Abstract
Increasing evidence shows that microRNAs play an important role in kidney disease. However, functions of long noncoding RNAs (lncRNAs) in kidney diseases remain undefined. We have previously shown that TGF-β1 plays a diverse role in renal inflammation and fibrosis and Smad3 is a key mediator in this process. In this study, we used RNA-sequencing to identify lncRNAs related to renal inflammation and fibrosis in obstructive nephropathy induced in Smad3 wild-type and knockout mice. We found that Arid2-IR was a Smad3-associated lncRNA as a Smad3 binding site was found in the promoter region of Arid2-IR and deletion of Smad3 abolished upregulation of Arid2-IR in the diseased kidney. In vitro knockdown of Arid2-IR from tubular epithelial cells produced no effect on TGF-β-induced Smad3 signaling and fibrosis but inhibited interleukin-1β-stimulated NF-κB-dependent inflammatory response. In contrast, overexpression of Arid2-IR promoted interleukin-1β-induced NF-κB signaling and inflammatory cytokine expression without alteration of TGF-β1-induced fibrotic response. Furthermore, treatment of obstructed kidney with Arid2-IR shRNA blunted NF-κB-driven renal inflammation without effect on TGF-β/Smad3-mediated renal fibrosis. Thus, Arid2-IR is a novel lncRNA that functions to promote NF-κB-dependent renal inflammation. Blockade of Arid2-IR may represent a novel and specific therapy for renal inflammatory disease.
Introduction
Increasing evidences show that noncoding RNAs (ncRNAs), including small ncRNAs and various classes of long ncRNAs (lncRNAs), play a critical role in kidney disease.1,2 In the past decade, many disease-associated microRNAs have been identified and reported as therapeutic targets for kidney disease.1,2,3 However, the specificity of miRNAs in the kidney disease has been challenged because a single miRNA is able to regulate multiple target genes and, in turn, a single gene can also be regulated by several miRNAs. Thus, the off-target effects of miRNAs hinder the application of microRNA therapy clinically.
The lncRNA, defined by nonprotein coding transcripts >200 nucleotides, is a new class of ncRNAs and has been found to be pervasively transcripted in the genome.4 Although the molecular mechanisms of lncRNAs remain largely unclear, lncRNAs may execute as signals, decoys, guides, and scaffolds in their biological functions.5 Recent studies indicated that lncRNAs involve in a variety of diseases and oncogenesis.6,7 However, the expression profile and functions of lncRNAs in kidney disease remain largely undefined. By using the high-throughput RNA-sequencing (RNA-Seq), we recently identified a number of lncRNAs that are differentially expressed in mouse models of chronic kidney disease.8 A recent study found that an lncRNA Xist was significantly upregulated in both tubular epithelial and glomerular cells in an experimental mouse model of membranous nephropathy and in urine samples from patients with different types of glomerular nephritis.9 These results suggest that lncRNAs may be biomarkers for kidney diseases, but functional role of lncRNAs in kidney disorders remains largely undefined.
It is now well accepted that TGF-β1 plays a diverse role in renal fibrosis and inflammation, two major pathological features in chronic kidney disease.10 In the context of fibrosis, TGF-β1 mediates renal fibrosis positively by activating its downstream mediator Smad3, which is negatively regulated by an inhibitor Smad7.10,11 In inflammatory response, TGF-β1 exerts to suppress renal inflammation by upregulating renal Smad7 but promotes inflammation via a Smad3-dependent mechanism.10 The diverse actions of TGF-β1/Smad signaling in renal inflammation are supported by the findings that deletion of Smad7 enhances, but overexpression of Smad7 blocks Smad3 signaling, thereby inhibiting renal inflammation and fibrosis.12,13 The role of Smad3 in renal inflammation and fibrosis is further supported by the finding that mice null for Smad3 are protected against renal inflammation and fibrosis in unilateral ureteral obstructive nephropathy (UUO) and angiotensin II-induced hypertensive nephropathy.14,15 The complexity and distinct roles of TGF-β/Smads in renal inflammation and fibrosis suggest that treatment of kidney disease should aim to correct the imbalance of TGF-β/Smad signaling or directly target the Smad-associated genes related to inflammation and/or fibrosis, rather than to block the general effect of TGF-β1.
