Abstract
Localization of Argonaute2 (AGO2) protein—an essential component for the processing of small interfering RNA (siRNA)-directed RNA interference (RNAi) in RNA-induced silencing complex (RISC) in nuage of rat spermatogenic cells—was evaluated by immunofluorescence microscopy (IFM) and immunoelectron microscopy (IEM). AGO2 was shown, for the first time, to be localized to four previously classified types of nuage: irregularly shaped perinuclear granules (ISPGs), intermitochondrial cement (IMC), satellite bodies (SBs), and chromatoid bodies (CBs). Dual IEM staining for AGO2/Maelstrom (MAEL) protein or AGO2/MIWI protein demonstrated that AGO2 is colocalized with MAEL or MIWI proteins in these types of nuage. Dual IFM and IEM staining of AGO2/lysosomal-associated membrane protein 2 (LAMP2) showed that CB in round spermatids are in contact with and surrounded by LAMP2-positive vesicles, whereas nuage in pachytene spermatocytes are not. Taken together, our findings indicate that: (i) AGO2 in pachytene spermatocytes functions in ISPGs, IMC, and SBs; (ii) AGO2 in round spermatids functions in CBs, and that CBs are associated with lysosomal compartments.
Keywords: electron microscopy, lysosome, Spermatogenesis, nuage, argonaute2, chromatoid body, immunofluorescence microscopy
Introduction
Argonaute (AGO) proteins are an evolutionarily conserved family whose members silence gene expression through RNA interference (RNAi) pathways. Members of the family can be divided into AGO and PIWI proteins. AGO subfamily members bind small interfering RNAs (siRNAs) and microRNAs (miRNAs), whereas PIWI subfamily members bind PIWI-interacting RNAs (piRNAs). Both siRNAs and miRNAs are cut from double-stranded RNA precursors by RNase III enzymes (e.g., Dicer) in the cytoplasm. AGO-small RNA complexes, termed RNA-induced silencing complexes (RISCs), can repress gene transcription, target mRNAs for degradation, or block mRNA translation (Cenik and Zamore 2011). Four members of the AGO subfamily (AGO1–AGO4) are known in humans, and five members are known in mice (Peters and Meister 2007). AGO2 is responsible for mRNA cleavage activity and is essential for embryonic development in mice. Mutations within a cryptic ribonuclease H domain of AGO2 inactivate RISCs.
The AGO subfamily is expressed in all mammalian cells, whereas the PIWI subfamily is expressed only in germ cells (Sasaki et al. 2003; Meister et al. 2004). A subset of piRNAs derived from repeat sequences are amplified through interplay between two PIWI proteins, termed the “ping-pong cycle” (Brennecke et al. 2007). The ping-pong mechanism occurs in PIWI proteins of primordial mouse testis but not those of adult testis (Beyret et al. 2012). piRNAs give rise to a piRNA-induced silencing complex (piRISC) in germ cell lines of many animal species. piRISC protects the integrity of the genome from invasion by transposable elements through silencing of these elements (Carthew 2006). During spermatogenesis, the maintenance of genomic integrity depends on silencing of transposable elements in pachytene spermatocytes undergoing meiosis (Aravin et al. 2007; Siomi et al. 2011).
In the fetal piRNA pathway of mouse testis, MILI and MIWI2 proteins are localized in two types of germinal cytoplasmic granules (Aravin et al. 2009): (i) pi-bodies (equivalent to IMC); (ii) piP-bodies containing P-body components (e.g., GW182, DCP1a, DDX6/p54) and XRN1 proteins. The latter type is equivalent to satellite bodies (SBs) in spermatocytes and most likely to chromatid bodies (CBs) in round spermatids.
Nuage is a germ cell-specific organelle discovered over a century ago. It was first observed in spermatogenic cells as a perinuclear granule stained by basic dyes, termed “chromatoid body” (CB). Recent morphological studies utilizing electron microscopy have shown that CB is a nuage component in spermatids, and that intermitochondrial cement (IMC) is another nuage component found in spermatocytes (Yokota 2008). Nuage in rat spermatogenic cells was classified into six types by Russell and Frank on the basis of ultrastructure and relationships to other organelles (1978). Our previous study of nuage of rat spermatogenic cells using immunocytochemical localization of various nuage-resident proteins demonstrated that four of the six types (irregularly-shaped perinuclear granules [ISPGs], IMC, SBs, and CBs) contain nuage proteins, whereas the other two do not (Yokota 2012a). The four nuage types in the former group appear during restricted stages of spermatogenic cell development (Table 1); ISPGs, IMC, and SBs appear exclusively in pachytene spermatocytes from stages VI to XIII, stages VI to XIII, and stages IX to XI, respectively; CBs appear in spermatids from steps 1 to 10 (Onohara et al. 2010; Onohara and Yokota 2012; Yonetamari et al. 2012; Yokota and Onohara 2013; Takebe et al. 2013; Kawahara et al. 2013). ISPGs, IMC, and SBs are dispersed into the cytosol during meiosis. Following meiosis, nuage reappears as a single organelle: a CB in haploid spermatids (Onohara et al. 2010). CBs are localized initially in the apical cytoplasm of round spermatids together with Golgi complex, subsequently move caudally to the cytoplasm to surround the base of the flagellum in elongated spermatids, and finally form the annulus (Fawcett et al. 1970), in which only DDX6 has been detected as a nuage protein (Kawahara et al. 2013).
Table 1.
AGO2 Staining Intensity of Each Nuage Structure in Spermatocytes and Spermatids during the Developing Cycle.
| Nuage | Stage |
I | II-III | IV | V | VI | VII | VIII | IX | X | XI | XII | XIII | XIV |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell type | ||||||||||||||
| ISPG | Spermatocyte1 | ++ | ++ | +++ | +++ | +++ | +++ | +++ | ++++ | ++ | + | +/- | +/- | + |
| IMC | Spermatocyte2 | - | - | - | - | + | ++ | ++ | +++ | ++ | + | - | - | - |
| SB | Spermatocyte4 | - | - | - | - | - | - | - | ++ | ++ | + | - | - | - |
| CB | Spermatid3 | ++ | +++ | +++ | ++++ | +++ | ++ | ++ | + | + | +/- | - | - | - |
ISPG, irregularly shaped perinuclear granules, are contained only in pachytene spermatocytes; 2IMC, intermitochondrial cement, is also contained in pachytene spermatocytes; 3CBs, chromatid bodies, are present only in spermatids at steps 1–11; 4SBs, satellite bodies, are observed only in pachytene spermatocytes at stage IX–XI. Signal intensity: -, no signal; +/-, very weak or no signal; +, weak; ++, intermediate; +++, strong; ++++, very strong.
