Abstract
Obesity induces chronic, low-grade inflammation, which increases the risk of colon cancer. We investigated the preventive effects of Bardoxolone methyl (BARD) on high-fat diet (HFD)-induced inflammation in a mouse colon. Male C57BL/6J mice (n=7) were fed a HFD (HFD group), HFD plus BARD (10 mg/kg) in drinking water (HFD/BARD group), or normal laboratory chow diet (LFD group) for 21 weeks. In HFD mice, BARD reduced colon thickness and decreased colon weight per length. This was associated with an increase in colon crypt depth and the number of goblet cells per crypt. BARD reduced the expression of F4/80 and CD11c but increased CD206 and IL-10, indicating an anti-inflammatory effect. BARD prevented an increase of the intracellular pro-inflammatory biomarkers (NF-қB, p NF-қB, IL-6, TNF-α) and cell proliferation markers (Cox2 and Ki67). BARD prevented fat deposition in the colon wall and prevented microbial population changes. Overall, we report the preventive effects of BARD on colon inflammation in HFD-fed mice through its regulation of macrophages, NF-қB, cytokines, Cox2 and Ki67, fat deposition and microflora.
Keywords: Bardoxolone methyl, Colon, High-fat diet, Inflammation
Introduction
Obesity increases the risk of developing colon cancer (Liu et al. 2012; Bardou et al. 2013). Studies have shown that the incidence of colon cancer is correlated with body size and body weight (Kono et al. 1999; Bassett et al. 2010). The risk of developing colon cancer is doubled in overweight or obese people as compared with healthy people with normal weight (Giovannucci et al. 1995). Diet-induced obese mice have high levels of inflammation and an increased risk of carcinogenesis (Erdelyi et al. 2009). Colon cancer is the third-most diagnosed cancer in the world (Ferlay et al. 2015); because of this, preventing obesity-associated colon cancer is important.
Consumption of HFD is a key factor in the development of obesity, which increases fat deposition and causes changes to the intestinal structure and morphology. Increased fat deposition has been observed in the intestine of diet-induced obese mice and is a risk factor for inflammatory bowel disease (Douglass et al. 2012; Paik et al. 2013). The chronic consumption of a HFD can affect colon crypts, which is accompanied by a disruption to the colon epithelium, as well as reduced goblet cell number, and increased epithelial cell proliferation (Bird and Stamp 1986; Paturi et al. 2010).
Inflammation is another risk factor for colon cancer (Giugliano et al. 2006; Day et al. 2013; Fang et al. 2013). It has been suggested that gut inflammation and oxidative stress induced by a HFD have carcinogenic effects in mice (Erdelyi et al. 2009). For instance, a HFD increases nuclear factor-κ light-chain enhancer of activated B cells (NF-қB) activation in the epithelial cells of the small intestine and colon in mice (Ding et al. 2010; Kim et al. 2012a). Moreover, a HFD elevates tumor necrosis factor alpha (TNF-α) protein by 72% in mouse colonic mucosa (Liu et al. 2012). A HFD also increases colon cancer cell proliferation, as observed by an increase in the level of nuclear antigen Ki67 (Ki67) expression (Park et al. 2012). Ki67 is a cell proliferation marker used to assist in the diagnosis of colon cancer (Shepherd et al. 1988; Takano et al. 1996; Nabi et al. 2008). Moreover, overweight people have a higher level of Cox2 mRNA in the colon as compared with people with weight in the normal range (Delage et al. 2007). A HFD promotes colitis in mice, which is associated with a shortened colon and an elevated level of Cox2 (Kim et al. 2012a). Compared with mice fed a normal diet, those fed a HFD are more susceptible to carcinogen-induced colon tumorigenesis. In particular, a HFD increases the development of polyps and aberrant crypt foci in colon cancer mouse models (Baltgalvis et al. 2009; Padidar et al. 2012). Furthermore, the gut microbiota composition correlates to body-weight gain, as well as to the local and systemic inflammation associated with human obesity (Verdam et al. 2013). Strategies to modulate gut microflora may help to prevent the risk of colon-associated complications in obesity.
Bardoxolone methyl (BARD) is a synthetic pentacyclic triterpenoid compound and a derivative of oleanolic acid. In other reports, this compound and its analog RTA 405 are effective in the suppression of body weight in obese humans and body fat in obese mice, respectively (Manenti et al. 2012; Chin et al. 2013). Anti-inflammatory, anti-obesity and anti-cancer effects of BARD have been demonstrated in preclinical and clinical studies (Liby et al. 2009; Saha et al. 2010; Tran et al. 2013; Wu et al. 2014). BARD and its analog 2-cyano-3,12-dioxooleana-1,9-dien-28-oic acid-9,11-dihydro-trifluoroethyl amide are immunomodulators and inhibitors of NF-қB signalling and various inflammatory mediators (ie TNF-α, Cox2) (Deeb et al. 2007; Aminzadeh et al. 2014; Choi et al. 2014). BARD suppresses the colitis-associated colon inflammation in mice and protects human colon epithelial cells from radiation-induced neoplastic transformation (Eskiocak et al. 2010; Choi et al. 2014). In this study, we investigated the cellular mechanism of BARD in the colons of mice fed a HFD by assessing the expression of various inflammatory and anti-inflammatory mediators, the degree of fat deposition and the changes in colon microbiota.
Materials & Methods
Experimental Procedures on Animals
Male, 12-week-old, C57BL/6J mice were obtained from the Animal Resource Centre (Perth, WA). After 1 week acclimatization, the mice were divided into three groups (n=7): (1) mice fed a normal lab chow diet (LFD group, Fat/Protein/Carbohydrate = 5:18:77 as % energy fat) (Vella Stock Feeds, Doonside, NSW); (2) mice fed a high-fat diet (HFD group, Fat/Protein/Carbohydrate = 40:15:45 as % energy fat) (SF11-095, Speciality Feeds, WA); and (3) mice fed the same HFD and supplemented with BARD (CAS: 218600-53-4, Shanghai Haoyuan Chemexpress Co., Ltd) in drinking water at dose of 10 mg/kg body weight (HFD/BARD group). It has been previously shown that a HFD (over 20 weeks) induces significant intestinal inflammation in C57BL/6J mice (Axling et al. 2012; Shen et al. 2014). We therefore chose 21 week of BARD intervention to observe the preventive effects of this compound on HFD-induced colon inflammation. We used BARD at dose 10 mg/kg body weight based on a previous study in which BARD mixed in the diet has potent anti-inflammatory and anti-tumor effects in mice (Liby et al. 2009; Wu et al. 2014). Since our aim was to study obesity, we chose to supplement BARD into the drinking water so as to avoid BARD from potentially reducing the palatability of the HF diet, which may influence food intake. We have previously reported that oral BARD administration at a dose of 10 mg/kg can suppress body weight in HFD mice (Camer et al. 2015b; Dinh et al. 2015b). At week 21 of treatment, all mice were sacrificed for tissue collection. Whole colons were washed in phosphate-buffered saline and cut longitudinally. Part of the colon was kept frozen at -80°C for oil-red O staining of neutral fat while the remaining part of the colon was rolled and fixed in 4% paraformaldehyde. The fixed tissues were embedded in paraffin for histology and immunohistochemistry. The cecal content was collected and stored at -80°C for an analysis of microbial population. All procedures were approved by the Animal Ethics Committee of the University of Wollongong, NSW, Australia, and complied with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes. The approval ID for this study is AE12/15.
Histology and Immunohistochemistry
Paraffin-embedded colons were cut to 5-µm sections and stained with hematoxylin and eosin (POCD Scientific, Artarmon, NSW). Histological photographs of the stained sections were taken using a Leica microscope (Leica Microsystems, Wetzlar, Germany). ImageJ 1.46r software (http://imagej.nih.gov/ij/download.html) was used for measurements of the average crypt length and the number of goblet cells per crypt. Crypt lengths were measured using a ×10 objective and dimensions were calculated using a graticule (μm). A total of 42 individual crypts from 7 mice (6 crypts per mouse) in each group was used for measurement of colon crypt lengths, as described in previous studies (Steinbrecher et al. 2002; Li et al. 2007). The number of goblet cells per crypt was counted from 42 individual crypts in each group (n=6 crypts per mouse; n=7 mice per group) in accordance with a previous study (Taraban et al. 2011). Quantification was performed in a blind, randomized order.
To determine the effect of BARD on fat deposition in mouse colons, colons were stained with oil-red O. Briefly, frozen colon tissue was mounted in optimum cutting temperature medium, cut at 10 μm, and fixed in 10% formalin for 10 min. Colons were washed with distilled water and incubated in 100% propylene glycol for 2 min. The sections were then incubated with 0.5% oil-red O (Sigma-Aldrich Pty. Ltd, Sydney, NSW, Australia) at 60°C for 7 min. Following this, all sections were directly washed with 85% propylene glycol for 1 min, and washed twice with distilled water. The stained sections were then counterstained with hematoxylin (POCD Scientific, Artarmon, NSW), washed thoroughly with tap water, and cover-slipped with mounting medium for imaging. Six fields per sample (n=7) (×10) was used to measured oil-red O staining by ImageJ 1.46r software.
We used immunohistochemistry to determine the expression of different inflammatory and proliferative biomarkers in the colon crypts, which is in accordance with a top-down morphogenesis of colorectal tumorigenesis described in a previous report (Shih et al. 2001). The antibodies used in this study have been previously employed and are reliable biomarkers for detecting gut inflammation and carcinogenesis in humans and mice (Charalambous et al. 2009; Vitor et al. 2009; Steinhagen et al. 2012; Göranzon et al. 2013). The immunohistochemical procedure was based on our previous work (Dinh et al. 2015a). The primary antibodies used for immunohistochemical staining included: anti-F4/80 (ab6640) at 1:1000, anti-CD11c (ab33483) at 1:500 or anti-CD206 (ab64693) at 1:3000, anti-IL-10 (ab34843) at 1:400, anti-Ki67 (ab15580) at 1:500 from Abcam Inc (Cambridge, MA); NF-қBp65 (sc-109) at 1:100, phosphorylated NF-қBp65 (sc-33020) at 1:100, IL-6 (sc-7920) at 1:100, IL-1β (sc-7884) at 1:100, TNF-α (sc-8301) at 1:100 from Santa Cruz Biotechnology (Dallas, TX). Secondary antibodies were used at 1:250 dilution: rabbit anti-rat IgG biotinylated secondary antibody (ab6733), goat anti-Armenian hamster IgG H&L biotin (ab5744), and goat anti-rabbit IgG H&L biotin (ab6720), from Abcam Inc. Negative controls for all immunostaining were carried out by omitting the primary antibody. Within each group, six fields per sample (n=7) were used to quantify the morphological staining of all biomarkers. CD11c (×20), CD206 (×20), phosphorylated NF-kBp65 (×40), and Ki67 were quantified as the percent of positive cells, whereas F4/80, NF-kBp65, Cox2, IL-1β, IL-6, TNF-α, IL-10 (×20) were quantified as the percentage positive area per image.
Real-time PCR (qRT-PCR) was used to quantify microbial strains from cecal content. One hundred mg of cecal content from each mouse was used for genomic DNA extraction with Isolate DNA kit (Bioline, Sydney, Australia). DNA concentration was determined using Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA).