By using RNA-Seq, we recently identified 151 Smad3-associated lncRNAs that are differentially expressed in the UUO kidney of Smad3 wild-type (WT) and knockout (KO) mice. Of them, 94 lncRNAs were significantly upregulated in the Smad3 WT kidney but downregulated in the UUO kidney of Smad3 KO mice.8 A novel lncRNA np_28496 is the most highly expressed lncRNAs in the UUO kidney.8 In this study, we further characterized the lncRNAnp_28496 and found that it was located within the intron region of the Arid2 gene. We thus named it as lncRNAArid2-IR. Furthermore, the functional role of Arid2-IR and the therapeutic potential by targeting the Arid2-IR in renal fibrosis and inflammation were investigated.
Results
Identification of Arid2-IR as a Smad3-associated lncRNA in the UUO kidney
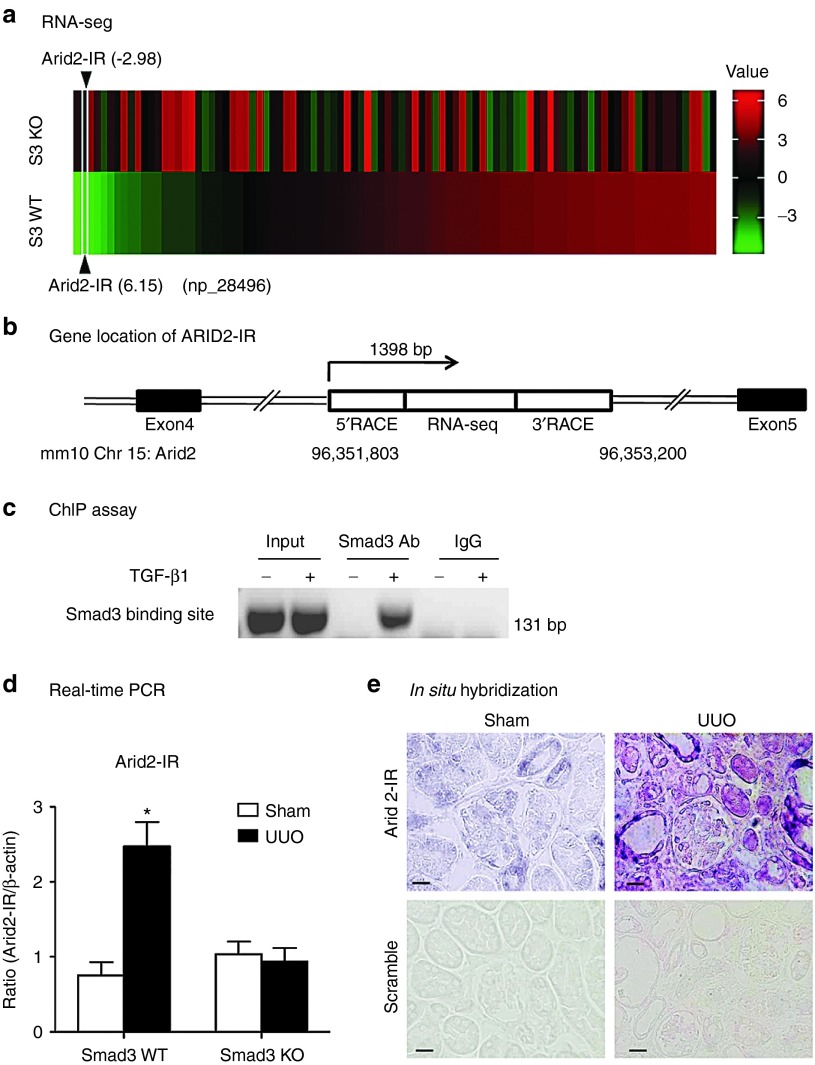
To identify novel Smad3-associated lncRNAs related to renal inflammation and fibrosis, the high-throughput RNA-Seq technique was used to analyze the expression of lncRNAs in the UUO kidney of Smad3 KO/WT mice. In the earlier study, we found that in comparison to Smad3 WT mice, 151 lncRNAs in the UUO kidney were significantly altered in Smad3 KO mice.8 Among them, 94 were upregulated in Smad3 WT but downregulated in the Smad3 KO UUO kidneys (Figure 1a). A novel lncRNA np_28496 was one of these Smad3-associated lncRNAs, which was highly upregulated in the UUO kidney of Smad3 WT but downregulated in Smad3 KO (Figure 1a). It was located within the intron region between the fourth and fifth exons of Arid2 on the chromosome 15 of the mouse genome (Figure 1b). Therefore, we named it as a lncRNA Arid2-IR. We then used the rapid amplification of cDNA ends (RACE) to amplify the full length of Arid2-IR. Results shown in the Supplementary Figure S1a, clearly demonstrated a1398bp transcript (Chr 15: 96,351,803–96,353,200).We next examined whether the promoter region of Arid2-IR had potential Smad3 binding sites by using rVista 2.0 (http://www.rvista.dcode.org/).16 As shown in the Supplementary Figure S1b there was a Smad binding site in a highly conserved region 1.6 kb upstream (Chr 15:96,350,182–96,350,191) of Arid2-IR. Sequence analysis revealed a Smad3 response element (AGACATCAT) located in this region, which is conserved between mouse and human (see Supplementary Figure 1c). Chromatin immunoprecipitation (ChIP) also clearly demonstrated the interaction between Smad3 and the Arid2-IR promoter (Figure 1c), indicating that Arid2-IR may be a transcriptional target of Smad3. Consistent with the result shown in RNA-seq, real-time PCR analysis also revealed that Arid2-IR was significantly upregulated in the UUO kidney of Smad3 WT but not in Smad3 KO mice (Figure 1d). In situ hybridization detected that Arid2-IR was weakly expressed by glomerular epithelial cells, tubular epithelial cells, and vascular smooth muscle cells, which was markedly upregulated in the UUO kidney, particularly in those with severe tubulointerstitial damage (Figure 1e).
Figure 1.
Characterization of Arid2-IR in kidney. (a) Heat map of expression profile of 94 lncRNAs in the UUO kidney detected by RNA-seq. Note that lncRNAs which upregulated in Smad3 wild-type (S3 WT, green) are downregulated in Smad3 knockout (S3 KO, red) mice. (b) Gene location of Arid2-IR in Chromosome 15 of mouse genome. (c) ChIP assay shows that Smad3 physically binds Arid2-IR promoter in response to TGF-β1 (5 ng/ml for 24 hours). (d) Real-time PCR detects the expression of Arid2-IR in Smad3 WT/KO UUO kidney. (e) In situ hybridization. Representative kidney tissue sections show that Arid2-IR is weakly expressed by glomerular and tubular epithelial cells in the normal mouse kidney, which is largely upregulated in the UUO kidney at day 5, particularly in those with highly dilated tubules. Each bar represents the mean ± SEM for groups of six mice. *P < 0.05 versus sham-operated mice. Scale bar = 50 µm.
Knockdown of Arid2-IR has no effect on TGF-β-induced Smad3 signaling and renal fibrosis but inhibits interleukin-1β (IL-1β)-induced NF-κB activation and renal inflammation in vitro.
