Abstract
Oncolytic viruses (OVs) have shown promising clinical activity when administered by direct intratumoral injection. However, natural barriers in the blood, including antibodies and complement, are likely to limit the ability to repeatedly administer OVs by the intravenous route. We demonstrate here that for a prototype of the clinical vaccinia virus based product Pexa-Vec, the neutralizing activity of antibodies elicited by smallpox vaccination, as well as the anamnestic response in hyperimmune virus treated cancer patients, is strictly dependent on the activation of complement. In immunized rats, complement depletion stabilized vaccinia virus in the blood and led to improved delivery to tumors. Complement depletion also enhanced tumor infection when virus was directly injected into tumors in immunized animals. The feasibility and safety of using a complement inhibitor, CP40, in combination with vaccinia virus was tested in cynomolgus macaques. CP40 pretreatment elicited an average 10-fold increase in infectious titer in the blood early after the infusion and prolonged the time during which infectious virus was detectable in the blood of animals with preexisting immunity. Capitalizing on the complement dependence of antivaccinia antibody with adjunct complement inhibitors may increase the infectious dose of oncolytic vaccinia virus delivered to tumors in virus in immune hosts.
Introduction
Oncolytic viruses (OVs) are multi-mechanistic therapeutics that can cause tumor debulking by direct oncolysis, deliver therapeutic transgenes, trigger vascular disruption, and critically induce antitumor immunity.1 To date, the successful clinical development of OVs has been largely as loco-regional therapeutics administered by direct injection into tumor beds.2,3 While this approach provides localized tumor destruction and the potential for the generation of systemic antitumor immunity,4,5,6,7 it does not take advantage of the ability of viruses to infect and destroy metastatic tumors. In preclinical models of systemic disease, the effectiveness of intravenous administration of OVs to virus naive animals has been demonstrated in a variety of tumor models.8,9 In cancer patients, however, the development of OVs as intravenous agents has been slower, in large part due to concerns about being able to dose past preexisting neutralizing antibodies or to deliver multiple doses of virus in patients developing an antiviral immune response.
Complement is a key component of the innate immune system's first line of defense, acting to target foreign pathogens for opsonization, neutralization, phagocytosis, and clearance from the circulatory system.10 Antibody-mediated complement activation is likely of particular importance for therapeutic vaccinia viruses as a large proportion of today's cancer patients were vaccinated during the smallpox eradication campaign. Indeed, as early as the 1950s, it was shown that complement could enhance the neutralizing capacity of antibodies induced by smallpox vaccination.11,12,13 Postvaccination era evaluation of residual protective immunity identified the persistence of antibodies against many vaccinia virus proteins by ELISPOT, immunoblot, and ELISAs; however, these provided weak neutralizing or no neutralizing activity in vitro in the absence of complement.14,15,16,17 We hypothesized that complement is integral to the function of antivaccinia antibody and that inactivation of complement could lead to improved survival of oncolytic vaccinia virus in the blood of hosts with preexisting viral immunity.
The complement C3 molecule provides an attractive therapeutic target since it sits at the axis of the three activation pathways and is the gateway to the terminal complement pathway. Compstatin is a 13 amino acid cyclic peptide that was selected from a phage display library for binding affinity to human and nonhuman primate C3 and C3b.18 Since its discovery, several analogs with improved pharmacodynamic and pharmacokinetic properties have been developed, with the analog CP40 emerging as the lead clinical candidate.19,20
We provide evidence here that in virus immune animal models, complement inhibition improves intravenous vaccinia virus delivery to tumors. We show that CP40 inhibited antibody-mediated virus neutralization in blood samples collected from immune cancer patients. Furthermore, in immune cynomolgus macaques, CP40 enhanced the infectious half-life of vaccinia virus in the circulation following intravenous administration.
Results
Antibody-mediated vaccinia virus neutralization is complement dependent
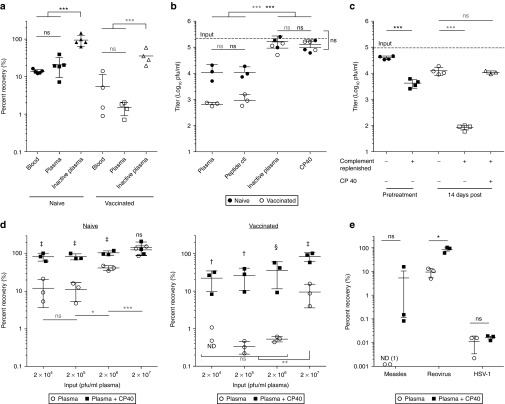
We undertook a components analysis to assess the sensitivity of Wyeth strain vaccinia virus to neutralizing factors in whole human blood from healthy volunteers who were either naive to the virus or vaccinated during childhood. Virus was incubated with whole blood, or fractions thereof, and infectious virus quantified by plaque assay. The anticoagulant Refludan was used as it does not interfere with the complement cascade.21 A concentration of 2 × 105 pfu/ml was used to mimic the clinical dose of 1 × 109 pfu in an estimated blood volume of 5 l that is required to facilitate tumor delivery in patients treated by intravenous infusion.22 As shown in Figure 1a, virus neutralization was approximately equal in whole blood and plasma, suggesting that the primary factors leading to loss of infectivity were plasma components. We found that in plasma samples from naive individuals, vaccinia virus was inactivated by up to 90%, but this inhibition could be reversed by heat inactivation of complement. Plasma from vaccinated individuals inhibited vaccinia virus with up to 99% of infectivity lost. Notably, heat inactivation of plasma from immunized individuals restored infectivity to ~20% of maximum. To confirm this complement dependence, we used the specific C3 inhibitor, CP40. Pretreatment of plasma with CP40 completely abrogated complement and antibody-mediated neutralization, with no detectable difference in viral recovery from the plasma of vaccinated versus naive donors (Figure 1b).
Figure 1.
Human complement and antibody lead to the neutralization of vaccinia virus. (a) Assessment of vaccinia virus neutralization in vitro in human blood samples from vaccinia naive or immunized healthy donors. Infectious virus was quantified by plaque assay following a 1 hour (37 °C) incubation at 2 × 105 pfu/ml in whole blood, plasma or heat inactivated plasma (n = 4–5 donors per immune status). (b) Plasma samples were heat inactivated or treated with CP40 (25 μmol/l) or the inactive peptide control prior to incubation with vaccinia virus (2 × 105 pfu/ml) (n = 3 donors per immune status). (c) Heat inactivated serum samples from four patients enrolled in a Pexa-Vec clinical was replenished with media or plasma from a naive donor as a source of complement. Naive plasma was pretreated with CP40 (25 μmol/l) when noted. Virus was incubated with complement and antibody at a concentration of 2 × 105 pfu/ml. (d) Vaccinia virus neutralization in naive (left) or vaccinated (right) plasma at virus concentrations ranging from 2 × 104 to 2 × 107 pfu/ml. Plasma was pretreated with CP40 (25 μmol/l) when noted (n = 3 donors per immune status). The comparisons between plasma and plasma + CP40 at each dose are indicated by (‡P < 0.001, †P < 0.01, §P < 0.05, ns = P > 0.05). (e) Measles Edmonston, reovirus, and HSV-1 were incubated with plasma (untreated or pretreated with CP40) and infectious virus quantified by plaque assay (n = 3 donors per virus). Data are represented as group means ± SD. Each dot represents a donor. ND, not detected. (***P < 0.001, **P < 0.01, *P < 0.05, ns = P > 0.05).