AGO2 was reported to be present in CBs (Kotaja 2006), but its status in ISPGs, IMC, and SBs remains unclear. We now demonstrate that AGO2 is present in all four types of nuage. AGO2 is localized in small clear vesicles surrounding CBs, which have lysosomal features. These vesicles are not found in the other types of nuage. Our findings suggest that ISPGs, IMC, and SBs are subcellular sites for AGO2 function, whereas CBs are related to AGO2 function and the lysosomal pathway.
Materials & Methods
Animals
Japanese white rabbits (3–4 kg) and Wistar rats (250–300 g) were obtained from Kyudo Co. (Tosu, Japan) and maintained on standard diets and water ad libitum. All experiments were performed in accordance with the Guidelines for Animal Experiments, Nagasaki International University.
Antibodies and Related Probes
A synthetic 14-amino acid sequence (VQVHQDTLRTMYFA) at the C-terminus of the rat AGO2 protein was conjugated with maleimide-activated egg albumin (Sigma-Aldrich Japan; Tokyo, Japan) through an additional cysteine at the N-terminus. This AGO2 polypeptide-albumin conjugate (200 μg peptide/animal) was emulsified with complete or incomplete Freund’s adjuvant and injected intracutaneously into the backs of rabbits. Peptide-specific antibody was purified by affinity chromatography on a peptide-conjugated CH-Sepharose 4B column (GE Healthcare; Tokyo, Japan). Guinea pig antibody vs mouse MAEL (MAELSTROM protein; a nuage component) was produced in our laboratory (Takebe et al. 2013). Rabbit anti-mouse MIWI (PIWIL1) antibody (G82) was from Cell Signaling Technology Japan (Tokyo, Japan). Mouse anti-LAMP2 antibody was kindly donated by Dr. Kenji Akasaki (Fukuyama University, Japan). Alexa 568-conjugated goat anti-rabbit IgG, goat anti-guinea pig IgG, and Alexa 488-conjugated goat anti-mouse IgG were from Molecular Probes (Eugene, OR). Horseradish peroxidase (HRP)-labeled pig antibody vs rabbit IgG was from Dako (Tokyo, Japan). Colloidal gold particles (diameters 5-, 8.5-, and 25-nm) were prepared as described previously (Yokota 2012b) and conjugated with Protein A/G/L (BioVision Inc.; Milpitas, CA).
Western Blotting Analysis of Anti-AGO2 Antibody
Rat testes were dissected and homogenized in 5 mM MOPS-KOH buffer (pH 7.4) containing 0.25 M sucrose, 1 mM ethylenediaminetetraacetic acid (EDTA), 1 mM phenylmethylsulfonyl fluoride (PMSF), and a cocktail of protease inhibitors (Sigma-Aldrich; St. Louis, MO) that included leupeptin, pepstatin, aprotinin, and antipain (medium A). The homogenate was centrifuged at 800 ×g for 10 min. The resulting supernatant was centrifuged at 10,000 ×g for 20 min, and the resulting pellet (mitochondrial fraction) was suspended in a small volume of medium A. The supernatant was centrifuged at 100,000 ×g for 1 hr in a Beckman ultracentrifuge using an SW 41 swing rotor. The resulting pellet was suspended in medium A and used as microsomal fraction, while the supernatant was used as cytosol fraction. Cell fractions were stored at -70°C. Protein concentrations were determined by the bicinchoninic acid method (Pierce Chemical; Rockford, IL) using bovine serum albumin (BSA) as a standard. Protein concentrations of the fractions were adjusted to 1 mg/ml, and fractions were mixed with one volume (vol) of SDS-PAGE sample buffer containing 0.1 vol of 0.3 M iodoacetamide, and heated in boiling water for 5 min. Samples (each 10 μg) were analyzed by western blotting using affinity-purified AGO2-specific antibody. For neutralization of the antibody, anti-AGO2 serum was pre-incubated with the peptide used as antigen at 500 μg/ml for 1 hr, and then used for western blotting at a 1:5000 dilution. The molecular mass of AGO2 protein was determined using prestained protein markers (Nippon Genetics Europe GmbH; Dueren, Germany).
Immunofluorescence Microscopy (IFM) using Frozen Sections
Rat testes were embedded in Tissue-Tek (Sakura Finetek Co.; Tokyo, Japan) and frozen in isopentane cooled by liquid nitrogen. Frozen sections (6-µm thick) were cut with a Leitz cryostat (Leica Instruments; Nussloch, Germany) and placed on silicon-coated glass slides. Sections were sequentially fixed in 4% paraformaldehyde diluted in 0.1 M HEPES-KOH (pH 7.4) for 15 min at room temperature, dried, treated with 0.1% Triton X-100, 0.2% Saponin in PBS for 15 min, incubated in blocking solution containing 2% fish gelatin and 15 mM NaN3 in PBS for 30 min, incubated with anti-AGO2 antibody (1 μg/ml) for 1 hr at room temperature, washed with PBS, and finally incubated with Alexa 568-labeled goat anti-rabbit IgG or anti-rabbit IgG solution with 3 μM DAPI (Sigma-Aldrich USA) for 30 min at room temperature. As controls, antisera absorbed with peptide-albumin conjugates or pre-immune sera were used for the primary reaction, followed by Alexa 568-labeled secondary antibodies. For double staining of AGO2 and MAEL, frozen sections were incubated with a mixture of rabbit anti-AGO2 and guinea pig anti-MAEL antibodies, followed by 30 min incubation with a mixture of Alexa 568-labeled goat anti-rabbit IgG or Alexa 488-labeled anti-guinea pig IgG containing 3 μM DAPI or Hoechst 33258 (Sigma-Aldrich) at room temperature. Processed sections were examined by IFM (model Eclipse E600; Nikon; Tokyo). Images were merged using Adobe Photoshop ver7.0 (Adobe Systems Inc.; San Jose, CA) for visualization of cell contours. Stages of seminiferous tubules were evaluated based on size and shape of DAPI-stained spermatocytes and spermatids, according to the cycle described by Russell et al. (1990).
IFM using Semi-thin Sections
Rat testes were cut into small tissue blocks in ice-cold fixative consisting of 1% glutaraldehyde, 0.01% CaCl2, and 0.05 M HEPES-KOH buffer (pH 7.4) and kept in the same fixative for 1 hr at 4°C. The tissue blocks were dehydrated by a graded ethanol series at 4°C and embedded in Epon 812, which was then polymerized for 20 hr at 60°C. Semi-thin sections (250-nm thick) were cut by diamond knife on a Reichert Ultracut R microtome and mounted onto silicon-coated glass slides. Sections were pretreated as described previously (Haraguchi and Yokota 2002). In brief, sections were treated with 10% sodium ethoxide to remove epoxy resin, digested with 0.05% trypsin, treated with borohydride, incubated in blocking solution containing 2% fish gelatin and 15 mM NaN3 in PBS, and incubated overnight with a mixture of rabbit anti-AGO2 antibody and mouse anti-LAMP2 antibody. Rabbit IgG and mouse IgG were visualized, respectively, by Alexa 568-labeled goat anti-rabbit IgG and Alexa 488-labeled goat anti-mouse IgG.
