Abstract
Shear-resistant adhesion and extravasation of disseminated cancer cells at the target organ is a crucial step in hematogenous metastasis. We found that the vascular adhesion molecule E-selectin preferentially promoted the shear-resistant adhesion and transendothelial migration of the estrogen receptor (ER)–/CD44+ hormone-independent breast cancer cells, but not of the ER+/CD44-/low hormone-dependent breast cancer cells. Coincidentally, CD44+ breast cancer cells were abundant in metastatic lung and brain lesions in ER– breast cancer, suggesting that E-selectin supports hematogenous metastasis of ER–/CD44+ breast cancer. In an attempt to prevent hematogenous metastasis through the inhibition of a shear-resistant adhesion of CD44+ cancer cells to E-selectin-expressing blood vessels on the premetastatic niche, an E-selectin targeted aptamer (ESTA) was developed. We demonstrated that a single intravenous injection of ESTA reduced metastases to a baseline level in both syngeneic and xenogeneic forced breast cancer metastasis models without relocating the site of metastasis. The effect of ESTA was absent in E-selectin knockout mice, suggesting that E-selectin is a molecular target of ESTA. Our data highlight the potential application of an E-selectin antagonist for the prevention of hematogenous metastasis of ER–/CD44+ breast cancer.
Introduction
Breast cancer is the second most common malignancy among women in western countries. Distant organ metastasis is the major cause of breast cancer-related mortality. Approximately 30% of women experience a recurrence within 5 years, with a 56% chance of distant metastasis,1 primarily to the bones, lung, brain, and liver.2,3,4 The incidence of visceral metastasis is higher in estrogen receptor (ER)– breast cancer than in other subtypes.5 After the development of visceral metastasis, the chance of a complete eradication of disease with currently available therapies is slim.6 The existing therapeutic options for metastatic breast cancer are mostly palliative and are associated with limited benefits. Therefore, a new strategy to control metastasis development is needed.
Distant metastasis occurs through either the lymph nodes or the blood stream. Lymph node involvement has been used as an independent prognostic factor in breast cancer, but ~30% of women develop distant metastases without axillary lymph node metastasis,7 indicating that hematogenous metastasis is an important contributor to mortality in breast cancer. Hematogenous metastasis is a multistep, sequential process that requires (i) local invasion by the primary tumor, (ii) intravasation, (iii) anoikis resistance, (iv) adhesion to blood vessels of the target organ, (v) transmigration/extravasation through the vascular endothelium of the target organ, and (vi) colonization at the target organ.8,9 All of these can be rate-limiting processes, and incompletion of any of these processes leads to failure metastasis. Recent studies suggest that disseminated circulating cancer cells successively adhere to and extravasate at the premetastatic niche.10,11 Thus, the circulation is an interface between the primary tumor and the target organ, and the vascular endothelium at the premetastatic niche represents a vital gateway for disseminated cancer cells to enter the target organ. Therefore, the premetastatic niche is an excellent therapeutic target for the prevention of hematogenous metastasis.
E-selectin (CD62E, ELAM-1, or LECAM-2) is expressed exclusively on the vessel surface in response to inflammatory cytokines.12 E-selectin mediates shear-resistant adhesion of circulating cells to the vessel surface via physical interaction with its ligand present on the circulating cells.13,14 This interaction results in a catch bond that switches from rolling adhesion of the circulating cells to firm adhesion along the endothelium mediated by further interactions with other adhesion molecules. Some cancer cells utilize the same adhesion cascade for their adhesion and transmigration.15,16 Although E-selectin expression is tightly controlled under normal conditions, its expression on the vessel surface is induced at distant organs at the premetastatic stage in response to secreted soluble factors from the primary tumor, rendering the distant target organ susceptible to hematogenous metastasis.10,11,17 The interaction of metastatic cancer cells with endothelial cells via E-selectin induces bidirectional signaling that results in increased endothelial permeability through the dissociation of VE-cadherin/β-catenin, in turn accelerating the transendothelial migration.18 Because of the role of E-selectin in the adhesion cascade,19,20 we aimed to develop a preventive approach using a thiophosphate backbone modified aptamer (thioaptamer) against E-selectin targeted aptamer (ESTA) to block hematogenous metastases of ER– breast cancer cells.
Results
E-selectin preferentially enhances the shear-resistant adhesion of ER– breast cancer cells to endothelial cells
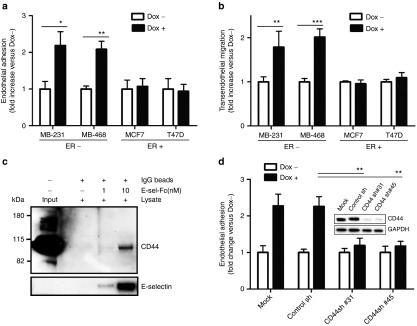
To understand the involvement of E-selectin in hematogenous metastasis, flow adhesion and transendothelial migration assays were performed using two ER– basal-like/CD44+ breast cancer cell lines, MDA-MB-231 and MDA-MB-468, two ER+ luminal-type/CD44low/– breast cancer cell lines, MCF-7 and T-47D, and a Tet-on inducible E-selectin human microvessel endothelial cell line (HMVEC).21 To exclusively investigate the role of E-selectin on endothelium, E-selectin expression was induced by doxycycline at a level similar to that induced by TNF-α.21 The shear-resistant adhesion of breast cancer cells to endothelial cells was examined under physiological flow conditions. The shear-resistant adhesion of MDA-MB-231 and MDA-MB-468 to E-selectin-expressing HMVEC increased 2.2-fold (P = 0.016) and 2.1-fold (P = 0.007), respectively (Figure 1a). In contrast, induction of E-selectin expression on endothelial cells did not trigger the shear-resistant adhesion of ER+ breast cancer cell lines MCF-7 and T-47D (1.1- and 0.9-fold, Figure 1a). The adhesion of MDA-MB-231 to E-selectin-expressing HMVEC was highest at 1 dyn/cm2, steeply declined at 2 dyn/cm2, and was abolished at 3 dyn/cm2 (see Supplementary Figure S1), suggesting that the E-selectin dependent adhesion of ER– breast cancer cells occurs at low shear stress if other adhesion molecules are not expressed. Similarly, E-selectin expression on HMVEC enhanced the transendothelial migration of MDA-MB-231 and MDA-MB-468 cells 1.8-fold (P = 0.002) and 2.0-fold (P = 0.0004), respectively. However, it did not enhance migration of MCF-7 or T-47D cells (0.9- and 1.1-fold, Figure 1b). These data suggest that E-selectin preferentially enhances the shear-resistant adhesion and transendothelial migration of ER– breast cancer cells, but not of ER+ cells. Further, these data indicate that binding partners for E-selectin are present on ER– breast cancer cells, but not on ER+ cells. We focused on CD44 as a potential E-selectin ligand present on cancer cells, because the hormone dependency of breast cancer is inversely correlated with CD44 status, i.e., ER– breast cancer cells are CD44+ and ER+ cells are CD44-/low (see Supplementary Figure S2). Physical interactions between CD44 and E-selectin were examined by performing a pull-down assay. The data demonstrated that E-selectin directly interacts with CD44, as detected by increased CD44 pull-down with an increased amount of E-selectin-Fc chimeric protein (Figure 1c and Supplementary Figure S3). Further, the functional knockdown of CD44 expression using CD44 shRNAs resulted in a reduction of the shear-resistant adhesion of two stably transfected MDA-MB-231 clones (CD44Sh31 and CD44Sh45) by 84% (P = 0.007) and 86% (P = 0.009), respectively, when compared with the control shRNA (Figure 1d). Collectively, these results suggest that E-selectin enhanced the shear-resistant adhesion and transendothelial migration of ER–/CD44+ breast cancer cells.