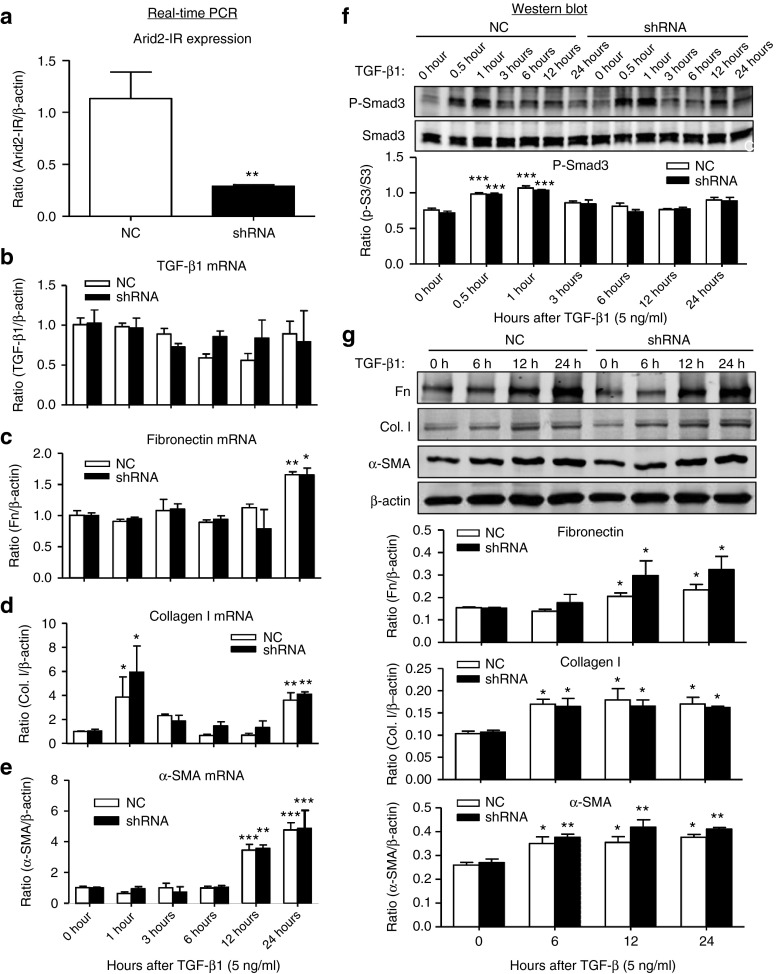
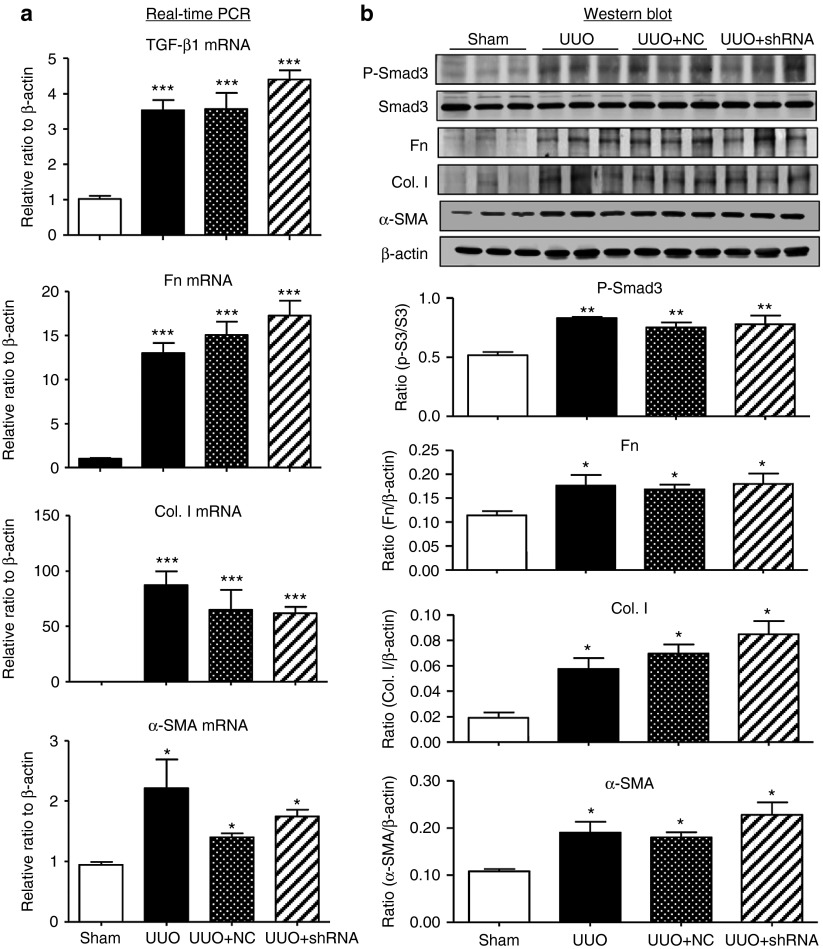
To study the functional role and potential mechanisms of Arid2-IR in renal fibrosis and inflammation, knockdown of Arid2-IR was carried out in vitro in mouse tubular epithelial cells (mTECs) by transient transfection with Arid2-IR siRNAs. Real-time PCR showed that transient transfection of the Arid2-IR siRNA significantly downregulated Arid2-IR in mTECs (see Supplementary Figure S2a). We then cloned Arid2-IR siRNA sequence into a pSuper.puro vector to construct a stable Arid2-IR shRNA expressing plasmid (see Supplementary Figure S2b) and transfected this expressing plasmid into mTECs to establish a stable Arid2-IR knockdown mTECs. Real-time PCR detected that mTECs expressing Arid2-IR shRNA largely reduced Arid2-IR expression when compared with those transfected with negative control (NC) shRNA plasmid (Figure 2a). To investigate the functional role of Arid2-IR in renal fibrosis, TGF-β1 at a dose of 5 ng/ml was added into the mTECs. Real-time PCR and western blot analysis clearly demonstrated that knockdown of Arid2-IR from mTECs did not alter the fibrotic effect of TGF-β1 including expression of TGF-β1, fibronectin, collagen type 1, α-smooth muscle actin (α-SMA), and phosphorylation of Smad3 (Figure 2b–g). Furthermore, we also found that overexpression of Arid2-IR in mTECs did not alter TGF-β1-induced fibrotic response (see Supplementary Figure S3).
Figure 2.
Knockdown of Arid2-IR in mTECs has no effect on TGF-β1-induced fibrotic responses in vitro. (a) Real-time PCR detects that mTECs stably transfected with the Arid2-IR-shRNA pSuper.puro vector (shRNA) show a large reduction in expression of Arid2-IR when compared with those transfected with NC shRNA-pSuper.puro vector (NC). **P < 0.01versus NC. (b–e) mRNA levels of TGF-β1, fibronectin (Fn), collagen I (Col. I), and α-SMA expression by real-time PCR. (f) Phosphorylation levels of Smad3 in response to TGF-β1 (5 ng/mL). (g) Expression levels of fibronectin (Fn), collagen I (Col. I), and α-SMA expression by western blot analysis. Each bar represents the mean ± SEM for at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus time 0.
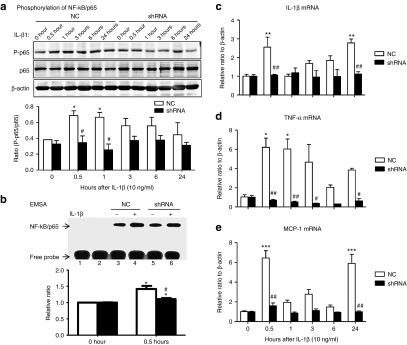
We then examined if Arid2-IR has a role in renal inflammation in mTECs with or without overexpressing Arid2-IR shRNA. As shown in Figure 3, western blot, electrophoretic mobility shift assay, and real-time PCR analysis revealed that knockdown of Arid2-IR from mTECs blunted IL-1β (10 ng/mL)-induced phosphorylation and DNA binding activity of NFκB/p65, thereby inhibiting a marked upregulation of proinflammatory cytokines (IL-1β, tumor necrosis factor (TNF)-α) and chemotactic factor (monocyte chemotactic protein 1 (MCP-1)) in response to IL-1β stimulation. To further confirm the promoter role of Arid2-IR in NF-κB-dependent renal inflammation, we overexpressed Arid2-IR in mTECs and found that overexpression of Arid2-IR was capable of sustaining IL-1β-induced NF-κB signaling and IL-1β, TNFα, and MCP-1 expression (see Supplementary Figure S4).
Figure 3.
Knockdown of Arid2-IR in mTECs inhibits IL-1β-induced inflammatory response in vitro. mTECs that stably express Arid2-IR shRNA were stimulated with IL-1β (10 ng/ml) over the period of time (0–24 hours) and examined for inflammatory responses by western blot and real-time PCR. (a). Phosphorylation of NF-κB/p65; (b). NF-κB DNA binding activity. mTECs were stably transfected with Arid2-IR or NC shRNA and were analyzed by electrophoretic mobility shift assay. After treated with or without IL-1β (10 ng/ml) for 30 minutes, cell nuclear extracts were collected (lane 3–6) and incubated in the Biotin-labeled NF-κB probe. Lane 1: no protein NC, lane 2: mutant NF-κB probe. (c–e) mRNA levels of IL-1β, TNF-α, and MCP-1 expression by real-time PCR. Each bar represents the mean ± SEM for at least three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus time 0; #P < 0.05, ##P < 0.01 versus NC. Each bar represents the mean ± SEM for three independent experiments.*P < 0.05 versus time 0, #P < 0.05versus NC.
Gene Transfer of Arid2-IR shRNA has no effect on TGF-β/Smad3-mediated renal fibrosis but inhibits NF-κB-driven renal inflammation in a mouse model of obstructive nephropathy.
