ca2+ oscillations and impaired NFATc1 activation.58,60 Consistently, RBP-J has been identified as an inhibitor of osteoclastogenesis by its ability to interfere with RANKL-induced Ca2+ oscillations.61 Moreover, pharmacological inhibition of CaM or calcineurin and silencing NFATc1, downstream of Ca2+ signaling, can all block osteoclast differentiation. Collectively, these studies support a critical for Ca2+ oscillations in osteoclastogenesis.
Given the critical influence of [Ca2+]i in osteoclast differentiation, we initially proposed that RGS proteins might play a key role in osteoclastogenesis by regulating Ca2+ oscillations. We found that RGS10 and RGS12 are specifically induced by RANKL during osteoclastogenesis.66,68 RGS10 silencing inhibits osteoclastogenesis by blocking [Ca2+]i levels in vitro, and deletion of RGS10 lead to a severe osteopetrosis.66,67 Mechanistically, we revealed that RGS10 competitively interacts with Ca2+/CaM and IP3 in a Ca2+-dependent manner to activate PLC to evoke Ca2+ oscillations for NFATc1 activation and thereby promotes osteoclast differentiation. Similar to RGS10, we also found that RGS12 deficiency could also inhibit osteoclast differentiation by interfering with Ca2+ oscillations.68 However, unlike RGS10, RGS12 does not bind to Ca2+/CaM and IP3 in a Ca2+-dependent manner during osteoclastogenesis, RGS12 can induce calcium influxes via activation of CaSR. These results support the notion that RGS proteins may regulate Ca2+ during osteoclast precursors in different ways to maintain a threshold level of Ca2+ which is required to osteoclastogenesis (Table 1).
Table 1.
RGS Proteins in the Regulation of Osteoblasts and Osteoclasts
| RGS Family Member | Gene Function in Bone Cells | Mechanism of Function in Bone Cells |
|---|---|---|
| RGS2 | Promote osteoblast differentiation and function in bone formation | Regulation of PTH and Ca2+ signaling |
| Axin | Negatively regulate osteoblast differentiation and bone formation | Regulation of canonical Wnt signaling |
| RGS5 | Promote osteoclast differentiation | Regulation of PTH levels |
| RGS10 | Regulate osteoclast differentiation | Regulation of Ca2+ signaling and NFATc1 activation |
| RGS12 | Regulate osteoclast differentiation | Regulation of Ca2+ oscillation via calcium channel and NFATc1 activation |
| RGS8 | Inhibit osteoclast differentiation | Regulation of acid-sensing OGR1/NFATc1 signaling pathway |
6. GPCR/RGS SIGNALING IN SKELETAL DISORDERS
A variety of GPCRs, including Fz, GPR68, PTH1R, and CaSR, have been reported to play essential roles in skeletal development and/or bone homeostasis.16,21 Hence, deletions or mutations of certain GPCRs have resulted in severe skeletal defects in mice. Also, many human diseases have been linked to defective GPCR signaling. As such, many drugs have been designed to target GPCRs for treating certain bone diseases. Nonetheless, whereas the roles of GPCRs in skeletal diseases have been highly studied, our understanding of RGS proteins in skeletal disorders is limited. Given the established roles of RGS proteins in regulating GPCR signaling, the mechanisms by which GPCRs cause skeletal diseases are likely to involve RGS proteins. Below, we provided some examples of skeletal diseases of defective GPCR signaling with emphasis into the aspects of signaling that are or may be regulated by RGS proteins.
6.1 PTH1R and GPCR 48 in Skeletal Development and Diseases
The roles of PTH and PTHrP, through activation of PTH1R, in bone are very complex. Whereas continuous administration of PTH or PTHrP results in bone loss from increased osteoclast activity, their intermittent administration triggers an increase in bone density from enhanced bone formation.79,80 PTHR1 is expressed in bone and kidney and plays a critical role in Ca2+ homeostasis by activating the Gαq–PLC signaling pathway. We have already discussed that Ca2+ is critical for both osteoblast and osteoclast differentiation. PTH1R can also activate the Gαs–AC–cAMP signaling pathway. Notably, PTH and PTHrP can stimulate PTH1R on osteoblasts to increase the ratio of RANKL/OPG (osteoprotegrin), which in turn promotes osteoclastogenesis. OPG is a natural inhibitor of osteoclastogenesis which functions by competing with RANKL for RANK binding. Mice deficient in OPG display an osteoporosis phenotype from enhanced osteoclast formation and function, whereas transgenic mice with OPG overexpression have resulted in osteopetrosis from defective osteoclastogenesis.97,98 The roles of PTH1R signaling in bone development and homeostasis have been established via numerous mice models in which this pathway is perturbed which results in severe skeletal defects.21 Hence, the loss-of-function of PTH1R has been linked to Blomstrand osteochondrodyspasis type, a severe form of dwarfism, in humans.99 Moreover, ligand-independent activation of PTH1R has been reported to cause Jansen’s metaphyseal chondrodysplasia, a disease that is characterized mainly by short stature, normal ephiphyseal plates but disorganized metaphyseal regions, hypercalcemia, and hypophosphatemia. Also, defects in PTH1R can cause enchondromatosis and primary failure of tooth eruption in humans.100
Similar to PTH1R, but in a less complex manner, deletion of GPCR 48, a newly discovered glycoprotein hormone receptor subfamily of GPCRs, have been shown to affect skeletal development by inhibiting osteoblast differentiation while promoting osteoclast differentiation.101 Interestingly, GPCR 48 can also activate the cAMP signaling pathway to reduce the expression level of Atf4 which leads to downregulation of Atf4 target genes in osteoblasts. Given that both PTH1R and GPCR48 activate the cAMP pathway which can be regulated by certain RGS proteins through attenuation of Gαs–AC signaling, RGS proteins are likely to play a key role in regulating PTH1R and GPCR48 in both skeletal physiology and disease. This notion is underscored by a recent report demonstrating that generation of an engineered GPCR with constitutive active Gs in mice can induce a dramatic anabolic skeletal response by 9 weeks of age, which is associated with an increase in bone density, bone volume, and osteoblast markers.102 So, the inability of the engineered GPCR to be inactivated by specific RGS proteins may be responsible for these bone effects under the constitutive activation of the Gs. Interestingly, this anabolic skeletal response was not observed during the first 4-week postnatal lives, suggesting that RGS protein may regulate bone formation in a temporal fashion. Further studies are needed to address this issue.