Figure 1.
E-selectin induces shear-resistant adhesion and transendothelial migration via interaction between E-selectin and CD44. (a) Effect of E-selectin on the shear-resistant adhesion of breast cancer cells to endothelial cells under physiological flow. A monolayer of Tet-on inducible E-selectin endothelial cells was grown on the chamber and incubated with doxycycline (1,500 ng/ml) for 5 hours. The shear-resistant adhesion of breast cancer cell lines (1 × 105 cells/ml) was tested at 37 °C for 5 minutes at 1 dyn/cm2. Cancer cells adherent to endothelial cells were counted microscopically (final magnification ×100) and compared +doxycycline versus –doxycycline. (b) Effect of E-selectin on transendothelial migration. Cancer cells that migrated through the E-selectin-inducible HMVEC monolayer were compared +doxycycline versus –doxycycline. (c) E-selectin physically interacts with CD44. Pull-down assays were performed using IgG magnetic beads coupled with two different concentration of recombinant human E-selectin-Fc chimera (1 and 10 nmol/l) or IgG beads alone. The beads were incubated with cell lysate isolated from MDA-MB-231 and the pull-down was analyzed by western blot using antipan-CD44 and anti-E-selectin antibodies. (d) CD44 mediates the shear-resistant adhesion of breast cancer cells to E-selectin-expressing endothelial cells. Two stable MDA-MB-231 clones that are transfected with shRNA against CD44 or control shRNA were infused into a flow chamber grown with E-selectin expressing HMVEC at 1 dyn/cm2. The data represent mean ± SD. Statistical significance was determined by Student's t-test.
CD44 is highly abundant in distant metastatic lesions of ER– breast cancer
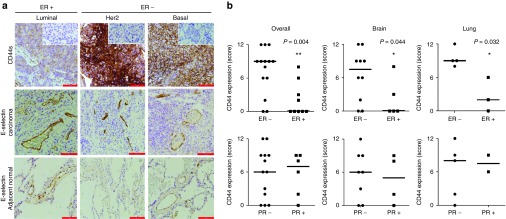
To seek further evidence for the involvement of CD44 in hematogenous metastasis of breast cancer, we evaluated CD44 expression using 23 metastatic breast tumors in the brain and lung (see Supplementary Table S1). Pathologic evaluation was performed by two independent pathologists (LZ and WZ). As a negative control, carcinoma tissues were stained with normal IgG. The expression of CD44 was significantly higher in ER– tumors than in ER+ tumors in the unadjusted analysis (P = 0.006, Figure 2a,b). In addition, the expression of CD44 was significantly higher in ER– metastatic breast tumors in lung (P = 0.032) and brain (P = 0.044), respectively (Figure 2b). However, no significant correlation was found between CD44 expression and PR status. E-selectin expression was highly upregulated in carcinoma tissue and noncancerous lung tissues adjacent to metastatic lung lesion (Figure 2a). These data may partly support preferential shear-resistant adhesion and transendothelial migration of CD44+ breast cancer cells to E-selectin-expressing endothelial cells and provide a further rationale for the therapeutic use of a functional blocker of E-selectin for the prevention of organ metastasis in ER– breast cancer.
Figure 2.
Immunohistochemical analyses of CD44 in metastatic breast tumor. (a) CD44 expression in metastatic breast tumor in the lung and E-selectin expression in tumor and adjacent lung were analyzed by immunohistochemistry. The images were captured at a final magnification of ×200 for CD44 and ×100 for E-selectin. The scale bars represent 100 and 200 μm, respectively. Inset shows a negative control that was probed with secondary antibody alone. (b) CD44 expression in metastatic breast tumor is inversely correlated with ER statuses, but not with PR. CD44 expression was analyzed semiquantitatively in a blinded fashion using a grading system from 0 to 12. The bars represent median. Statistical significance was determined by Mann–Whitney unadjusted test.
ESTA inhibits shear-resistant adhesion of breast cancer cells to endothelial cells
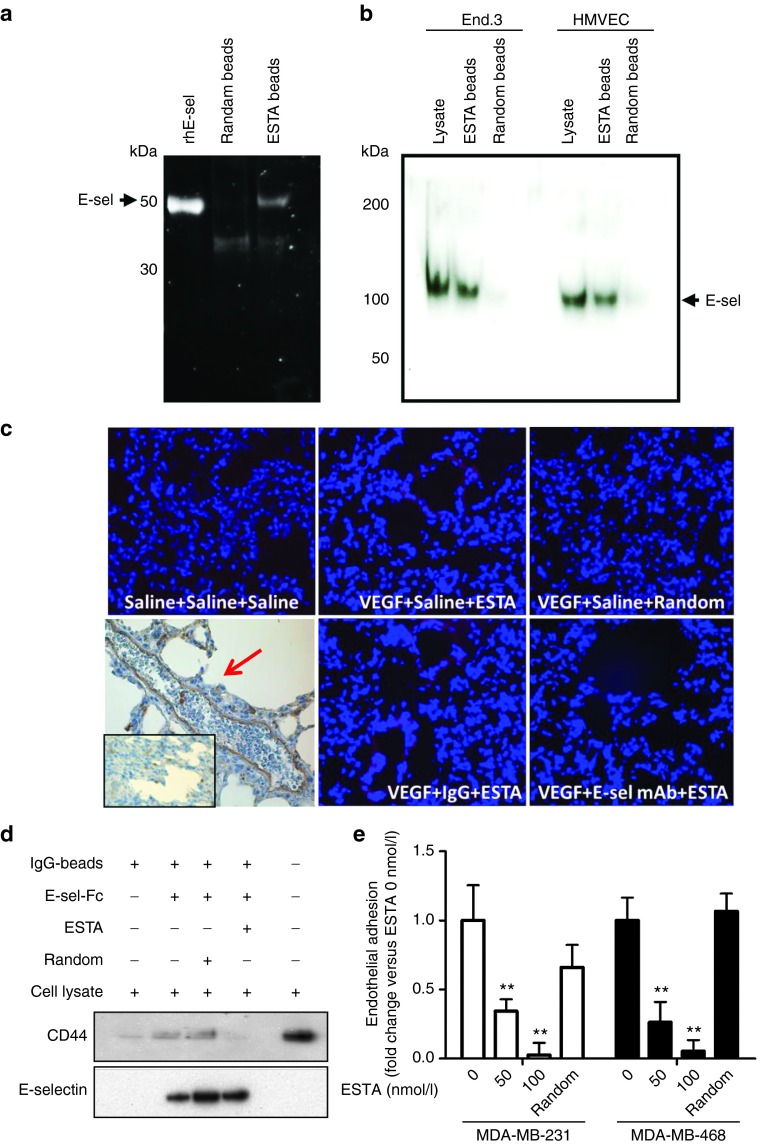
We previously reported a thioaptamer against E-selectin (ESTA) as a high-affinity functional E-selectin blocker (KD = 47 nmol/l, IC50 = 63–80 nmol/l).21 We further confirmed specific binding of ESTA to E-selectin. We found that the external domain of recombinant human E-selectin protein bound to ESTA-conjugated silica beads, whereas no binding was detected in random aptamer-conjugated beads (Figure 3a). Similarly, ESTA-conjugated beads pulled down 110 kDa E-selectin protein from plasma membrane fractions isolated from HMVEC and mouse endothelial cell lines (End.3), whereas random aptamer conjugated beads did not pull down E-selectin (Figure 3b). In addition, we found that an i.v. injection of Cy3-labeled ESTA (100 µg in 100 µl saline) accumulated to lung capillaries of mice that were preconditioned with VEGF; however, preinjection of E-selectin monoclonal antibody blocked Cy3-ESTA binding to vessel walls (Figure 3c). Intravenous injection of the same amount of random aptamer did not exhibit visible binding to the lung capillaries (Figure 3c). Furthermore, the results from the pull-down assay showed that ESTA completely abrogated the physical interaction between CD44 and E-selectin-Fc chimera, whereas the random aptamer did not interfere with this interaction (Figure 3d and Supplementary Figure S4). Finally, preincubation of E-selectin-expressing HMVEC