For Quantitative real-time PCR (qPCR), 20 ng DNA was used per PCR reaction (20 µl). PCR was performed using SensiFast™ SYBR No-ROX reagents (Bioline). Different primers were used to quantify dominant bacterial strains, including the Gram-positive Bifidobacteria, Lactobacilli, and the Gram-negative Bacteroides-Prevotella, which were purchased from Sigma-Aldrich (Sydney, Australia) (Table 1). Quantification was carried out using Lightcycler 480 real-time PCR system (F. Hoffmann-La Roche Ltd, Switzerland), with a two-step PCR protocol. The PCR conditions were 95°C for 3 min, followed by 40 cycles of 95°C for 5 sec, 60°C for 10 sec, and 72°C for 10 sec. A positive value was determined when a crossing point (CP) was smaller than 35 (Durant et al. 2009). Relative bacterial population was quantified as per the Livak method (2-ΔΔT) using universal primer values as a reference (Condezo-Hoyos et al. 2014).
Table 1.
Primers for qPCR.
| Microbiota | Primer Sequence (5’ to 3’) | References |
|---|---|---|
| All bacteria | Universal F: ACTCCTACGGGAGGCAGCAG Universal R: ATTACCGCGGCTGCTGG |
(Vincent et al. 2013) |
| Bacteroides-Prevotella spp | F: GAGAGGAAGGTCCCCCAC R: CGCTACTTGGCTGGTTCAG |
(Cani et al. 2008) |
| Lactobacillus spp. | F: GAGGCAGCAGTAGGGAATCTTC R:GGCCAGTTACTACCTCTATCCTTCTTC |
(Delroisse et al. 2008) |
| Bifidobacterium spp | F: CGCGTCTGGTGTGAAAG R: CCCCACATCCAGCATCCA |
(Delroisse et al. 2008) |
F: forward primer, R: reverse primer.
Statistical Analysis
Sample size in the present study was adopted from a previous study (Gu et al. 2013) and confirmed by a power calculation using JMP software (SAS Institute Inc., Cary, NC). We used SPSS 19 package (SPSS, Chicago, IL) to perform a one-way analysis of variance test, and a least significant difference post-hoc analysis for data analysis. We used the Pearson’s test for correlation among the histological and immunohistochemical staining parameters. All values are expressed as mean ± SEM. Statistical significance was considered at p<0.05.
Results
BARD Improves Morphology in the Colons of HFD-fed Mice
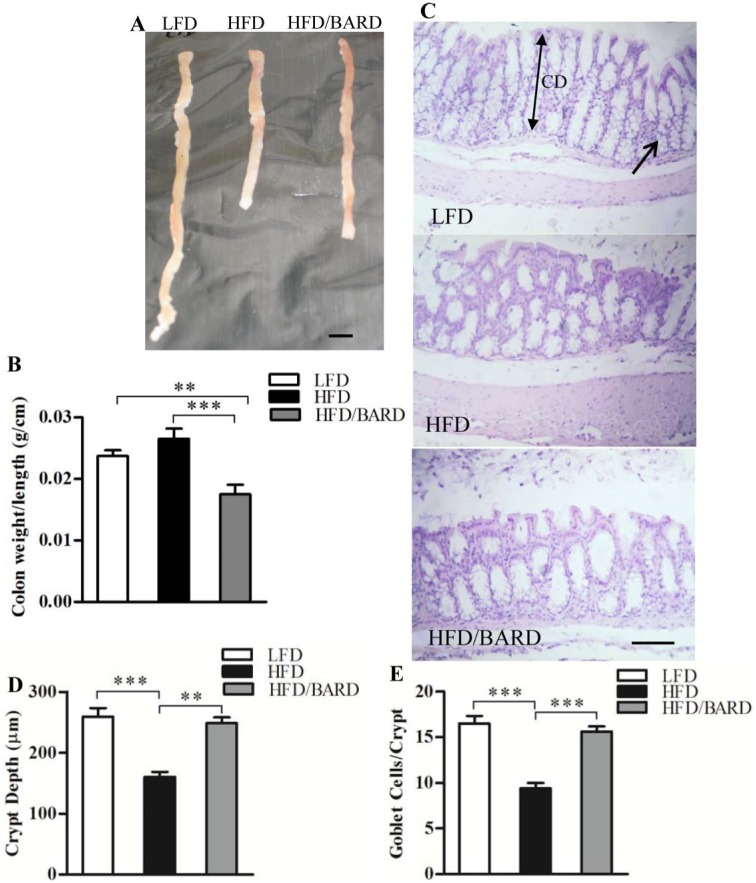
HFD mice had shorter colons, as compared with LFD and HFD mice administered BARD (Fig. 1A). HFD/BARD mice had significantly reduced colon weight per colon length (0.017 g/cm; Fig. 1B). This is 26% (p<0.01) lower than the LFD mice (0.024 g/cm) and 34% (p<0.001) lower than the HFD control mice (0.026 g/cm).
Figure 1.
Effect of bardoxolone methyl (BARD) on colon morphology in mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A) Mouse colons. (B) Colon weight per length (g/cm) (n=7). (C) Hematoxylin and eosin stained sections (×10). The arrow indicates goblet cells, whereas the double-headed arrow indicates the colon crypt depth (CD). (D) Crypt depth (µm). (E) Number of goblet cells per crypt. All data are presented as mean ± SEM. A total of 42 crypts from 7 mice in each group were used for the analysis of crypt depth and number of goblet cells per crypt. **p<0.01; ***p<0.001. Scale (A) 1 cm; (C) 100 µm.
We investigated the effect of BARD on colon crypt depth and on the number of goblet cells per crypt in HFD mice by histological staining (Fig. 1C). Colon crypt depth in HFD mice was 38% (p<0.001) shorter than that in LFD mice (Fig. 1D). Compared with the HFD control, HFD/BARD mice had longer colon crypts (+55%, p<0.001), similar to the LFD mice. Similarly, the HFD mice had half as many goblet cells per crypt in the colon as LFD mice and HFD/BARD mice (p<0.001) (Fig. 1E). These data demonstrate that BARD improves colon morphology in HFD mice.
BARD Suppresses Infiltration of Inflammatory Macrophages in the Colons of HFD-fed Mice
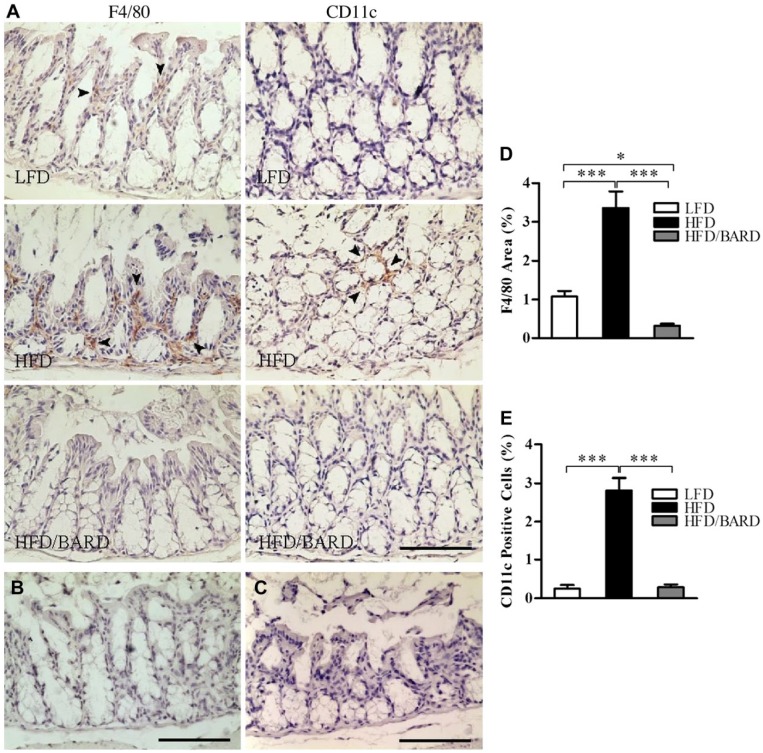
The effect of BARD on macrophage infiltration was determined through the expression of F4/80 (total) and CD11c (M1 or inflammatory macrophage phenotype) immunostaining in the colon crypts (Fig. 2A–2C). HFD mice had 3.4% F4/80-positive area compared with 1.1% in LFD mice (Fig. 2D). Compared with the HFD control, HFD/BARD mice had significant reduction in F4/80-positive area (p<0.001). Similarly, the HFD mice had an increased level of CD11c-positive cells (2.8%), whereas the number of CD11c cells was reduced in the colons of both HFD/BARD and FD mice (Fig. 2E).
Figure 2.
Effect of bardoxolone methyl (BARD) on the expression of F4/80 and CD11c macrophages in the colon crypts of mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A) F4/80- and CD11c-stained sections (×20). (B) Negative F4/80-stained section (×20). (C) Negative CD11c-stained section (×20). The arrowheads indicate F4/80 and CD11c staining (brown). (D) F4/80-positive area (%). (E) CD11c-positive cells (%). Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. *p<0.05, ***p<0.001. Scale, 100 µm.
BARD Suppresses NF-қB Activation and Pro-inflammatory Cytokines in the Colons of HFD-fed Mice
We investigated the effect of BARD on NF-қB activation by assessing protein expression of total NF-қBp65 and its phosphorylation (pNF-қBp65) in the colon crypts (Fig. 3A–3D). The NF-қBp65-positive area in the colon crypts of HFD mice was 14.8%, which was significantly higher than that in the LFD (6%) and HFD/BARD (4.6%) mice (Fig. 3E; p<0.001). HFD mice had more pNF-қBp65 positive cells (36%) in the colon crypts as compared with LFD and HFD/BARD mice, which had reduced proportion of pNF-қBp65-stained cells (Fig. 3F; p<0.001). This reduction of NF-қBp65 by BARD was correlated with a reduction in phosphorylated (p)NF-қBp65 levels (Fig. 3G). These results therefore demonstrate the anti-inflammatory effect of BARD through a suppression of NF-қB activity.
Figure 3.
Effect of bardoxolone methyl (BARD) on the expression of NF-қB in the colon crypts of mice fed a low-fat diet (LFD), a high-fat diet (HFD), or high-fat diet supplemented with BARD (HFD/BARD). (A) NF-қBp65-stained sections (×20). (B) Phosphorylated NF-қBp65-stained sections (×40). (C) Negative NF-қBp65-stained section (×20). (D) Negative phosphorylated NF-қBp65-stained section (×40). The arrowheads indicate NF-қBp65-positive areas and phosphorylated NF-қBp65 positive cells (brown). (E) NF-қBp65-positive area (%). (F) Phosphorylated NF-қBp65-positive cells (%). (G) Correlation between NF-қBp65 and phosphorylated NF-Bp65. Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM ***p<0.001. NF-қBp65: p65 nuclear factor-κ light-chain enhancer of activated B cells; pNF-қBp65: phosphorylated NF-қBp65. Scale (A, C) 100 µm; (B, D) 50 µm.