This led us to investigate the role that complement plays in amplifying the effect of anamnestic antibody in patients treated with Pexa-Vec (pexastimogene devacirepvec; JX-594) (ClinicalTrials.gov Identifier NCT01394939). Serum samples were collected from cancer patients prior to treatment and 2 weeks after their first infusion and subsequently heat inactivated to be used as a source of antibody. Virus neutralization was evaluated after incubation with this serum with or without the addition of plasma from a naive donor as a replenished source of complement. As shown in Figure 1c, the antibody boosted by Pexa-Vec treatment mediated enhanced neutralization of vaccinia virus; however, this activity was substantially mitigated by complement inhibition.
It has been shown clinically through dose escalation of Pexa-Vec that there exists in patients natural barriers to intravenous delivery to tumor beds that can be overcome at a breakthrough dose.22 We characterized vaccinia virus neutralization at doses above and below this dose (2 × 104–2 × 107 pfu/ml). In plasma from naive donors (Figure 1d, left panel), neutralization declined at higher virus concentrations with no detectable neutralization at 2 × 107 pfu/ml. However, in naive plasma samples pretreated with CP40, viral recovery was almost 100% at all dose levels. In vaccinated plasma samples (Figure 1d, right panel), a threshold effect was observed whereby between 0.1 and 1% of virus was recovered at dose levels between 2 × 104 and 2 × 106 pfu/ml. However, at the dose of 2 × 107 pfu/ml, a statistically significant increase in recovery was observed. Consistent with our previous results, elevated levels of infectious virus could be recovered at all doses if complement was inhibited with CP40.
We also assessed the neutralizing capacity of immune plasma on other clinical candidate oncolytic viruses in the presence or absence of CP40. While complement inhibition did not affect viral neutralization in HSV-1 seropositive plasma, it enhanced the recovery of measles Edmonston and reovirus (Figure 1e). Measles Edmonston was shown to be very sensitive to complement and antibody. A clear biologic trend was associated with complement inhibition in measles samples; however, due to the variability within the donors, it was not statistically significant.
Viral neutralization is mediated via the membrane attack complex
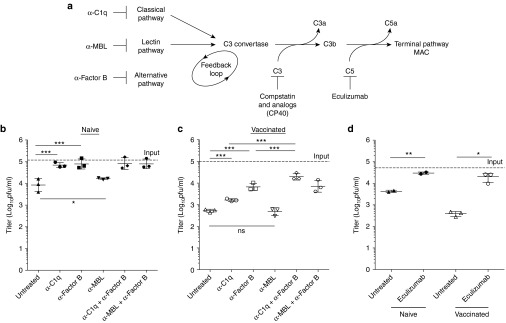
We selectively inhibited individual components of the activation pathways of the complement cascade to establish their contribution to vaccinia virus neutralization. In plasma from naive donors, inhibition of either the classical or alternative pathways resulted in no detectable virus neutralization (Figure 2b,c). This indicated that both the classical or alternative pathways could lead to complement activation and viral neutralization in vitro. Neutralization in immune plasma was inhibited with monoclonal antibodies to the classical and alternative pathways, and this was maximized when used in combination, highlighting a role for the alternative pathway as a positive amplification loop (Figure 2b). Specifically, we observed a 3-fold and 13-fold increase with the anti-C1q and anti-Factor B antibodies, respectively, and a 38-fold increase in titer when used in combination. Inhibition of the mannose binding lectin (MBL) pathway using an anti-MBL antibody did not have a large impact on the viability of vaccinia virus in human plasma (Figure 2b,c). A mannose-binding lectin assay validated the functional inhibition of the MBL pathway (Supplementary Figure S1a). The clinically approved monoclonal antibody, eculizumab, directed against C5, was used to inhibit the terminal pathway. In both naive and immune plasma, inhibition of the terminal pathway abrogated virtually all viral neutralization, leading to a mean 7-fold and 52-fold increase in titer, respectively (Figure 2d). To validate the effect of the terminal pathway on vaccinia virus particles in the presence of antiviral antibodies and complement, we carried out sucrose density centrifugation of virus preparations. Native viral particles subjected to centrifugation banded on a sucrose gradient in fractions 12 and 13 (Supplementary Figure S1b). In contrast, detergent or plasma treated viral particles were detected in fractions 1–4, consistent with complement- and antibody-mediated destruction of vaccinia virus.
Figure 2.
Mechanistic characterization of complement and antibody mediated neutralization of vaccinia virus. (a) Complement pathway schematic and targets of inhibitory antibodies. Complement activation pathway dissection in naive (b) or immune (c) plasma. Selective inhibition of the various pathways of the complement cascade demonstrates their contribution to viral neutralization. Plasma from naive and immune donors was pretreated with 500 μg/ml 1379 (anti-Factor B) or 100 μg/ml P1H10 (anti-C1q) or 60 μg/ml F38 (anti-MBL) or 50 μg/ml of eculizumab (anti-C5) (d) to allow for binding. Vaccinia virus neutralization over the course of a 1 hour incubation period at 37 °C (2 × 105 pfu/ml) was subsequently assessed by plaque assay (n = 3 donors per immune status with the exception of naive eculizumab samples n = 2). Data are represented as group means ± SD. Each dot represents a donor. (***P < 0.001, **P < 0.01, *P < 0.05, ns = p > 0.05).
Complement depletion of Fischer rats enhances virus delivery to tumor sites in the presence of neutralizing antibodies
As CP40 is a primate-specific inhibitor, to evaluate the effects of complement depletion in vivo in a tumor bearing rat model, we employed cobra venom factor (CVF). To first validate that the rat model mimicked our human findings, we assessed virus neutralization in vitro. Vaccinia virus was shown to be sensitive to rat complement, and the antiviral antibodies were neutralizing in only the presence of complement (Supplementary Figure S2a). CVF-mediated complement depletion was confirmed by immunoblot against the C3 protein (Supplementary Figure S2b). Therefore, in the rat, the complement dependence of the anti-vaccinia antibody neutralization closely mirrored the effects seen in human blood samples.