6.2 CaSR in Skeletal Development and Disease
As discussed above, CaSR can sense and respond to extracellular Ca2+ concentrations to regulate calcium homeostasis. Under elevated levels of plasma calcium, CaSR is activated, which in turn inhibit the release of PTH from the parathyroid gland. Moreover, CaSR can also function as a sensor for other cations such as lanthanum (La3+), gadolinium (Gd3+), barium (Ba2+), strontium (Sr2+), beryllium (Be2+), and magnesium (Mg2+).103,104 Mice deficient in CaSR (CaSR−/−) display severe rickets, characterized by defective hypertrophic chondrocytes, impaired growth plate calcification, disordered mineral deposition, excessive osteoid accumulation, and prolonged mineralization lag time in metaphyseal bone.105 Also, the CaSR−/−mice developed hyperparathyroidism, hypercalcemia, and dwarfism as well as increased bone mineralization and resorption. To elucidate the mechanism of CaSR functions in bone, Dvorak and colleagues generated a transgenic mouse with constitutively active CaSR in mature osteoblasts driven by the osteocalcin promoter.106 These mice show an increase in RANKL expression by osteoblasts which trigger an increase in osteoclast formation and bone resorption. These transgenic mice also show an increase in bone formation. Consistently, CaSR can upregulate PTHrP in osteoblasts to promote osteoblast and osteoclast differentiation in part via an increase in extracellular Ca2+ levels and RANKL upregulation.16
In line with the mouse studies, inactivating mutations of the CaSR gene have been reported to cause many diseases in humans, including the familial hypocalciuric hypercalcemia (FHH) and neonatal severe hyperparathyroidism (NSHPT).107,108 NSHPT can cause a life-threatening hypercalcemia and defectively mineralized skeleton, whereas FHH can show mild-to-moderate hypercalcemia. Moreover, mutations that activate CaSR can cause autosomal dominant hypocalcemia (ADH) in humans. ADH patients display low-to-normal levels of PTH and hypercalciuria with hypocalcemia but no overt skeletal deformities. Ligand binding to CaSR can activate the PLC signaling pathway in both osteoblasts and osteoclasts, presumably via the activation of Gαq, to increase intracellular calcium levels. A role for RGS proteins in regulating the functions of CaSR in the skeletal system was demonstrated by our reports that RGS10 and RGS12 can cause skeletal defects by interfering with Ca2+ oscillations via the PLC pathways and Ca2+ influxes through the calcium channel, respectively.66–68 More studies are needed to explicitly address the roles of other RGS in regulating Ca2+ signaling in skeletal physiology and bone disorders.
6.3 The Canonical Wnt Signaling Pathway in Bone Disease
Wnt signaling pathway is a critical regulator of many development processes, including skeletal development.32 In addition, Wnt signaling has also been associated with many bone diseases. Wnt signaling is a very complicated pathway that includes the canonical Wnt–β-catenin, the noncanonical Wnt-planar cell polarity, and the Wnt–calcium (Wnt–Ca2+) pathways. Of the three Wnt signaling components, however, the canonical Wnt–Ca2+ pathway has been shown to be the major component of the Wnt signaling that regulates bone cells. So, activation of Wnt signaling prevents β-catenin degradation through obliteration of the multiprotein β-catenin destruction complex and thus increases the β-catenin level in the cytoplasm, from where it is translocated in the nucleus to induce gene expression for cell differentiation and function (Fig. 3).
6.3.1 Axin in Bone Development and Bone Disease
Axin is a key component of the β-catenin destruction complex.109,110 The role of axin in suppressing the canonical Wnt–β-catenin signaling pathway has been addressed above (Fig. 4). Consistent with these findings, targeted deletion of axin in mice causes abnormal skull development, a phenotype resembling craniosynostosis in humans, resulting from an increase in calvarial osteoblast development.50–52 These mutant mice also display defective bone ossification from enhanced osteoblast proliferation and differentiation. The skeletal anomalies of the axin-deficient mice were shown to be mediated through enhanced activation of β-catenin. This finding is in agreement with that of Yan et al. who demonstrated that axin also functions as a negative regulator of bone remodeling in adult mice and promotes osteoblastogenesis through the β-catenin–BMP2/4–Osx signaling pathway.50 Taken together, these findings demonstrate a crucial role for this RGS protein in regulating the canonical Wnt–β-catenin signaling pathway in skeletal development and bone disease. Given the central of axin in the regulating Wnt signaling, we further examined the role of Wnt in skeletal development and bone disease.
6.3.2 Mouse Models of Wnt Signaling in Skeletal Homeostasis
The role of the canonical Wnt–β-catenin in bone development and homeostasis is underscored by the findings from many mouse models in which this signaling pathway is disturbed which lead to skeletal deformities.32 Whereas mutations that enhance the Wnt–β-catenin signaling pathway in osteoblasts can trigger an increase in bone mineralization, its inactivation in the osteoblastic lineage cells can result in a decrease in bone mass. For instance, alteration of the Wnt10b gene, one type of Wnt ligand, has established it as a positive regulator of bone homeostasis by enhancing osteoblast differentiation in adult bone.111–113 Conversely, deletion of Wls, a chaperone required for the secretion of Wnt proteins, causes severe osteoporosis in adult mice through impairment of osteoblast differentiation and enhancement of osteoclast differentiation.114 Furthermore, mice with mutations in the LRP5 and/or LPR6 have been shown to recapitulate human diseases of defective Wnt signaling. Hence, global LRP5 deficiency or its targeted deletion in osteocytes has recapitulated the osteoporotic low-bone formation phenotype of humans with loss-of-function mutations in LRP5 or LRP6.115–117 Conversely, introduction of the human high-bone mass gain-of-function mutations in LRP5 β-propeller 1 has induced a similar phenotype in mice when expressed ubiquitously or selectively in the limbs or in the cells of the osteoblastic lineage.32 Further studies have identified many endogenous enhancers of Wnt signaling, such as four R-spondin (RSPO) proteins which bind to Fz and possibly LRP6 to induce β-catenin activation and endogenous inhibitors of Wnt signaling such as sclerostin and dickkopf (DKK1) which can bind to LRP5 or LRP6 to inhibit Wnt signaling.32 The Wnt signaling in bone is complex, and our discussion here only highlights some aspects of the canonical Wnt–β-catenin signaling pathway and its relevance to bone disease.
