Abstract
Spinal cord injury (SCI) frequently provokes serious detrimental outcomes because neuronal regeneration is limited in the central nervous system (CNS). Thus, the creation of a permissive environment for transplantation therapy with neural stem/progenitor cells (NS/PCs) is a promising strategy to replace lost neuronal cells, promote repair, and stimulate functional plasticity after SCI. Macrophages are important SCI-associated inflammatory cells and a major source of secreted factors that modify the lesion milieu. Here, we used conditional medium (CM) from bone marrow-derived M1 or M2 polarized macrophages to culture murine NS/PCs. The NS/PCs showed enhanced astrocytic versus neuronal/oligodendrocytic differentiation in the presence of M1- versus M2-CM. Similarly, cotransplantation of NS/PCs with M1 and M2 macrophages into intact or injured murine spinal cord increased the number of engrafted NS/PC-derived astrocytes and neurons/oligodendrocytes, respectively. Furthermore, when cotransplantated with M2 macrophages, the NS/PC-derived neurons integrated into the local circuitry and enhanced locomotor recovery following SCI. Interesting, engrafted M1 macrophages promoted long-distance rostral migration of NS/PC-derived cells in a chemokine (C-X-C motif) receptor 4 (CXCR4)-dependent manner, while engrafted M2 macrophages resulted in limited cell migration of NS/PC-derived cells. Altogether, these findings suggest that the cotransplantation of NS/PCs together with polarized macrophages could constitute a promising therapeutic approach for SCI repair.
Introduction
Because of the limited capacity of the adult central nervous system (CNS) to undergo repair following traumatic damage, spinal cord injury (SCI) remains a devastating disease with poor functional outcomes.1 Cell transplantation therapy is a promising approach for promoting repair and functional plasticity after SCI.2 NS/PCs are regarded as particularly advantageous for transplantation therapy.3,4 Manipulation of the microenvironment after SCI is considered necessary to facilitate the differentiation of engrafted NS/PCs into neurons.5,6
Inflammation is a critical pathological process that leads to secondary damage after SCI.7,8 Macrophages, like microglia, can be polarized under appropriate conditions into at least two main subpopulations of cells, M1 macrophages (classically activated, proinflammatory macrophages) and M2 macrophages (alternatively activated, anti-inflammatory macrophages),9,10,11 which can lead to neuroinflammation having detrimental or beneficial effects after SCI.12,13,14 Although some reports show that IL-4-activated microglia induce NS/PC differentiation into oligodendrocytes, and IFN-γ-activated microglia induce NS/PC differentiation into neurons, it is still unknown how polarized macrophages mechanistically trigger the differentiation of NS/PCs into specific progeny cells, either in vitro or in vivo.15
The engagement of the engrafted NS/PCs at injured sites is key to the success of cell transplantation therapy after SCI.2,3 The proinflammatory microenvironment and several chemokines and their receptors (e.g., monocyte chemoattractant protein-1, chemokine (C-X-C motif) ligand 12 (CXCL12)/chemokine (C-X-C motif) receptor 4 (CXCR4)), as well as inflammatory cytokines (e.g., IFN-γ and TNF-α) of the injured spinal cord influence the migration of both transplanted and endogenous NS/PCs toward the lesion site.16,17,18,19 We propose that polarized macrophages are the source of at least some of these promigratory factors.
This study analyzed (i) the differentiation of NS/PCs in the presence of soluble factors secreted by M1 or M2 macrophages in vitro and in vivo and (ii) the migration of engrafted NS/PCs cotransplanted with M1 or M2 macrophages in a murine SCI model. The results demonstrate that modification of the spinal cord environment by polarized macrophages together with NS/PC-mediated neurogenesis is an exciting new combinatorial approach for the treatment of SCI.