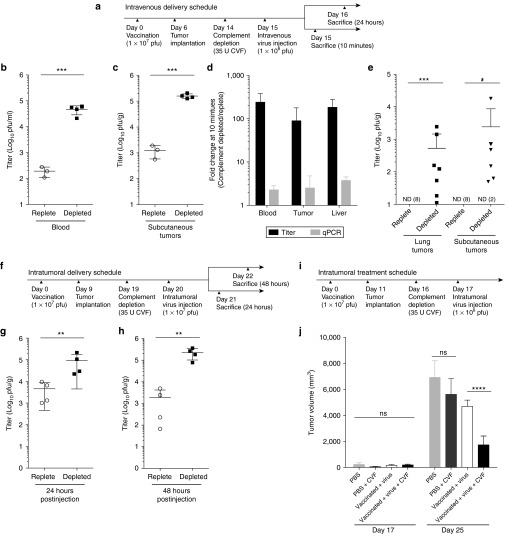
Rats were vaccinated (1 × 107 pfu), and once neutralizing antivaccinia titers were established, animals were treated intravenously with a single dose of oncolytic vaccinia virus (1 × 108 pfu). In half the animals, the virus therapy was carried out following complement depletion with CVF (Figure 3a). An early time point after tail vein injection of virus (10 minutes) was chosen to simultaneously measure live virus in the blood and early delivery to subcutaneous mammary adenocarcinoma tumors. A greater than 100-fold increase in mean infectious virus titer was observed in the blood of vaccinated complement-depleted animals, compared to their complement-replete counterparts (Figure 3b). A corresponding increase in delivery of infectious virus to tumors was observed in complement-depleted animals 10 minutes postinjection (Figure 3c). To further examine the mechanism associated with the increased detection of live virus following complement depletion, vaccinia virus genomes were quantified in tumors, blood, and liver by quantitative real-time PCR. Although titer increased substantially following complement depletion, only a minor change was observed in viral genomes in each organ. This suggests that while we observed a reduction in viral neutralization, the distribution of virus particles remained unchanged (Figure 3d). This increased delivery of infectious virus was able to infect and persist in the tumor. Specifically, at 24 hours after intravenous virus administration, infectious virus could only be recovered from subcutaneous tumors or tumor nodules in the lungs if complement was depleted at the time of virus administration (Figure 3e). Consistent with the effects observed in the blood and tumors, we observed a concomitant increase in delivery of infectious virus to the liver 10 minutes postinjection in complement-depleted rats. However, replicating virus could not be recovered from livers 24 hours after virus administration, irrespective of complement status (Supplementary Figure S2c), suggesting that complement inhibition did not increase infection of normal organs. In virus naive rats, complement depletion did not affect the stability of infectious virus in the blood after intravenous administration of virus (Supplementary Figure S2d). Similarly, complement depletion did not have a detectable effect on the titer of infectious virus recovered from the tumors of naive rats 48 hours posttreatment (Supplementary Figure S2e).
Figure 3.
Complement depletion in vaccinia virus immune Fischer rats leads to improved delivery and infection of tumors. Vaccinia virus (1 × 108 pfu) was delivered intravenously to F344 Fischer rats bearing bilateral 13762 MAT B III tumors, according the schedule in (a). As per the treatment groups, rats were vaccinated intravenously with 1 × 107 pfu and depleted of complement with 35 U of CVF. Infectious virus in the blood (b) and in subcutaneous tumors (c) 10 minutes post virus administration was quantified by plaque assay (n = 3–4 rats per group). (d) The fold change in titer and total genome content of blood, subcutaneous tumors, and liver is expressed as the ratio of complement depleted relative to complement replete samples. (e) Subcutaneous and lung tumor titers 24 hours after an intravenous dose of oncolytic vaccinia virus (n = 7–8 per group). Vaccinia virus (1 × 107 pfu) was delivered intratumorally to rats bearing bilateral tumors, according the schedule in (f). As per the treatment groups, rats were vaccinated intravenously with 1 × 107 pfu and depleted of complement with 35 U of CVF. Subcutaneous tumor titers are shown for animals sacrificed 24 (g) and 48 (h) hours post virus administration (n = 4 per group). Rats were vaccinated with 1 × 107 pfu vaccinia virus or untreated, then vaccinia virus (1 × 108 pfu) or PBS was delivered intratumorally to complement replete or depleted bearing subcutaneous tumors (i) and tumor volume determined at the indicated time points in (j). Data are represented as group means ± SD. Each dot represents a rat (***P < 0.001, **P < 0.01, *P < 0.05, ns = P > 0.05). PBS, phosphate-buffered saline, CVF, cobra venom factor.
To examine the effect that complement and antibody exerts on virus neutralization in the tumor microenvironment, immune rats were implanted with subcutaneous tumors and treated with vaccinia virus by intratumoral injection with or without complement depletion, as outlined in Figure 3f. Remarkably, tumors from complement-depleted animals contained a mean 20- and 117-fold more live virus than their complement-replete counterparts at 24 and 48 hours after virus administration, respectively (Figure 3g,h). In contrast, complement depletion had no detectable effect on the infection of tumors of naive animals following intratumoral injection (Supplementary Figure S2f,g).
Lastly, we evaluated the effect of the combinatorial strategy on tumor burden. Animals were vaccinated and treated with either phosphate-buffered saline (PBS) or vaccinia virus intratumorally (1 × 108 pfu) with or without CVF, according to the schedule in Figure 3i. While complement depletion in the context of PBS treatment had no impact on tumor growth, complement depletion of vaccinated animals treated with vaccinia virus provided enhanced tumor control at an early time point (Figure 3j).
Transient complement inhibition in cynomolgus macaques is a safe strategy that increases the stability of vaccinia virus in the blood
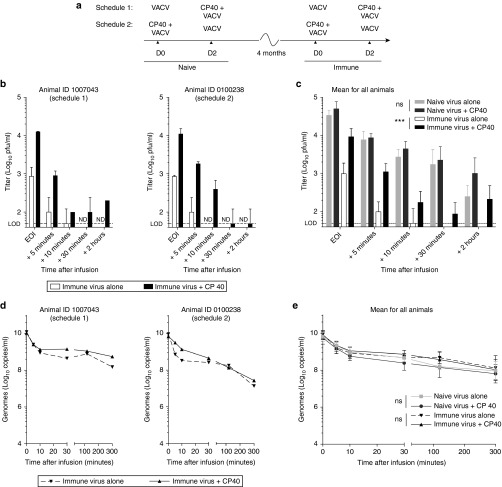
We conducted a feasibility study in cynomolgus macaques to evaluate the use of CP40 to transiently inhibit complement in the context of an intravenous infusion of oncolytic vaccinia virus. Four macaques were treated according to the schedule outlined in Figure 4a. Each animal received a dose of virus as a single agent (1 × 108 pfu infused over 30 minutes), and a dose of virus with a CP40 pretreatment (2 mg/kg bolus), separated by 2 days. This regimen was first performed when the animals were naive and 4 months later when they were immune to the virus. Vaccinia virus was shown to be sensitive to nonhuman primate (NHP) complement and the antiviral antibody response mounted by 3 months after virus treatment was demonstrated to be neutralizing only in the presence of complement (Supplementary Figure S3). Animals were randomly assigned to two groups with opposite treatment schedules, such that half the animals received single agent virus first and half received virus in combination with CP40 first. No difference was observed as a consequence of the order in which treatments were given.
Figure 4.