Immunoelectron Microscopy (IEM)
Rat testes were cut into small tissue blocks by razor blade in ice-cold fixative consisting of 4% paraformaldehyde, 0.2% glutaraldehyde, 0.01% CaCl2, and 0.05 M HEPES-KOH buffer (pH 7.4), and kept in the same fixative for 1 hr at 4°C. The tissue blocks were dehydrated by a graded ethanol series at -20°C and embedded in LR White, which was then polymerized under UV light at -20°C. Thin sections were cut by diamond knife on a Reichert Ultracut R microtome and mounted on nickel grids. For single labeling, grids were immersed in droplets of 2% fish gelatin diluted in PBS for 30 min followed by rabbit anti-AGO2 antibody or mouse anti-LAMP2 antibody, and then incubated 30 min with protein A/G/L-gold probes (15- or 25-nm). Dual labeling of AGO2-MAEL, AGO2-MIWI, or AGO2-LAMP2 was carried out as follows: (i) one side of the section was exposed to one of the two antibodies by floating the grid on a droplet of the antibody and then on protein A/G/L probe with large gold particles; (ii) the other side of the section was exposed to the other antibody followed by protein A/G/L probe with small gold particles. As a control, antigen-absorbed antibodies or non-immune sera were used for primary immunoreaction, followed by protein A/G/L-gold probes. Sections were contrasted with uranyl acetate and lead citrate and examined by IEM (model H7650, Hitachi; Tokyo) at an acceleration voltage of 80 kV. Seminiferous tubule stages and spermatid steps were evaluated based on the cycle described by Russell et al. (1990).
Results
Specificity of Anti-AGO2 Antibody
The anti-AGO2 antibody established in our laboratory gave a single band with a molecular mass of 97 kDa (Fig. 1), similar to that (97.3 kDa) calculated from the amino acid sequence using the UniProtKB database. This band was observed in all tested cell fractions. AGO2 staining of the mitochondrial fraction (Fig. 1, lane 3) appeared to be stronger than that of whole extract of testis (Fig. 1, lane 2). However, we confirmed by electron microscopy that the mitochondrial fraction contains not only mitochondrial but also CB, IMC, and the other testicular cell-specific granules (data not shown). The AGO2 signal in the mitochondrial fraction (Fig. 1, lane 3) appeared to be derived from some of CBs and IMC, which were co-sedimented in this fraction. The signal in the microsomal fraction (Fig. 1, lane 4) appeared to arise from ISPGs and SBs, which were co-sedimented in this fraction. Antibody specificity was confirmed by neutralization of the antibody with the peptide used as the antigen (Fig. 1, lane 7).
Figure 1.
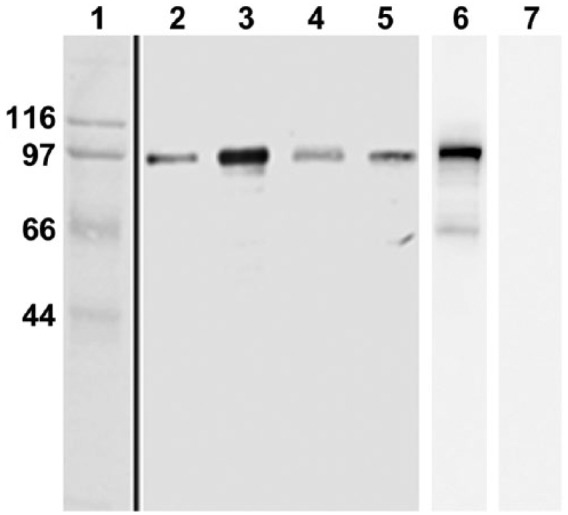
Immunoblotting of cell fractions from rat testis with anti-AGO2 antibody. Lane 1, molecular markers (mass numbers shown at left). Lane 2, whole extract of testis. Lanes 3, 6 and 7, mitochondrial fraction. Lane 4, microsomal fraction. Lane 5, cytosol fraction. Affinity purified anti-AGO2 antibody is used for lanes 2–5. Mitochondrial fractions are blotted with serum preincubated with PBS alone (lane 6) or with the peptide used as antigen (lane 7).
IFM Evaluation of AGO2 Localization in Rat Spermatogenic Cells
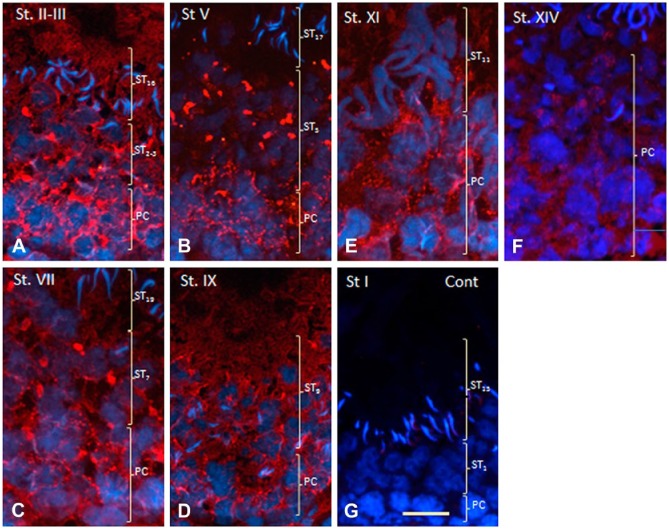
Localization of AGO2 in CBs of squash preparations or dried-down slides of distinct spermatocyte developmental stages was reported by Kotaja et al. (2006). We attempted to demonstrate for the first time the in situ localization of AGO2 in testis tissue sections. AGO2 was detected in pachytene spermatocytes and round spermatids. Two types of AGO2 staining patterns were observed in the cytoplasm of pachytene spermatocytes: weak diffuse staining and small granular or filamentous staining (Fig. 2A–2F). Diffuse staining was seen in all spermatocyte stages. Granular or filamentous staining of similar intensity was seen in spermatocytes from stages I to X, but the intensity declined in the subsequent stages (Fig. 2F). In round spermatids, large, irregularly-shaped granules were stained strongly, and weak diffuse staining was also observed (Fig. 2A–2F). Elongated spermatids showed diffuse staining but not granular staining. Neither type of staining was seen in the control sections (Fig. 2G).
Figure 2.