Results
Macrophage polarization state is maintained over time in culture after withdrawal of polarizing factors
Bone marrow-derived macrophages (BMDMs) were obtained from bone marrow hematopoietic stem cells after induction in vitro with macrophage colony-stimulating factor. Fluorescence-activated cell sorting analysis showed that nearly 99% of the stimulated bone marrow stem cells expressed F4/80, a specific marker of macrophages (Supplementary Figure S1a). After stimulation of the BMDMs with lipopolysaccharide (LPS) plus IFN-γ to yield M1 polarization or IL-4 to yield M2 polarization states, BMDM-derived M1 and M2 macrophages appeared flat with cellular extensions under phase contrast microscopy (Supplementary Figure S1b). Most M1 macrophages assumed a rounded shape, while M2 macrophages exhibited an elongated spindle-like shape. On the other hand, unstimulated BMDMs without LPS, IFN-γ, or IL-4 yielded unpolarized M0 macrophages with an irregular polygonal morphology (Supplementary Figure S1b). The morphological changes of the polarized M1 and M2 macrophages were similar to those previously reported by McWhorter.20
To further confirm the macrophage polarization state of the BMDM-differentiated cells, flow cytometry was used to determine surface antigen expression(F4/80, CD86, and CD206), quantitative polymerase chain reaction (qPCR) and western blotting analyses were conducted to evaluate the expression of iNOS and CD86, established specific markers of M1 macrophages, and arginase 1 (Arg1) and CD206, established specific markers of M2 macrophages9,11,21 in macrophages exposed to LPS/IFN-γ or IL-4 polarizing stimuli during the first 24 hours of culture. Flow cytometrical analysis revealed that macrophages activated with LPS/ IFN-γ had increased expression of CD86 (approximately 43% of F4/80-positive cells were CD86 positive), whereas macrophages activated with IL-4 had increased expression of CD206 (approximately 84% of F4/80-positive cells were CD206 positive) (Supplementary Figure S1c). Consequently, macrophages stimulated with LPS/IFN-γ expressed high mRNA levels of inos and cd86, as well as il1β, il6, il12, tnfa,irf5, and ifng, but not arg1 or cd206 (Supplementary Figures S1d and S2a–f). Conversely, cells stimulated with IL-4 expressed high mRNA levels of arg1 and cd206, in addition to il4, il10, and tgfb, but not inos or cd86 (Supplementary Figures S1e and Figure S2g–l). The protein expression level of iNOS but not of Arg1 was significantly higher in macrophages stimulated with LPS/IFN-γ, while the protein level of Arg1 was significantly higher in macrophages stimulated with IL-4 (Supplementary Figure S1h). These results suggest that BMDMs were sufficiently polarized to become M1 or M2 macrophages after 24-hour stimulation with LPS/IFN-γ or IL-4, as previously reported.21
To harvest the conditional medium (CM) from unpolarized M0 and polarized M1/M2 macrophages without exogenous LPS/IFN-γ or IL-4, we cultivated the polarized M1 and M2 macrophages for 24 hours, as described above. The medium of the polarized M1 and M2 macrophages, as well as that of the unpolarized M0 macrophages, was then changed to neurobasal medium without polarizing factors and the macrophages were subjected again to another 24 hours of culture.
Next, we verified that the polarized M1/M2 macrophages retained their polarized phenotype after the second 24 hours of culture using flow cytometry, qPCR and western blot analyses to assess the expression levels of markers specific to the polarized states (Supplementary Figure S1c–h). About 38% cells were still F4/80+CD86+ among LPS/IFN-γ-activated macrophages. By contrast, 26% of cells were F4/80+CD206+ among IL-4-activated macrophages (Supplementary Figure S1c). Addittionally, the polarized M1 and M2 macrophages still expressed high mRNA levels of inos/cd86 (Supplementary Figure S1f) and arg1/cd206 (Supplementary Figure S1g), respectively, relative to the control, unpolarized M0 macrophages. In addition, the M1 and M2 macrophages continued to secrete high protein levels of iNOS and Arg1 into the culture medium (Supplementary Figure S1h). The culture medium was collected from the cells during the second 24-hour culture period and used as M0-CM, M1-CM, and M2-CM for further experiments, as described below. Supplementary Figure S1c–h shows that the M1 and M2 macrophages maintained their polarized phenotype in vitro even after withdrawal of the polarizing triggers, at least for some time.