Complement inhibition in a cynomolgus macaque model stabilizes vaccinia virus in the blood of immune animals. Vaccinia virus (1 × 108 pfu) was infused using a central venous line over the course of 30 minutes. As per the treatment schedule in a, animals received a bolus intravenous dose of CP40 (2 mg/kg) immediately prior to virus treatment. Infectious virus in the blood was measured by plaque assay on samples taken at various time points after the end of the infusion (EOI). Blood titers for two representative immune animals are shown in b and are represented as technical replicate means ± SD. Mean titers for all naive and immune animals are shown in c and are represented as group means ± SD (n = 4 macaques). Genome content of blood collected at various time points after the EOI, as measured by qPCR using primers against the viral gene E3L. Genome concentrations in the blood are shown for two representative animals are shown in d and are represented as technical replicate means ± SD. Mean concentrations for all naive and immune animals in e and are represented as group means ± SD (n = 4 macaques). VACV, vaccinia virus, ND, not detected, LOD, limit of detection (***P < 0.001, ns = P > 0.05).
Our primary objective was to determine if complement inhibition reduced antibody- and complement-mediated virus neutralization. Infectious virus in the blood was quantified by plaque assay. In naive animals, no effect of CP40 on the concentration of infectious virus in the blood was detected (Figure 4c and Supplementary Figure S4a). In contrast, profound differences in the levels of circulating infectious virus were observed in immune animals when pretreated with CP40 (Figure 4b,c and Supplementary Figure S4a). If CP40 was administered, peak titers at the end of the infusion were elevated (mean 9.93-fold increase; range: 5.8–14.8) and approached levels observed when animals were naive. While infectious virus in the blood disappeared within 10–30 minutes after the infusion in immune animals, this was prolonged up to 2 hours with CP40 pretreatment. In agreement with our earlier studies of complement depletion in rats, we did not see a change in the clearance rate of viral genomes from the circulation with CP40. (Figure 4d,e and Supplementary Figure S4b). The quantification of infectious virus and total genome content in the blood for two additional animals is shown in Supplementary Figure S4. Consistent with previously published dose finding studies,20 CP40 inhibition of complement was validated by hemolysis assay (data not shown).
While we saw the predicted effect on live virus in the blood, we did not observe adverse events that could be attributed to complement inhibition. Blood chemistry was evaluated on the day of infusion at several time points before and after treatment and did not indicate renal, hepatic, or pancreatic dysfunction. Complete blood counts indicated transient changes in lymphocyte, neutrophil, and monocyte counts, but these changes occurred both with and without CP40 (Supplementary Figure S5). Mild fevers were observed in some naive animals; however, the incidence of fever on the day of infusion was not increased by complement inhibition. Fevers were not observed in immune animals on the day of infusion (Supplementary Table S1). Pock lesions were observed on naive animals but not on immune animals and resolved without complications. A cytokine array was performed on plasma samples taken on the day of infusion. Although the analysis was complicated by the time frame of repeat dosing with or without CP40, cytokine expression did not suggest that complement inhibition modified pro- or anti-inflammatory cytokine profiles (Supplementary Figure S6).
Discussion
In this study, we demonstrate that the persistent antibody that is associated with smallpox vaccination retains a robust ability to induce vaccinia virus neutralization that is dependent on complement activation, and, as a corollary, that complement inhibition can suppress the neutralizing effects of preexisting humoral immunity. In vitro modeling of virus neutralization indicated that preexisting immunity from smallpox vaccination presented an important hurdle, resulting in the neutralization of up to 99% of virus. Moreover, humoral immunity is a dynamic barrier to oncolytic viruses, as observed by the elevated antibody titers boosted by Pexa-Vec treatment. In vivo, complement depletion improved infection of tumors in immune animals when virus was delivered both systemically and intratumorally. In a nonhuman primate model, we found CP40-mediated complement inhibition to be a safe and efficacious method to increase the stability of virus in the blood. These findings provide a strong rationale for a complement intervention strategy in both single administrations of oncolytic vaccinia virus to preimmune individuals and multidosing treatment schedules that are faced with increasing titers of antibody.
Clinical evidence demonstrates that the best therapeutic responses are associated with higher doses of virus. In a cohort of patients with advanced hepatocellular carcinoma treated by direct intratumoral injection with either low dose (1 × 108 pfu) or high dose (1 × 109 pfu) Pexa-Vec, survival duration was significantly related to dose (14.1 months on high dose compared to 6.7 months on the low dose). Another mechanism of action of OV therapy, the phenomenon of acute tumor vascular disruption by means of infection of tumor-associated endothelial cells, has also been associated with the administration of higher doses of virus. These studies suggest that strategies to increase the effective dose delivered to neoplastic sites would lead to better outcomes in the clinic.
Indeed, vaccinia virus possesses two independent mechanisms that confer reduced sensitivity to complement-mediated neutralization: the vaccinia complement protein (VCP) and the formation of the extracellular enveloped virus (EEV) virion. VCP possesses factor I co-factor activity and inhibits the formation of the C3 convertase.23 Consequently, VCP has been shown to reduce complement-enhanced antibody-mediated neutralization in vitro.24 However, infection and replication is required to produce VCP, a primarily secreted protein.25 Vaccinia virus produces two different infectious virions: the intracellular mature virus (IMV) and the enveloped virion (EEV). The EEV particle is wrapped in an additional membrane and acquires host cell complement-regulatory proteins that protect it from complement-mediated destruction.26 However, the fragility of the EEV membrane precludes its use as an infusion product. While both these adaptations may help to traffic virus to metastatic sites following a primary local infection, they are not advantageous to an initial infusion of oncolytic vaccinia virus comprised primarily of IMV particles.
A phase 1 dose escalation trial of intravenously delivered oncolytic vaccinia virus (Pexa-Vec,) demonstrated a pharmacokinetic barrier to infection of tumor sites, whereby only doses above 1.5 × 107 pfu/kg achieved detectable tumor infection.22 The pattern of delivery was highly suggestive of a transient saturation of the mechanisms that inactivate the virus dose in the bloodstream. We also observed a threshold effect in vitro whereby the proportion of live virus recovered was maximized by increasing the ratio of virus to volume of plasma (Figure 1d). We speculate that in circumstances where the amount of antibody is fixed, the stoichiometric requirement of multiple aggregated Fc tails to bind one C1q molecule is increasingly difficult to meet at higher virus concentrations.27 This experiment shows that the most efficient way to increase the effective dose is not to increase the dose of virus administered, but to reduce neutralization by blunting the functionality of the antibody.
A complement-intervention strategy may be applicable to other oncolytic vectors, such as Reolysin and MV-NIS (Figure 1e). While HSV-1 is sensitive to complement, and depletion has been shown to improve the systemic delivery of HSV-1 to brain tumors of naive rats,28 here we showed that the antibody-mediated HSV-1 neutralization is not enhanced by complement inhibition. We demonstrated that the antibody from healthy human donors against measles and reovirus exhibits some degree of complement dependence. The general population possesses a high degree of seropositivity to these viruses, and the level of anamnestic antibody has been shown to dramatically increase following treatment with these oncolytic viruses.29,30 Both these OV candidates are being evaluated in intravenous delivery clinical settings and may be enhanced by complement inhibition. Promising interim clinical results have been reported with MV-NIS for the treatment of multiple myeloma, a patient population in which the levels of functional antibody titers were shown to be very low.29 Concurrent complement inhibition may enable the expansion of the patient cohort to one in which live virus can be administered systemically without sustaining a prohibitive degree of neutralization.