Immunofluorescence staining of AGO2 protein in seminiferous tubules of rat testis at various developmental stages. Nuclei are stained with DAPI. PC and ST (in parentheses) indicate (respectively) areas where pachytene spermatocytes or spermatids are distributed. (A) Stages II to III. Fine vesicular and diffuse staining is observed in the cytoplasm of PCs and STs at steps 2 to 3 (ST2-3) and ST16. (B) Stage V. Staining pattern similar to that in (A) is seen in the cytoplasm of PC, but large irregularly-shaped granules are stained in spermatids at ST5. No staining is seen in spermatids at ST17. (C) Stage VII. Similar staining is observed in PCs and STs at ST7. (D) Stage IX. Small granular and diffuse staining is seen in the cytoplasm of both PCs and STs at ST9. (E) Stage XI. Fine granular staining is observed in the cytoplasm of PCs but not in STs at ST11. (F) Stage XIV. Granular staining is almost gone, but weak diffuse staining is still present in PCs. (G) Immunohistochemical control section of a stage I seminiferous tubule. No staining is seen in PCs or in STs at ST1 or ST15. Scale, 25 μm.
A dual-staining technique was used to investigate the possible colocalization of AGO2 with nuage protein MAEL (Soper et al. 2008; Takebe et al. 2013). Most granules in the cytoplasm of pachytene spermatocytes (Fig. 3: PCs) and of round spermatids [Fig. 3: STs at steps 2-3 (ST2-3), ST5, ST8] were stained for MAEL (Fig. 3A–3L). Some granules in pachytene spermatocytes were positive only for AGO2 (Fig. 3A–3I: arrows). In all stages of pachytene spermatocytes, cytoplasm per se was stained for AGO2 but not for MAEL. In the cytoplasm of pachytene spermatocytes at stages IX–XI and of elongated spermatids at steps 9–11, AGO2 and MAEL were colocalized in small granules (Fig. 3J–3L: arrowheads). In the controls, no staining for either AGO2 or MAEL was observed (data not shown).
Figure 3.
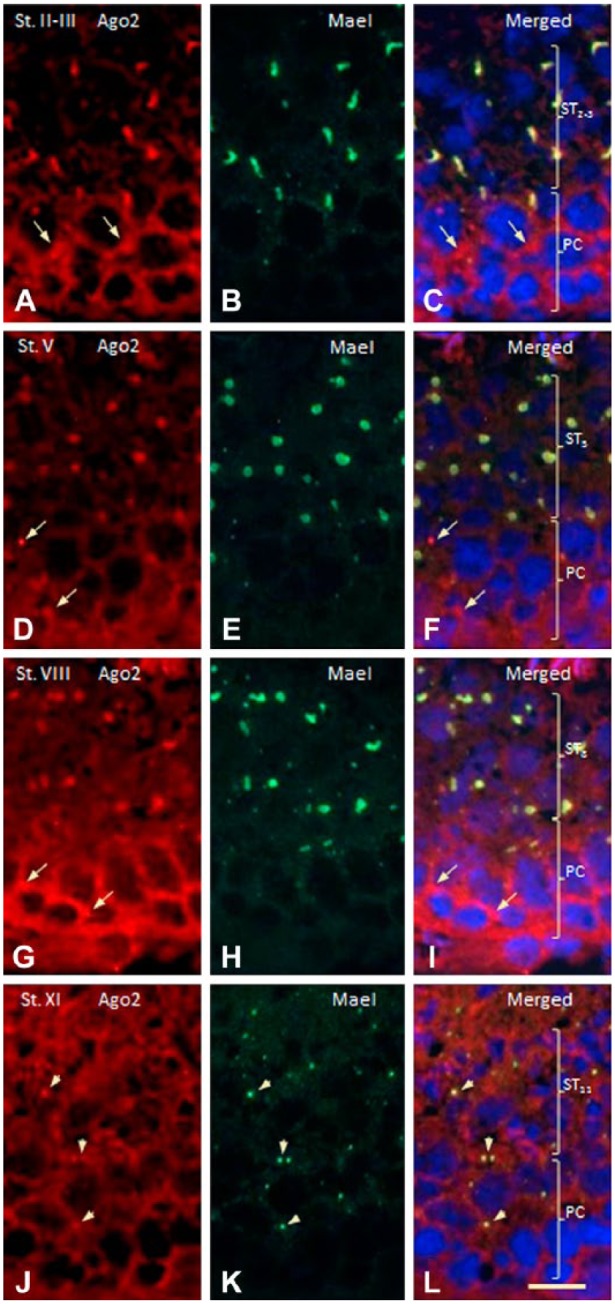
Dual immunofluorescence staining for AGO2 (red) and MAEL (green) proteins in rat seminiferous tubules at various developmental stages. Nuclei are stained with Hoechst 33258. PC and ST (in parentheses) indicate (respectively) areas where pachytene spermatocytes or spermatids are distributed. (A–C) Stages II-III. Irregularly-shaped granules located in spermatids (ST) are stained for both AGO2 and MAEL. The area in PCs diffusely stained for AGO2 is not stained for MAEL. (D–F) Stage V. Granules in STs are stained for both proteins. Some small granules in PCs (arrows) are stained for AGO2 but not for MAEL. (G–I) Stage VIII. Granules in STs are stained for both proteins. Some granules in PCs (arrows) are stained for AGO2 but not for MAEL. (J–L) Strong diffuse staining for AGO2 is seen in the cytoplasms of PCs and STs. Cytoplasm of PC is also weakly stained for MAEL. Small granules in PCs and STs are stained for both proteins. Scale, 10 μm.
IEM Evaluation of AGO2 Localization in Rat Spermatogenic Cells
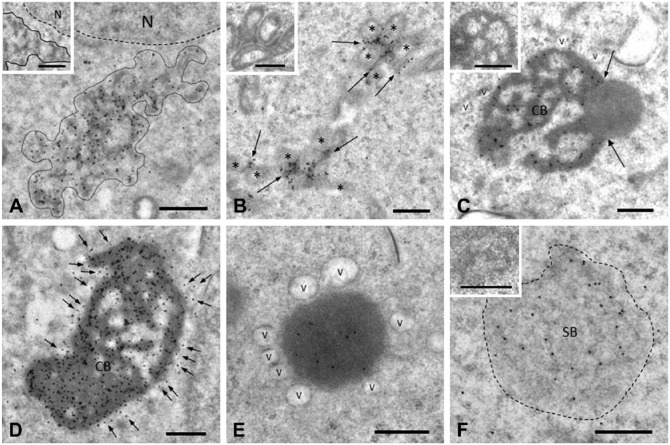
Nuage structures of rat testis were classified into four types (ISPGs, IMC, SB, and CB) in our previous study (Yokota 2012a). In the present study, we used gold labeling and IEM to evaluate the localization of AGO2 in these four types. Strong signals for AGO2 were found in nuage structures of pachytene spermatocytes and round spermatids, whereas weak signals were scattered in cytoplasm and nucleoplasm of spermatocytes. For ISPGs, gold particles indicating AGO2 sites were associated with electron-dense material, particularly in the periphery (Fig. 4A). IMC was strongly labeled for AGO2 (Fig. 4B). CBs were variably labeled in round spermatids depending on the developmental step; the strongest labeling was observed for step 5 (Fig. 4C, 4D). Gold labeling was concentrated in dense material but surrounding small clear vesicles were also AGO2-positive (Fig. 4D). Late CBs in elongated spermatids, which moved to the cytoplasm around the base of the flagellum, were weakly labeled, and surrounding clear vesicles were AGO2-negative (Fig. 4E). AGO2 was localized in SBs. Gold labeling was associated with a dense thread-like material (Fig. 4F). No signals were observed in the controls (Fig. 4, inserts).