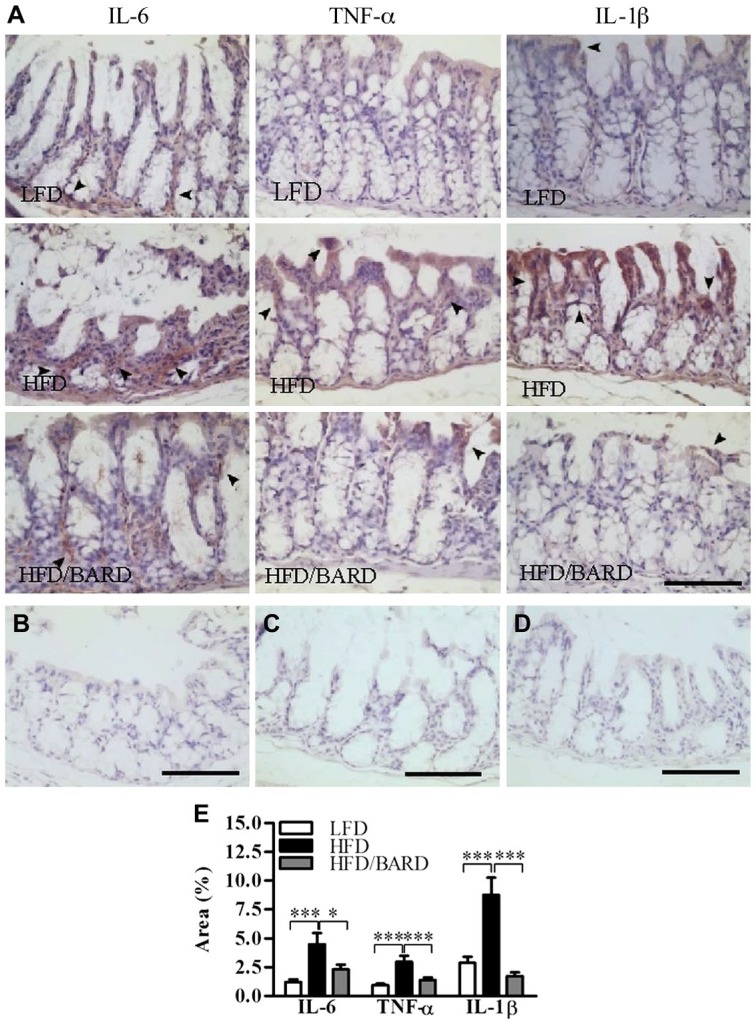
We further investigated the effect of BARD on the expression of pro-inflammatory cytokines within the colon crypts (Fig. 4A–4D). In LFD mice, percent of positive areas of pro-inflammatory cytokines were 1.2%, 0.9% and 2.9% for IL-6, TNF-α, IL-1β, respectively (Fig. 4E). In contrast, HFD had higher percentage of positive areas of IL-6 (4.5 %), TNF-α (3%), and IL-1β (8.8%). HFD mice administered BARD had a reduced percent of pro-inflammatory cytokine-positive areas, by 2.3% for IL-6, 1.4% for TNF-α, and 1.8% for IL-1β. The reduction in pro-inflammatory cytokine-positive staining in response to BARD was also correlated with decreased levels of total F4/80 and CD11c macrophages (Table 2).
Figure 4.
Effect of bardoxolone methyl (BARD) on the expression of pro-inflammatory cytokines in colon crypts of mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A) IL-6-, TNF-α-, and IL-1β-stained sections (×20). (B) Negative IL-6-stained section (×20). (C) Negative TNF-α-stained section (×20). (D) Negative IL-1β-stained section (×20). The arrowheads indicate IL-6-, TNF-α-, and IL-1β-positive areas (brown). (E) IL-6-, TNF-α-, and IL-1β-positive area (%). Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. *p<0.05, ***p<0.001. IL-6: interleukin 6; IL-1β: interleukin 1 beta; TNF-α: tumor necrosis factor-alpha. Scale, 100 µm.
Table 2.
Correlation between Macrophages and Cytokines.
TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-1β: interleukin 1 beta; F4/80: marker of total macrophage; CD11c: marker of M1 macrophage phenotype; p: significance level, *p<0.05, **p<0.01, ***p<0.001.
BARD Suppresses Cox2 Protein Expression in the Colons of HFD-fed Mice
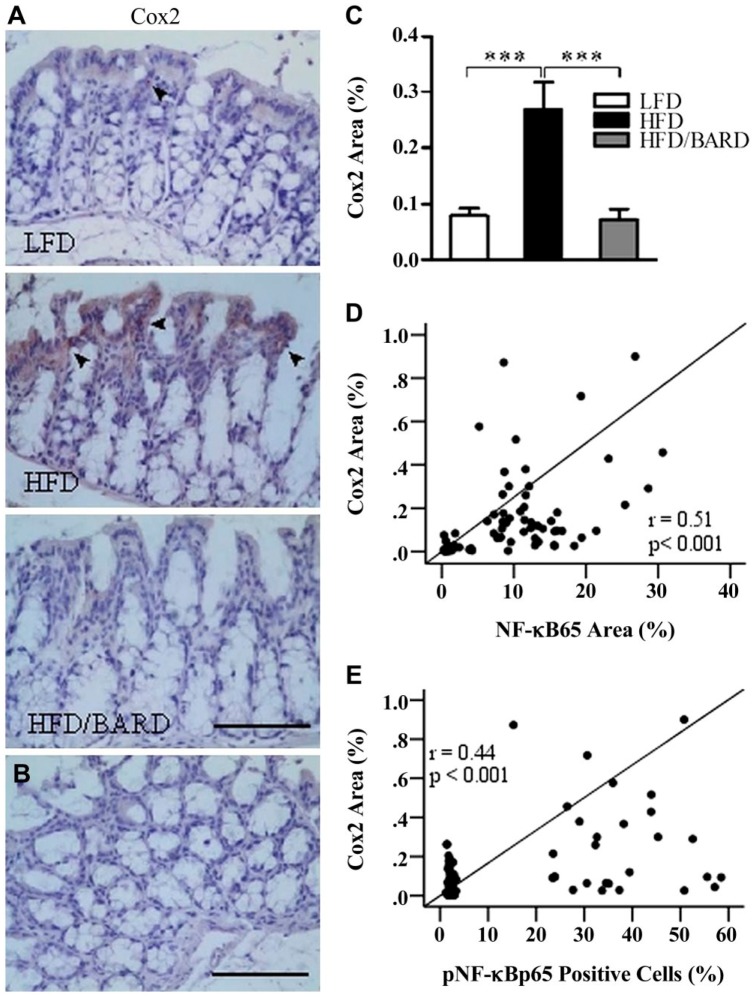
We investigated the effect of BARD on the expression of Cox2 protein in the colon crypts, which is an inflammatory and proposed cancer marker in gut epithelial cells (Fig. 5A and 5B). Compared with mice fed a LFD, those fed a HFD were positive for Cox2 immunostaining (0.3% positive area; Fig. 5C). Similar to LFD mice, HFD/BARD mice were almost negative for Cox2 staining. The reduced level of Cox2 protein in HFD/BARD mice also correlated with the reduced levels of total and phosphorylated NF-қBp65 (Fig. 5D and 5E, respectively).
Figure 5.
Effect of bardoxolone methyl (BARD) on the expression of Cox2 in the colon crypts of mice fed a low-fat diet (LFD), a high-fat diet (HFD), and a high-fat diet supplemented with BARD (HFD/BARD). (A) Cox2-stained sections (×20). (B) Negative Cox2-stained section (×20). The arrowheads indicate Cox2-positively stained areas (brown). (C) Cox2-positive area (%). (D) Correlation between Cox2 and NF-қBp65. (E) Correlation between Cox2 and pNF-қBp65. Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. ***p<0.001. Cox2: cyclooxygenase 2; NF-қBp65: p65 nuclear factor-κ light-chain enhancer of activated B cells; pNF-қBp65: phosphorylated NF-қBp65. Scale, 100 µm.
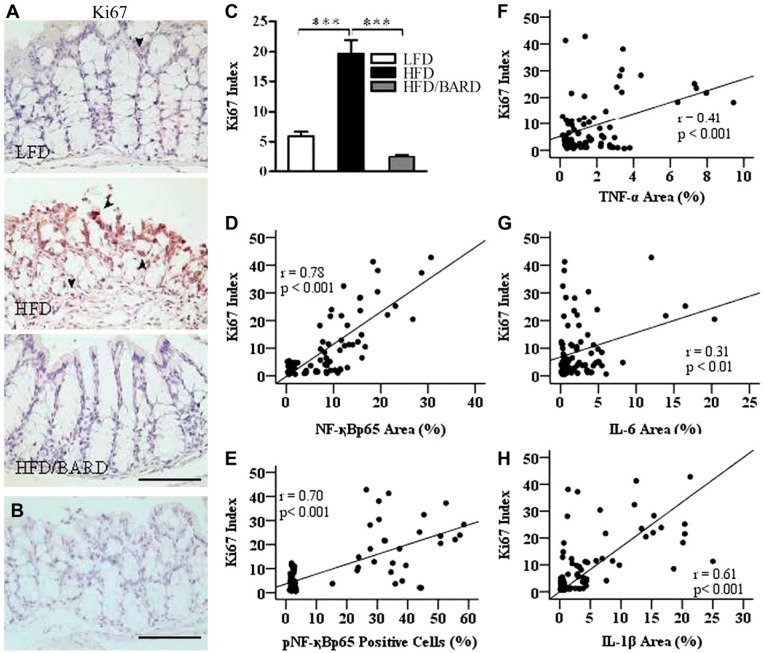
BARD Suppresses the Ki67 Index in the Colons of HFD-fed Mice
Figure 6A shows a significant change in the expression of Ki67 immunoactivity in the colon epithelial cells among the three groups of mice (Fig. 6A and 6B). In Fig. 6C, colons of HFD mice had 19.6% Ki67-positive cells as compared with 5.9% for LFD and 2.5% in HFD/BARD mice (p<0.001). The percent of Ki67-positive cells in HFD/BARD mice was comparable with that of LFD mice, suggesting that BARD has an anti-proliferative effect. There was a moderate correlation between Ki67 expression and the levels of total and phosphorylated NF-қB (Fig. 6D and 6E, respectively) and pro-inflammatory cytokines TNF-α, IL-6, and IL-1β (Fig. 6F, 6G and 6H, respectively).
Figure 6.
Effect of bardoxolone methyl (BARD) on the expression of Ki67 in colon epithelial cells in mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A) Ki67-stained sections (×20). (B) Negative Ki67-stained section (×20). The arrowheads indicate brown nuclear staining for Ki67. (C) Ki67-positive cells (%). (D) Correlation between Ki67 and total NF-қBp65. (E) Correlation between Ki67 and pNF-қBp65. (F) Correlation between Ki67 and TNF-α. (G) Correlation between Ki67 and IL-6. (H) Correlation between Ki67 and IL-1β. Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. ***p<0.001. NF-қBp65: p65 nuclear factor-κ light-chain enhancer of activated B cells; pNF-қBp65: phosphorylated NF-қBp65; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-1β: interleukin 1 beta. Scale, 100 µm.