cells with 50 nmol/l ESTA resulted in an inhibition of the shear-resistant adhesion of ER–/CD44+ breast cancer of 2.9-fold inhibition, P = 0.009, in MDA-MB-231 cells, and 3.8-fold inhibition, P = 0.003, in MDA-MB-468 cells (Figure 3e). Preincubation of doxycycline treated HMVEC with 100 nmol/l ESTA abolished the E-selectin-dependent adhesion to a level similar to the adhesion to untreated HMVEC at all shear stress levels (see Supplementary Figure S5). The random aptamer did not interfere with the E-selectin-dependent adhesion (Figure 3e and Supplementary Figure S5). ESTA up to 500 nmol/l was not cytotoxic to either HMVEC endothelial21 or MDA-MB-231 breast cancer cells (see Supplementary Figure S6), suggesting that ESTA is a noncytotoxic functional antagonistic ligand for both human and mouse E-selectin.
Figure 3.
ESTA is a functional antagonistic ligand of E-selectin. (a) Physical interaction of ESTA with recombinant human E-selectin. ESTA or random aptamer conjugated silica beads were incubated with the external domain of rhE-selectin. The ESTA-conjugated silica beads were incubated with SDS sample buffers to release the protein, separated by SDS–PAGE, and visualized by SYPRO RUBY. Arrows indicate the rhE-selectin. (b) Physical interaction of ESTA with human and mouse plasma membrane E-selectin. Plasma membrane proteins were isolated from human (HMVEC) and mouse (End.3) endothelial cell lines to test the binding to the ESTA or random aptamer conjugated silica beads. Arrows indicate the E-selectin (110 kDa) extracted from plasma membranes. (c) The binding of ESTA to E-selectin expressing lung capillaries. Six-week-old athymic nu/nu mice were preconditioned with i.v. injection of VEGF (100 ng). E-selectin expression in the lung capillaries was confirmed by immunohistochemistry as indicated by the red arrow. Three hours after VEGF or saline injection, the mice were intravenously injected with either saline, rat antimouse E-selectin monoclonal antibody, or rat IgG (100 µg). Three hours later, the mice were intravenously injected with Cy3-labeled ESTA (100 µg). Tissues were harvested 12 hours later and 6-µm frozen sections of lung tissue were counterstained with Hoechst 33342. (d) ESTA inhibits physical interaction of CD44 and E-selectin. IgG magnetic beads coupled with rhE-selectin-FC chimera were preincubated with ESTA (50 nmol/l) for 30 minutes and then incubated with MDA-MB-231 cell lysate (500 µg) at 4 °C for 1 hour. After a brief wash with lysis buffer, protein was eluted from the beads and subjected to western blot. (e) ESTA inhibits the shear-resistant adhesion of CD44+ breast cancer cells to E-selectin expressing HMVEC. A monolayer of E-selectin inducible Tet-on HMVEC grown on a flow chamber was incubated with doxycycline for 5 hours and then pretreated with ESTA (50 or 100 nmol/l) or random aptamer (100 nmol/l) for 30 minutes at 37 °C. The adhesion of CD44+ breast cancer cells (MDA-MB-231 and MDA-MB-468 at 105cells/ml) was infused into a chamber at 1 dyn/cm2 for 5 min and the cancer cell adhesion to HMVEC was counted and compared with that of untreated HMVEC (0 nmol/l). The data represent mean ± SD. Statistical significance was determined by nonparametric Student's t-test.
ESTA inhibits hematogenous metastasis
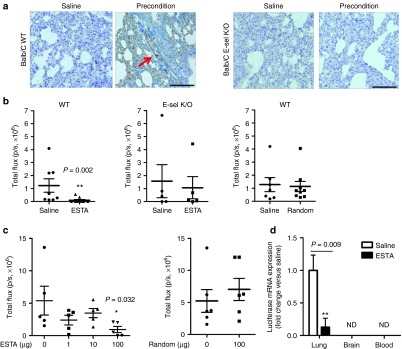
On the basis of the E-selectin-specific antagonistic effect of ESTA in vitro, we next evaluated the antimetastatic effect of ESTA in vivo. To mimic the premetastatic niche, 6-week-old female Balb/C mice (n = 8–10) were preconditioned by successive intraperitoneal injections of conditioned medium.11 Immunohistochemical analysis showed that preconditioning of mice induced E-selectin expression in blood vessels in the lung, whereas E-selectin expression was absent in saline control mice and E-selectin K/O mice (Figure 4a). We found that a single i.v. injection of ESTA via tail vein significantly reduced metastases of 4T1 cells by 92.2% in wild-type Balb/C mice (P = 0.002, Figure 4b). However, the inhibition of metastasis was not observed when the same amount of control random aptamer (100 µg) was injected into wild-type Balb/C mice or when ESTA was injected into E-selectin K/O mice (Figure 4b). This suggests that ESTA exerts its effect through the functional blockade of E-selectin. A dose escalation study was performed using the xenogeneic model. A single bolus i.v. injection of ESTA into female athymic nude mice preconditioned with VEGF confirmed that a single dose of 100 μg of ESTA was effective for the prevention of metastasis formation (Figure 4c). The same dose of control random aptamer showed no effect on metastasis development (Figure 4c). To further determine whether ESTA-mediated inhibition of metastasis to the lung causes a redirection of metastatic site, the fate of intravenously injected 4T1-luc cells was determined by performing qRT-PCR. Twelve days after i.v. injection, luciferase mRNA was not detectable in blood, indicating that the injected cancer cells were cleared within 12 days. The reduction of lung metastasis by ESTA did not result in a relocation of metastasis to the brain, another common metastatic site of ER– breast cancer (Figure 4d).
Figure 4.