Figure 4.
IEM staining for AGO2 in various nuage structures. (A) ISPGs. Gold particles showing AGO2 labeling are associated with a dense material that comprises ISPGs (bordered by solid line). (B) IMC. AGO2 signals (arrows) are present on IMC between mitochondria (*). (C) CB from early round spermatids. AGO2 signals are associated with dense worm-like structures that comprise CBs. The dense material is sometimes attached to less dense material that is AGO2-negative (arrows). Small clear vesicles (v) are usually attached to CBs. (D) CBs from step 5 spermatids. Strong AGO2 signals are seen on dense material. Many clear vesicles surrounding CBs (arrows) are also AGO2-positive. (E) Late CB from step 9–11 spermatids. AGO2 signals are weak. No labeling is seen in vesicles (v) surrounding CBs. (F) SB. Moderate AGO2 signals are associated with dense material network. No AGO2 signals are seen in IEM controls (inserts in all panels). Scale, 0.5 μm.
Our previous studies have shown that nuage proteins, including BRUNOL2 (Yonetamari et al. 2012), DDX4 (Onohara et al. 2010), DDX6 (Kawahara et al. 2013), DDX25 (Onohara and Yokota 2012), MAEL (Takebe et al. 2013), and NANOS1 (Yokota and Onohara, 2013), are present in spermatogenic cell-specific structures, as described by Russell and Frank (1978) and Clermont et al. (1993): granulated bodies, reticulated bodies, mitochondria-associated granules, ribosome aggregates, puffs, and neck particles (Yokota 2012a). In the present study, AGO2 was not detected in any of these non-nuage structures (data not shown).
Dual IEM Labeling of AGO2/MAEL and AGO2/MIWI
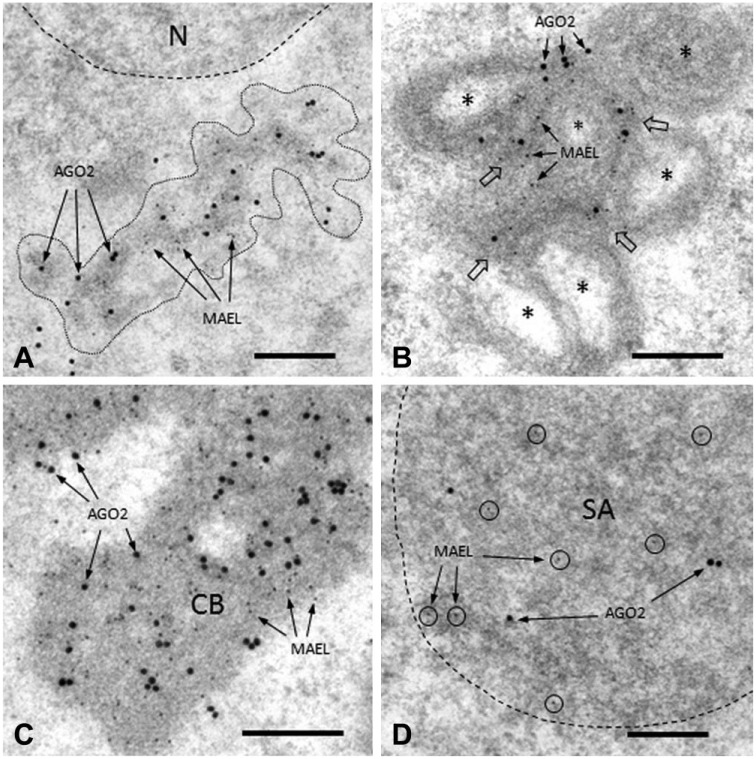
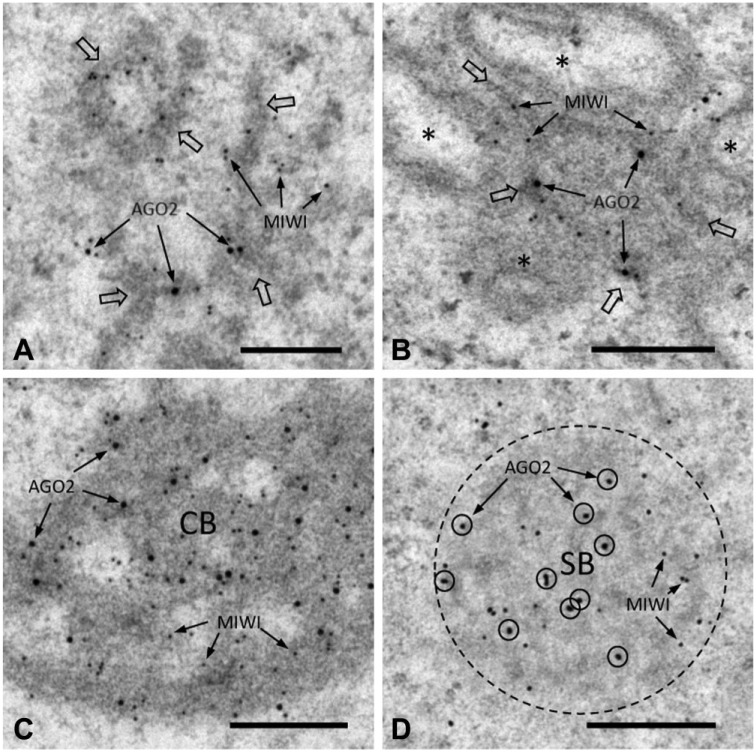
MAEL protein is a known nuage component (Soper et al. 2008; Aravin et al. 2009; Takebe et al. 2013). We used a dual-labeling technique to evaluate the colocalization of AGO2 with MAEL in four nuage structures. The two proteins were colocalized in ISPGs (Fig. 5A), IMC (Fig. 5B), CBs (Fig. 5C), and SBs (Fig. 5D). In the present study, we also observed AGO2/ MIWI colocalization in ISPGs, IMC, CBs, and SBs (Fig. 6A–6D). The two proteins were associated with dense material in each of these cases. In controls, no signals were detected in nuage structures (data not shown).