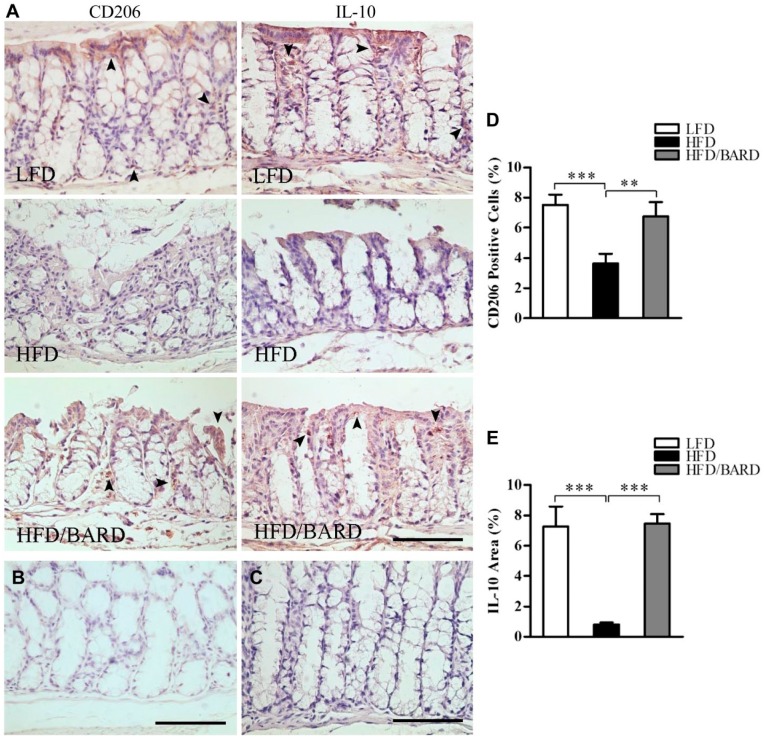
BARD Increases M2 Macrophage and Anti-inflammatory Cytokines in the Colons of HFD-fed Mice
We further investigated the effects of BARD on the expression of anti-inflammatory macrophages (M2 macrophage phenotype) and anti-inflammatory cytokines by assessing the level of epithelial CD206-positive cells and the IL-10-positive area in colon crypts, respectively (Fig. 7A–7C). The percentage of CD206-positive cells in HFD mice were 3.6%, which is half that of the LFD mice (7.5%) (p<0.001) (Fig. 7D). However, the percentage of CD206-positive cells was higher in HFD/BARD mice (6.7%) as compared with HFD mice (p<0.01). In Fig. 7E, the percentage of IL-10-positive area in HFD mice was 0.8%, which is lower than that in LFD mice (7.3%) and in HFD/BARD (7.5%) (p<0.001). The increase in M2 macrophages and IL-10 proteins in the colon crypts suggests a potent anti-inflammatory effect of BARD in HFD-fed mice.
Figure 7.
Effect of bardoxolone methyl (BARD) on the expression of M2 macrophages and anti-inflammatory cytokine IL-10 in colon crypts mice fed a low-fat diet (LFD), a high-fat diet (HFD), and a high-fat diet supplemented with BARD (HFD/BARD). (A) CD206- and IL-10-stained sections (×20). (B) Negative CD206-stained section (×20). (C) Negative IL-10-stained section (×20). The arrowheads indicate CD206-positive cells and IL-10-positive areas (brown). (D) CD206-positive cells (%). (E) IL-10-positive area (%). Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. **p<0.01, ***p<0.001. IL-10: interleukin 10. Scale, 100 µm.
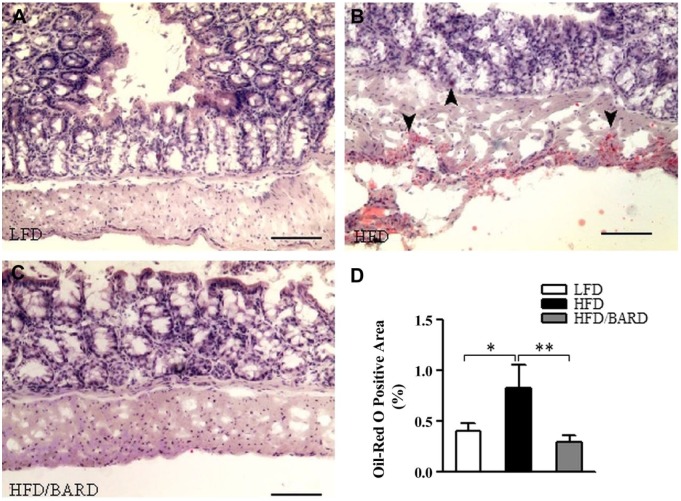
BARD Prevents Fat Deposition in the Colon of HFD-fed Mice
Figure 8A–8C shows oil-red O staining of fat in the colons of HFD-fed mice. HFD mice had 0.8% oil-red O-positive areas in the colon wall and crypts, which is twice as high as that of LFD mice (p<0.05; Fig. 8D). Compared with HFD mice, HFD/BARD mice had reduced oil-red O-positive staining (p<0.01), similar to LFD mice. Table 3 shows that BARD reduced the amount of fat deposition in the colons of HFD-fed mice, which correlated with the reduction of F4/80 macrophages (p=0.0001), pNF-қBp65 (p=0.0165), TNF-α (p=0.0337). Reduced fat deposition by BARD also correlated with the increase in the anti-inflammatory marker IL-10 (p=0.0226). These data suggest that HFD-induced fat deposition may contribute to inflammatory progression in the mouse colon and that BARD can prevent such effects.
Figure 8.
Effect of bardoxolone methyl (BARD) on fat deposition in the colon mucosa and wall of mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A–C) Oil-red O-stained sections (×10). (D) Oil-red O-positive areas (%). Six fields per sample (n=7) were used for statistical analysis. All data are presented as mean ± SEM. *p<0.05, **p<0.01. Scale, 100 µm.
Table 3.
Correlation between Adiposity and Inflammatory Modulators.
| Pearson Correlation |
||
|---|---|---|
| Measures | r | p |
| F4/80 | 0.414 | 0.0001 |
| CD11c | 0.148 | 0.1992 |
| CD206 | −0.178 | 0.1142 |
| NF-қB | 0.093 | 0.4252 |
| pNF-қB | 0.262 | 0.0165 |
| TNF-α | 0.238 | 0.0337 |
| IL-1β | 0.176 | 0.1128 |
| IL-6 | −0.033 | 0.7710 |
| Cox2 | −0.094 | 0.4023 |
| Ki67 | 0.194 | 0.0852 |
| IL-10 | −0.256 | 0.0226 |
F4/80: marker of total macrophage; CD11c: marker of M1 macrophage phenotype; CD206: marker of M2 macrophage phenotype; NF-қB: nuclear factor-κ light-chain enhancer of activated B cells; pNF-қB: phosphorylated NF-қB; TNF-α: tumor necrosis factor alpha; IL-6: interleukin 6; IL-1β: interleukin 1 beta; IL-10: interleukin 10; Ki67: nuclear antigen Ki67; Cox2: cyclooxygenase 2; r: Pearson correlation coefficient; p: significance level, p<0.05.
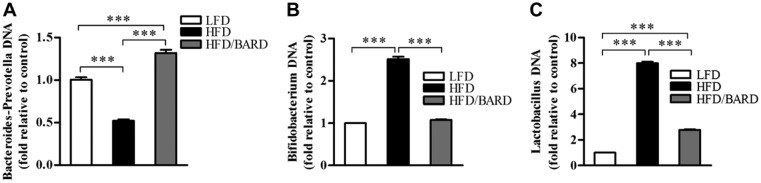
BARD Prevents the Alteration of Gut Microbiota in HFD Mice
To investigate the effect of BARD on gut microbiota, relative DNA abundance of Gram-positive bacteria Bifidobacterium sp., Lactobacillus spp., and Gram-negative bacteria Bacteroides-Prevotella spp. was determined by real-time PCR. The relative DNA abundance of Bacteroides-Prevotella spp. was significantly reduced in HFD-fed mice, which was half that of LFD mice (Fig. 9A; p<0.001). BARD significantly increased the relative DNA abundance of Bacteroides-Prevotella spp., which was 3-fold higher than that in HFD mice (p<0.001). The relative abundance of Bidifobacterium spp. in HFD/BARD mice was similar to the DNA abundance in LFD mice (Fig. 9B; p<0.001), and significantly upregulated compared to mice on HFD (p<0.001). The HFD also increased the relative DNA abundance of Lactobacillus spp., which is 8-fold higher than that in LFD mice (Fig. 9C; p<0.001). HFD/BARD mice had reduced DNA level of Lactobacillus spp. compared with HFD mice (p<0.001). In Table 4, the reduction in the populations of Bidifobacterium spp. and Lactobacillus spp. in response to BARD supplementation correlated with the decrease in inflammatory biomarkers (NF-қBp65, pNF-қBp65, TNF-α, IL-1β, IL-6), Cox2, and Ki67 and the increase in the anti-inflammatory cytokine IL-10. Conversely, BARD induced an increase in the Gram negative Bacteroides-Prevotella spp. population, and this correlated with the increase in IL-10 and the decrease in inflammatory biomarkers. These data therefore demonstrate that HFD disturbs the gut microbiota population, which can be prevented by BARD.
Figure 9.
Effect of bardoxolone methyl (BARD) on relative DNA abundance of fecal bacteria in mice fed a low-fat diet (LFD), a high-fat diet (HFD), or a high-fat diet supplemented with BARD (HFD/BARD). (A) Relative DNA abundance of Bacteroides-Prevotella spp. (B) Relative DNA abundance of Bifidobacterium spp. (C) Relative DNA abundance of Lactobacillus spp. All data are presented as mean ± SEM (n=7). ***p<0.001.
Table 4.
Correlation between Fecal Microbiota and Inflammatory Biomarkers in Mouse Colons.
| Measures | Bifidobacterium spp. | Bacteroides-Prevotella spp. | Lactobacillus spp. |
|---|---|---|---|
| NF-қB | 0.61** | − 0.77*** | 0.53* |
| pNF-қB | 0.96*** | − 0.86*** | 0.93*** |
| TNF-α | 0.65** | − 0.55* | 0.59** |
| IL-1β | 0.61** | − 0.66** | 0.54* |
| IL-6 | 0.52* | − 0.35* | 0.52* |
| Cox2 | 0.54* | − 0.49** | 0.53* |
| Ki67 | 0.80*** | − 0.88*** | 0.73*** |
| IL-10 | − 0.78*** | 0.72*** | − 0.79*** |
NF-қB: nuclear factor-κ light-chain enhancer of activated B cells; pNF-қB: phosphorylated NF-қB; TNF-α: tumor necrosis factor-alpha; IL-6: interleukin 6; IL-1β: interleukin 1 beta; IL-10: interleukin 10; Ki67: nuclear antigen Ki67; Cox2: cyclooxygenase 2; p: significance level, *p<0.05, **p<0.01, ***p<0.001.