ESTA inhibits the development of hematogenous metastasis. (a) The induction of E-selectin expression on the pulmonary capillary after preconditioning in Balb/C wild type mice (left), but not in E-selectin K/O mice (right). Scale bar represents 100 µm. (b) ESTA inhibits the development of metastasis in a syngeneic mouse model of breast cancer metastasis. Six-week-old female Balb/C or E-selectin K/O mice were preconditioned for a week with i.p. injections of conditioned media. The mice then received a single i.v. injection of ESTA (100 µg), random aptamer (100 µg), or saline, followed by 4T1-Luc cells (3 × 104). Lung metastasis formation was measured by bioluminescent image 3 weeks after cancer cell inoculation. The data represent mean ± SEM. Statistical significance was determined by nonparametric Mann–Whitney test. (c) Effective dose of ESTA for the prevention of hematogenous metastasis. Six-week-old female athymic nu/nu mice (n = 5–6) was preconditioned with i.v. injection of VEGF. Two hours later, the mice were injected with either saline, various amounts of ESTA (1–100 ng), or random aptamer (100 ng), and then with MDA-MB-231-Luc (3 × 104/100 µl saline). Bioluminescence was measured 3 weeks later. The data represent mean ± SEM. Statistical significance was determined by Mann–Whitney test. (d) Blockade of lung metastasis by ESTA does not cause a relocation of metastasis. Whole organs (lung and brain) and blood were collected 12 days after cancer cell injection. Luciferase mRNA was detected by 80 cycles of qRT-PCR and normalized by GAPDH. The data represent mean ± SD. Statistical significance was determined by Student's t-test.
We used MDA-MB-231-TGL human breast cancer cell line (ER–/CD44+ ) to further validate the effect of ESTA. Preconditioning the mice with VEGF induced the expression of E-selectin on vessels in the lung (Figure 3c) and resulted in an increase of lung metastasis that was 5.6-fold greater than in mice without preconditioning (6.3 × 105 ± 3.3 × 105 versus 3.5 × 106 ± 1.6 × 106, P = 0.014, Figure 5a). Similar to the results from the syngeneic model, a single i.v. injection of ESTA via tail vein reduced metastasis formation to baseline levels that were equivalent to those in mice without preconditioning, 3.5 × 106 ± 1.6 × 106 versus 9.7 × 105 ± 5.7 × 105, P = 0.038 (Figure 5a). The effect of ESTA was observed only in preconditioned mice. No effect was noted in the mice without preconditioning (Figure 5a), indirectly supporting the target-specific nature of ESTA therapy. In contrast, ESTA did not prevent metastasis formation in CD44 knocked-down MDA-MB-231 cells, suggesting the involvement of CD44 in hematogenous metastasis at the premetastatic stage (Figure 5b). Histopathologic evaluation of metastatic colony numbers yielded patterns similar to the bioluminescence images. Of note, ESTA injection did not affect the overall size of metastatic colonies. However, the metastatic colonies of MDA-MB-231-CD44sh cells were smaller than those of MDA-MB-231 cells, further implicating a role of CD44 in colonization (see Supplementary Figure S7). We kept the mice up to 6 weeks after the cancer cell injection, but did not find evidence of relocation of metastasis by bioluminescent imaging (data not shown). The i.v. injection of ESTA did not have an effect on mouse body weight (see Supplementary Figure S8a,b). Overall, our data indicate that ESTA effectively inhibits organ metastases of ER–/CD44+ breast cancer cells via the functional blockade of E-selectin without relocation of metastatic site.
Figure 5.
ESTA prevents metastasis formation of human breast cancer cells. Female athymic nu/nu mice (n = 7–8) were preconditioned with i.v. injection of VEGF-A 2 hours before i.v. injection of ESTA. The mice were then injected with MDA-MB-231-TGL cells (3 × 104 cells in 100 µl saline) (a) or MDA-MB-231-TGL-CD44sh cells (b) via tail vein at 6-hour intervals. ESTA did not prevent lung metastasis of MDA-MB-231-TGL-CD44sh. FFPE sections of lung tissues (5 μm) were stained with hematoxylin and eosin. The number of lung metastasis colonies was counted under the light microscope at a final magnification of ×100. The data were normalized by area. The data represent mean ± SEM. Statistical significance was determined by Mann–Whitney test.
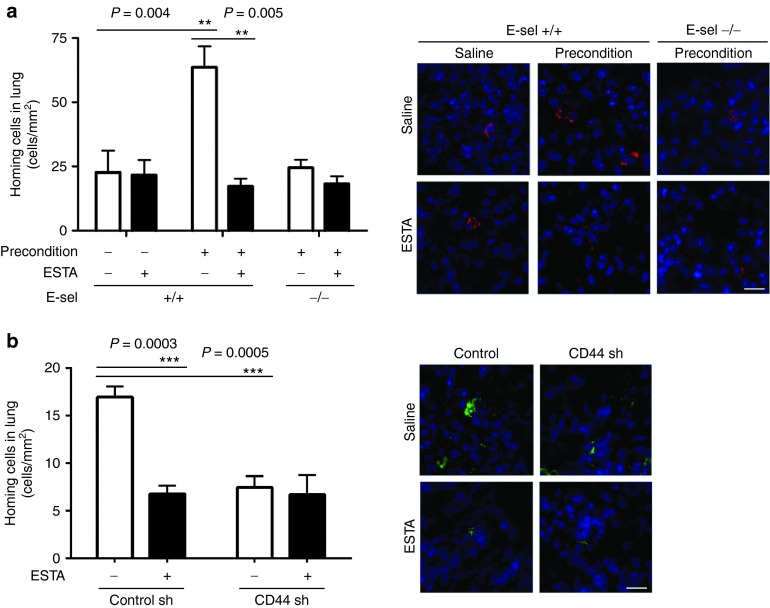
ESTA inhibits cancer cell homing
To validate whether ESTA inhibits the homing of cancer cells, in vivo homing assays were performed 12 hours after i.v. injection of cancer cells. The homing of 4T1-mC cells to the lung was increased 2.8-fold when the mice were preconditioned (22.7 ± 8.5 versus 63.6 ± 8.2, P = 0.004). However, homing was reduced to the basal level following a single injection of ESTA, and in preconditioned E-selectin K/O mice (Figure 6a). As expected, ESTA showed no effect in E-selectin K/O mice, suggesting that the effect of ESTA is limited to E-selectin. In addition, homing of MDA-MB-231-CD44sh remained at a level similar to that in ESTA-treated control mice (Figure 6b). Overall, our data indicate that ESTA inhibits the homing of CD44+ breast cancer cells in the circulation through the inhibition of E-selectin.
Figure 6.
ESTA blocks the homing of cancer cells to the lung. (a) ESTA inhibits the homing of mCherry-expressing 4T1 murine breast cancer cells. Twelve hours later, the mice were perfused with warm saline to eliminate remaining circulating cells and the lungs were harvested. (b) ESTA has no effect on the homing of MDA-MB-231-TGL-CD44sh cells to the lung. Twelve hours after cancer cell injection, the lungs were harvested. The 12-μm frozen sections of lung tissue were counterstained with Hoechst 33342. The homing of cancer cells was assessed by counting fluorescent cells in the lung parenchyma (cell count/area). The scale bars represent 20 μm. The data represent mean ± SD. Statistical significance was determined by Student's t-test.