6.3.3 Bone Diseases of Defective Wnt Signaling
Many human diseases have been reported to be associated with abnormal Wnt signaling leading to skeletal deformities.32 Some of these diseases result from LRP5 mutations. Loss-of-function mutations in LPR5 can lead to osteoporosis-pseudoglioma syndrome, an autosomal recessive disorder characterized by severe juvenile-onset osteoporosis from decreased bone formation and congenital or juvenile-onset blindness, and other diseases with similar phenotypes.118–120 Gain-of-function mutations in LRP5 can cause high bone-mass syndrome from increased bone formation. Notably, gain-of-function mutations of the LRP5 gene can decrease LPR5 binding with sclerostin and DKK1, which in turn promote the activation of the Wnt–β-catenin signaling pathway. Other mutations have shown to affect the Wnt signaling by targeting sclerostin.121,122 Indeed, sclerostin deficiency can cause sclerosteosis and Van Buchen disease, two autosomal recessive disorders characterized by bone overgrowth from excessive bone formation mostly in skull and mandible.123 These human reports provide strong evidence that the Wnt–β-catenin signaling pathway is critical for skeletal homeostasis and bone development. Also, inactivating mutations that affect the Wnt signaling cause severe skeletal deformities.
6.4 RGS Proteins in Inflammatory Bone Disease
Excessive osteoclast formation and/or activity are responsible for the tooth loss associated with many oral diseases including periodontitis. Many RGS proteins including RGS10 can promote osteoclasts differentiation, and proinflammatory factors, such as tumor necrosis factor alpha (TNFα), interleukin 1 (IL-1), and IL-6, can potently influence osteoclast differentiation and function.124–126 In fact, the trio of RANKL, TNFα, and IL-1 are known to be strongly associated with bone loss in many oral diseases.126–129 We reasoned that given its role in osteoclasts, RGS10 might also play a role in boss induced by inflammatory conditions like periodontitis. Toward this end, we employed the adeno-associated virus (AAV)-mediated gene silencing strategy to deplete RGS10 (AAV-shRNA-RGS10) in an established mouse model of bacteria-induced periodontal disease. Our data revealed that RGS10 silencing can protect mice from both the inflammation and bone loss associated with the periodontal disease. We showed that the number of dendritic cells, T cells, and osteoclasts were all decreased in the disease mice-treated with AAV-shRNA-RGS10 as compared to control littermates. Notably, the expression of osteoclast markers and proinflammatory markers were also reduced in the periodontal lesions. These results indicate that RGS10 can regulate both osteoclast differentiation and inflammatory bone diseases.
Our finding is in agreement with that of a previous report which showed that human monocyte-derived dendritic cells constitutively express high levels of RGS2, RGS10, RGS14, RGS18, and RGS19, but low levels of RGS3 and RGS13.38 Activation of toll-like receptor 3 (TLR3) or TLR4 on these dendritic cells can stimulate the expression of RGS1, RGS16, and RGS20 and attenuate the expression levels of RGS18 and RGS14 without modifying other RGS proteins. These results indicate that TLR signaling can affect GPCR signaling by altering the expression of RGS proteins. The role of RGS in macrophages was addressed by Lee and colleagues who demonstrated that macrophages deficient in RGS10 (RGS10−/−) generate higher levels of TNF, IL-1β, and IL-12p70 in response to LPS treatment and also exhibited higher cytotoxicity on dopaminergic MN9D neuroblastoma cells.130 Moreover, the authors found that Rgs10−/−macrophages display dysregulated M1 responses along with blunted M2 alternative activation responses. While our finding on the role of RGS10 in periodontitis may seem in disagreement with that of this study, these discrepancies may stem from the complex nature of RGS protein functions which can regulate GPCR signaling in a temporal- and cell-specific manner. Nonetheless, more studies are needed to fully investigate the role RGS10 in inflammatory conditions. We anticipate that RGS10, aside from its reported role in periodontitis, may also carry critical roles in other inflammatory bone diseases like rheumatoid arthritis.
7. CONCLUSION AND PERSPECTIVES
Our understanding of the roles of RGS proteins in the skeletal system remains mostly unexplored aside from a few studies that have demonstrated essential roles for some RGS proteins in bone formation and/or homeostasis as well as bone diseases through their regulatory effects on osteoblasts, chondrocytes, and/or osteoclasts. Whereas these limited numbers of studies have helped improve our understanding of GPCR–G proteins–RGS signaling in bone biology, much work remains to be done in this area. Besides, GPCR signaling controls many physiological processes, so impacting the functions of RGS proteins in the skeletal system may undesirably have impacts on other organ systems or vice versa. Consequently, it will be critical to address this issue as we further our understanding of RGS proteins on bone biology with the hope of developing better therapy for bone loss by specifically and efficiently targeting GPCR signaling. Indeed, GPCR signaling has been and is likely to remain a valuable target for many disease states, including allergy, ulcers and reflux, pain, high blood pressure, migraine headache, nausea, schizophrenia, and depression. Notably, therapy targeting sclerostin is being examined for treating osteoporosis.32 Most importantly, through their regulatory roles on GPCR signaling and their cell-specific and -selectivity patterns of functions, targeting RGS proteins may also evolve as an effective and potent therapeutic option for bone loss associated with bone disorders of either excessive osteoclast function or attenuated osteoblast activity.