Discussion
BARD inhibits colon cancer cell growth by inducing apoptosis, which is associated with NF-қB suppression (Gao et al. 2010). Furthermore, a mouse study has suggested that BARD can treat colitis-associated colon cancer as it reduces colon tumors and inflammation-driven colon carcinogenesis (TNF-α, IL-6, IL-1β, Cox2) (Choi et al. 2014). The present study investigated the anti-inflammation and anti-cell proliferation effects of BARD in the colons of mice fed a HFD. Fat deposition and microbial population changes were also determined along with these changes in the mouse colon.
In the present study, chronic HFD (for 21 weeks) feeding in mice led to a shortened colon, associated with a reduced length of colon crypts and a reduced number of goblet cells per crypt, which could be reversed by BARD. Consistent with our result, mice administered HFD for 35 weeks have shortened colon and crypt lengths, which is associated with the upregulation of inflammation and cancer-associated genes (Deol et al. 2015). However, mice fed a HFD for 10 and 15 weeks have been reported to have elongated colon crypts as compared with the LFD group (Bird and Stamp 1986). Also, mice on an acute HFD for 30 days have normal colon crypt length, similar to that of control mice (Basson et al. 2004). It appears that a HFD causes changes in mouse colon crypt length depending on the duration of exposure to a HFD. Moreover, the loss of goblet cells in the colon has been implicated in tissue injury in a colitic mouse model (Xiao et al. 2007). HFD induces goblet cell elimination, crypt destruction, and the infiltration of inflammatory cells in the colons of chemical-induced colitic rats (Morampudi et al. 2014). In the present study, the normalisation of colon crypts by BARD indicates its effectiveness in the prevention of HFD-induced changes in colon morphology and inflammation. The effects of BARD on colon crypts in our study are consistent with a previous study showing that BARD increases colon length and reduces colon thickness in colitis-associated mice (Choi et al. 2014).
Macrophage infiltration is a common characteristic of inflammation in the colons of diet-induced obese mice (Erdelyi et al. 2009; Lam et al. 2012; Liu et al. 2012). We found an increased level of F4/80 and CD11c immunohistochemical staining in the colons of HFD-fed mice, which could be suppressed by BARD. These findings in the colon coincide with observations in white and brown adipose tissue of mice fed a HFD or a HFD plus BARD (Dinh et al. 2015a; Dinh et al. 2015b). The modulating effects of BARD on infiltrating macrophages are consistent with the immunomodulation function of most triterpenes (Honda et al. 1997; Gao et al. 2008; Ikeda et al. 2008; Kulkarni et al. 2013). Colitic mice fed a HFD develop severe inflammation in the colon accompanied by an exaggerated level of infiltrating macrophages (Teixeira et al. 2011). F4/80 and CD11c macrophages are found in the colon, and an increased level of these cells is often seen in inflammatory colons and in carcinogenesis (Takada et al. 2010; Rivollier et al. 2012; Hume and Gordon In Press). These results suggest a potential role of BARD in preventing tumorigenesis and cancer.
In inflamed intestinal mucosa, activated NF-қB is highly expressed in macrophages and epithelial cells (Rogler et al. 1998). As well as reducing macrophage invasion, BARD also reduces total and phosphorylated NF-қB proteins. NF-қB is an important target of anti-cancer drugs, and an increasing number of studies have demonstrated BARD’s effect in reducing NF-қB activation (Pergola et al. 2011). Similar effects have been observed with ursolic acid triterpene, which inhibits the NF-қB activation in human intestinal epithelial cells and peritoneal macrophages in IL-10-deficient mice (Chun et al. 2014). This is consistent with known inhibitory effects of most pentacyclic triterpenes (Takada and Aggarwal 2003; Liby and Sporn 2012; Hwang et al. 2014). From this data, we have shown that NF-қB plays an essential role in HFD-induced colon inflammation and we suggest that BARD is an effective chemo-preventive agent.
The increased activation of NF-қB in the colon of HFD-fed mice was associated with an elevated level of pro-inflammatory cytokines (Kim et al. 2012a). Studies have shown that BARD induces anti-inflammatory effects via modulating the pro-inflammatory response (Auletta et al. 2010), as found in the present study. A high level of TNF-α enhances the development of inflammatory bowel disease and colon cancer in mice and rats (Sands and Kaplan 2007; Jain and Bird 2010; Flores et al. 2012). The inhibition of this cytokine is important for preventing intestinal inflammation (Kojouharoff et al. 1997; Popivanova et al. 2008). TNF-α, IL-6, and IL-1β have been identified as a cluster of inflammatory markers that increase in colon cancer (Ong et al. 2010; Klampfer 2011). The suppressive effect of BARD on these pro-inflammatory cytokines is consistent with most pentacyclic triterpenoids; for instance, supplementation with ursanic, oleanic, and lupanic triterpenoids in T84 colon carcinoma cells strongly suppresses the inflammatory genes associated with inflammatory bowel diseases (Mueller et al. 2013). A reduction in the level of pro-inflammatory cytokines is correlated with a reduction in the number of infiltrating macrophages. This indicates that BARD’s suppression of pro-inflammatory cytokines may occur partly through modulation of immune cells. This is consistent with betulinic acid triterpene, which reduces TNF-α expression in mice (Oliveira Costa et al. 2014).
Inflammatory cells have an elevated level of Cox2 (Masferrer et al. 1994). We observed a reduction in Cox2 protein expression in HFD mice also given BARD. In one study on an LPS-exposed macrophage cell line, oleanolic acid and ursolic acid derivatives were shown to suppress Cox2 protein and mRNA expression (Suh et al. 1998). Similarly, a synthetic oleanolic acid analog, 18alpha-olean-12-ene-3beta-23,28-triol, was previously shown to reduce Cox2 expression in human colon HT-29 cancer cells (Janakiram et al. 2008). A BARD analog, RTA dh404, reduced renal Cox2 expression, and reduced phosphorylated NF-қBp65 in rats with chronic kidney disease (Aminzadeh et al. 2014). Similar to RTA dh404, we found that a reduction in Cox2 protein is correlated with a reduction in total and phosphorylated NF-қBp65. Previously, BARD has been found to suppress NF-қB leading to a reduction in Cox2 in human leukemia cell lines (Shishodia et al. 2006). Another study has shown that the combined treatment of triterpenoid α and β-amyrin reduces the immunohistochemical staining level of Cox2 and phosphorylated NF-қBp65 in the colons of colitic mice (Vitor et al. 2009). Similar effects have been described for other triterpenes including maslinic acid and betulinic acid (Takada and Aggarwal 2003; Hsum et al. 2011). Cox2 inhibitors (such as celecoxib) have been used for the primary prevention of colon tumors and suppression of adipose inflammation in HFD-induced obese rats (Sheng et al. 1997; Half and Arber 2009; Hsieh et al. 2010). These results thus demonstrate the anti-inflammatory effect of BARD on Cox2 suppression and NF-қB regulation.
A HFD increases Ki67 in various tissues, including the prostate gland and the small intestine in mice (Petit et al. 2007; De Wit et al. 2008; Benesh et al. 2013). We found that a HFD increases Ki67 (a cell proliferation marker) in the epithelial cells of colon crypts in mice, and that BARD can prevent this HFD-induced increase in Ki67. A similar effect has been observed in azoxymethane-induced colon cancer mice, as shown by a reduced number of tumors and Ki67 expression (Choi et al. 2014). Increased Ki67 expression has been consistently observed in colon tumors in humans and mice (Evans et al. 2006; Vinuesa et al. 2012). Epithelial Ki67 as a biomarker has been used to investigate some cancers and colorectal polyps (Deckert et al. 1989; Aubert et al. 2002; Aslan et al. 2005; Nabi et al. 2008). The upregulation of Ki67-positive proliferating cells reflects a failure of epithelial cells to repress DNA synthesis during their migration to the crypt surface, which leads to adenoma formation and tumorigenesis (Deschner and Lipkin 1975; Shih et al. 2001). A cell culture study on human colon crypts has shown that Ki67 in normal tissue is expressed in the basal crypt, which is undetectable after 3–5 days of the crypt cycle; however, Ki67-positive cells with budding structures are continuously and extensively expressed in adenomatous crypts (Dame et al. 2014). The increased Ki67 index in the shortened crypts in the present study likely reflects the HFD-associated promotion of an adenomatous environment in the mouse colon. These results are consistent with a previous study showing the protective role of BARD in colonic epithelial cells (Kim et al. 2012b). Overall, increased Ki67, NF-қB, and pro-inflammatory cytokines in the present study suggest a risk of tumorigenesis in HFD-induced obesity. Furthermore, the reduced Ki67 index and the lower level of inflammatory modulators by BARD, which is associated with an improvement in the colon crypts to resemble that of normal, LFD controls, suggests anti-proliferative effects and regulation of colon epithelial homeostasis, inflammation and tumorigenesis by BARD.
M2 macrophages in colon lamina propria are known to release the anti-inflammatory cytokine IL-10 in response to gut stimuli such as microbiota (Rivollier et al. 2012). CD206 is a biomarker of M2. This study shows that a HFD reduces CD206 and IL-10 expression, which can be reversed by BARD. It is reported that a high level of IL-10 and CD206 causes resistance to inflammatory bowel disease (Bain et al. 2013). M2 macrophages reduce colitis through IL-10 expression in mice (Zhu et al. 2014). Several studies have demonstrated the anti-inflammatory effects of pentacyclic triterpenes through the induction of IL-10 (Chen et al. 2012; Martín et al. 2014). Ursolic acid, α- and β-amyrin increase the expression of IL-10 protein in mouse colons (Vitor et al. 2009; Jang et al. 2014). Our data are also consistent with the known anti-cancer effects of BARD (Gao et al. 2010; Hong et al. 2012; Choi et al. 2014).
We investigated the effect of oral BARD on lipid deposition in the colons of HFD-fed mice. HFD-induced fat accumulation in the colon wall and mucosa were reduced by BARD. We have previously observed the reducing effects of oral BARD on liver, white and brown adipose tissue in HFD-fed mice (Camer et al. 2015a; Dinh et al. 2015a; Dinh et al. 2015b; Dinh et al. 2015c). An increased lipid accumulation has been observed in the intestinal mucosal of HFD-induced and genetically-induced obese mice, as assessed by increased oil-red O staining of neutral fat and excess triacylglycerol (Douglass et al. 2012). The present data therefore suggests the potential of BARD in preventing HFD-induced fat deposition in the intestine.
A previous study has implicated an overflow of dietary fat to the distal intestine as a causative factor for microbiota alterations in the gut, contributing to obesity and metabolic syndrome (de Wit et al. 2012). We found that BARD prevents the alteration of microbiota in HFD mice, with an increase in Bacteroides-Prevotella spp. and a reduction in Bidifobacterium spp. and Lactobacillus spp. The presence of Bacteroides-Prevotella spp. protects humans from inflammation, and induces obesity resistance (De Filippo et al. 2010). A calorie-restricted diet prevented the growth of both Bidifobacterium spp. and Lactobacillus spp., species whose presence tends to that accompany excessive weight loss in overweight adolescents (Santacruz et al. 2009). BARD has been identified as a suppressor of body weight, with a significant weight loss effect in obese people and in patients with chronic kidney disease (Manenti et al. 2012). Additionally, we have previously reported the preventive effect of BARD in HFD-induced body weight in mice (Dinh et al. 2015a; Dinh et al. 2015b). Therefore, the effect of BARD treatment in preventing an alteration to gut microbiota induced by a HFD in obese mice may contribute to BARD’s ability to prevent HFD diet-induced obesity.