Figure 5.
Dual IEM labeling for AGO2 and MAEL proteins in nuage structures in rat spermatogenic cells. Large (25-nm diameter) and small (5-nm) gold particles correspond to AGO2 and MAEL proteins, respectively. (A) ISPGs (bordered by dotted line). AGO2 and MAEL signals are associated with dense material (ISPGs). (B) IMC. AGO2 and MAEL signals (arrows) are seen in dense material (IMC), as indicated by the large open arrows, between mitochondria (*). (C) Part of a CB. Strong AGO2 and MAEL signals are seen in dense material (CB). (D) Part of a SB (bordered by dotted line). AGO2 and MAEL signals are seen in SB. Scale, 0.25 μm.
Figure 6.
Dual IEM labeling for AGO2 and MIWI proteins in nuage structures. Large (25-nm diameter) and small (5- or 8.5-nm) gold particles correspond to AGO2 and MIWI proteins, respectively. (A) ISPGs (indicated by large open arrows). AGO2 and MIWI signals are colocalized (arrows) in dense material (ISPGs). (B) IMC (indicated by large open arrows). AGO2 and MIWI signals (arrows) are seen in dense material (IMC) between mitochondria (*). (C) CB. AGO2 and MIWI signals are closely associated with dense material (CB). (D) SB (bordered by dotted line). AGO2 (circled) and MIWI signals are seen in SB. Scale, 0.25 μm.
Altered AGO2 Labeling Intensity in Spermatogenic Cells during Spermatogenesis
Altered AGO2 labeling intensity (quantified using arbitrary units based on IEM data) in the four types of nuage structure during spermatogenesis is summarized in Table 1. AGO2 signals in ISPGs increased in parallel with their development, peaking in pachytene spermatocytes at stage IX and declining sharply thereafter. Signals in IMC appeared in pachytene spermatocytes at stage VI, peaked at stage IX, and then declined; no signals were detected after stage XII. AGO2 signals in CBs were seen in spermatids at step 1, increased rapidly, peaked at step 5, and then decreased gradually, with no signals were detected after step 12. Signals in SBs appeared in pachytene spermatocytes at stage IX and disappeared by stage XII.
Relationships of CB with Lysosomal Compartments
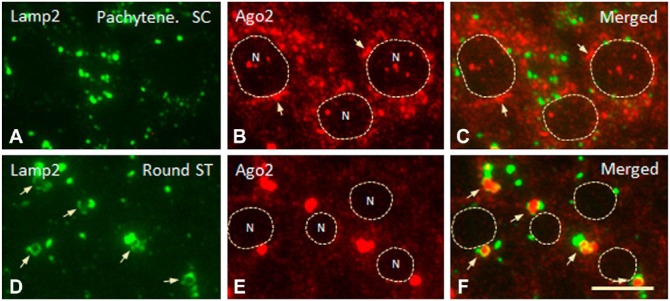
AGO2 was detected in clear vesicles surrounding CBs. We demonstrated previously that these vesicles contain a lysosomal membrane protein (LAMP2) and the lysosomal proteases cathepsin D and H (Haraguchi et al. 2005). Here, we used a high-resolution IFM technique on 250-nm sections to evaluate the localization of AGO2 and LAMP2 in nuage. In pachytene spermatocytes, LAMP2-positive small granules were not in contact with small-round or short strand-shaped AGO2-positive granules (Fig. 7A–7C). In round spermatids, in contrast, LAMP2-positive granules were round- or torus-shaped, and many of them were in contact with AGO2-positive granules, which were often located in the central area of the torus-shaped granules (Fig. 7D–7F). These observations suggest that a CB is surrounded by small lysosomal components.
Figure 7.
Dual IFM labeling for LAMP2 (green) and AGO2 (red) proteins in spermatogenic cells. (A–C) Staining for LAMP2 and AGO2 in pachytene spermatocytes (SC) at stage V. Small granules stained (separately) for the two proteins are not in close contact with each other. Strand-shaped structures (arrows) in contact with nuclei (N) are AGO2-positive. Punctate staining for AGO2 is seen in nuclei. (D–F) LAMP2 and AGO2 staining in round spermatids (ST) at step 5. LAMP2-positive granules (arrows) are in close contact with and frequently surround large AGO2-positive granules. No punctate staining for AGO2 is seen in nuclei (N, surrounded by dotted line). Scale,10 μm.
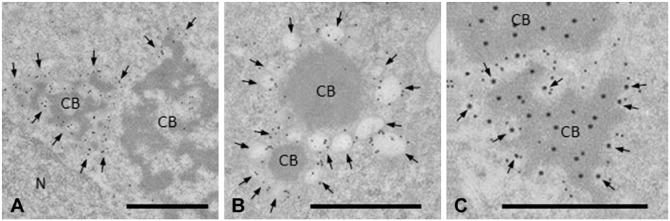
To test this possibility, we performed IEM single staining of LAMP2 and dual staining of LAMP2 and AGO2. Small vesicles attached to or surrounding CBs were LAMP2-positive (Fig. 8). CBs in round spermatids were partially surrounded by LAMP2-positive vesicles, whereas CBs in elongated spermatids were smaller and completely covered by vesicles (Fig. 8A, 8B). Dual IEM staining showed that AGO2 was localized in both CBs and LAMP2-positive vesicles (Fig. 8C).
Figure 8.
IEM staining of LAMP2 protein in vesicles surrounding CBs. (A) CBs in step 5 spermatids. The CB to the left is surrounded by LAMP2-positive vesicles, whereas the CB to the right is in contact with only a few vesicles (arrows). (B) Late CBs in step 9 spermatids. CBs are notably smaller and homogeneous, and almost entirely surrounded by LAMP2-positive vesicles (arrows). (C) Dual labeling of CBs for LAMP2 (small gold particles) and AGO2 (large particles). AGO2 is colocalized with LAMP2 in vesicles (arrows). N, nucleus. Scale, 1 μm.
Discussion
Localization of AGO2 in Spermatogenic Cells
Immunofluorescence staining revealed that AGO2 is localized in cytoplasmic matrix (cytosol) and in granular compartments (of various shapes and sizes) in pachytene spermatocytes. In round spermatids, AGO2 staining was observed in irregularly shaped granules. Dual staining of AGO2 and MAEL (a typical nuage protein) showed colocalization of these two proteins in granules in round spermatids, suggesting that these granules are CBs. It was unclear whether the two proteins were similarly colocalized in granules in pachytene spermatocytes, because MAEL in these granules was weakly stained by immunofluorescence. Our previous study (Takebe et al. 2013) demonstrated positive IEM staining of nuage in pachytene spermatocytes. In the present study, we therefore applied IEM to dual staining of AGO2 and MAEL. We observed clear colocalization of the two proteins in ISPGs, IMC, and SBs in pachytene spermatocytes, and in CBs of round spermatids. AGO2 was similarly colocalized with MIWI in ISPGs, IMC, and SBs in pachytene spermatocytes, and in CBs in round spermatids. These findings suggest that these nuage components are subcellular sites of AGO2 functioning in spermatogenic cells.