Acknowledgments
Research reported in this publication was supported by the National Institute of Athritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under AR-055307 and R01-AR-44741 to Y.P.L.
References
- 1.Feng X. Chemical and biochemical basis of cell-bone matrix interaction in health and disease. Curr Chem Biol. 2009;3(2):189–196. doi: 10.2174/187231309788166398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyce BF, Rosenberg E, de Papp AE, Duong le T. The osteoclast, bone remodelling and treatment of metabolic bone disease. Eur J Clin Invest. 2012;42(12):1332–1341. doi: 10.1111/j.1365-2362.2012.02717.x. [DOI] [PubMed] [Google Scholar]
- 3.Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone. 2007;40(2):251–264. doi: 10.1016/j.bone.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 4.Karsenty G. Transcriptional control of skeletogenesis. Annu Rev Genomics Hum Genet. 2008;9:183–196. doi: 10.1146/annurev.genom.9.081307.164437. [DOI] [PubMed] [Google Scholar]
- 5.Karsenty G, Oury F. Biology without walls: the novel endocrinology of bone. Annu Rev Physiol. 2012;74:87–105. doi: 10.1146/annurev-physiol-020911-153233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Provot S, Schipani E. Molecular mechanisms of endochondral bone development. Biochem Biophys Res Commun. 2005;328(3):658–665. doi: 10.1016/j.bbrc.2004.11.068. [DOI] [PubMed] [Google Scholar]
- 7.Teitelbaum SL. Bone resorption by osteoclasts. Science. 2000;289(5484):1504–1508. doi: 10.1126/science.289.5484.1504. [DOI] [PubMed] [Google Scholar]
- 8.Zhao Q, Shao J, Chen W, Li YP. Osteoclast differentiation and gene regulation. Front Biosci. 2007;12:2519–2529. doi: 10.2741/2252. [DOI] [PubMed] [Google Scholar]
- 9.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 10.Feng X, McDonald JM. Disorders of bone remodeling. Annu Rev Pathol. 2011;6:121–145. doi: 10.1146/annurev-pathol-011110-130203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jules J, Ashley JW, Feng X. Selective targeting of RANK signaling pathways as new therapeutic strategies for osteoporosis. Expert Opin Ther Targets. 2010;14(9):923–934. doi: 10.1517/14728222.2010.511179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page RC. Milestones in periodontal research and the remaining critical issues. J Periodontal Res. 1999;34(7):331–339. doi: 10.1111/j.1600-0765.1999.tb02262.x. [DOI] [PubMed] [Google Scholar]
- 13.Goronzy JJ, Weyand CM. Rheumatoid arthritis. Immunol Rev. 2005;204:55–73. doi: 10.1111/j.0105-2896.2005.00245.x. [DOI] [PubMed] [Google Scholar]
- 14.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001;358(9285):903–911. doi: 10.1016/S0140-6736(01)06075-5. [DOI] [PubMed] [Google Scholar]
- 15.Kimple AJ, Bosch DE, Giguere PM, Siderovski DP. Regulators of G-protein signaling and their Galpha substrates: promises and challenges in their use as drug discovery targets. Pharmacol Rev. 2011;63(3):728–749. doi: 10.1124/pr.110.003038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keinan D, Yang S, Cohen RE, Yuan X, Liu T, Li YP. Role of regulator of G protein signaling proteins in bone. Front Biosci. 2014;19:634–648. doi: 10.2741/4232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCudden CR, Hains MD, Kimple RJ, Siderovski DP, Willard FS. G-protein signaling: back to the future. Cell Mol Life Sci. 2005;62(5):551–577. doi: 10.1007/s00018-004-4462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacol Ther. 2007;116(3):473–495. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zalewska M, Siara M, Sajewicz W. G protein-coupled receptors: abnormalities in signal transmission, disease states and pharmacotherapy. Acta Pol Pharm. 2014;71(2):229–243. [PubMed] [Google Scholar]
- 20.Louwette S, Van Geet C, Freson K. Regulators of G protein signaling: role in hematopoiesis, megakaryopoiesis and platelet function. J Thromb Haemost. 2012;10(11):2215–2222. doi: 10.1111/j.1538-7836.2012.04903.x. [DOI] [PubMed] [Google Scholar]
- 21.Wu M, Deng L, Zhu G, Li YP. G protein and its signaling pathway in bone development and disease. Front Biosci. 2010;15:957–985. doi: 10.2741/3656. [DOI] [PubMed] [Google Scholar]
- 22.Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiol Rev. 1999;79(4):1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- 23.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 24.Doupnik CA, Davidson N, Lester HA, Kofuji P. RGS proteins reconstitute the rapid gating kinetics of gbetagamma-activated inwardly rectifying K+ channels. Proc Natl Acad Sci U S A. 1997;94(19):10461–10466. doi: 10.1073/pnas.94.19.10461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.De Vries L, Zheng B, Fischer T, Elenko E, Farquhar MG. The regulator of G protein signaling family. Annu Rev Pharmacol Toxicol. 2000;40:235–271. doi: 10.1146/annurev.pharmtox.40.1.235. [DOI] [PubMed] [Google Scholar]
- 26.Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacol Rev. 2002;54(3):527–559. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- 27.Xie GX, Palmer PP. How regulators of G protein signaling achieve selective regulation. J Mol Biol. 2007;366(2):349–365. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Terzi D, Stergiou E, King SL, Zachariou V. Regulators of G protein signaling in neuropsychiatric disorders. Prog Mol Biol Transl Sci. 2009;86:299–333. doi: 10.1016/S1877-1173(09)86010-9. [DOI] [PubMed] [Google Scholar]