Discussion
Distant visceral metastasis is the primary cause of mortality from many types of cancer. Inflammation has been recognized as a cue for the initiation of hematogenous metastasis.16,22 In response to soluble factors secreted from the primary tumor, a subpopulation of bone marrow-derived hematopoietic progenitor cells creates a premetastatic niche and inflames nearby vessels.11,23 The vascular adhesion cascade at the vessels around the premetastatic niche is activated as characterized by E-selectin expression (Figures 3c and 4a; ref. 10), thereby assisting in the successive shear-resistant adhesion and transendothelial migration of circulating cancer cells to the target organs. Therefore, vascular inflammation at distant organ cooperates with the primary tumor and creates a supportive and permissive milieu at the premetastatic stage. In view of this cooperative behavior of normal host tissues, we explored the possibility of preventing organ metastasis through blockade of an environmental co-operator expressing on the vessel surface (i.e., E-selectin) as depicted in Figure 7.
Figure 7.
A working model of adhesion blockade. Cancer cells locally invade in the primary tumor and intravasate. E-selectin expression is induced at the vessel surface of the premetastatic niche of distant organs. Disseminated cancer cells make an initial contact with the vessel surface at the premetastatic niche through interaction with E-selectin. Successful firm adhesion of cancer cells to the vessel surface lead to extravasation/homing at distant organ. ESTA binds to E-selectin and blocks the interaction with CD44+ (HCELL) cancer cell surface. The blockade of shear-resistant adhesion of cancer cells to the vessel surface reduces the chance of homing.
In an attempt to block the shear-resistant adhesion of circulating cancer cells to the premetastatic niche, we developed an antagonistic thioaptamer against ESTA, which has ≥10,000× higher affinity and ≤1,000× lower IC50 compared with the E-selectin natural ligand sialyl-Lewisx (sLex) (KD = 47 nmol/l versus 100–2000 µmol/l, IC50 = 67–80 nmol/l versus 100–750 µmol/l; ref. 21). Our data demonstrate that ESTA effectively inhibited the shear-resistant adhesion of ER–/CD44+ breast cancer cells in vitro. To our surprise, a single i.v. injection of ESTA effectively prevented hematogenous metastasis to a baseline level in vivo (Figures 4, 5, and 6). Although this strategy was effective in blocking the adhesion/homing of circulating tumor cells in experimental forced metastasis models when ESTA was intravenously administered 6 hours before cancer cell injection, we believe that the masking of the E-selectin function at the premetastatic niche by ESTA is a temporal effect. The plasma T1/2 of ESTA was 3.09 minutes following a single injection (see Supplementary Figure S9a); however, ESTA was widely distributed to the tissue in preconditioned mice. The lung exhibited the highest accumulation of ESTA (17.2-fold) 6 hours after the i.v. bolus injection (see Supplementary Figure S9b). These data suggest that ESTA remains in the tissues at least for 6 hours after i.v. injection. Nevertheless, daily injections were necessary for the inhibition of hematogenous metastasis in the primary tumor model (see Supplementary Figure S10). Thus, aptamer backbone modification, such as PEGylation to improve PK parameters, is essential for realistic clinical application for metastasis prevention.
sLex and sLeA differentially glycosylate membrane proteins of circulating cells; the glycosylated form of CD44 (HCELL) is a ligand for E-selectin.14,24 We found that shear-resistant adhesion of breast cancer cells via E-selectin was differentially regulated depending on ER/CD44 statuses (Figure 1a). Because both CD44 expression and sLex synthesis are reduced or absent in ER+ breast cancers,25 it is possible that the adhesive strength of ER+ breast cancer cells to E-selectin is weaker than that of ER–. Our data showed that shear-resistant adhesion of ER+ cells was not E-selectin-dependent, but appeared to be P-selectin-dependent (Figure 1a and Supplementary Figure S11).26 CD44 has been successfully used as a surrogate stem cell marker.27 However, the role of CD44 in the primary tumor has been controversial, as it is reported to have dual physiological functions as either a tumor promoter or a suppressor.27,28 Recent studies showed that the presence of CD44+ circulating tumor cells was associated with poor prognosis.29,30,31 Similarly, our data indicated that CD44 expression in metastatic lung and brain lesions was higher in ER– than in ER+ breast cancer (Figure 2), and CD44 knockdown reduced shear-resistant adhesion in vitro, homing in vivo, and metastasis development, suggesting a role of CD44 as a mediator of hematogenous metastasis via the interaction with vascular E-selectin (Figures 1d, 5b, and 6b). Several E-selectin ligands have been reported to be expressed in different cancer cells.32,33,34,35,36 We and other group found that CD44v is a direct binding partner of E-selectin in MDA-MB-231 cells,37 whereas another reported the lack of CD44v binding to E-selectin because of the absence of CD44v expression in the same cell line.33 Although the current study did not identify the CD44v isoform, this question should be addressed further. Alternative splicing of human CD44 is highly complex through nine variable exons (v2–v10) that can yield a different set of glycoproteins with potentially different functions.38,39 The further involvement of multiple factors, including tumor type, tumor progression,40 cell type, and cell culture conditions,41 which affect the CD44 splicing pattern, adds an extra layer of complexity. Considering the complexity of alternative splicing, the question regarding which variant form of CD44 serves as HCELLv further remains to be elucidated on the standardization of experimental conditions and methods of detection.
To exclusively focus on hematogenous metastasis and rule out effects from the primary tumor, we used forced metastasis mouse models in this study. We were initially concerned that the inhibition of metastasis by ESTA could result in redirection of the metastatic site to organs other than the lung. However, cancer cells were cleared by day 12 after injection, and we did not detect obvious metastasis at other organs (Figure 4d). Although the models that we used in this study primarily develop lung metastases following i.v. injection of cancer cells via the tail vein,7,42 it is important to understand whether ESTA has the same inhibitory effect on metastasis to other organs. Hematogenous metastasis is complex and may be differentially regulated in each organ by multiple factors, such as hydrodynamics or vascular structure.8 Capillary sizes and structures significantly influence the chance of physical occlusions of cancer cells within small capillaries.17,43 In addition, wall shear stress, which is largely determined by flow rate, is different in each organ, as exemplified by a slower flow rate in bone marrow than in high endothelial venules.44 Differential flow dynamics, in conjunction with expression of adhesion molecules on vessels in each organ, may coordinately define the probability of margination and adhesion of circulating cancer cells to the endothelial wall under a gravitational field.45,46
The targeting strategy at the vessel surface of the premetastatic niche of target organs for the prevention of hematogenous metastasis differs considerably from conventional therapeutic interventions or chemoprevention strategies. Blocking a gateway using noncytotoxic functional blockers at distant organs may be a useful complementary therapy in combination with neoadjuvant therapy or management of metastasis prophylactically after the completion of adjuvant chemotherapy for disease management. A preventive therapy for cancer metastasis has not yet emerged. A challenge to implementing such a preventive strategy is to determine when to treat and who should be treated. Therefore, using predictive biomarkers to stratify patients who are likely to benefit from prophylaxis is important. The results of our study suggest that the status of CD44/HCELL in the primary tumor, as well as circulating tumor cells, might provide a basis for stratifying patients with ER– breast cancer for potential response to E-selectin-targeted therapy.
Materials and Methods
Human specimens. Human specimens were obtained and handled in accordance with an IRB-approved protocol at the University of Oklahoma Health Sciences Center.