In conclusion, this study shows that mice on a chronic HFD have colon inflammation, as assessed by the upregulation of inflammatory biomarkers in the colon epithelium. This was shown by an increase in M1 macrophage invasion and an elevated level of NF-қB, pro-inflammatory cytokines, Cox2 and Ki67. Suppressing these inflammatory markers is important for the prevention of colon inflammation. BARD decreased the expression of pro-inflammatory cytokines, Cox2 and Ki67 while increasing the expression of the anti-inflammatory marker IL-10. This may relate to the anti-cancer effects observed in an existing study (Choi et al. 2014). Moreover, BARD prevents fat deposition in the colon wall of HFD mice, and this is accompanied by a normalisation of colon microbiota. The outcome of the present study suggests a need for further investigations using BARD to prevent inflammatory disease and obesity-associated colon inflammation and colon cancer. This study suggests a future application of BARD in the prevention of obesity-associated colon disorder in humans. However, the use of BARD should be prohibited in patients with advanced chronic kidney disease and those susceptible to cardiovascular disease to avoid possible heart failure-related side effects, as observed in previous clinical trials (Chin et al. 2014). Further studies on the molecular mechanisms of chronic kidney diseases and cardiovascular disease in animal models will also help to understand the potential side effects of BARD, which may assist in the future applications of BARD in human patients.
Acknowledgments
We would like to thank Ms Linda Cohen for her editorial revision of this manuscript.
Footnotes
Author Contributions: CD carried out the experiments, data analysis and statistical analysis and wrote the manuscript. YY, AS and XH were responsible for the experiment design and helped interpret the results. QZ has contributed to the discussion section and helped interpret the results. PZ contributed to microbial quantification. All authors have contributed to and approved the final manuscript.
Competing Interests: The authors declared no potential competing interests with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- Aminzadeh MA, Reisman SA, Vaziri ND, Khazaeli M, Yuan J, Meyer CJ. (2014). The synthetic triterpenoid RTA dh404 (CDDO-dhTFEA) restores Nrf2 activity and attenuates oxidative stress, inflammation, and fibrosis in rats with chronic kidney disease. Xenobiotica 44:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan DL, Gulbahce HE, Pambuccian SE, Manivel JC, Jessurun J. (2005). Ki-67 immunoreactivity in the differential diagnosis of pulmonary neuroendocrine neoplasms in specimens with extensive crush artifact. Am J Clin Pathol 123:874-878. [DOI] [PubMed] [Google Scholar]
- Aubert S, Wacrenier A, Leroy X, Devos P, Carnaille B, Proye C, Wemeau JL, Lecomte-Houcke M, Leteurtre E. (2002). Weiss system revisited: a clinicopathologic and immunohistochemical study of 49 adrenocortical tumors. Am J Surg Pathol 26:1612-1619. [DOI] [PubMed] [Google Scholar]
- Auletta JJ, Alabran JL, Kim BG, Meyer CJ, Letterio JJ. (2010). The synthetic triterpenoid, CDDO-Me, modulates the proinflammatory response to in Vivo lipopolysaccharide challenge. J Interferon Cytokine Res 30:497-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axling U, Olsson C, Xu J, Fernandez C, Larsson S, Ström K, Ahrné S, Holm C, Molin G, Berger K. (2012). Green tea powder and Lactobacillus plantarum affect gut microbiota, lipid metabolism and inflammation in high-fat fed C57BL/6J mice. Nutr Metabolism 9:1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bain CC, Scott CL, Uronen-Hansson H, Gudjonsson S, Jansson O, Grip O, Guilliams M, Malissen B, Agace WW, Mowat AM. (2013). Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol 6:498-510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltgalvis KA, Berger FG, Peña MMO, Davis JM, Carson JA. (2009). The interaction of a high-fat diet and regular moderate intensity exercise on intestinal polyp development in ApcMin/+ mice. Cancer Prev Res 2:641-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardou M, Barkun AN, Martel M. (2013). Obesity and colorectal cancer. Gut 62:933-947. [DOI] [PubMed] [Google Scholar]
- Bassett JK, Severi G, English DR, Baglietto L, Krishnan K, Hopper JL, Giles GG. (2010). Body size, weight change, and risk of colon cancer. Cancer Epidemiol Biomarkers Prev 19:2978-2986. [DOI] [PubMed] [Google Scholar]
- Basson MD, Yu CF, Gomez R, Bashir O, Goodlad RA. (2004). Effects of fiber and fat on murine proximal colonic mucosal proliferation and crypt depth. Nutr Res 24:901-908. [Google Scholar]
- Benesh EC, Humphrey PA, Wang Q, Moley KH. (2013). Maternal high-fat diet induces hyperproliferation and alters Pten/Akt signaling in prostates of offspring. Sci Rep 3:3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird RP, Stamp D. (1986). Effect of a high fat diet on the proliferative indices of murine colonic epithelium. Cancer Lett 31:61-67. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Dinh CH, Wang H, Cheng L, Huang XF. (2015a). Bardoxolone methyl prevents insulin resistance and the development of hepatic steatosis in mice fed a high-fat diet. Mol Cell Endocrinol 412:36-43. [DOI] [PubMed] [Google Scholar]
- Camer D, Yu Y, Szabo A, Fernandez F, Dinh CHL, Huang X-F. (2015b). Bardoxolone methyl prevents high-fat diet-induced alterations in prefrontal cortex signalling molecules involved in recognition memory. Progress in Neuro-Psychopharmacology and Biological Psychiatry 59:68-75. [DOI] [PubMed] [Google Scholar]
- Cani P, Bibiloni R, Knauf C, Waget A, Neyrinck A, Delzenne N, Burcelin R. (2008). Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57:1470-1481. [DOI] [PubMed] [Google Scholar]
- Charalambous MP, Lightfoot T, Speirs V, Horgan K, Gooderham NJ. (2009). Expression of COX-2, NF-κB-p65, NF-κB-p50 and IKKα in malignant and adjacent normal human colorectal tissue. Br J Cancer 101:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Wan Y, Zhou T, Li J, Wei Y. (2012). Ursolic acid attenuates lipopolysaccharide-induced acute lung injury in a mouse model. Immunotherapy 5:39-47. [DOI] [PubMed] [Google Scholar]
- Chin M, Lee C-YI, Chuang J-C, Bumeister R, Wigley WC, Sonis ST, Ward KW, Meyer C. (2013). Bardoxolone methyl analogs RTA 405 and dh404 are well tolerated and exhibit efficacy in rodent models of type 2 diabetes and obesity. Am J Physiol Renal Physiol 304:1438-1446. [DOI] [PubMed] [Google Scholar]
- Chin MP, Wrolstad D, Bakris GL, Chertow GM, De Zeeuw D, Goldsberry A, Linde PG, Mccullough PA, Mcmurray JJ, Wittes J, Meyer CJ. (2014). Risk factors for heart failure in patients with type 2 diabetes mellitus and stage 4 chronic kidney disease treated with bardoxolone methyl. J Card Fail 20:953-958. [DOI] [PubMed] [Google Scholar]
- Choi SH, Kim B-G, Robinson J, Fink S, Yan M, Sporn MB, Markowitz SD, Letterio JJ. (2014). Synthetic triterpenoid induces 15-PGDH expression and suppresses inflammation-driven colon carcinogenesis. J Clin Invest 124:2472-2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun J, Lee C, Hwang SW, Im JP, Kim JS. (2014). Ursolic acid inhibits nuclear factor-κB signaling in intestinal epithelial cells and macrophages, and attenuates experimental colitis in mice. Life Sci 110:23-34. [DOI] [PubMed] [Google Scholar]
- Condezo-Hoyos L, Mohanty IP, Noratto GD. (2014). Assessing non-digestible compounds in apple cultivars and their potential as modulators of obese faecal microbiota in vitro. Food Chem 161:208-215. [DOI] [PubMed] [Google Scholar]
- Dame MK, Jiang Y, Appelman HD, Copley KD, Mcclintock SD, Aslam MN, Attili D, Elmunzer BJ, Brenner DE, Varani J, Turgeon DK. (2014). Human colonic crypts in culture: segregation of immunochemical markers in normal versus adenoma-derived. Lab Invest 94:222-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day SD, Enos RT, Mcclellan JL, Steiner JL, Velázquez KT, Murphy EA. (2013). Linking inflammation to tumorigenesis in a mouse model of high-fat-diet-enhanced colon cancer. Cytokine 64:454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691-14696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit N, Bosch-Vermeulen H, De Groot P, Hooiveld G, Bromhaar M, Jansen J, Müller M, Van Der Meer R. (2008). The role of the small intestine in the development of dietary fat-induced obesity and insulin resistance in C57BL/6J mice. BMC Med Genomics 1:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit N, Derrien M, Bosch-Vermeulen H, Oosterink E, Keshtkar S, Duval C, De Vogel-Van Den Bosch J, Kleerebezem M, Müller M, Van Der Meer R. (2012). Saturated fat stimulates obesity and hepatic steatosis and affects gut microbiota composition by an enhanced overflow of dietary fat to the distal intestine. Am J Physiol Gastrointest Liver Physiol 303:G589-G599. [DOI] [PubMed] [Google Scholar]
- Deckert M, Reifenberger G, Wechsler W. (1989). Determination of the proliferative potential of human brain tumors using the monoclonal antibody Ki-67. J Cancer Res Clin Oncol 115:179-188. [DOI] [PubMed] [Google Scholar]
- Deeb D, Gao X, Dulchavsky SA, Gautam SC. (2007). CDDO-me induces apoptosis and inhibits Akt, mTOR and NF-κB signaling proteins in prostate cancer cells. Anticancer Res 27:3035-3044. [PubMed] [Google Scholar]
- Delage B, Rullier A, Capdepont M, Rullier E, Cassand P. (2007). The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers. Nutr J 6:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delroisse JM, Boulvin AL, Parmentier I, Dauphin RD, Vandenbol M, Portetelle D. (2008). Quantification of Bifidobacterium spp. and Lactobacillus spp. in rat fecal samples by real-time PCR. Microbiol Res 163:663-670. [DOI] [PubMed] [Google Scholar]
- Deol P, Evans JR, Dhahbi J, Chellappa K, Han DS, Spindler S, Sladek FM. (2015). Soybean oil is more obesogenic and diabetogenic than coconut oil and fructose in mouse: potential role for the liver. PLoS ONE 10:e0132672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschner EE, Lipkin M. (1975). Proliferative patterns in colonic mucosa in familial polyposis. Cancer 35:413-418. [DOI] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NMJ, Magness S, Jobin C, Lund PK. (2010). High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 5:e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C, Szabo A, Yu Y, Camer D, Zhang Q, Wang H, Huang X-F. (2015a). Bardoxolone methyl prevents fat deposition and inflammation in brown adipose tissue and enhances sympathetic activity in mice fed a high-fat diet. Nutrients 7:4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh CHL, Szabo A, Camer D, Yu Y, Wang H, Huang X-F. (2015b). Bardoxolone methyl prevents fat deposition and inflammation in the visceral fat of mice fed a high-fat diet. Chem Biol Interact 229:1-8. [DOI] [PubMed] [Google Scholar]