- 29.Traynor J. mu-Opioid receptors and regulators of G protein signaling (RGS) proteins: from a symposium on new concepts in mu-opioid pharmacology. Drug Alcohol Depend. 2012;121(3):173–180. doi: 10.1016/j.drugalcdep.2011.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wieland T, Lutz S, Chidiac P. Regulators of G protein signalling: a spotlight on emerging functions in the cardiovascular system. Curr Opin Pharmacol. 2007;7(2):201–207. doi: 10.1016/j.coph.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Hurst JH, Hooks SB. Regulator of G-protein signaling (RGS) proteins in cancer biology. Biochem Pharmacol. 2009;78(10):1289–1297. doi: 10.1016/j.bcp.2009.06.028. [DOI] [PubMed] [Google Scholar]
- 32.Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–192. doi: 10.1038/nm.3074. [DOI] [PubMed] [Google Scholar]
- 33.Miles RR, Sluka JP, Santerre RF, et al. Dynamic regulation of RGS2 in bone: potential new insights into parathyroid hormone signaling mechanisms. Endocrinology. 2000;141(1):28–36. doi: 10.1210/endo.141.1.7229. [DOI] [PubMed] [Google Scholar]
- 34.Thirunavukkarasu K, Halladay DL, Miles RR, Geringer CD, Onyia JE. Analysis of regulator of G-protein signaling-2 (RGS-2) expression and function in osteoblastic cells. J Cell Biochem. 2002;85(4):837–850. doi: 10.1002/jcb.10176. [DOI] [PubMed] [Google Scholar]
- 35.Eszlinger M, Holzapfel HP, Voigt C, Arkenau C, Paschke R. RGS 2 expression is regulated by TSH and inhibits TSH receptor signaling. Eur J Endocrinol. 2004;151(3):383–390. doi: 10.1530/eje.0.1510383. [DOI] [PubMed] [Google Scholar]
- 36.Riekenberg S, Farhat K, Debarry J, et al. Regulators of G-protein signalling are modulated by bacterial lipopeptides and lipopolysaccharide. FEBS J. 2009;276(3):649–659. doi: 10.1111/j.1742-4658.2008.06813.x. [DOI] [PubMed] [Google Scholar]
- 37.Lee HK, Yeo S, Kim JS, et al. Protein kinase C-eta and phospholipase D2 pathway regulates foam cell formation via regulator of G protein signaling 2. Mol Pharmacol. 2010;78(3):478–485. doi: 10.1124/mol.110.064394. [DOI] [PubMed] [Google Scholar]
- 38.Shi GX, Harrison K, Han SB, Moratz C, Kehrl JH. Toll-like receptor signaling alters the expression of regulator of G protein signaling proteins in dendritic cells: implications for G protein-coupled receptor signaling. J Immunol. 2004;172(9):5175–5184. doi: 10.4049/jimmunol.172.9.5175. [DOI] [PubMed] [Google Scholar]
- 39.Giorelli M, Livrea P, Defazio G, et al. Interferon beta-1a counteracts effects of activation on the expression of G-protein-coupled receptor kinases 2 and 3, beta-arrestin-1, and regulators of G-protein signalling 2 and 16 in human mononuclear leukocytes. Cell Signal. 2002;14(8):673–678. doi: 10.1016/s0898-6568(02)00011-6. [DOI] [PubMed] [Google Scholar]
- 40.Zayzafoon M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J Cell Biochem. 2006;97(1):56–70. doi: 10.1002/jcb.20675. [DOI] [PubMed] [Google Scholar]
- 41.Klein RF, Nissenson RA, Strewler GJ. Pertussis toxin inhibits hormonal stimulation of bone resorption in fetal rat limb bones. J Pharmacol Exp Ther. 1991;258(3):877–881. [PubMed] [Google Scholar]
- 42.Ko JK, Choi KH, Kim IS, Jung EK, Park DH. Inducible RGS2 is a cross-talk regulator for parathyroid hormone signaling in rat osteoblast-like UMR106 cells. Biochem Biophys Res Commun. 2001;287(4):1025–1033. doi: 10.1006/bbrc.2001.5692. [DOI] [PubMed] [Google Scholar]
- 43.Abou-Samra AB, Juppner H, Force T, et al. Expression cloning of a common receptor for parathyroid hormone and parathyroid hormone-related peptide from rat osteoblast-like cells: a single receptor stimulates intracellular accumulation of both cAMP and inositol trisphosphates and increases intracellular free calcium. Proc Natl Acad Sci U S A. 1992;89(7):2732–2736. doi: 10.1073/pnas.89.7.2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silve CM, Hradek GT, Jones AL, Arnaud CD. Parathyroid hormone receptor in intact embryonic chicken bone: characterization and cellular localization. J Cell Biol. 1982;94(2):379–386. doi: 10.1083/jcb.94.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Homme M, Schaefer F, Mehls O, Schmitt CP. Differential regulation of RGS-2 by constant and oscillating PTH concentrations. Calcif Tissue Int. 2009;84(4):305–312. doi: 10.1007/s00223-009-9222-1. [DOI] [PubMed] [Google Scholar]
- 46.Koh J, Dar M, Untch BR, et al. Regulator of G protein signaling 5 is highly expressed in parathyroid tumors and inhibits signaling by the calcium-sensing receptor. Mol Endocrinol. 2011;25(5):867–876. doi: 10.1210/me.2010-0277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miao D, Tong XK, Chan GK, Panda D, McPherson PS, Goltzman D. Parathyroid hormone-related peptide stimulates osteogenic cell proliferation through protein kinase C activation of the Ras/mitogen-activated protein kinase signaling pathway. J Biol Chem. 2001;276(34):32204–32213. doi: 10.1074/jbc.M101084200. [DOI] [PubMed] [Google Scholar]
- 48.Brown EM. Mechanisms underlying the regulation of parathyroid hormone secretion in vivo and in vitro. Curr Opin Nephrol Hypertens. 1993;2(4):541–551. doi: 10.1097/00041552-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 49.Liang Y, Li C, Guzman VM, et al. Identification of a novel alternative splicing variant of RGS5 mRNA in human ocular tissues. FEBS J. 2005;272(3):791–799. doi: 10.1111/j.1742-4658.2004.04516.x. [DOI] [PubMed] [Google Scholar]
- 50.Yan Y, Tang D, Chen M, et al. Axin2 controls bone remodeling through the beta-catenin-BMP signaling pathway in adult mice. J Cell Sci. 2009;122(pt 19):3566–3578. doi: 10.1242/jcs.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gwak J, Hwang SG, Park HS, et al. Small molecule-based disruption of the axin/beta-catenin protein complex regulates mesenchymal stem cell differentiation. Cell Res. 2012;22(1):237–247. doi: 10.1038/cr.2011.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Minear S, Leucht P, Jiang J, et al. Wnt proteins promote bone regeneration. Sci Transl Med. 2010;2(29):29ra30. doi: 10.1126/scitranslmed.3000231. [DOI] [PubMed] [Google Scholar]