Previous studies differ in the specific actors of the complement cascade that are reported to be required for vaccinia virus neutralization.31,32 We felt that it was important to resolve this discrepancy in order to determine which complement inhibitors may have the most clinical value. In contrast to the results of Magge et al., we demonstrated that inhibition of C1 and C3 prevented viral neutralization in both naive and immune plasma samples. Additionally, with the use of eculizumab, we have confirmed that C5 is required for viral neutralization and that the membrane attack complex is likely responsible for virolysis (Figure 2). Our findings suggest that the C1 esterase inhibitor and eculizumab may also offer some benefit to the delivery of oncolytic vaccinia virus.
Other approaches for shielding virus from neutralization and preventing unwanted cellular interactions, like serotype exchange and pseudotyping33,34 or polymer coating,35 require extensive engineering or postproduction modifications. In contrast, pharmacological inhibition of complement with short half-life peptide inhibitors is an off-the-shelf strategy that gives strict temporal control over complement activity on the day of administration. The Compstatin analogs offer an optimal point of intervention in the complement cascade since they target C3 and are thus able to quench initiation and prevent further amplification.19 With a half-life of just under 12 hours, CP40 will allow for the rebound of complement activity by the time that early released virus particles are produced.20 Short-term complement inhibition, provides enhanced delivery of infectious virus without precluding advantageous complement-mediated tumor clearance mechanisms such as antibody-mediated complement-dependent cytotoxicity as has been demonstrated following Pexa-Vec treatment that occur late after virus treatment (4 to 8 weeks posttreatment).36
The rat studies demonstrate the feasibility of complement disruption as a tool to reduce viral neutralization. Although infectious titer in the blood and the tumor was increased (Figure 3b,c), we did not alter the rapid nonspecific clearance that accounts for the majority of the dose (Figure 3d). It should be noted that there are many additional barriers to the delivery of therapeutic viruses to tumors, including poor tumor uptake and heterogeneous distribution within the tumor.37,38 Nonetheless, the proportion of the virions that reach the tumor intact and infectious can be increased by complement inhibition.
Several differences between the human and mouse complement systems have been reported that limit the utility of the mouse model in the context of this study. Low hemolytic activity of complement from all inbred mouse strains relative to rat or human has been documented39 and may be partially attributed to a C4 polymorphism that precludes the formation of a functional C5 convertase.40 More recently, an unspecified classical pathway inhibitor has been discovered in mouse serum, but not human or rat serum, and inhibits IgG-dependent complement-dependent cytotoxicity.41 It has also been identified that the immunodominant IMV antigens differ between humans and mice. While H3 is greatly immunodominant to L1 in vaccinated humans, H3 and L1 are codominant in mice.16 Moreover, mouse monoclonal antibodies targeting the L1 protein were shown to be neutralizing independently of complement whereas antibodies targeting the D8, H3, and A27 surface proteins were only neutralizing in the presence of supplemented complement.42 The predominance of human antivaccinia antibodies to target nonneutralizing epitopes supports the profile of complement fixing antibodies that we observed in human plasma. Taken together with our findings of the similarity to humans, we believe that the rat model has greater predictive value for the preclinical development of oncolytic vaccinia virus. Conclusions drawn from mice with regard to anti-vaccinia antibody and complement must be carefully weighed with the limitations of the model.
To circumvent the neutralizing factors in the blood and increase infection of tumors, direct intratumoral injection has been the clinically preferred route of administration. In fact, the tumor microenvironment can be a highly perfused region and complement plays an important role in the neoplastic development process.43,44 We have demonstrated that even for intratumoral dosing of immune animals, it is important to inhibit complement (Figure 3g,h). These findings call in to question the degree to which intratumoral dosing treatment regimens are able to overcome preexisting antibodies and suggests that the benefit of repeat dosing may be independent of oncolysis.
Oncolytic viruses represent a versatile class of multi-modality therapeutics. Although local administration has demonstrated therapeutic efficacy in the clinic, disseminated metastatic disease requires systemic dosing. In addition to presenting a barrier to intravenous administration, complement and antibody act in the tumor microenvironment to prevent the infection of cancer cells. We have demonstrated a window of opportunity whereby delivery and infection of tumors can be dramatically improved in virus-immune hosts through complement inhibition strategies. We suggest that adjunct complement inhibition may be essential going forward with oncolytic vaccinia virus and other oncolytic vectors that generate complement-fixing antibody.
Materials and Methods
Cell culture and virus. The U2OS, Vero, L929, and HeLa cell lines were purchased from the American Type Culture Collection (Manassas, VA) and maintained in complete Dulbecco's Modified Eagle's medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). The 13762 MAT B III (CRL-1666) rat mammary adenocarcinoma cell line was purchased from the American Type Culture Collection and maintained in McCoy's 5A medium modified (ATCC, Manassas, VA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT). The oncolytic vaccinia virus Wyeth strain TK- gfp was previously described.8,45 HSV-1 (ICP0 null mutant n212)46 was a gift from Dr Karen Mossman, McMaster University. Reolysin was a gift from Dr Patrick Lee (Dalhousie University). Measles Edmonston was purchased from American Type Culture Collection (Manassas, VA).
Human blood neutralization experiments. This study protocol was approved by the Ottawa Hospital Research Ethics Board, and all volunteers gave informed consent. Donors were stratified into immunological groups based on self-reported vaccination status. Human blood was collected from healthy donors by venipuncture into glass serum collection vacutainer tubes (BD Biosciences, Mississauga, Ontario, Canada) and treated immediately with Refludan21 at a final concentration of 50 μg/ml. Blood was spun at 800g for 10 minutes to obtain plasma. Plasma aliquots were incubated for 30 minutes at 56 °C to inactivate complement. Blood or fractions thereof were incubated for 1 hour at 37 °C with vaccinia virus at range of doses between 2 × 104–2 × 107 pfu/ml. The infectious virus remaining was quantified by plaque assay on U2OS cells. To investigate the complement pathway, the Compstatin analog CP4020 was preincubated for 15 minutes at 37 °C at a final concentration of 25 μmol/l prior to virus inoculation. Alternatively, the neutralizing monoclonal antibodies 3F8 (ref. 47) mouse anti-human MBL, P1H10 mouse anti-human C1q, 1379 mouse-anti human Factor B,48 or eculizumab were preincubated for 15 minutes at 37 °C with plasma at concentrations of 60, 100 or 500 or 50 μg/ml, respectively.