- Dinh CHL, Szabo A, Yu Y, Camer D, Wang H, Huang X-F. (2015c). Bardoxolone methyl prevents mesenteric fat deposition and inflammation in high-fat diet mice. ScientificWorldJournal 2015:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass JD, Malik N, Chon S-H, Wells K, Zhou YX, Choi AS, Joseph LB, Storch J. (2012). Intestinal mucosal triacylglycerol accumulation secondary to decreased lipid secretion in obese and high fat fed mice. Front Physiol 3:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durant JF, Fonteyne PA, Richez P, Marot L, Belkhir L, Tennstedt D, Gala JL. (2009). Real-time PCR and DNA sequencing for detection and identification of Trichophyton rubrum as a cause of culture negative chronic granulomatous dermatophytosis. Med Mycol 47:508-514. [DOI] [PubMed] [Google Scholar]
- Erdelyi I, Levenkova N, Lin EY, Pinto JT, Lipkin M, Quimby FW, Holt PR. (2009). Western-style diets induce oxidative stress and dysregulate immune responses in the colon in a mouse model of sporadic colon cancer. J Nutr 139:2072-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskiocak U, Kim SB, Roig AI, Kitten E, Batten K, Cornelius C, Zou YS, Wright WE, Shay JW. (2010). CDDO-me protects against space radiation-induced transformation of human colon epithelial cells. Radiat Res 174:27-36. [DOI] [PubMed] [Google Scholar]
- Evans C, Morrison I, Heriot AG, Bartlett JB, Finlayson C, Dalgleish AG, Kumar D. (2006). The correlation between colorectal cancer rates of proliferation and apoptosis and systemic cytokine levels; plus their influence upon survival. Br J Cancer 94:1412-1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Seki T, Tsukamoto T, Qin H, Yin H, Liao L, Nakamura H, Maeda H. (2013). Protection from inflammatory bowel disease and colitis-associated carcinogenesis with 4-vinyl-2,6-dimethoxyphenol (canolol) involves suppression of oxidative stress and inflammatory cytokines. Carcinogenesis 34:2833-2841. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386. [DOI] [PubMed] [Google Scholar]
- Flores MBS, Rocha GZ, Damassouza DM, Osriocosta F, Dias MM, Ropelle ER, Camargo JA, De Carvalho RB, Carvalho HF, Saad MJA, Carvalheira JBC. (2012). Obesity-induced increase in tumor necrosis factor-α leads to development of colon cancer in mice. Gastroenterology 143:741-753.e744. [DOI] [PubMed] [Google Scholar]
- Gao X, Deeb D, Danyluk A, Media J, Liu Y, Dulchavsky SA, Gautam SC. (2008). Immunomodulatory activity of synthetic triterpenoids: inhibition of lymphocyte proliferation, cell-mediated cytotoxicity, and cytokine gene expression through suppression of NF-κB. Immunopharmacol Immunotoxicol 30:581-600. [DOI] [PubMed] [Google Scholar]
- Gao X, Deeb D, Hao J, Liu Y, Arbab AS, Dulchavsky SA, Gautam SC. (2010). Synthetic triterpenoids inhibit growth, induce apoptosis and suppress pro-survival Akt, mTOR and NF-κB signaling proteins in colorectal cancer cells. Anticancer Res 30:785-792. [PMC free article] [PubMed] [Google Scholar]
- Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. (1995). Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Intern Med 122:327-334. [DOI] [PubMed] [Google Scholar]
- Giugliano D, Ceriello A, Esposito K. (2006). The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol 48:677-685. [DOI] [PubMed] [Google Scholar]
- Göranzon C, Kumawat AK, Hultgren-Hörnqvist E, Tysk C, Eriksson S, Bohr J, Nyhlin N. (2013). Immunohistochemical characterization of lymphocytes in microscopic colitis. J Crohns Colitis 7:e434-e442. [DOI] [PubMed] [Google Scholar]
- Gu M, Fan S, Liu G, Guo L, Ding X, Lu Y, Zhang Y, Ji G, Huang C. (2013). Extract of wax gourd peel prevents high-fat diet-induced hyperlipidemia in C57BL/6 mice via the inhibition of the PPARγ pathway. Evid Based Complement Alternat Med 2013:342561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Half E, Arber N. (2009). Colon cancer: preventive agents and the present status of chemoprevention. Expert Opin Pharmacother 10:211-219. [DOI] [PubMed] [Google Scholar]
- Honda T, Finlay HJ, Gribble GW, Suh N, Sporn MB. (1997). New enone derivatives of oleanolic acid and ursolic acid as inhibitors of nitric oxide production in mouse macrophages. Bioorg Med Chem Lett 7:1623-1628. [Google Scholar]
- Hong DS, Kurzrock R, Supko JG, He X, Naing A, Wheler J, Lawrence D, Eder JP, Meyer CJ, Ferguson DA, Mier J, Konopleva M, Konoplev S, Andreeff M, Kufe D, Lazarus H, Shapiro GI, Dezube BJ. (2012). A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res 18:3396-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh P, Lu K, Chiang C, Chen C. (2010). Suppressive effect of COX2 inhibitor on the progression of adipose inflammation in high-fat-induced obese rats. Eur J Clin Invest 40:164-171. [DOI] [PubMed] [Google Scholar]
- Hsum YW, Yew WT, Hong PLV, Soo KK, Hoon LS, Chieng YC, Mooi LY. (2011). Cancer chemopreventive activity of maslinic acid: Suppression of COX-2 expression and inhibition of NF-KB and AP-1 activation in raji cells. Planta Med 77:152-157. [DOI] [PubMed] [Google Scholar]
- Hume DA, Gordon S. (1985). The mononuclear phagocyte system of the mouse defined by immunohistochemical localisation of antigen F4/80. Springer Netherlands. [DOI] [PubMed] [Google Scholar]
- Hwang Y-J, Song J, Kim H-R, Hwang K-A. (2014). Oleanolic acid regulates NF-κB signaling by suppressing MafK expression in RAW 264.7 cells. BMB Rep 47:524-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Murakami A, Ohigashi H. (2008). Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr Food Res 52:26-42. [DOI] [PubMed] [Google Scholar]
- Jain SS, Bird RP. (2010). Elevated expression of tumor necrosis factor-α signaling molecules in colonic tumors of Zucker obese (fa/fa) rats. Int J Cancer 127:2042-2050. [DOI] [PubMed] [Google Scholar]
- Janakiram N, Indranie C, Malisetty S, Jagan P, Steele V, Rao C. (2008). Chemoprevention of colon carcinogenesis by oleanolic acid and its analog in male F344 rats and modulation of COX-2 and apoptosis in human colon HT-29 cancer cells. Pharmaceut Res 25:2151-2157. [DOI] [PubMed] [Google Scholar]
- Jang SE, Jeong JJ, Hyam SR, Han MJ, Kim DH. (2014). Ursolic acid isolated from the seed of cornus officinalis ameliorates colitis in mice by inhibiting the binding of lipopolysaccharide to toll-like receptor 4 on macrophages. J Agric Food Chem 62:9711-9721. [DOI] [PubMed] [Google Scholar]
- Kim K-A, Gu W, Lee I-A, Joh E-H, Kim D-H. (2012a). High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the TLR4 signaling pathway. PLoS ONE 7:e47713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SB, Pandita RK, Eskiocak U, Ly P, Kaisani A, Kumar R, Cornelius C, Wright WE, Pandita TK, Shay JW. (2012b). Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc Natl Acad Sci USA 109:E2949-E2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfer L. (2011). Cytokines, inflammation and colon cancer. Curr Cancer Drug Targets 11:451-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojouharoff G, Hans W, Obermeler F, Männel DN, Andus T, Schölmerich J, Gross V, Falk W. (1997). Neutralization of tumour necrosis factor (TNF) but not of IL-1 reduces inflammation in chronic dextran sulphate sodium-induced colitis in mice. Clin Exp Immunol 107:353-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono S, Handa K, Hayabuchi H, Kiyohara C, Inoue H, Marugame T, Shinomiya S, Hamada H, Onuma K, Koga H. (1999). Obesity, weight gain and risk of colon adenomas in Japanese men. JPN J Cancer Res 90:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni AA, Thatcher TH, Hsiao H-M, Olsen KC, Kottmann RM, Morrissette J, Wright TW, Phipps RP, Sime PJ. (2013). The triterpenoid CDDO-Me inhibits Bleomycin-induced lung inflammation and fibrosis. PLoS ONE 8:e63798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YY, Ha CWY, Campbell CR, Mitchell AJ, Dinudom A, Oscarsson J, Cook DI, Hunt NH, Caterson ID, Holmes AJ, Storlien LH. (2012). Increased gut permeability and microbiota change associate with mesenteric fat inflammation and metabolic dysfunction in diet-induced obese mice. PLoS ONE 7:e34233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Lin JE, Chervoneva I, Schulz S, Waldman SA, Pitari GM. (2007). Homeostatic control of the crypt-villus axis by the bacterial enterotoxin receptor guanylyl cyclase c restricts the proliferating compartment in intestine. Am J Pathol 171:1847-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby K, Risingsong R, Royce DB, Williams CR, Ma T, Yore MM, Sporn MB. (2009). Triterpenoids CDDO-methyl ester or CDDO-ethyl amide and rexinoids LG100268 or NRX194204 for prevention and treatment of lung cancer in mice. Cancer Prev Res 2:1050-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liby KT, Sporn MB. (2012). Synthetic oleanane triterpenoids: multifunctional drugs with a broad range of applications for prevention and treatment of chronic disease. Pharmacol Rev 64:972-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Brooks RS, Ciappio ED, Kim SJ, Crott JW, Bennett G, Greenberg AS, Mason JB. (2012). Diet-induced obesity elevates colonic TNF-α in mice and is accompanied by an activation of Wnt signaling: a mechanism for obesity-associated colorectal cancer. J Nutr Biochem 23:1207-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manenti L, Allinovi M, Vaglio A, Allegri L, Gnappi E, Simonetti G, Vilalta R, Lapeyraque A-L, Gruppo R, Sherwinter J, Smith J, Thornburg C, Jungraithmayr T, Wuehl E, Al-Akash S, Davin JC, Macher MA, Langman C, Camacho Diaz JA, Chin M, Goldsberry M, Angie Hebbar S, Meyer C, Audhya P, Toto R, Warnock D, Pergola P, Imai E, Haneda M, Ito S, Kobayashi F, Yamasaki T, Chan J, Makino H. (2012). HUS and diabetic nephropathy. Nephrol Dial Transplant 27:ii11-ii13. [Google Scholar]