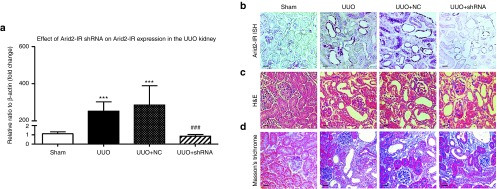
The differential role of Arid2-IR in renal fibrosis and inflammation was further determined in a mouse model of UUO by delivering the Arid2-IR shRNA expressing plasmid into the kidney using a well-established ultrasound-microbubble-mediated gene transfer technique as described earlier.3,13 Results shown in Figure 4a,b revealed that ultrasound-microbubble-mediated Arid2-IR shRNA transfer markedly reduced levels of Arid2-IR in the UUO kidney when compared with the kidney treated with or without NC plasmid. Consistent with the in vitro finding, knockdown of the Arid2-IR from the UUO kidney did not alter the levels of renal fibrosis as determined by both H&E and Masson's trichrome staining, although numbers of mononuclear cells infiltrating the tubulointerstitium were reduced (Figure 4c,d). Quantitative real-time PCR and western blot analysis confirmed this finding and revealed that knockdown of Arid2-IR from the UUO kidney did not alter levels of TGF-β/Smad3 signaling and renal fibrosis including expression of fibronectin, collagen I, and α-SMA at both mRNA and protein levels (Figure 5).
Figure 4.
Effect of ultrasound-microbubble-mediatedArid2-IR shRNA gene transfer on expression of Arid2-IR and changes in renal histology in a mouse model of UUO. (a) Real-time PCR shows that levels of Arid2-IR are largely upregulated in the UUO kidney, which is normalized by ultrasound-microbubble-mediated Arid2-IR shRNA transfer. (b) In situ hybridization shows that a marked upregulation of Arid2-IR by the damaged tubular epithelial cells is inhibited by ultrasound-microbubble-mediated Arid2-IR shRNA transfer. (c) H&E stained sections. (d) Masson's trichrome stained sections. Each bar represents the mean ± SEM for groups of six to eight mice; ***P < 0.001 versus sham-operated mice; ###P < 0.001 versus control treatment (UUO + NC). Scale bar = 100 µm.
Figure 5.
Knockdown of Arid2-IR by ultrasound-microbubble-mediated Arid2-IR shRNA gene transfer produces no effect on renal fibrosis in a mouse model of UUO. (a) Real-time PCR shows that ultrasound-mediated Arid2-IR shRNA therapy produces no effect on mRNA expression of TGF-β1, fibronectin (Fn), collagen I (Col. I), and α-SMA in the UUO kidney. (b) Western blot analysis shows that knockdown of Arid2-IR by Arid2-IR shRNA do not alter levels of phospho-Smad3 and expression of fibronectin, collagen I, and α-SMA in the UUO kidney. Each bar represents the mean ± SEM for groups of six to eight mice. *P < 0.05, ***P < 0.001 versus sham-operated mice.
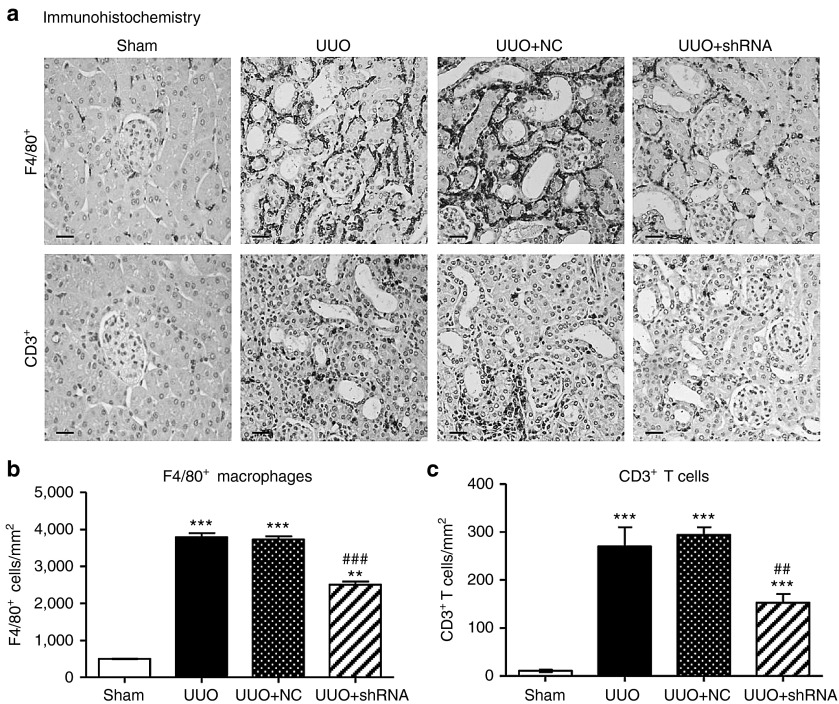
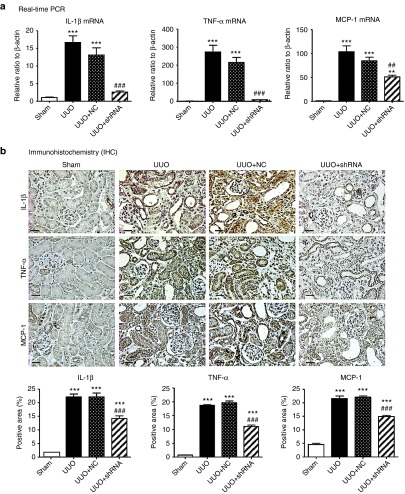
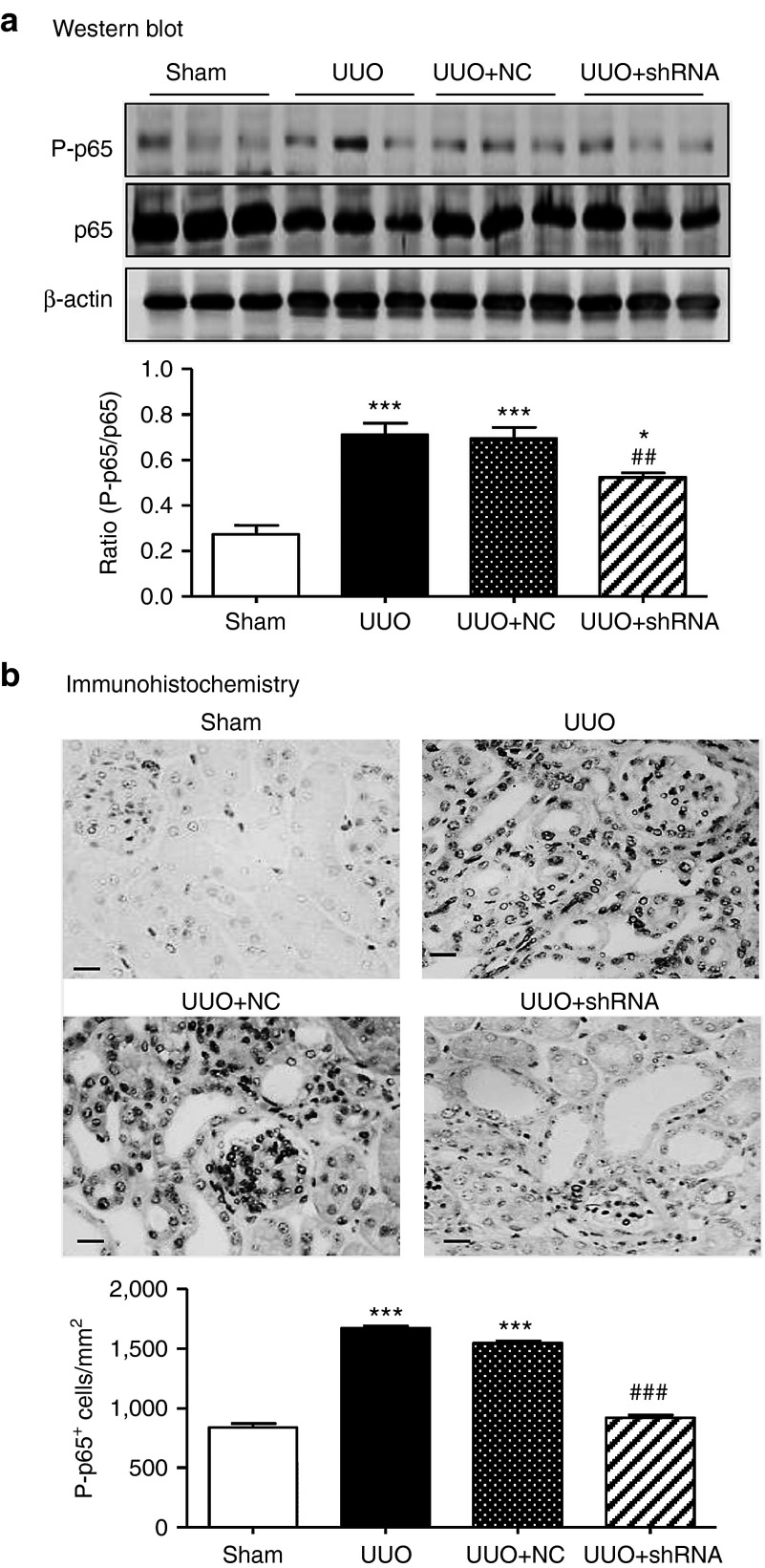
Inflammation is another pathological feature of UUO and thus we examined whether knockdown of Arid2-IR can influence renal inflammation in the UUO kidney. Immunohistochemistry showed that mice treated with Arid2-IR shRNAs exhibited a marked reduction in numbers of F4/80+macrophages and CD3+ T cells infiltrating the tubulointerstitium of the UUO kidney (Figure 6).This was associated with a marked inhibition of IL-1β, TNF-α, and MCP-1 expression as determined at the mRNA level by real-time PCR and at the protein level by immunohistochemistry (Figure 7). Consistent with the in vitro finding as shown in Figure 3a, inhibition of renal inflammation by knocking down Arid2-IR in the UUO kidney was associated with inactivation of NF-κB signaling as demonstrated by a marked reduction in levels of phosphorylated NF-κB/p65 and phosphorylated NF-κB/p65 nuclear translocation in the UUO kidney (Figure 8).