In order to assess anti-vaccinia antibody from hyperimmune individuals, leftover serum was collected from patients enrolled in JX594-CRC019 (ClinicalTrials.gov Identifier IDNCT01394939). This study protocol was approved by the Ottawa Hospital Research Ethics Board and all patients gave informed consent. The serum was heat inactivated and combined with serum-free media or plasma collected from a naive donor as a source of complement and incubated with vaccinia virus at a ratio of 2 × 105 pfu/ml. The infectious virus remaining was quantified by plaque assay on U2OS cells. Neutralization of HSV-1, Reovirus, and Measles Edmonston were performed at a ratio of 2 × 106 pfu/ml. Plasma samples were incubated with CP40 (25 μmol/l) or DMEM for 15 minutes at 37 °C prior to a 1-hour incubation at 37 °C in the presence of virus. HSV-1 and measles were titered on Veros and reovirus titered on L929 cells.
Fluorochrome linked immunoassay. Activation of the MBL-dependent lectin pathway was assessed in human serum incubated with mAb 3F8 at 10 μg/ml as previously described.49
Sucrose gradient separation of viral proteins. Vaccinia virus was incubated with PBS, PBS with detergent (1% Triton X-100) or plasma from an immune donor at a ratio of 2 × 107 pfu/ml for 1 hour at 37 °C. Samples were overlaid on continuous 5–75% sucrose gradients and spun at 25,000 rpm in an SW41Ti rotor for 35 minutes at 4 °C. The gradient was collected in 16 fractions.
Immunoblotting. Plasma or viral proteins were resolved on 4–12% polyacrylamide gels (BioRad, Hercules, CA) and transferred to nitrocellulose membranes (Amersham GE Healthcare Lifesciences, Baie d'Urfe, Quebec, Canada). Membranes were incubated for 1 hour at room temperature or at 4 °C overnight with the following antibodies and dilutions: 1:100 rabbit anti-rat C3 (Cedarlane, Burlington, ON; CL7334AP); rabbit polyclonal antibody to Vaccinia (Quartett, Berlin, Germany; 1220100715). Membranes were incubated with goat antirabbit horseradish peroxidase-conjugated IgG (Jackson ImmunoResearch Laboratories, West Grove, PA) for 1 hour at room temperature. Proteins were detected using Supersignal West Pico chemiluminescent substrate (Pierce Biotechnology, Rockford, IL) followed by exposure to X-ray film (Fuji Photo Film, Tokyo, Japan).
Animal studies
Rat model. Female F344 Fischer rats weighing 100–150 g were purchased from Charles River (Wilmington, MA). All animals were housed in pathogen-free conditions, and all studies conducted were in accordance with the guidelines of the Animal Care Veterinary Service facility of the University of Ottawa. Tumors were established by injecting 5 × 105–1 × 106 13762 MAT B III cells subcutaneously unilaterally or bilaterally in the left and right flanks or 3 × 105 cells intravenously by tail vein injection. Tumors were treated intravenously with 1 × 108 pfu of vaccinia virus, and 10 minutes or 24 hours postinfection, the animals were sacrificed and the tumors resected. Tumors were also treated with an intratumoral injection of 1 × 107 pfu, and animals were sacrificed either 24 or 48 hours after administration. For the tumor control experiment, 1 × 108 pfu of vaccinia virus was injected intratumorally. Tumor were measured with calipers, and tumor volumes calculated using the formula v = (w2 × l)/2. Blood was collected by cardiac puncture into EDTA vacutainer tubes for analysis of in vivo neutralization. Alternatively, blood was collected with serum tubes and treated with Refludan to assess in vitro neutralization. Viral neutralization was assessed in vitro in plasma at a concentration of 2 × 105 pfu/ml. For the depletion of complement, 35 U of CVF (Quidel, San Diego, CA) was administered intraperitoneally 24 hours prior to virus delivery. A second dose of 35 U was administered intraperitoneally 24 hours after virus if animals were to be sacrificed 48 hours after virus administration. Tumors and livers were collected and immediately frozen. Tissues were homogenized in PBS and titrated on U2OS cells.
Cynomolgus macaque model. Healthy male and female cynomolgus macaques weighing 3–6 kg were obtained from Primus BioResources (Vaudreuil-Dorion, Quebec, Canada) and Charles River (Montreal, Quebec, Canada). All NHP studies were performed under a protocol approved by the Animal Resource Centre, University Health Network, Toronto, ON, Canada.
To facilitate intravenous injection and blood sampling, a vascular access port was surgically implanted into the right flank with the venous line inserted into the right femoral/iliac vein. Animals were allowed to recover from surgery for several weeks prior to the initiation of the study. Macaques were randomized across schedule groups such that a male and female were assigned to each group. Animals received two doses of vaccinia virus (1 × 108 pfu infused over 30 minutes) in the naive setting (day 0 and day 2), a booster dose (1 × 108 pfu 2 months post naive treatment), and two doses in the immune setting (day 0, day 2). According to their group schedule, animals received a bolus dose of CP40 (2 mg/kg) immediately prior to their virus infusion either on day 0 or day 2. Blood samples were collected immediately before and at various time points after the infusion of virus (end of infusion, +5 minutes, +10 minutes, +30 minutes, +2 hours, +5 hours). EDTA-treated whole-blood samples were frozen for subsequent analysis by quantitative PCR and titration of infectious virus on U2OS cells. EDTA- and Refludan-treated blood samples were spun at 800g for 5 minutes to obtain plasma, and immediately frozen until further analysis. Rectal temperature was measured immediately before and at various time points after the infusion of virus (end of infusion, +5 minutes, +10 minutes, +30 minutes, +2 hours, +5 hours). Cell blood count (CBC) profiles were determined using a Hemavet 950FS on samples collected immediately before the infusion of virus, at the end of the infusion, +2 hours and + 5 hours after the infusion. Blood biochemistry was analyzed using a Vetscan VS2 on samples collected immediately before the infusion of virus, at the end of the infusion, +2 hours and +5 hours after the infusion.
Ex vivo neutralization. Blood was collected from healthy cynomolgus macaques using the anticoagulant Refludan (50 μg/ml). Plasma was heat inactivated and combined with plasma from a naive animal as a source of complement (ratio 5:1 naive plasma to heat inactivated plasma). Viral neutralization was assessed in vitro in plasma at a concentration of 2 × 105 pfu/ml. Samples were incubated for 1 hour at 37 °C and infectious virus remaining quantified by plaque assay.
Cytokine array. A MILLIPLEX MAP nonhuman primate magnetic bead cytokine array (Millipore, Bedford MA) was performed according to the manufacturer's instructions by the Princess Margaret Genomics Centre, Toronto, Ontario. Samples were read with Luminex 100, and data was analyzed using Bio-plex Manager 6.0 (BioRad, Mississauga, Ontario, Canada). OBS Concentrations were measured in pg/ml.
Quantitative PCR. DNA was isolated from whole blood collected in EDTA tubes, liver or tumor samples using the DNeasy kit (Qiagen, Germantown, MD) according to the manufacture's protocol. Quantitative PCR was performed on viral DNA extracted from whole blood or tissues using the SYBR Green PCR Master Mix (Qiagen, Germantown, MD) on a RotorGene RG3000A (Corbett Research, Mortlake, New South Wales, Australia). Primers targeting the E3L gene were used to quantify viral genomes (TCCGTCGATGTCTACACAGG; ATGTATCCCGCGAAAAATCA). DNA isolated from purified viral stock was used as a standard where molecular weight was used to give an estimate of the number of copies per μg of DNA.