Cell lines and culture conditions. The human breast cancer cell lines MDA-MB-231, MDA-MB-468, T-47D, and MCF-7, and the murine breast cancer cell line 4T1 were purchased from ATCC. MDA-MB-231 carrying luciferase and GFP reporters (MDA-MB-231-TGL) was a kind gift from Dr. Massague at Memorial Sloan Kettering Cancer Center.47 CD44 knocked-down MDA-MB-231 and its control were established by lentivirus infection with shRNA (Santa Cruz Biotechnology, Santa Cruz, CA). 4T1-Luc and 4T1-mC were established by lentivirus infection of firefly luciferase and mCherry, respectively (Capital Biosciences, Rockville, MD). E-selectin-expressing Tet-on inducible human microvascular endothelial cells (HMVEC) were developed.21 E-selectin open reading frame was purchased from OriGene and cloned into pTRE-Tight-BI-RFP (Clontech, Mountain View, CA). HMVEC cells were stably transfected with an rtTA plasmid (Clontech) and the positive rtTA clone was further stably transfected with pTRE-Tight-BI-hE-selectin-RFP. The stable clones expressing DsRed on treatment with 1,000 ng of doxycycline were enriched by FACS sorter. The cells were cultured in endothelial basal medium-2 (Lonza, Basel, Switzerland) supplemented with 2% Tet-approved FBS (Clontech). E-selectin expression was induced for 5 hours with 1500 µg/ml of doxycycline.21 All cells were cultured in 5% CO2 humid chambers at 37 °C.
Flow adhesion assay. HMVEC were grown to confluence on the flow chamber (Ibidi, Martinsried, Germany) coated with collagen I and fibronectin. After incubation with doxycycline for 5 hours, cancer cells (105 cells/ml) were infused into a flow chamber at 1–4 dyn/cm2 shear stress for 5 minutes at 37 °C. The unbound cells were washed off with Dulbecco's modified Eagle medium containing 1% FBS. Using a light microscope at final magnification ×100, the number of cells adherent to endothelial cells was determined by counting cells on at least three random fields.
Transendothelial cell migration assay. HMVEC were grown to confluence on the 12.0-µm pore-size transwell plate (Millipore, Billerica, MA). Cancer cells were suspended in serum-free Dulbecco's modified Eagle medium and seeded onto the upper chamber (104 cells). The cancer cells that migrated to the lower chamber were stained by Diff Quick (Polysciences, Cambridge, MA) and counted under a light microscope.
Animal model. All animal housing and handling procedures were in accordance with institutional guidelines at Thomas Jefferson University and the University of Oklahoma. For syngeneic models, 6-week-old female Balb/C mice (Jackson Laboratory, Bar Harbor, ME) or E-selectin knockout mice (C.129S4-Seletm1Dmil/J; Jackson Laboratory) were injected intraperitoneally with saline or preconditioned for 7–10 days with conditioned media collected from 4T1 breast cancer cells. After preconditioning, the mice were then intravenously injected with 100 µg dose of ESTA or saline once. Six hours later, 4T1-luc murine breast cancer cells (3 × 104 in 100 µl saline) were intravenously injected via tail vein. Three to four weeks after cancer cell injection, metastasis formation was measured by bioluminescent imaging (Caliper Life Sciences, Hopkinton, MA) as photon flux following retro-orbital injection of 100 µl D-luciferin (15 mg/ml). Images were visualized using Living Image software (Caliper Life Sciences). For xenogeneic models, 6-week-old female athymic nu/nu mice (n = 7–8, Taconic Farm, Germantown, NY) were intravenously injected with 100 µl of saline or 100 ng of vascular endothelial growth factor (VEGF)-A via tail vein for preconditioning. Two hours later, the mice were intravenously injected with 100 µg ESTA or saline once. Four hours later, mice were injected human MDA-MB-231-TGL cells or MDA-MB-231-TGL-CD44 shRNA (3 × 104 in 100 µl saline) 4 hours later via tail vein. Three weeks after cancer cell injection, metastasis formation was measured by bioluminescent imaging.
In vivo homing assay. Preconditioned female mice (n = 3) were intravenously injected with ESTA ~6 hours before intravenous (i.v.) injection of a stable clone of mCherry-expressing 4T1 murine breast cancer cells (3 × 104) or eGFP-expressing MDA-MB-231 human breast cancer cells transfected with CD44shRNA or its control. Twelve hours later, the mice were perfused with warm saline to eliminate residual cancer cells in the circulation. Then, the organs were harvested, the 12-μm frozen sections of tissue were stained with Hoechst 33342, and the number of fluorescent cells was counted under a fluorescent microscope (Leica DM2500 microscope, Leica Microsystems, Buffalo Grove, IL).
Aptamer synthesis. ESTA (5′-CGCTCGGA*TCGA*TA*A*GCTTCGA*TCCCA*CTCTCCCGTTCA*CTTCTCCTCA*CGTCA*CGGA*TCCTCTA*GA*GCA*CTG-3′) and random aptamer (5′-CCCACTTA*TCGTCCCTTAA*TGA*GTTTA*CTCGCA*CACCGGACAGCCGTCGGATGGCTGGATCCG*TAGCGGTCCGG-3′) were chemically synthesized in a DNA synthesizer (Expedite 8909, Applied Biosystems, Foster City, CA) using the standard phosphoramidite chemistry as described earlier. The 5′ end of the ESTA was coupled with Cy3 phosphoramidite. Aptamers were quickly heat denatured and cooled to form tertiary structure. Aptamer-conjugated beads were generated by direct chemical synthesis on 5 µm silica beads.
Reverse transcription and real-time polymerase chain reaction. RNA (1 µg) was reverse transcribed using random hexamer primers and SuperScript III First-Strand Synthesis Supermix (Life Technologies, Carlsbad, CA). qPCR reactions (80 cycles) were performed using TaqMan gene expression master mix (Life Technologies) and Biorad CFX96 Touch Real-Time PCR Detection System (Biorad, Hercules, CA). Luciferase and mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers of TaqMan gene expression assay were purchased from Life Technologies. Data is presented as delta – Ct relative to an endogenous control, GAPDH.
In vitro binding assays. For the beads-binding assay, the ESTA or random aptamer-conjugated silica beads were incubated with 1 µg of recombinant human E-selectin (R&D systems, Minneapolis, MN) in phosphate-buffered saline (PBS) supplemented with 5 mmol/l MgCl2 for 30 minutes at RT. After a quick washing with PBS, the silica beads were incubated with SDS sample buffer at 95 °C to release the protein, then were separated by SDS–PAGE, and visualized by SYPRO RUBY to detect rhE-selectin. Plasma membrane protein was isolated from human (HMVEC) and mouse (End.3) endothelial cell lines that were treated with TNF-α. A total protein (10 µg) was incubated with ESTA or random aptamer-conjugated silica beads in lysis buffer for 1 hour at 4 °C. After a quick washing with binding buffer, the bound protein was eluted with SDS sample buffer for 5 minutes at 95 °C, separated by SDS–PAGE. The gels were subjected to Western blotting to detect E-selectin. For the pull-down assay, MDA-MB-231 cells were lysed in a lysis buffer (50 mmol/l Tris–HCl pH 7.5, 150 mmol/l NaCl, 1% Triton X-100, 1 mmol/l ethylenediaminetetraacetic acid, 1% protease inhibitor (Roche, Indianapolis, IN), 1% phosphatase inhibitor cocktail (Calbiochem, San Diego, CA) on ice for 10 minutes, and centrifuged at 14,000 ×g for 5 minutes. The human IgG magnetic beads were incubated with an increasing amount of the recombinant human E-selectin-Fc chimeric proteins (R&D systems) in PBS supplemented with 5 mmol/l MgCl2 at room temperature for 1 hour. After a quick wash with PBS, the E-selectin-Fc coupled magnetic beads were incubated with cell lysate at 4 °C for 1 hour. After washing three times with lysis buffer, the bound protein was eluted in SDS buffer. The binding between E-selectin and CD44 was detected by Western blot using anti-CD44 antibody. The equal amount of E-selectin–Fc coupling per reaction was confirmed by E-selectin antibody.