Figure 6.
Ultrasound-microbubble-mediated Arid2-IR shRNA gene transfer inhibits infiltration of F4/80+ macrophages and CD3+ T cells in the UUO kidney. (a) Immunohistochemistry shows that there is a marked increase in F4/80+ macrophages and CD3+ T cells infiltrating the tubulointerstitium of the UUO kidney, which is largely blocked by treatment with Arid2-IR shRNA. (b) Quantitation of F4/80+ macrophages infiltrating the tubulointerstitum. (c) Quantitation of CD3+ T cells infiltrating the tubulointerstitum. Each bar represents the mean ± SEM for groups of six to eight mice. **P < 0.01, ***P < 0.001 versus sham-operated mice; ##P < 0.01, ###P < 0.001 versus control treatment (UUO + NC). Scale bar = 100 µm.
Figure 7.
Ultrasound-microbubble-mediated Arid2-IR shRNA gene transfer blocks proinflammatory cytokines and chemokine MCP-1 expression in the UUO kidney. (a) Real-time PCR analysis of IL-1β, TNF-α, and MCP-1 mRNA expression. (b) Immunohistochemistry and quantitative analysis of IL-1β, TNF-α, and MCP-1 expression. Each bar represents the mean ± SEM for groups of six to eight mice. **P < 0.01, ***P < 0.001 versus sham-operated mice; ##P < 0.01, ###P < 0.001 versus control treatment (UUO + NC). Scale bar = 100 µm.
Figure 8.
Ultrasound-microbubble-mediated Arid2-IR shRNA gene transfer blocks NF-κB signaling in the UUO kidney. (a) Western blot analysis of NF-κB/p65 phosphorylation. (b) Immunohistochemistry and quantitative analysis of phospho-NF-κB/p65 nuclear translocation in the UUO kidney. Each bar represents the mean ± SEM for groups of six to eight mice. *P < 0.01, ***P < 0.001 versus sham-operated mice; ##P < 0.01, ###P < 0.001 versus control treatment (UUO + NC). Scale bar = 50 µm.
Discussion
LncRNAs have been shown to play a regulatory role in controlling gene expression. In patients with IgA-negative mesangial proliferative glomerulonephritis, thousands of lncRNAs and protein-coding genes are differentially expressed.17 However, the function of lncRNAs in kidney disease remains undefined. By using the next generation sequencing, we recently identified a number of Smad3-associated lncRNAs that are related to renal inflammation and fibrosis in mouse models of immune-related (anti-glomerular basement membrane glomerulonephritis) or nonimmune-related (UUO) kidney diseases,8 suggesting that TGF-β/Smad3-associated lncRNAs may have regulatory roles in renal fibrosis and inflammation. In this study, we found that the Arid2-IR is one of the most highly upregulated lncRNAs in the UUO kidney of WT mice with progressive renal fibrosis and inflammation, which is largely suppressed in the diseased kidney of Smad3 KO mice. We also found that knockdown of Arid2-IR from mTECs or from the UUO kidney did not influence TGF-β/Smad3 signaling and renal fibrosis but inhibited activation of NF-κB signaling and renal inflammation in vitro and in vivo. Thus, Arid2-IR may play a functional role in renal inflammation and may be as a therapeutic target for inflammatory kidney disease.