Statistics. Where possible, statistical analyses were performed on log-transformed values. When data points fell below the limit of detection, the value of half the limit of detection was used for statistical comparisons however was reported as ND (not detected) for graphical purposes. Statistical analyses were performed using GraphPad Prism (GraphPad, La Jolla, CA) and the R statistical software (Foundation for Statistical Computing, Vienna, Austria). Two-tailed unpaired Student's t-tests were performed for the comparison of two groups. Linear mixed effects models were fitted for experiments with repeated measures data using treatment condition, vaccination status, or time point as fixed effects, and donor as the random effect. Model fit was compared for models generated using pooled or unpooled levels of fixed effects to determine statistical significance. P values < 0.05 were considered significant (*P ≤ 0.05, **P ≤ 0.01, ***P < 0.001, ****P < 0.0001).
SUPPLEMENTARY MATERIAL Figure S1. Validation of mechanistic analysis of vaccinia virus neutralization by complement and antibody. Figure S2. Supporting data from the Fischer rat model. Figure S3. Validation of complement and antibody mediated neutralization of vaccinia virus in macaque plasma. Figure S4. Titer and genome analysis for all animals. Figure S5. Complete blood cell counts for animals on the day of infusion. Figure S6. Cytokine profile on the day of infusion. Table S1. Fever incidence on the day of infusion.
Acknowledgments
We thank S. Avery (UHN Animal Resource Centre, Toronto, Ontario, Canada) for assisting with nonhuman primate housing and experiments. We also thank K. Yates and E. MacDonald for technical assistance with the rat experiments. We are grateful to M. Morrissey for assistance with the MBL assay. We thank D. Vaillant for assistance in the preparation of virus as well as members of the Bell, Auer, Atkins, and Diallo laboratories for feedback on this project.
J.C.B. is supported by the Terry Fox Research Foundation, the Ontario Institute for Cancer Research, and the Ottawa Regional Cancer Foundation. J.A.M. is supported by the Ontario Institute for Cancer Research. J.D.L. is supported by the National Institutes of Health grants AI068730 and the European Community's Seventh Framework Programme under grant agreement no. 602699 (DIREKT). L.E. is the recipient of a Canadian Institute for Health Research Canada Graduate Scholarship.
J.D.L. is the inventor of a patent and/or patent applications that describe the use of complement inhibitors for therapeutic purposes and the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors for clinical applications. C.J.B. and D.K. are employees of SillaJen. The other authors declare no conflict of interest.
L.E., J.C.B., S.A.A., J.A.M., J.D.L., G.L.S., C.J.B., D.K., H.A., R.C.A., J.M.T., K.A.P., C.S.I., and C.G.L. were involved in the conception and design of experiments. L.E., M.M., and S.A.A. conducted in vitro experiments. C.T.S, L.E., and T.F. were involved in the rat experiments. L.E., S.A.A., D.H., A.G., R.L., S.F., J.A.M., and J.C.B. were involved in the nonhuman primate experiment. Statistical analysis was performed by L.E. and aided by C.S.F. The manuscript was written and reviewed by L.E., J.C.B., C.J.B, C.G.L., M.M., J.D.L, S.A.A., and J.A.M.
Supplementary Material
References
- Kirn, DH and Thorne, SH (2009). Targeted and armed oncolytic poxviruses: a novel multi-mechanistic therapeutic class for cancer. Nat Rev Cancer 9: 64–71. [DOI] [PubMed] [Google Scholar]
- Vacchelli, E, Vitale, I, Tartour, E, Eggermont, A, Sautès-Fridman, C, Galon, J et al. (2013). Trial watch: anticancer radioimmunotherapy. Oncoimmunology 2: e25595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miest, TS and Cattaneo, R (2014). New viruses for cancer therapy: meeting clinical needs. Nat Rev Microbiol 12: 23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, BH, Hwang, T, Liu, TC, Sze, DY, Kim, JS, Kwon, HC et al. (2008). Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol 9: 533–542. [DOI] [PubMed] [Google Scholar]
- Heo, J, Reid, T, Ruo, L, Breitbach, CJ, Rose, S, Bloomston, M et al. (2013). Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med 19: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, TH, Moon, A, Burke, J, Ribas, A, Stephenson, J, Breitbach, CJ et al. (2011). A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol Ther 19: 1913–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingemar Andtbacka, RH, Collichio, FA, Amatruda, T, Senzer, NN, Chesney, J, Delman, KA et al. (2013). OPTiM: a randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C and IV melanoma. J Clin Oncol 31. [Google Scholar]
- Kim, JH, Oh, JY, Park, BH, Lee, DE, Kim, JS, Park, HE et al. (2006). Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther 14: 361–370. [DOI] [PubMed] [Google Scholar]
- Stojdl, DF, Lichty, BD, tenOever, BR, Paterson, JM, Power, AT, Knowles, S et al. (2003). VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4: 263–275. [DOI] [PubMed] [Google Scholar]
- Ricklin, D, Hajishengallis, G, Yang, K and Lambris, JD (2010). Complement: a key system for immune surveillance and homeostasis. Nat Immunol 11: 785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, C, Nomura, M, Kitaoka, M, Takeuchi, Y and Kimura, M (1968). Complement requirement of the neutralizing antibody appearing after immunization with smallpox vaccine. Jpn J Microbiol 12: 256–259. [DOI] [PubMed] [Google Scholar]
- McCarthy, K and Germer, WD (1952). Two heat-labile factors in normal sera which neutralize variola virus. Br J Exp Pathol 33: 529–536. [PMC free article] [PubMed] [Google Scholar]
- Takabayashi, K and McIntosh, K (1973). Effect of heat-labile factors on the neutralization of vaccinia virus by human. Infect Immun 8: 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence, SJ, Lottenbach, KR, Newman, FK, Buller, RM, Bellone, CJ, Chen, JJ et al. (2007). Antibody responses to vaccinia membrane proteins after smallpox vaccination. J Infect Dis 196: 220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarlund, E, Lewis, MW, Hansen, SG, Strelow, LI, Nelson, JA, Sexton, GJ et al. (2003). Duration of antiviral immunity after smallpox vaccination. Nat Med 9: 1131–1137. [DOI] [PubMed] [Google Scholar]
- Benhnia, MR, McCausland, MM, Su, HP, Singh, K, Hoffmann, J, Davies, DH et al. (2008). Redundancy and plasticity of neutralizing antibody responses are cornerstone attributes of the human immune response to the smallpox vaccine. J Virol 82: 3751–3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty, S, Felgner, P, Davies, H, Glidewell, J, Villarreal, L and Ahmed, R (2003). Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol 171: 4969–4973. [DOI] [PubMed] [Google Scholar]