In the fetal piRNA pathway of mouse testis, MILI and MIWI2 proteins are localized in two types of germinal cytoplasmic granules (Aravin et al. 2009). The first type is pi-bodies having the MILI-TDRD1 module of the piRNA pathway. The second type is piP-bodies having the MIWI2-TDRD9-MAEL module of the piRNA pathway and containing P-body components (e.g., GW182, DCP1a, DDX6/p54, and XRN1 proteins). Morphologically, pi-bodies and piP-bodies correspond (respectively) to IMC and SBs observed in the present study. Satellite bodies (SB; also known as sponge bodies) were shown in early studies (Fawcett et al. 1970; Russell and Frank 1978) to differ from the perinuclear granules that we term ISPGs based on their EM morphology and stage of emergence in the cycle (Russell et al. 1990). ISPGs appear mainly in pachytene spermatocytes at stages VI to XII, whereas SBs appears mainly at stages IX to X (Takebe et al. 2013). In adult testis, ISPGs are far larger and more numerous than SBs. ISPGs are thus discrete granules clearly distinguishable from SBs and the other nuage structures. The role of ISPGs in the piRNA pathway remains to be elucidated.
AGO2 has a ribonuclease H domain, and mutations in this domain result in the inactivation of RISC, suggesting that the slicer activity of AGO2 provides the catalytic engine for RNA interference (Liu et al. 2007). Localization of AGO2, as revealed by the present study, suggests that siRISC and/or miRISC are localized to IMC, ISPG, and SBs of pachytene spermatocytes, and to CBs of round spermatids. They, in addition to piRISC, may contribute to RNA silencing/processing pathways in those locations.
Relationships among Nuage and Lysosomal Compartments
We previously reported localization of various nuage proteins in spermatogenic cells (Yokota 2012a). In the present study, only AGO2 showed positive staining in the clear vesicles surrounding CBs in round spermatids. It is unclear why nuage proteins other than AGO2 were not detected in these vesicles. Under IFM, torus-shaped vesicles were stained for both AGO2 and LAMP2. Typical lysosomes in spermatogenic cells are larger than the clear vesicles, are located primarily near the nucleus, and are stained for LAMP2 but not for AGO2. In pachytene spermatocytes under EM, ISPGs, IMC, and SBs were not surrounded by LAMP2-positive vesicles. In round and elongated spermatids, CBs were in contact with and surrounded by LAMP2-positive vesicles containing the lysosomal proteases cathepsin D and H (Haraguchi et al. 2005).
The lysosomal system is an intracellular digestion machinery (Mortimore and Pösö 1984; Dunn Jr. 1994) that functions in cooperation with the ubiquitin/proteasome system (Hershko and Ciechanover 1992; Hochstrasser 1995). Our present findings suggest that (i) nuage (CB) in spermatids is closely associated with the lysosomal digestion machinery, whereas nuage structures (ISPGs, IMC, SB) in pachytene spermatocytes are not, and (ii) ISPGs, IMC, and SBs in pachytene spermatocytes are sites at which nuage proteins function (only), whereas CBs in spermatids are sites at which nuage proteins function and are simultaneously degraded.
We previously demonstrated that the CB has several “aggresomal markers”, including Hsp70, ubiquitin, vimentin, and proteasome subunits (Haraguchi et al. 2005). Aggresomes are subcellular foci for the degradation of misfolded or unnecessary proteins by the ubiquitin/proteasome system or by autophagy (for review see Kopito and Sitia 2000; Garcia-Mata et al. 2002; Wójcik and DeMartino 2003). Unnecessary proteins generated within CBs in round and elongated spermatids may be degraded by the lysosomal system as well as the ubiquitin/proteasome system. Our 2005 study also showed that proteins associated with the nucleus, cytosol, and mitochondria are present in CBs (Haraguchi et al. 2005). In a mouse study, Amigo et al. (2012) reported that SCaMC-1L, a subgroup of ATP-Mg/Pi carriers, is expressed exclusively in male germ cells, is regulated during spermatogenesis, and is found in CBs in round spermatids and IMC in spermatocytes, as well as in mitochondria. In transient expression experiments, SCaMC-1L in COS-7 cells formed aggregates that displayed aggresome-like features similar to those of CBs. The results of the present study suggest that protein/RNA degradation pathways seem to be associated with the CB and could be involved in the degradation of CB material. Such nuage-mediated degradation of proteins presumably does not occur in pachytene spermatocytes because nuage in these cells has no relationship with lysosomal compartments or proteasomes.
In conclusion, AGO2 in pachytene spermatocytes is localized to ISPGs, IMC, SBs, nucleus, and cytosol, whereas AGO2 in spermatids is localized to CBs and cytosol. AGO2 is colocalized with other nuage proteins (MAEL, MIWI) in both spermatocytes and spermatids. These nuage components appear to be AGO2 functional sites. AGO2 also colocalizes with the lysosomal membrane protein LAMP2 in small vesicles that contact and surround CBs but not ISPGs, IMC, or SBs. Our findings suggest that CBs are related to the lysosomal pathway, whereas ISPGs, IMC, and SBs are not.
Acknowledgments
The authors are grateful to Dr. S. Anderson for English editing of the manuscript.
Footnotes
Author Contributions: YF performed the western blotting, immunofluorescence microscopy, YO carried out the preparation of rabbit antibody to AGO2, HF designed the study and drafted the manuscript, SY designed the study, performed immunofluorescence and immunoelectron microscopy and drafted the manuscript. All authors have read and approved the final manuscript.
Competing Interests: The authors declared no potential competing interests with respect to the research, authorship, and/or publication of this article.
Funding: This study was supported in part by research funding from Nagasaki International University, grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology (17570158 to SY, 25840119 to YF, 26460086 to HF), and by the Science Research Promotion Fund of the Promotion and Mutual Aid Corporation for Private Schools of Japan.