It is now well accepted that TGF-β1/Smad3 is a key pathway in the pathogenesis of renal fibrosis in both experimental and human kidney diseases.10 In addition, deletion of Smad3 impairs the immunity and attenuates renal and cardiac inflammation in the UUO kidney and in angiotensin II-induced hypertensive kidney and heart disease,14,15,18 suggesting a functional role for Smad3 in inflammation. It is also reported that Smad3 may be critical for TGF-β-mediated chemotaxis, infiltration of monocytes and local cytokine release.19,20 Recent studies demonstrated that Smad3 can be activated by many inflammatory pathways including the IL-1β-NF-κB and Ras pathway.21,22 In this study, we found that the promoter region of Arid2-IR contained a Smad3 binding site and deletion of Smad3 gene completely blocked upregulation of Arid2-IR in the UUO kidney, suggesting a positive regulatory role for Smad3 in Arid2-IR expression during renal inflammation. Interestingly, although Arid2-IR was upregulated in the UUO kidney of Smad3 WT mice, it did not have a regulatory role in TGF-β/Smad3-mediated renal fibrosis because knockdown of Arid2-IR did not influence TGF-β/Smad3 signaling and fibrosis but inhibited activation of NF-κB signaling and renal inflammation in vitro and in vivo. The differential effects of Arid2-IR in renal fibrosis and inflammation suggest that Arid2-IR may be a downstream mediator of Smad3 in renal inflammation because mice null for Smad3 are protected against Smad3-mediated fibrosis and NF-κB-driven inflammation in a number of renal and cardiac diseases.14,15,18 The promoter role for Arid2-IR in NF-κB-dependent renal inflammation, but not in TGF-β-mediated renal fibrosis, was further confirmed by overexpressing Arid2-IR to enhance IL-1β-induced NF-κB signaling and inflammatory cytokine expression without alteration of TGF-β1-induced fibrosis response by mTECs in vitro. Overall, although the regulatory mechanisms of TGF-β/Smad3 in renal inflammation remain largely unclear, results from this study suggest that Smad3 may act by stimulating the Arid2-IR-NF-κB-dependent mechanism to mediate renal inflammation.
It should be pointed out that although the discovery of functional lncRNAs represents a new layer of complexity in the molecular architecture of human disease,4,5,6,7 this understanding of lncRNA functions and mechanisms during the disease process remains limited. The majority of human ncRNAs are expressed in a tissue-specific manner and some of their expression level is relatively rare. In this study, although we identified a potential role of Arid2-IR in renal inflammation in a mouse model of UUO and in vitro, the precise mechanisms whereby Arid2-IR interacts with the NF-κB pathway to regulate renal inflammation remain largely unknown. It is reported that lncRNAs can regulate the expression of genes in close genomic proximity (cis-acting regulation) or target distant transcriptional activators or repressors (trans-acting) via a variety of mechanisms.23,24,25 Arid2 is a host gene of Arid2-IR; a subunit of the polybromo and BRG1-associated factor chromatin-remodeling complex, which facilitates ligand-dependent transcriptional activation by nuclear receptors and plays an essential role in the development and tissue-specific gene expression in individual differentiation programs.26 It has been shown that Arid2 functions as a tumor suppressor gene in hepatocellular carcinoma and melanoma.27,28 This study added new evidence for Arid2-IR in renal inflammation via the NF-κB-dependent pathway. However, the precise molecular mechanisms between Arid2-IR and NF-κB pathway-related signaling proteins remain unknown and require further investigation.
In this study, we also provided new evidence for the use of noninvasive ultrasound-microbubble–mediated technique to effectively deliver lncRNA Arid2-IR into the kidney. By using this technique, we are able to deliver a number of genes such as Smad7, miR-21, miR-29b, and miR-433 into both normal and diseased kidney in a noncell type-specific manner without detectable side effects.3,13,29,30,31,32 We now reported here to effectively transfect the Arid2-IR shRNA into the mouse kidney to inhibit Arid2-IR-mediated renal inflammation in the UUO kidney. The mechanism by which ultrasound-mediated Arid2-IR shRNA transfer may largely be attributed to the local ultrasound-mediated microbubble cavitation as described earlier. The ability of knocking down Arid2-IR to inhibit renal inflammation in a mouse model of UUO suggests that targeting the lncRNA Arid2-IR may represent a novel and specific therapy for kidney disease.
In conclusion, this study demonstrates that Arid2-IR is a TGF-β/Smad3-associated lncRNA and functions to promote NF-κB-driven renal inflammation without effect on TGF-β/Smad3-mediated renal fibrosis in a mouse model of obstructive nephropathy and in vitro. Results from this study suggest that actions of lncRNAs are specific and targeting lncRNAs may represent a novel and specific therapy for kidney disease.
Materials and Methods
RNA-Seq. Kidney tissues of Smad3 KO/WT (n = 2 in each group) were collected for RNA-Seq from the sham-operated and UUO kidneys at day 5 as described earlier.8 The experimental procedures were approved by the Animal Experimentation Ethics Committee at the Chinese University of Hong Kong.
Mouse model of UUO and ultrasound-mediated gene transfer of Arid2-IRshRNA plasmids. A mouse model of UUO was induced in male C57BL/6J mice at 8 weeks of age (20–22 g body weight) and an Arid2-IR shRNA expressing plasmid was transfected into the left kidney as described earlier.3,30,31,32 In brief, before the left ureter was ligated, groups of six to eight mice received the mixed solution (200 μl/mouse) containing either the Arid2-IR shRNA-pSuper.puro vector or NC shRNA-pSuper.puro vector (200 μg/mouse) and lipid microbubbles (Sonovue, Bracco, Milan, Italy) at a ratio of 1:1 (vol/vol) via the tail vein injection as described earlier.3,30,31,32 Immediately after injection, an ultrasound transducer (Therasonic, Electro-Medical Supplies, Wantage, UK) was directly placed on the skin of the back against the left kidney with a pulse-wave output of 1 MHz at 2 W/cm2 for a total of 5 minutes. Kidney tissues were harvested at day 5 after the ultrasound treatment. In addition, groups of 6–8 sham-operated and UUO mice without ultrasound treatment were used as controls. The experimental procedures were performed following the approved protocol by the Animal Experimentation Ethics Committee at the Chinese University of Hong Kong.
RACE cDNA amplification. SMARTer RACE cDNA Amplification Kit (Clontech, CA) was used for a rapid amplification of 5′ and 3′ end of Arid2-IR. The primer for 5′ and 3′ RACE were listed in Supplementary Table S1. cDNA reverse-transcription from the kidney tissue of UUO kidney was used as the template for RACE PCR according to the user manual. Advantage 2 Polymerase Mix (Clontech, CA) was used for RACE PCR amplification.
Vector construction. To construct the Arid2-IR shRNA plasmid, Arid2-IR or NC shRNA primers (listed in Supplementary Table S1) were annealed and cloned into pSuper.puro vector Oligoengine at HindIII and BglII sites. To construct the Arid2-IR overexpression vector, the full length of Arid2-IR was amplified by using PremixTaq (Takara, Japan) and then inserted into pcDNA3.1 vector at the NheI and EcoRI sites. The sequences were confirmed by DNA-sequencing.