- Ricklin, D and Lambris, JD (2008). Compstatin: a complement inhibitor on its way to clinical application. Adv Exp Med Biol 632: 273–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano, AM, Ricklin, D, Huang, Y, Reis, ES, Chen, H, Ricci, P et al. (2014). Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood 123: 2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu, H, Ricklin, D, Bai, H, Chen, H, Reis, ES, Maciejewski, M et al. (2013). New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology 218: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexborn, F, Engberg, AE, Sandholm, K, Mollnes, TE, Hong, J and Nilsson Ekdahl, K (2009). Hirudin versus heparin for use in whole blood in vitro biocompatibility models. J Biomed Mater Res A 89: 951–959. [DOI] [PubMed] [Google Scholar]
- Breitbach, CJ, Burke, J, Jonker, D, Stephenson, J, Haas, AR, Chow, LQ et al. (2011). Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477: 99–102. [DOI] [PubMed] [Google Scholar]
- Sahu, A, Isaacs, SN, Soulika, AM and Lambris, JD (1998). Interaction of vaccinia virus complement control protein with human complement proteins: factor I-mediated degradation of C3b to iC3b1 inactivates the alternative complement pathway. J Immunol 160: 5596–5604. [PubMed] [Google Scholar]
- Isaacs, SN, Kotwal, GJ and Moss, B (1992). Vaccinia virus complement-control protein prevents antibody-dependent complement-enhanced neutralization of infectivity and contributes to virulence. Proc Natl Acad Sci USA 89: 628–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotwal, GJ, Isaacs, SN, McKenzie, R, Frank, MM and Moss, B (1990). Inhibition of the complement cascade by the major secretory protein of vaccinia virus. Science 250: 827–830. [DOI] [PubMed] [Google Scholar]
- Vanderplasschen, A, Mathew, E, Hollinshead, M, Sim, RB and Smith, GL (1998). Extracellular enveloped vaccinia virus is resistant to complement because of incorporation of host complement control proteins into its envelope. Proc Natl Acad Sci USA 95: 7544–7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diebolder, CA, Beurskens, FJ, de Jong, RN, Koning, RI, Strumane, K, Lindorfer, MA et al. (2014). Complement is activated by IgG hexamers assembled at the cell surface. Science 343: 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda, K, Wakimoto, H, Ichikawa, T, Jhung, S, Hochberg, FH, Louis, DN et al. (2000). Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant. J Virol 74: 4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, SJ, Federspiel, MJ, Peng, KW, Tong, C, Dingli, D, Morice, WG et al. (2014). Remission of disseminated cancer after systemic oncolytic virotherapy. Mayo Clin Proc 89: 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, CL, Twigger, KR, Vidal, L, De Bono, JS, Coffey, M, Heinemann, L et al. (2008). Characterization of the adaptive and innate immune response to intravenous oncolytic reovirus (Dearing type 3) during a phase I clinical trial. Gene Ther 15: 911–920. [DOI] [PubMed] [Google Scholar]
- Magge, D, Guo, ZS, O'Malley, ME, Francis, L, Ravindranathan, R and Bartlett, DL (2013). Inhibitors of C5 complement enhance vaccinia virus oncolysis. Cancer Gene Ther 20: 342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leddy, JP, Simons, RL and Douglas, RG (1977). Effect of selective complement deficiency on the rate of neutralization of enveloped viruses by human sera. J Immunol 118: 28–34. [PubMed] [Google Scholar]
- Roberts, DM, Nanda, A, Havenga, MJ, Abbink, P, Lynch, DM, Ewald, BA et al. (2006). Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 441: 239–243. [DOI] [PubMed] [Google Scholar]
- Miest, TS, Yaiw, KC, Frenzke, M, Lampe, J, Hudacek, AW, Springfeld, C et al. (2011). Envelope-chimeric entry-targeted measles virus escapes neutralization and achieves oncolysis. Mol Ther 19: 1813–1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlisle, RC, Di, Y, Cerny, AM, Sonnen, AF, Sim, RB, Green, NK et al. (2009). Human erythrocytes bind and inactivate type 5 adenovirus by presenting Coxsackie virus-adenovirus receptor and complement receptor 1. Blood 113: 1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, MK, Breitbach, CJ, Moon, A, Heo, J, Lee, YK, Cho, M et al. (2013). Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci Transl Med 5: 185ra63. [DOI] [PubMed] [Google Scholar]
- Miller, AC and Russell, SJ (2014). Heterogeneous delivery is a barrier to the translational advancement of oncolytic virotherapy for treating solid tumors. Virus Adapt Treat 6: 11–31. [Google Scholar]
- Carlisle, R, Choi, J, Bazan-Peregrino, M, Laga, R, Subr, V, Kostka, L et al. (2013). Enhanced tumor uptake and penetration of virotherapy using polymer stealthing and focused ultrasound. J Natl Cancer Inst 105: 1701–1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, GL and Mattes, MJ (1989). Mouse strains with typical mammalian levels of complement activity. J Immunol Methods 125: 147–158. [DOI] [PubMed] [Google Scholar]
- Ebanks, RO and Isenman, DE (1996). Mouse complement component C4 is devoid of classical pathway C5 convertase subunit activity. Mol Immunol 33: 297–309. [DOI] [PubMed] [Google Scholar]
- Ratelade, J and Verkman, AS (2014). Inhibitor(s) of the classical complement pathway in mouse serum limit the utility of mice as experimental models of neuromyelitis optica. Mol Immunol 62: 104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaever, T, Meng, X, Matho, MH, Schlossman, A, Li, S, Sela-Culang, I et al. (2014). Potent neutralization of vaccinia virus by divergent murine antibodies targeting a common site of vulnerability in L1 protein. J Virol 88: 11339–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pio, R, Ajona, D and Lambris, JD (2013). Complement inhibition in cancer therapy. Semin Immunol 25: 54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markiewski, MM, DeAngelis, RA, Benencia, F, Ricklin-Lichtsteiner, SK, Koutoulaki, A, Gerard, C et al. (2008). Modulation of the antitumor immune response by complement. Nat Immunol 9: 1225–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parato, KA, Breitbach, CJ, Le Boeuf, F, Wang, J, Storbeck, C, Ilkow, C et al. (2012). The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol Ther 20: 749–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfilippo, CM and Blaho, JA (2006). ICP0 gene expression is a herpes simplex virus type 1 apoptotic trigger. J Virol 80: 6810–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, H, Wakamiya, N, Suzuki, Y, Hamonko, MT and Stahl, GL (2002). Identification of human mannose binding lectin (MBL) recognition sites for novel inhibitory antibodies. Hybrid Hybridomics 21: 25–36. [DOI] [PubMed] [Google Scholar]
- Thurman, JM, Kraus, DM, Girardi, G, Hourcade, D, Kang, HJ, Royer, PA et al. (2005). A novel inhibitor of the alternative complement pathway prevents antiphospholipid antibody-induced pregnancy loss in mice. Mol Immunol 42: 87–97. [DOI] [PubMed] [Google Scholar]
- Walsh, MC, Shaffer, LA, Guikema, BJ, Body, SC, Shernan, SK, Fox, AA et al. (2007). Fluorochrome-linked immunoassay for functional analysis of the mannose binding lectin complement pathway to the level of C3 cleavage. J Immunol Methods 323: 147–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.