Western blotting. Proteins were separated by a precast 4–12% Bis–Tris gel (Invitrogen, Carlsbad, CA) with the NuPAGE system (Invitrogen) and transferred to a 0.2-µm polyvinylidene difluoride membrane. The membrane was probed with primary antibodies, rabbit antihuman CD44 (Cell Signaling, #3578, Danvers, MA) and E-selectin (Santa Cruz, #SC-14011), and anti-GAPDH (Millipore, #AB2302), and then horseradish peroxidase-conjugated secondary antibody. It was developed using Supersignal West ECL substrate system (Thermo Scientific, Rockford, IL). Chemiluminescence was detected using FluorChem M imager (ProteinSimple, Santa Clara, CA).
Aptamer clearance. Following a single i.v. bolus administration of Cy3-ESTA (100 µg in 100 µl of saline) into 8-week-old ICR mice (n = 3), whole blood was collected by cardiac puncture at 2, 5, 10, 20, 30, 60, 120, 180, and 360 minutes after the injection. The fluorescence intensity in the plasma (50 µl) was measured using a fluorimeter at 544/590 nm (excitation/emission wavelengths) to determine the pharmacokinetics parameters of ESTA. Plasma was also collected from saline-injected mice and used as a baseline. For tissue distribution, DNA was extracted from organs with phenol/chloroform/isoamyl alcohol (pH 8.0). An equal amount of DNA (7 pg) was used for qPCR analysis using the primer pair that corresponds to the ESTA sequence: forward primer (5′-CGCTCGGATC GATAAGCTT-3′) and reverse primer (5′-CAGTGCTCTAGAGGATC-3′). qPCR was run in triplicate on CFX96 Touch Real-Time PCR Detection. Data were summarized as fold change over saline injected.
Statistical analysis. All in vitro experiments were carried out in triplicate and repeated at least twice independently. The results were presented as mean ± SD. The comparisons between each group were made using GraphPad Prism statistical software to provide a 95% confidential level. P-values of ≤0.05 were considered statistically significant and expressed as *P < 0.05; **P < 0.01; ***P < 0.001. For the correlation of CD44 and ER or progesterone receptor, data was analyzed using an unadjusted Mann–Whitney's test and an adjusted van Elteren test with metastasis as the stratification factor. In addition, the correlation of CD44 with ER or progesterone receptor was analyzed using Mann–Whitney's test separately for brain or lung metastasis.
SUPPLEMENTARY MATERIAL Figure S1. Shear-resistant adhesion of MDA-MB-231 on E-selectin inducible HMVEC under various shear stress. Figure S2. ER status is inversely correlated with CD44. Figure S3. The original data that corresponds to Fig. 1c. Figure S4. The original data that corresponds to Fig. 3d. Figure S5. Blockade of adhesion of MDA-MB-231 to E-selectin expressing HMVEC under various shear stress. Figure S6. MDA-MB-231 breast cancer cells were cultured in DMEM supplemented with 10% FBS containing 50, 100, or 500 nM ESTA for 24 hours. Figure S7. Metastatic colony development in the lung. Figure S8. Body weight of mice used in Fig. 5. Figure S9. Pharmaceutical parameter of a single bolus intravenous injection of ESTA. Figure S10. Daily injection of ESTA inhibits the lung metastasis in the mouse model of primary breast tumor. Figure S11. Shear-resistant adhesion of MCF-7 is P-selectin dependent. Table S1. Clinical characteristics of tissues.
Acknowledgments
This work was supported by the Department of Defense (W81XWH-11-1-0238), the National Institutes of Health (1R01CA160271-01A1), the American Cancer Society (IRG-08-060-04), and the Pennsylvania Breast Cancer Coalition to T.T., and U54CA151668 to D.G.G. The authors thank the Pathology and Imaging Core Facilities at the University of Oklahoma. D.G.G. is a founder of AM Biotechnologies, which has licensed thioaptamer selection technologies.
Supplementary Material
References
- Coleman, RE and Rubens, RD (1987). The clinical course of bone metastases from breast cancer. Br J Cancer 55: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disibio, G and French, SW (2008). Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med 132: 931–939. [DOI] [PubMed] [Google Scholar]
- Patanaphan, V, Salazar, OM and Risco, R (1988). Breast cancer: metastatic patterns and their prognosis. South Med J 81: 1109–1112. [PubMed] [Google Scholar]
- Swenerton, KD, Legha, SS, Smith, T, Hortobagyi, GN, Gehan, EA, Yap, HY et al. (1979). Prognostic factors in metastatic breast cancer treated with combination chemotherapy. Cancer Res 39: 1552–1562. [PubMed] [Google Scholar]
- Smid, M, Wang, Y, Zhang, Y, Sieuwerts, AM, Yu, J, Klijn, JG et al. (2008). Subtypes of breast cancer show preferential site of relapse. Cancer Res 68: 3108–3114. [DOI] [PubMed] [Google Scholar]
- Solomayer, EF, Diel, IJ, Meyberg, GC, Gollan, C and Bastert, G (2000). Metastatic breast cancer: clinical course, prognosis and therapy related to the first site of metastasis. Breast Cancer Res Treat 59: 271–278. [DOI] [PubMed] [Google Scholar]
- Weigelt, B, Peterse, JL and van ‘t Veer, LJ (2005). Breast cancer metastasis: markers and models. Nat Rev Cancer 5: 591–602. [DOI] [PubMed] [Google Scholar]
- Chiang, AC and Massagué, J (2008). Molecular basis of metastasis. N Engl J Med 359: 2814–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantel, K and Brakenhoff, RH (2004). Dissecting the metastatic cascade. Nat Rev Cancer 4: 448–456. [DOI] [PubMed] [Google Scholar]
- Hiratsuka, S, Goel, S, Kamoun, WS, Maru, Y, Fukumura, D, Duda, DG et al. (2011). Endothelial focal adhesion kinase mediates cancer cell homing to discrete regions of the lungs via E-selectin up-regulation. Proc Natl Acad Sci USA 108: 3725–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan, RN, Riba, RD, Zacharoulis, S, Bramley, AH, Vincent, L, Costa, C et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438: 820–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua, MP (1993). Endothelial-leukocyte adhesion molecules. Annu Rev Immunol 11: 767–804. [DOI] [PubMed] [Google Scholar]
- Woodward, J (2008). Crossing the endothelium: E-selectin regulates tumor cell migration under flow conditions. Cell Adh Migr 2: 151–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thankamony, SP and Sackstein, R (2011). Enforced hematopoietic cell E- and L-selectin ligand (HCELL) expression primes transendothelial migration of human mesenchymal stem cells. Proc Natl Acad Sci USA 108: 2258–2263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, W (2008). Catch bonds in adhesion. Annu Rev Biomed Eng 10: 39–57. [DOI] [PubMed] [Google Scholar]
- Nguyen, DX, Bos, PD and Massagué, J (2009). Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9: 274–284. [DOI] [PubMed] [Google Scholar]