- 53.Yu HM, Jerchow B, Sheu TJ, et al. The role of Axin2 in calvarial morphogenesis and craniosynostosis. Development. 2005;132(8):1995–2005. doi: 10.1242/dev.01786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hay E, Laplantine E, Geoffroy V, et al. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling, osteoblast function, and bone formation. Mol Cell Biol. 2009;29(4):953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Regard JB, Cherman N, Palmer D, et al. Wnt/beta-catenin signaling is differentially regulated by Galpha proteins and contributes to fibrous dysplasia. Proc Natl Acad Sci U S A. 2011;108(50):20101–20106. doi: 10.1073/pnas.1114656108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Takayanagi H, Kim S, Koga T, et al. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3(6):889–901. doi: 10.1016/s1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 57.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(suppl):S67–S79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 58.Koga T, Inui M, Inoue K, et al. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428(6984):758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 59.Zou W, Kitaura H, Reeve J, et al. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176(6):877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mocsai A, Humphrey MB, Van Ziffle JA, et al. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A. 2004;101(16):6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li S, Miller CH, Giannopoulou E, Hu X, Ivashkiv LB, Zhao B. RBP-J imposes a requirement for ITAM-mediated costimulation of osteoclastogenesis. J Clin Invest. 2014;124(11):5057–5073. doi: 10.1172/JCI71882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao B, Grimes SN, Li S, Hu X, Ivashkiv LB. TNF-induced osteoclastogenesis and inflammatory bone resorption are inhibited by transcription factor RBP-J. J Exp Med. 2012;209(2):319–334. doi: 10.1084/jem.20111566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang X, Huang G, Luo X, Penninger JM, Muallem S. Role of regulator of G protein signaling 2 (RGS2) in Ca(2+) oscillations and adaptation of Ca(2+) signaling to reduce excitability of RGS2−/−cells. J Biol Chem. 2004;279(40):41642–41649. doi: 10.1074/jbc.M406450200. [DOI] [PubMed] [Google Scholar]
- 64.Brown EM. Role of the calcium-sensing receptor in extracellular calcium homeostasis. Best Pract Res Clin Endocrinol Metab. 2013;27(3):333–343. doi: 10.1016/j.beem.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 65.Lombardi G, Di Somma C, Rubino M, et al. The roles of parathyroid hormone in bone remodeling: prospects for novel therapeutics. J Endocrinol Invest. 2011;34(7 suppl):18–22. [PubMed] [Google Scholar]
- 66.Yang S, Chen W, Stashenko P, Li YP. Specificity of RGS10A as a key component in the RANKL signaling mechanism for osteoclast differentiation. J Cell Sci. 2007;120(pt 19):3362–3371. doi: 10.1242/jcs.008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang S, Li YP. RGS10-null mutation impairs osteoclast differentiation resulting from the loss of [Ca2+]i oscillation regulation. Genes Dev. 2007;21(14):1803–1816. doi: 10.1101/gad.1544107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yang S, Li YP. RGS12 is essential for RANKL-evoked signaling for terminal differentiation of osteoclasts in vitro. J Bone Miner Res. 2007;22(1):45–54. doi: 10.1359/jbmr.061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schiff ML, Siderovski DP, Jordan JD, et al. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature. 2000;408(6813):723–727. doi: 10.1038/35047093. [DOI] [PubMed] [Google Scholar]
- 70.Richman RW, Diverse-Pierluissi MA. Mapping of RGS12-Cav2.2 channel interaction. Methods Enzymol. 2004;390:224–239. doi: 10.1016/S0076-6879(04)90015-8. [DOI] [PubMed] [Google Scholar]
- 71.Richman RW, Strock J, Hains MD, et al. RGS12 interacts with the SNARE-binding region of the Cav2.2 calcium channel. J Biol Chem. 2005;280(2):1521–1528. doi: 10.1074/jbc.M406607200. [DOI] [PubMed] [Google Scholar]
- 72.Iwai K, Koike M, Ohshima S, et al. RGS18 acts as a negative regulator of osteoclastogenesis by modulating the acid-sensing OGR1/NFAT signaling pathway. J Bone Miner Res. 2007;22(10):1612–1620. doi: 10.1359/jbmr.070612. [DOI] [PubMed] [Google Scholar]
- 73.Tomura H, Mogi C, Sato K, Okajima F. Proton-sensing and lysolipid-sensitive G-protein-coupled receptors: a novel type of multi-functional receptors. Cell Signal. 2005;17(12):1466–1476. doi: 10.1016/j.cellsig.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Ludwig MG, Vanek M, Guerini D, et al. Proton-sensing G-protein-coupled receptors. Nature. 2003;425(6953):93–98. doi: 10.1038/nature01905. [DOI] [PubMed] [Google Scholar]
- 75.Huang C, Hydo LM, Liu S, Miller RT. Activation of choline kinase by extracellular Ca2+ is Ca(2+)-sensing receptor, Galpha12 and Rho-dependent in breast cancer cells. Cell Signal. 2009;21(12):1894–1900. doi: 10.1016/j.cellsig.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 76.Heino TJ, Chagin AS, Savendahl L. The novel estrogen receptor G-protein-coupled receptor 30 is expressed in human bone. J Endocrinol. 2008;197(2):R1–R6. doi: 10.1677/JOE-07-0629. [DOI] [PubMed] [Google Scholar]
- 77.Uzawa T, Hori M, Ejiri S, Ozawa H. Comparison of the effects of intermittent and continuous administration of human parathyroid hormone(1–34) on rat bone. Bone. 1995;16(4):477–484. doi: 10.1016/8756-3282(95)90194-9. [DOI] [PubMed] [Google Scholar]