Cell culture and establishment of a stable Arid2-IR knockdown mTEC. An mTEC (a gift from Dr. Jeffrey B. Kopp, NIH) was cultured in DMEM-F12 (Gibco, CA), supplemented with 5% FBS (Gibco, CA).To generate an Arid2-IR knockdown stable cell line, mTECs were stably transfected with Arid2-IR shRNA-pSuper.puro vector or NC shRNA-pSuper.puro vector using Lipofectamine LTX kit according to the manufacturer's instructions (Invitrogen, CA). Cells stably expressing the Arid2-IR shRNA were selected by addition of 4 μg/ml Puromycin (Gibco, CA) for 7 days.
To examine the role of Arid2-IR in renal fibrosis and inflammation, mTECs with or without expressing Arid2-IR or Arid2-IR shRNA were stimulated with TGF-β (5 ng/ml, R&D system, MN) or IL-1β (10 ng/ml, R&D system) for periods of 0, 0.5, 1, 3, 6, 12, and 24 hours. Effects of downregulating or overexpressing Arid2-IR on activation of TGF-β/Smad3 or NF-κB signaling and expression of fibrotic markers (TGF-β1, fibronectin, collagen I, α-SMA) and inflammatory cytokines (IL-1β, TNF-α, and MCP-1) were examined by real-time PCR and western blot analysis.
ChIP analysis. ChIP was performed by Transcription Factor ChIP kit according to the manufacturer's instructions as described earlier.30 In brief, mTECs pretreated with TGF-β1 were firstly cross-linked with 1% formaldehyde for 10 minutes at 37 °C, quenched with glycine, and then sonicated using a Bioruptor (Diagenode, Liège, Belgium) to generate 300–600 bp DNA fragments. Immunoprecipitation was performed with the antibody against Smad3, and a normal IgG was used as a control. Precipitated DNAs were detected by PCR using specific primers (see Supplementary Table S1).
Real-time PCR analysis. Total RNA was isolated from the cultured cells and kidney tissues using Trizol (Invitrogen, CA) according to the manufacturer's instructions. Real-time PCR was performed by SYBR Green Supermix using CFX96 PCR System (Bio-Rad, CA) as described earlier.8,30,31,32 The primers used in this study, including mouse Arid2-IR, TGF-β1, fibronectin, collagen I, α-SMA, IL-1β, TNF-α, MCP-1, and β-actin were described in Supplementary Table S1. The relative level of detected gene was normalized with the internal control β-actin and expressed as mean ± SEM.
In situ hybridization. To detect the expression pattern and location of Arid2-IR in the kidney, in situ hybridization was performed in normal and UUO kidneys with Digoxigenin-labeled probes (Roche Diagnostics, IN) as shown in Supplementary Table S1, following the established protocol as described earlier.30,31,32
Western blot analysis. Protein from kidney tissues and cultured cells was extracted using the radio immunoprecipitation assay (RIPA) lysis buffer. Western blot analysis was performed as described earlier.30,31,32 In brief, after blocking nonspecific binding with 5% bovine serum albumin (BSA), membranes were then incubated overnight at 4 °C with the primary antibody against fibronectin and α-SMA (DAKO, Carpentaria, CA), phospho-Smad3 (CST, Danvers, MA) and Smad3 (Santa Cruz Biotech, Santa Cruz, CA), collagens I (Southern Tech, Birmingham, AL), phospho-p65 (Ser536) and p65 (CST), and β-actin (Santa Cruz), followed by IRDye800-conjugated secondary antibody (Rockland Immunochemicals, Gilbertsville, PA). Signals were detected using the LiCor/Odyssey infrared image system (LI-COR Biosciences, Lincoln, NE), followed by quantitative analysis using the Image J program (http://www.imagej.nih.gov/ij/). The ratio for the protein examined was normalized against β-actin and expressed as the mean ± SEM.
Immunohistochemistry. Immunohistochemistry was performed in 4-μm-paraffin sections using a microwave-based antigen retrieval technique. The antibodies used in this study included F4/80 (MCA497, Serotec, Oxford, UK), CD3 (ab-16669, Abcam, Cambridge, MA), IL-1β (sc-7884, Santa Cruz), MCP-1(14–7996, eBioscience, San Diego, CA), TNF-α (sc-1351, Santa Cruz), and phospho-p65 (Ser536) (ab-86299, Abcam). After immunostaining, sections were counterstained with hematoxylin (except for phospho-p65). The percentage of positive staining areas were quantified using the Image-Pro Plus software (Media Cybernetics, Bethesda, MD) in 10 consecutive fields (×40), whereas positive cells for CD3+ and F4/80+ cells were counted under the ×40 power field of microscope in 10 random areas of kidney tissues using a 0.25-mm2 graticule fitted in the eyepiece of the microscope and expected as cells/mm2.
Electrophoretic mobility shift assay. Total numbers of 5 × 106 mTECs with stably expressing Arid2-IR shRNA-pSuper.puro vector or NC shRNA-pSuper.puro vector were treated with or without IL-1β (10 ng/ml, R&D system) for 30 minutes. The nuclear extract from cells was prepared by using kit (Thermoscientific, MA) according to the manufacturer's instructions. The nuclear extracts were incubated with biotin-labeled oligonucleotide probes for NF-κB/p65 (5′-AGT TGA GGG GAC TTT CCC AGG-3′). To verify the specificity of the shifted bands, cold competition analysis was performed using a 100-fold excess of unlabeled oligonucleotide (NF-κB) that was coincubated with the nuclear extracts for 10 minutes at room temperature before the addition of the biotin-labeled oligonucleotide probe. The reaction mixtures were then separated by 6.5% nondenaturing polyacrylamide gel electrophoresis at 100 V for 60 minutes, transferred onto a nitrocellulose membrane by electroblotting, and cross-linked for 20 minutes. After being blocked in the blocking buffer for 15 minutes at room temperature, sections were incubated with strepto-avidin conjugated HRP (1:300) for 15 minutes and signal was detected by using an ECL substrate system (Thermoscientific).
Statistical analysis. Data obtained from this study were expressed as mean ± SEM. Statistical analyses were performed using one-way analysis of variance followed by Newman–Keuls multiple comparison test from GraphPad Prism 5.0 (Graph Pad Software, San Diego, CA).
SUPPLEMENTARY MATERIAL Figure S1. Characterization of Arid2-IR. Figure S2. Knockdown of Arid2-IR by siRNA in mouse tubular epithelial cells (mTECs). Figure S3. Overexpression of Arid2-IR produces no effect on TGF-β1-induced fibrotic responses in mTECs. Figure S4. Overexpressing Arid2-IR inhibits IL-1β-induced NF-κB signaling and inflammatory cytokine expression by mTECs. Table S1. Primers and probes used in this study.
Acknowledgments
This study was supported by grants: the Major State Basic Research Development Program of China, 973 program, grant 2012CB5177005 (H.Y.L.) and the National Natural Science Foundation of China, Major Program grant 81130012 (X.Y.), National Natural Science Foundation grant 81100542 and 81470942 (Q.Z.), the Research Grant Council of Hong Kong grants GRF 468711, CUHK3/CRF/12R, TRS T12-402/13N (H.Y.L.), and the Focused Investment Scheme A program (H.Y.L.) from the Chinese University of Hong Kong.