- Chambers, AF, Groom, AC and MacDonald, IC (2002). Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2: 563–572. [DOI] [PubMed] [Google Scholar]
- Tremblay, PL, Auger, FA and Huot, J (2006). Regulation of transendothelial migration of colon cancer cells by E-selectin-mediated activation of p38 and ERK MAP kinases. Oncogene 25: 6563–6573. [DOI] [PubMed] [Google Scholar]
- Zollner, TM and Asadullah, K (2003). Selectin and selectin ligand binding: a bittersweet attraction. J Clin Invest 112: 980–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackstein, R (2011). The biology of CD44 and HCELL in hematopoiesis: the ‘step 2-bypass pathway' and other emerging perspectives. Curr Opin Hematol 18: 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann, AP, Somasunderam, A, Nieves-Alicea, R, Li, X, Hu, A, Sood, AK et al. (2010). Identification of thioaptamer ligand against E-selectin: potential application for inflamed vasculature targeting. PLoS One 5: e13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psaila, B and Lyden, D (2009). The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9: 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiratsuka, S, Watanabe, A, Aburatani, H and Maru, Y (2006). Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis. Nat Cell Biol 8: 1369–1375. [DOI] [PubMed] [Google Scholar]
- Jacobs, PP and Sackstein, R (2011). CD44 and HCELL: preventing hematogenous metastasis at step 1. FEBS Lett 585: 3148–3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julien, S, Ivetic, A, Grigoriadis, A, QiZe, D, Burford, B, Sproviero, D et al. (2011). Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer Res 71: 7683–7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederichs, J, Zeller, Y, Hafezi-Moghadam, A, Gröne, HJ, Ley, K and Altevogt, P (2000). The CD24/P-selectin binding pathway initiates lung arrest of human A125 adenocarcinoma cells. Cancer Res 60: 6714–6722. [PubMed] [Google Scholar]
- Louderbough, JM and Schroeder, JA (2011). Understanding the dual nature of CD44 in breast cancer progression. Mol Cancer Res 9: 1573–1586. [DOI] [PubMed] [Google Scholar]
- Lopez, JI, Camenisch, TD, Stevens, MV, Sands, BJ, McDonald, J and Schroeder, JA (2005). CD44 attenuates metastatic invasion during breast cancer progression. Cancer Res 65: 6755–6763. [DOI] [PubMed] [Google Scholar]
- Aktas, B, Tewes, M, Fehm, T, Hauch, S, Kimmig, R and Kasimir-Bauer, S (2009). Stem cell and epithelial-mesenchymal transition markers are frequently overexpressed in circulating tumor cells of metastatic breast cancer patients. Breast Cancer Res 11: R46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoropoulos, PA, Polioudaki, H, Agelaki, S, Kallergi, G, Saridaki, Z, Mavroudis, D et al. (2010). Circulating tumor cells with a putative stem cell phenotype in peripheral blood of patients with breast cancer. Cancer Lett 288: 99–106. [DOI] [PubMed] [Google Scholar]
- Baccelli, I, Schneeweiss, A, Riethdorf, S, Stenzinger, A, Schillert, A, Vogel, V et al. (2013). Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat Biotechnol 31: 539–544. [DOI] [PubMed] [Google Scholar]
- Shirure, VS, Reynolds, NM and Burdick, MM (2012). Mac-2 binding protein is a novel E-selectin ligand expressed by breast cancer cells. PLoS One 7: e44529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen, K, Liu, DQ, Guo, YL, Wang, C, Shan, J, Fang, M et al. (2008). CD44v4 is a major E-selectin ligand that mediates breast cancer cell transendothelial migration. PLoS One 3: e1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng, Y, Yeh, K, Takatani, T and King, MR (2012). Three to tango: MUC1 as a ligand for both E-selectin and ICAM-1 in the breast cancer metastatic cascade. Front Oncol 2: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gout, S, Morin, C, Houle, F and Huot, J (2006). Death receptor-3, a new E-selectin counter–receptor that confers migration and survival advantages to colon carcinoma cells by triggering p38 and ERK MAPK activation. Cancer Res 66: 9117–9124. [DOI] [PubMed] [Google Scholar]
- Dimitroff, CJ, Descheny, L, Trujillo, N, Kim, R, Nguyen, V, Huang, W et al. (2005). Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res 65: 5750–5760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirure, VS, Liu, T, Delgadillo, LF, Cuckler, CM, Tees, DF, Benencia, F et al. (2015). CD44 variant isoforms expressed by breast cancer cells are functional E-selectin ligands under flow conditions. Am J Physiol Cell Physiol 308: C68–C78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponta, H, Sherman, L and Herrlich, PA (2003). CD44: from adhesion molecules to signalling regulators. Nat Rev Mol Cell Biol 4: 33–45. [DOI] [PubMed] [Google Scholar]
- Prochazka, L, Tesarik, R and Turanek, J (2014). Regulation of alternative splicing of CD44 in cancer. Cell Signal 26: 2234–2239. [DOI] [PubMed] [Google Scholar]
- Bánky, B, Rásó-Barnett, L, Barbai, T, Tímár, J, Becságh, P and Rásó, E (2012). Characteristics of CD44 alternative splice pattern in the course of human colorectal adenocarcinoma progression. Mol Cancer 11: 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C, Kato, M, Shiue, L, Shively, JE, Ares, M Jr and Lin, RJ (2006). Cell type and culture condition-dependent alternative splicing in human breast cancer cells revealed by splicing-sensitive microarrays. Cancer Res 66: 1990–1999. [DOI] [PubMed] [Google Scholar]
- Kim, MY, Oskarsson, T, Acharyya, S, Nguyen, DX, Zhang, XH, Norton, L et al. (2009). Tumor self-seeding by circulating cancer cells. Cell 139: 1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron, MD, Schmidt, EE, Kerkvliet, N, Nadkarni, KV, Morris, VL, Groom, AC et al. (2000). Temporal progression of metastasis in lung: cell survival, dormancy, and location dependence of metastatic inefficiency. Cancer Res 60: 2541–2546. [PubMed] [Google Scholar]
- Mazo, IB and von Andrian, UH (1999). Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol 66: 25–32. [DOI] [PubMed] [Google Scholar]
- Decuzzi, P, Lee, S, Bhushan, B and Ferrari, M (2005). A theoretical model for the margination of particles within blood vessels. Ann Biomed Eng 33: 179–190. [DOI] [PubMed] [Google Scholar]
- Decuzzi, P, Lee, S, Decuzzi, M and Ferrari, M (2004). Adhesion of microfabricated particles on vascular endothelium: a parametric analysis. Ann Biomed Eng 32: 793–802. [DOI] [PubMed] [Google Scholar]
- Minn, AJ, Gupta, GP, Siegel, PM, Bos, PD, Shu, W, Giri, DD et al. (2005). Genes that mediate breast cancer metastasis to lung. Nature 436: 518–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.