- 78.Tam CS, Heersche JN, Murray TM, Parsons JA. Parathyroid hormone stimulates the bone apposition rate independently of its resorptive action: differential effects of intermittent and continuous administration. Endocrinology. 1982;110(2):506–512. doi: 10.1210/endo-110-2-506. [DOI] [PubMed] [Google Scholar]
- 79.Takeuchi Y. Treatment of osteoporosis with PTH. Clin Calcium. 2014;24(6):893–902. [PubMed] [Google Scholar]
- 80.Inoue D. Role for PTHrP in bone and cartilage metabolism. Clin Calcium. 2014;24(6):863–869. [PubMed] [Google Scholar]
- 81.Ueno Y, Shinki T, Nagai Y, Murayama H, Fujii K, Suda T. In vivo administration of 1,25-dihydroxyvitamin D3 suppresses the expression of RANKL mRNA in bone of thyroparathyroidectomized rats constantly infused with PTH. J Cell Biochem. 2003;90(2):267–277. doi: 10.1002/jcb.10623. [DOI] [PubMed] [Google Scholar]
- 82.Homme M, Schmitt CP, Himmele R, Hoffmann GF, Mehls O, Schaefer F. Vitamin D and dexamethasone inversely regulate parathyroid hormone-induced regulator of G protein signaling-2 expression in osteoblast-like cells. Endocrinology. 2003;144(6):2496–2504. doi: 10.1210/en.2002-0160. [DOI] [PubMed] [Google Scholar]
- 83.Cadigan KM, Peifer M. Wnt signaling from development to disease: insights from model systems. Cold Spring Harb Perspect Biol. 2009;1(2):a002881. doi: 10.1101/cshperspect.a002881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450(1):9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 85.Jenny A. Planar cell polarity signaling in the Drosophila eye. Curr Top Dev Biol. 2010;93:189–227. doi: 10.1016/B978-0-12-385044-7.00007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohn AD, Moon RT. Wnt and calcium signaling: beta-catenin-independent pathways. Cell Calcium. 2005;38(3–4):439–446. doi: 10.1016/j.ceca.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 87.Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 88.Im SH, Rao A. Activation and deactivation of gene expression by Ca2+/calcineurin-NFAT-mediated signaling. Mol Cells. 2004;18(1):1–9. [PubMed] [Google Scholar]
- 89.Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707–747. doi: 10.1146/annurev.immunol.15.1.707. [DOI] [PubMed] [Google Scholar]
- 90.Brown EM, Gamba G, Riccardi D, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–580. doi: 10.1038/366575a0. [DOI] [PubMed] [Google Scholar]
- 91.Garrett JE, Capuano IV, Hammerland LG, et al. Molecular cloning and functional expression of human parathyroid calcium receptor cDNAs. J Biol Chem. 1995;270(21):12919–12925. doi: 10.1074/jbc.270.21.12919. [DOI] [PubMed] [Google Scholar]
- 92.Luo X, Popov S, Bera AK, Wilkie TM, Muallem S. RGS proteins provide biochemical control of agonist-evoked [Ca2+]i oscillations. Mol Cell. 2001;7(3):651–660. doi: 10.1016/s1097-2765(01)00211-8. [DOI] [PubMed] [Google Scholar]
- 93.Dolmetsch RE, Lewis RS. Signaling between intracellular Ca2+ stores and depletion-activated Ca2+ channels generates [Ca2+]i oscillations in T lymphocytes. J Gen Physiol. 1994;103(3):365–388. doi: 10.1085/jgp.103.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392(6679):933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 95.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17(19):7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gu X, Spitzer NC. Distinct aspects of neuronal differentiation encoded by frequency of spontaneous Ca2+ transients. Nature. 1995;375(6534):784–787. doi: 10.1038/375784a0. [DOI] [PubMed] [Google Scholar]
- 97.Simonet WS, Lacey DL, Dunstan CR, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–319. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 98.Wu Y, Liu J, Guo H, et al. Establishment of OPG transgenic mice and the effect of OPG on bone microarchitecture. Int J Endocrinol. 2013;2013:125932. doi: 10.1155/2013/125932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smith EP, Boyd J, Frank GR, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- 100.Decker E, Stellzig-Eisenhauer A, Fiebig BS, et al. PTHR1 loss-of-function mutations in familial, nonsyndromic primary failure of tooth eruption. Am J Hum Genet. 2008;83(6):781–786. doi: 10.1016/j.ajhg.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Luo J, Zhou W, Zhou X, et al. Regulation of bone formation and remodeling by G-protein-coupled receptor 48. Development. 2009;136(16):2747–2756. doi: 10.1242/dev.033571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hsiao EC, Boudignon BM, Chang WC, et al. Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass. Proc Natl Acad Sci U S A. 2008;105(4):1209–1214. doi: 10.1073/pnas.0707457105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Theman TA, Collins MT. The role of the calcium-sensing receptor in bone biology and pathophysiology. Curr Pharm Biotechnol. 2009;10(3):289–301. doi: 10.2174/138920109787847538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chattopadhyay N, Brown EM. Cellular “sensing” of extracellular calcium (Ca(2+)(o)): emerging roles in regulating diverse physiological functions. Cell Signal. 2000;12(6):361–366. doi: 10.1016/s0898-6568(00)00082-6. [DOI] [PubMed] [Google Scholar]
- 105.Garner SC, Pi M, Tu Q, Quarles LD. Rickets in cation-sensing receptor-deficient mice: an unexpected skeletal phenotype. Endocrinology. 2001;142(9):3996–4005. doi: 10.1210/endo.142.9.8364. [DOI] [PubMed] [Google Scholar]
- 106.Dvorak MM, Chen TH, Orwoll B, et al. Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology. 2007;148(7):3156–3163. doi: 10.1210/en.2007-0147. [DOI] [PubMed] [Google Scholar]