Supplementary Material
References
- Chung, AC, Yu, X and Lan, HY (2013). MicroRNA and nephropathy: emerging concepts. Int J Nephrol Renovasc Dis 6: 169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen, JM, Haller, H and Thum, T (2011). MicroRNAs as mediators and therapeutic targets in chronic kidney disease. Nat Rev Nephrol 7: 286–294. [DOI] [PubMed] [Google Scholar]
- Li, R, Chung, AC, Dong, Y, Yang, W, Zhong, X and Lan, HY (2013). The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway. Kidney Int 84: 1129–1144. [DOI] [PubMed] [Google Scholar]
- Ponting, CP, Oliver, PL and Reik, W (2009). Evolution and functions of long noncoding RNAs. Cell 136: 629–641. [DOI] [PubMed] [Google Scholar]
- Wang, KC and Chang, HY (2011). Molecular mechanisms of long noncoding RNAs. Mol Cell 43: 904–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizzo, R, Almeida, MI, Colombatti, A and Calin, GA (2012). Long non-coding RNAs and cancer: a new frontier of translational research? Oncogene 31: 4577–4587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wapinski, O and Chang, HY (2011). Long noncoding RNAs and human disease. Trends Cell Biol 21: 354–361. [DOI] [PubMed] [Google Scholar]
- Zhou, Q, Chung, AC, Huang, XR, Dong, Y, Yu, X and Lan, HY (2014). Identification of novel long noncoding RNAs associated with TGF-β/Smad3-mediated renal inflammation and fibrosis by RNA sequencing. Am J Pathol 184: 409–417. [DOI] [PubMed] [Google Scholar]
- Huang, YS, Hsieh, HY, Shih, HM, Sytwu, HK and Wu, CC (2014). Urinary Xist is a potential biomarker for membranous nephropathy. Biochem Biophys Res Commun 452: 415–421. [DOI] [PubMed] [Google Scholar]
- Lan, HY (2011). Diverse roles of TGF-β/Smads in renal fibrosis and inflammation. Int J Biol Sci 7: 1056–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng, XM, Huang, XR, Chung, AC, Qin, W, Shao, X, Igarashi, P et al. (2010). Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, AC, Huang, XR, Zhou, L, Heuchel, R, Lai, KN and Lan, HY (2009). Disruption of the Smad7 gene promotes renal fibrosis and inflammation in unilateral ureteral obstruction (UUO) in mice. Nephrol Dial Transplant 24: 1443–1454. [DOI] [PubMed] [Google Scholar]
- Chen, HY, Huang, XR, Wang, W, Li, JH, Heuchel, RL, Chung, AC et al. (2011). The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential. Diabetes 60: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inazaki, K, Kanamaru, Y, Kojima, Y, Sueyoshi, N, Okumura, K, Kaneko, K et al. (2004). Smad3 deficiency attenuates renal fibrosis, inflammation, and apoptosis after unilateral ureteral obstruction. Kidney Int 66: 597–604. [DOI] [PubMed] [Google Scholar]
- Liu, Z, Huang, XR and Lan, HY (2012). Smad3 mediates ANG II-induced hypertensive kidney disease in mice. Am J Physiol Renal Physiol 302: F986–F997. [DOI] [PubMed] [Google Scholar]
- Loots, GG and Ovcharenko, I (2004). rVISTA 2.0: evolutionary analysis of transcription factor binding sites. Nucleic Acids Res 32(Web Server issue): W217–W221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui, W, Li, H, Ou, M, Tang, D and Dai, Y (2012). Altered long non-coding RNA expression profile in patients with IgA-negative mesangial proliferative glomerulonephritis. Int J Mol Med 30: 173–178. [DOI] [PubMed] [Google Scholar]
- Yang, X, Letterio, JJ, Lechleider, RJ, Chen, L, Hayman, R, Gu, H et al. (1999). Targeted disruption of SMAD3 results in impaired mucosal immunity and diminished T cell responsiveness to TGF-beta. EMBO J 18: 1280–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft, GS, Yang, X, Glick, AB, Weinstein, M, Letterio, JL, Mizel, DE et al. (1999). Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat Cell Biol 1: 260–266. [DOI] [PubMed] [Google Scholar]
- Huang, XR, Chung, AC, Yang, F, Yue, W, Deng, C, Lau, CP et al. (2010). Smad3 mediates cardiac inflammation and fibrosis in angiotensin II-induced hypertensive cardiac remodeling. Hypertension 55: 1165–1171. [DOI] [PubMed] [Google Scholar]
- Daly, AC, Vizán, P and Hill, CS (2010). Smad3 protein levels are modulated by Ras activity and during the cell cycle to dictate transforming growth factor-beta responses. J Biol Chem 285: 6489–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, JI, Lee, MG, Cho, K, Park, BJ, Chae, KS, Byun, DS et al. (2003). Transforming growth factor-beta1 activates interleukin-6 expression in prostate cancer cells through the synergistic collaboration of the Smad2, p38-NF-kappaB, JNK, and Ras signaling pathways. Oncogene 22: 4314–4332. [DOI] [PubMed] [Google Scholar]
- Guttman, M and Rinn, JL (2012). Modular regulatory principles of large non-coding RNAs. Nature 482: 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil, AM, Guttman, M, Huarte, M, Garber, M, Raj, A, Rivea Morales, D et al. (2009). Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proc Natl Acad Sci USA 106: 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ørom, UA, Derrien, T, Beringer, M, Gumireddy, K, Gardini, A, Bussotti, G et al. (2010). Long noncoding RNAs with enhancer-like function in human cells. Cell 143: 46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M, Zhao, H, Zhang, X, Wood, LD, Anders, RA, Choti, MA et al. (2011). Inactivating mutations of the chromatin remodeling gene ARID2 in hepatocellular carcinoma. Nat Genet 43: 828–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, F, Flowers, S and Moran, E (2012). Essential role of ARID2 protein-containing SWI/SNF complex in tissue-specific gene expression. J Biol Chem 287: 5033–5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, Z, Cui, K, Murray, DM, Ling, C, Xue, Y, Gerstein, A et al. (2005). PBAF chromatin-remodeling complex requires a novel specificity subunit, BAF200, to regulate expression of selective interferon-responsive genes. Genes Dev 19: 1662–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan, HY, Mu, W, Tomita, N, Huang, XR, Li, JH, Zhu, HJ et al. (2003). Inhibition of renal fibrosis by gene transfer of inducible Smad7 using ultrasound-microbubble system in rat UUO model. J Am Soc Nephrol 14: 1535–1548. [DOI] [PubMed] [Google Scholar]
- Qin, W, Chung, AC, Huang, XR, Meng, XM, Hui, DS, Yu, CM et al. (2011). TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29. J Am Soc Nephrol 22: 1462–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, X, Chung, AC, Chen, HY, Meng, XM and Lan, HY (2011). Smad3-mediated upregulation of miR-21 promotes renal fibrosis. J Am Soc Nephrol 22: 1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, HY, Zhong, X, Huang, XR, Meng, XM, You, Y, Chung, AC et al. (2014). MicroRNA-29b inhibits diabetic nephropathy in db/db mice. Mol Ther 22: 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.