- Martín R, Cordova C, San Román JA, Gutierrez B, Cachofeiro V, Nieto ML. (2014). Oleanolic acid modulates the immune-inflammatory response in mice with experimental autoimmune myocarditis and protects from cardiac injury. Therapeutic implications for the human disease. J Mol Cell Cardiol 72:250-262. [DOI] [PubMed] [Google Scholar]
- Masferrer JL, Zweifel BS, Manning PT, Hauser SD, Leahy KM, Smith WG, Isakson PC, Seibert K. (1994). Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci USA 91:3228-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morampudi V, Bhinder G, Wu X, Dai C, Sham HP, Vallance BA, Jacobson K. (2014). DNBS/TNBS colitis models: providing insights into inflammatory bowel disease and effects of dietary fat. J Vis Exp:e51297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Triebel S, Rudakovski O, Richling E. (2013). Influence of triterpenoids present in apple peel on inflammatory gene expression associated with inflammatory bowel disease (IBD). Food Chem 139:339-346. [DOI] [PubMed] [Google Scholar]
- Nabi U, Nagi AH, Sami W. (2008). Ki-67 proliferating index and histological grade, type and stage of colorectal carcinoma. J Ayub Med Coll Abbottabad 20:44-48. [PubMed] [Google Scholar]
- Oliveira Costa JF, Barbosa-Filho JM, De Azevedo, Maia GL, Guimarães ET, Meira CS, Ribeiro-Dos-Santos R, Pontes De, Carvalho LC, Soares MBP. (2014). Potent anti-inflammatory activity of betulinic acid treatment in a model of lethal endotoxemia. Int Immunopharmacol 23:469-474. [DOI] [PubMed] [Google Scholar]
- Ong ZY, Gibson RJ, Bowen JM, Stringer AM, Darby JM, Logan RM, Yeoh ASJ, Keefe DM. (2010). Pro-inflammatory cytokines play a key role in the development of radiotherapy-induced gastrointestinal mucositis. Radiat Oncol 5:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padidar S, Farquharson A, Williams L, Kearney R, Arthur J, Drew J. (2012). High-fat diet alters gene expression in the liver and colon: links to increased development of aberrant crypt foci. Dig Dis Sci 57:1866-1874. [DOI] [PubMed] [Google Scholar]
- Paik J, Fierce Y, Treuting PM, Brabb T, Maggio-Price L. (2013). High-fat diet-induced obesity exacerbates inflammatory bowel disease in genetically susceptible Mdr1a−/− male mice. J Nutr 143:1240-1247. [DOI] [PubMed] [Google Scholar]
- Park H, Kim M, Kwon GT, Lim DY, Yu R, Sung M-K, Lee KW, Daily JW, Park JHY. (2012). A high-fat diet increases angiogenesis, solid tumor growth, and lung metastasis of CT26 colon cancer cells in obesity-resistant BALB/c mice. Mol Carcinog 51:869-880. [DOI] [PubMed] [Google Scholar]
- Paturi G, Butts C, Monro J, Nones K, Martell S, Butler R, Sutherland J. (2010). Cecal and colonic responses in rats fed 5 or 30% corn oil diets containing either 7.5% broccoli dietary fiber or microcrystalline cellulose. J Agric Food Chem 58:6510-6515. [DOI] [PubMed] [Google Scholar]
- Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG. (2011). Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365:327-336. [DOI] [PubMed] [Google Scholar]
- Petit V, Arnould L, Martin P, Monnot M-C, Pineau T, Besnard P, Niot I. (2007). Chronic high-fat diet affects intestinal fat absorption and postprandial triglyceride levels in the mouse. J Lipid Res 48:278-287. [DOI] [PubMed] [Google Scholar]
- Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, Oshima M, Fujii C, Mukaida N. (2008). Blocking TNF-α in mice reduces colorectal carcinogenesis associated with chronic colitis. J Clin Invest 118:560-570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivollier A, He J, Kole A, Valatas V, Kelsall BL. (2012). Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med 209:139-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogler G, Brand K, Vogl D, Page S, Hofmeister R, Andus T, Knuechel R, Baeuerle PA, Schölmerich J, Gross V. (1998). Nuclear factor κB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology 115:357-369. [DOI] [PubMed] [Google Scholar]
- Saha PK, Reddy VT, Konopleva M, Andreeff M, Chan L. (2010). The triterpenoid 2-cyano-3,12-dioxooleana-1,9-dien-28-oic-acid methyl ester has potent anti-diabetic effects in diet-induced diabetic mice and leprdb/db mice. J Biol Chem 285:40581-40592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sands BE, Kaplan GG. (2007). The role of TNFα in ulcerative colitis. J Clin Pharmacol 47:930-941. [DOI] [PubMed] [Google Scholar]
- Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, Campoy C, Moreno LA, Veiga O, Redondo-Figuero C, Garagorri JM, Azcona C, Delgado M, García-Fuentes M, Collado MC, Sanz Y, Group ES. (2009). Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity 17:1906-1915. [DOI] [PubMed] [Google Scholar]
- Shen W, Wolf PG, Carbonero F, Zhong W, Reid T, Gaskins HR, Mcintosh MK. (2014). Intestinal and systemic inflammatory responses are positively associated with Sulfidogenic bacteria abundance in high-fat–fed male C57BL/6J mice. J Nutr 144:1181-1187. [DOI] [PubMed] [Google Scholar]
- Sheng H, Shao J, Kirkland SC, Isakson P, Coffey RJ, Morrow J, Beaucnamp RD, Dubois RN. (1997). Inhibition of human colon cancer cell growth by selective inhibition of cyclooxygenase-2. J Clin Invest 99:2254-2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd NA, Richman PI, England J. (1988). Ki-67 derived proliferative activity in colorectal adenocarcinoma with prognostic correlations. J Pathol 155:213-219. [DOI] [PubMed] [Google Scholar]
- Shih I-M, Wang T-L, Traverso G, Romans K, Hamilton SR, Ben-Sasson S, Kinzler KW, Vogelstein B. (2001). Top-down morphogenesis of colorectal tumors. Proc Natl Acad Sci U S A 98:2640-2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shishodia S, Sethi G, Konopleva M, Andreeff M, Aggarwal BB. (2006). A synthetic triterpenoid, CDDO-Me, inhibits IκBα Kinase and enhances apoptosis induced by TNF and chemotherapeutic agents through down-regulation of expression of Nuclear Factor κB-regulated gene products in human leukemic cells. Clin Cancer Res 12:1828-1838. [DOI] [PubMed] [Google Scholar]
- Steinbrecher KA, Wowk SA, Rudolph JA, Witte DP, Cohen MB. (2002). Targeted inactivation of the mouse guanylin gene results in altered dynamics of colonic epithelial proliferation. Am J Pathol 161:2169-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhagen E, Shia J, Markowitz AJ, Stadler ZK, Salo-Mullen EE, Zheng J, Lee-Kong SA, Nash GM, Offit K, Guillem JG. (2012). Systematic immunohistochemistry screening for lynch syndrome in early age-of-onset colorectal cancer patients undergoing surgical resection. J Am Coll Surg 214:61-67. [DOI] [PubMed] [Google Scholar]
- Suh N, Honda T, Finlay HJ, Barchowsky A, Williams C, Benoit NE, Xie QW, Nathan C, Gribble GW, Sporn MB. (1998). Novel triterpenoids suppress inducible nitric oxide synthase (iNOS) and inducible cyclooxygenase (COX-2) in mouse macrophages. Cancer Res 58:717-723. [PubMed] [Google Scholar]
- Takada Y, Aggarwal BB. (2003). Betulinic acid suppresses carcinogen-induced NF-κB activation through inhibition of IκBα kinase and p65 phosphorylation: abrogation of cyclooxygenase-2 and matrix metalloprotease-9. J Immunol 171:3278-3286. [DOI] [PubMed] [Google Scholar]
- Takada Y, Hisamatsu T, Kamada N, Kitazume MT, Honda H, Oshima Y, Saito R, Takayama T, Kobayashi T, Chinen H, Mikami Y, Kanai T, Okamoto S, Hibi T. (2010). Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10–producing regulatory macrophage subset. J Immunol 184:2671-2676. [DOI] [PubMed] [Google Scholar]
- Takano Y, Saegusa M, Ikenaga M, Mitomi H, Okayasu I. (1996). Apoptosis of colon cancer: Comparison with Ki-67 proliferative activity and expression of p53. J Cancer Res Clin Oncol 122:166-170. [DOI] [PubMed] [Google Scholar]
- Taraban VY, Slebioda TJ, Willoughby JE, Buchan SL, James S, Sheth B, Smyth NR, Thomas GJ, Wang ECY, Al-Shamkhani A. (2011). Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol 4:186-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Leonel A, Aguilar E, Batista N, Alves A, Coimbra C, Ferreira A, De Faria A, Cara D, Alvarez Leite J. (2011). The combination of high-fat diet-induced obesity and chronic ulcerative colitis reciprocally exacerbates adipose tissue and colon inflammation. Lipids Health Dis 10:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran K, Risingsong R, B.Royce D, Williams CR, Sporn MB, Pioli PA, Gediya LK, Njar VC, Liby KT. (2013). The combination of the histone deacetylase inhibitor vorinostat and synthetic triterpenoids reduces tumorigenesis in mouse models of cancer. Carcinogenesis 34:199-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdam FJ, Fuentes S, De Jonge C, Zoetendal EG, Erbil R, Greve JW, Buurman WA, De Vos WM, Rensen SS. (2013). Human intestinal microbiota composition is associated with local and systemic inflammation in obesity. Obesity 21:E607-E615. [DOI] [PubMed] [Google Scholar]
- Vincent M, Philippe E, Everard A, Kassis N, Rouch C, Denom J, Takeda Y, Uchiyama S, Delzenne NM, Cani PD, Migrenne S, Magnan C. (2013). Dietary supplementation with Agaricus blazei murill extract prevents diet-induced obesity and insulin resistance in rats. Obesity 21:553-561. [DOI] [PubMed] [Google Scholar]
- Vinuesa AG, Sancho R, García-Limones C, Behrens A, Ten Dijke P, Calzado MA, Muñoz E. (2012). Vanilloid receptor-1 regulates neurogenic inflammation in colon and protects mice from colon cancer. Cancer Res 72:1705-1716. [DOI] [PubMed] [Google Scholar]
- Vitor CE, Figueiredo CP, Hara DB, Bento AF, Mazzuco TL, Calixto JB. (2009). Therapeutic action and underlying mechanisms of a combination of two pentacyclic triterpenes, α- and β-amyrin, in a mouse model of colitis. Br J Pharmacol 157:1034-1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Liu X, Fan J, Chen W, Wang J, Zeng Y, Feng X, Yu X, Yang X. (2014). Bardoxolone methyl (BARD) ameliorates aristolochic acid (AA)-induced acute kidney injury through Nrf2 pathway. Toxicology 318:22-31. [DOI] [PubMed] [Google Scholar]
- Xiao H, Gulen MF, Qin J, Yao J, Bulek K, Kish D, Altuntas CZ, Wald D, Ma C, Zhou H, Tuohy VK, Fairchild RL, De La, Motte C, Cua D, Vallance BA, Li X. (2007). The Toll-interleukin-1 receptor member SIGIRR regulates colonic epithelial homeostasis, inflammation, and tumorigenesis. Immunity 26:461-475. [DOI] [PubMed] [Google Scholar]
- Zhu W, Yu J, Nie Y, Shi X, Liu Y, Li F, Zhang X-L. (2014). Disequilibrium of M1 and M2 macrophages correlates with the development of experimental inflammatory bowel diseases. Immunol Invest 43:638-652. [DOI] [PubMed] [Google Scholar]