References
- Amigo I, Traba J, Satrústegui J, del Arco A. (2012). SCaMC-1like a member of the mitochondrial carrier (MC) family preferentially expressed in testis and localized in mitochondria and chromatoid body. PLOS ONE 7:e40470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. (2007). Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316:744-747. [DOI] [PubMed] [Google Scholar]
- Aravin AA, van der Heijden GW, Castañeda J, Vagin VV, Hannon GJ, Bortvin A. (2009). Cytoplasmic compartmentalization of the fetal piRNA pathway in mice. PLOS Genetics 5:e1000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyret E, Liu N, Liu H. (2012). piRNA biogenesis during adult spermatogenesis in mice is independent of the ping-pong mechanism. Cell Res 22:1429-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Aravin AA, Stark A, Dus M, Kellis M, Sachidanandam R, Hannon G. (2007). Discrete small RNA-generating loci as transposon activity in drosophila. Cell 128:1089-1103. [DOI] [PubMed] [Google Scholar]
- Carthew RW. (2006). A new RNA dimension to genome control. Science 313:305-306. [DOI] [PubMed] [Google Scholar]
- Cenik ES, Zamore PD. (2011). Argonaute proteins. Current Biol 21:R446-R449. [DOI] [PubMed] [Google Scholar]
- Clermont Y, Oko R, Hermo L. (1993). ‘Cell biology of mammalian spermiogenesis’ in Desjardins C, Ewing LL, (eds). Cell and molecular biology of the testis. New York: Oxford University Press, pp. 332-376. [Google Scholar]
- Dunn WA., Jr.(1994). Autophagy and related mechanisms of lysosome-mediated protein degradation. Trends in Cell Biology 4:139-143. [DOI] [PubMed] [Google Scholar]
- Fawcett DW, Eddy EM, Phillips DM. (1970). Observations on the fine structure and relationships of the chromatoid body in mammalian spermatogenesis. Biol Reprod 2:129-153. [DOI] [PubMed] [Google Scholar]
- Garcia-Mata R, Gao Y-S, Sztul E. (2002). Hassles with taking out the garbage: aggravating aggresomes. Traffic 3:388-396. [DOI] [PubMed] [Google Scholar]
- Haraguchi CM, Yokota S. (2002). Immunofluorescence technique for 100-nm-thick semithin sections of Epon-embedded tissues. Histochem Cell Biol 117:81-85. [DOI] [PubMed] [Google Scholar]
- Haraguchi CM, Mabuchi T, Hirata S, Shoda T, Hoshi K, Akasaki K, Yokota S. (2005). Chromatoid bodies: aggresome-like characteristics and degradation sites for organelles of spermiogenic cells. J Histochem Cytochem 53:455-465. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. (1992). The ubiquitin system for protein degradation. Ann Rev Biochem 61:761-807. [DOI] [PubMed] [Google Scholar]
- Hochstrasser M. (1995). Ubiquitin, proteasomes, and the regulation of intracellular protein degradation. Current Opinion in Cell Biol 7:315-223. [DOI] [PubMed] [Google Scholar]
- Kawahara C, Yokota S, Fujita H. (2013). DDX6 localizes to nuage structures and the annulus of mammalian spermatogenic cells. Histochem Cell Biol 141:111-124. [DOI] [PubMed] [Google Scholar]
- Kopito RR, Sitia R. (2000). Aggresomes and Russell bodies. Symptoms of cellular indigestion? EMBO Rep 1:225-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotaja N, Lin H, Parvinen M, Sassone-Corsi P. (2006). Interplay PIWI/Argonaute protein MIWI and kinesin KIF17b in chromatoid bodies of male germ cells. J Cell Sci 119:2819-2825. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. (2004). Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15:185-197. [DOI] [PubMed] [Google Scholar]
- Mortimore GE, Pösö AR. (1984). Lysosomal pathway in hepatic protein degradation: regulatory role of amino acids. Federation Proc 43:1289-1294. [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song J-J, Hammond SM, Joshua-Tor L, Hannon GJ. (2007). Argonaute2 is the catalytic engine of mammalian RNAi. Science 305:1437-1441. [DOI] [PubMed] [Google Scholar]
- Onohara Y, Fujiwara T, Yasukochi T, Himeno M, Yokota S. (2010). Localization of mouse vasa homolog protein in chromatoid body and related nuage structures of mammalian spermatogenic cells during spermatogenesis. Histochem Cell Biol 133:627-639. [DOI] [PubMed] [Google Scholar]
- Peters L, Meister G. (2007). Argonaute proteins: mediators of RNA silencing. Molecular Cell 26:611-624. [DOI] [PubMed] [Google Scholar]
- Russell L, Frank B. (1978). Ultrastructural characterization of nuage in spermatocytes of the rat testis. Anat Rec 190:79-98. [DOI] [PubMed] [Google Scholar]
- Russell LD, Ettlin RA, Hikim APS, Clegg ED. (1990). Histological and histopathological evaluation of the testis. Florida: Cache River Press. [Google Scholar]
- Sasaki T, Shiohama A, Minoshima S, Shimizu N. (2003). Identification of eight members of the Argonaute family in the human genome. Genomics 82:323-330. [DOI] [PubMed] [Google Scholar]
- Siomi MC, Sato K, Pezic D, Aravin AA. (2011). PIWI-interacting small RNAs: the vanguard of genome defense. Nature Rev Mol Cell Biol 12:246-258. [DOI] [PubMed] [Google Scholar]
- Soper SF, van der Heijden GW, Hardiman TC, Gooheart M, Martin SL, de Boer P, Bortin A. (2008). Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 15:285-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebe M, Onohara Y, Yokota S. (2013). Expression of MAEL in nuage and non-nuage compartments of rat spermatogenic cells and colocalization with DDX4, DDX25 and MIWI. Histochem Cell Biol 140:169-181. [DOI] [PubMed] [Google Scholar]
- Wójcik C, DeMartino GN. (2003). Intracellular localization of proteasomes. Int J Biochem Cell Biol 35:579-589. [DOI] [PubMed] [Google Scholar]
- Yokota S. (2008). Historical survey on chromatoid body research, Acta Histochem Cytochem 41:65-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S. (2012a). Nuage proteins: their localization in subcellular structures of spermatogenic cells as revealed by immunoelectron microscopy. Histochem cell Biol 138:1-11. [DOI] [PubMed] [Google Scholar]
- Yokota S. (2012b). ‘Preparation of colloidal gold particles and conjugation to Protein A, IgG, F(ab’)2, and Streptavidine’ in Schwarzbach SD, Osafune T, (eds.) Immunoelectron microscopy. Methods and protocols. New York: Springer, pp. 109-119. [Google Scholar]
- Yokota S, Onohara Y. (2013). Expression sites of NANOS1 in rat spermatogenic cells as revealed by immunoelectron microscopy. Open J Cell Bio 2:1-10. [Google Scholar]
- Yonetamari H, Onohara Y, Yokota S. (2012). Localization of BRUNOL2 in rat spermatogenic cells as revealed by immunofluorescence and immunoelectron microscopic techniques. Open J Cell Biol 2:11-20. [Google Scholar]