- 107.Brown EM, Pollak M, Chou YH, Seidman CE, Seidman JG, Hebert SC. Cloning and functional characterization of extracellular Ca(2+)-sensing receptors from parathyroid and kidney. Bone. 1995;17(2 suppl):7S–11S. doi: 10.1016/8756-3282(95)00199-n. [DOI] [PubMed] [Google Scholar]
- 108.Pollak MR, Brown EM, Chou YH, et al. Mutations in the human Ca(2+)-sensing receptor gene cause familial hypocalciuric hypercalcemia and neonatal severe hyper-parathyroidism. Cell. 1993;75(7):1297–1303. doi: 10.1016/0092-8674(93)90617-y. [DOI] [PubMed] [Google Scholar]
- 109.Jho EH, Zhang T, Domon C, Joo CK, Freund JN, Costantini F. Wnt/beta-catenin/ Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway. Mol Cell Biol. 2002;22(4):1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gerlach JP, Emmink BL, Nojima H, Kranenburg O, Maurice MM. Wnt signalling induces accumulation of phosphorylated beta-catenin in two distinct cytosolic complexes. Open Biol. 2014;4(11):140120. doi: 10.1098/rsob.140120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bennett CN, Ouyang H, Ma YL, et al. Wnt10b increases postnatal bone formation by enhancing osteoblast differentiation. J Bone Miner Res. 2007;22(12):1924–1932. doi: 10.1359/jbmr.070810. [DOI] [PubMed] [Google Scholar]
- 112.Bennett CN, Longo KA, Wright WS, et al. Regulation of osteoblastogenesis and bone mass by Wnt10b. Proc Natl Acad Sci U S A. 2005;102(9):3324–3329. doi: 10.1073/pnas.0408742102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stevens JR, Miranda-Carboni GA, Singer MA, Brugger SM, Lyons KM, Lane TF. Wnt10b deficiency results in age-dependent loss of bone mass and progressive reduction of mesenchymal progenitor cells. J Bone Miner Res. 2010;25(10):2138–2147. doi: 10.1002/jbmr.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhong Z, Zylstra-Diegel CR, Schumacher CA, et al. Wntless functions in mature osteoblasts to regulate bone mass. Proc Natl Acad Sci U S A. 2012;109(33):E2197–E2204. doi: 10.1073/pnas.1120407109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kato M, Patel MS, Levasseur R, et al. Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. J Cell Biol. 2002;157(2):303–314. doi: 10.1083/jcb.200201089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clement-Lacroix P, Ai M, Morvan F, et al. Lrp5-independent activation of Wnt signaling by lithium chloride increases bone formation and bone mass in mice. Proc Natl Acad Sci U S A. 2005;102(48):17406–17411. doi: 10.1073/pnas.0505259102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cui Y, Niziolek PJ, MacDonald BT, et al. Lrp5 functions in bone to regulate bone mass. Nat Med. 2011;17(6):684–691. doi: 10.1038/nm.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gong Y, Slee RB, Fukai N, et al. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107(4):513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 119.Little RD, Carulli JP, Del Mastro RG, et al. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70(1):11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Boyden LM, Mao J, Belsky J, et al. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346(20):1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 121.Poole KE, van Bezooijen RL, Loveridge N, et al. Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J. 2005;19(13):1842–1844. doi: 10.1096/fj.05-4221fje. [DOI] [PubMed] [Google Scholar]
- 122.Brunkow ME, Gardner JC, Van Ness J, et al. Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet. 2001;68(3):577–589. doi: 10.1086/318811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Balemans W, Patel N, Ebeling M, et al. Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet. 2002;39(2):91–97. doi: 10.1136/jmg.39.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jules J, Shi Z, Liu J, Xu D, Wang S, Feng X. Receptor activator of NF-{kappa}B (RANK) cytoplasmic IVVY535-538 motif plays an essential role in tumor necrosis factor-{alpha} (TNF)-mediated osteoclastogenesis. J Biol Chem. 2010;285(48):37427–37435. doi: 10.1074/jbc.M110.149484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jules J, Zhang P, Ashley JW, et al. Molecular basis of requirement of receptor activator of nuclear factor kappaB signaling for interleukin 1-mediated osteoclastogenesis. J Biol Chem. 2012;287(19):15728–15738. doi: 10.1074/jbc.M111.296228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kikuta J, Yamaguchi M, Shimizu M, Yoshino T, Kasai K. Notch signaling induces root resorption via RANKL and IL-6 from hPDL cells. J Dent Res. 2014;94:140–147. doi: 10.1177/0022034514555364. [DOI] [PubMed] [Google Scholar]
- 127.Akiyama T, Miyamoto Y, Yoshimura K, et al. Porphyromonas gingivalis-derived lysine gingipain enhances osteoclast differentiation induced by tumor necrosis factor-alpha and interleukin-1beta but suppresses that by interleukin-17A: importance of proteolytic degradation of osteoprotegerin by lysine gingipain. J Biol Chem. 2014;289(22):15621–15630. doi: 10.1074/jbc.M113.520510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Meng S, Zhang L, Tang Y, et al. BET inhibitor JQ1 blocks inflammation and bone destruction. J Dent Res. 2014;93(7):657–662. doi: 10.1177/0022034514534261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. J Periodontol. 2005;76(11 suppl):2033–2041. doi: 10.1902/jop.2005.76.11-S.2033. [DOI] [PubMed] [Google Scholar]
- 130.Lee JK, Chung J, Kannarkat GT, Tansey MG. Critical role of regulator G-protein signaling 10 (RGS10) in modulating macrophage M1/M2 activation. PLoS One. 2013;8(11):e81785. doi: 10.1371/journal.pone.0081785. [DOI] [PMC free article] [PubMed] [Google Scholar]