M1-CM-treated NS/PCs are biased toward astrocytic differentiation in vitro, whereas M2-CM-treated NS/PCs are biased toward neuronal and oligodendrocytic differentiation
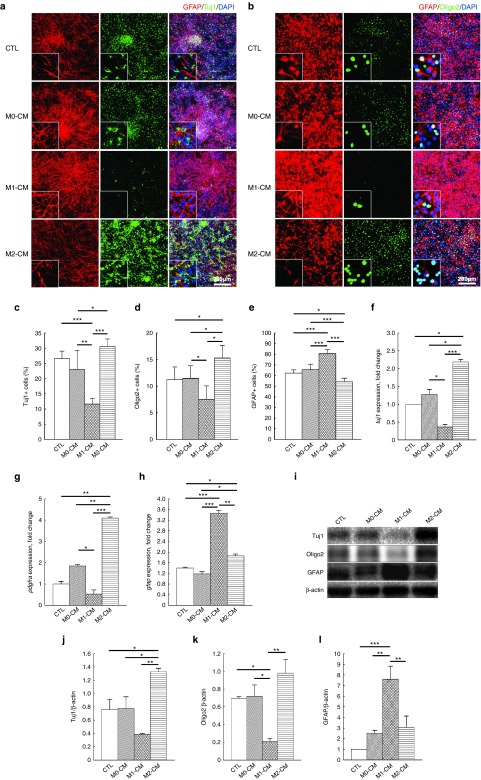
NS/PCs derived from the embryonic spinal cords of both wild-type (WT) and green fluorescent protein (GFP)-transgenic mice (TgGFP mice) were cultured to form neurospheres. The neurospheres expressed the specific markers of NS/PCs, Sox2, and nestin (Supplementary Figure S3a,b). NS/PCs were cultured with normal differentiation medium as the control group or with M0-CM, M1-CM, or M2-CM as the experimental groups. Tuj1, Oligo2/PDGFRα, and glial fibrillary acidic protein (GFAP) were used as specific markers of differentiated neurons, oligodendrocytes, and astrocytes, respectively (Figure 1a,b).
Figure 1.
Differentiation of neural stem/progenitor cells (NS/PCs) following stimulation with M0, M1- or M2-CM in vitro. (a,b) Representative immunofluorescence images of NS/PCs differentiated into Tuj1-, Oligo2-, and glial fibrillary acidic protein (GFAP)-positive (+) cells without (control, CTL) or with the assorted CM samples. The enlarged cells in the lower left-hand corner of each panel show the typical morphology for each culture. Space bars = 200 μm. (c–e) Percentage of Tuj1-, Oligo2-, and GFAP-positive cells differentiated from NS/PCs without (control) or with the assorted CM samples. (f–h) qPCR analysis of tuj1, pdgfra, and gfap, (i) western blotting of Tuj1, Oligo2, and GFAP (j–l) a densitometric quantitative analysis in cells differentiated from NS/PCs without (CTL) or with the assorted CM samples. Data in (c–h) and (j–l) were pooled from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001); data in (a, b, and i) are representative of three independent experiments.
Immunofluorescence staining showed that the penetration rates of the differentiated neural cells were similar in the control and M0-CM groups, where nearly 25% of the cells were Tuj1-postive, >10% were Oligo2-positive, and ~60% were GFAP-positive (Figure 1a–e). However, M1-CM-treated NS/PCs were far more biased toward differentiation into GFAP-positive cells than M2-CM-treated NS/PCs, while M2-CM-treated NS/PCs were more biased toward differentiation into Tuj1- and Oligo2-positive cells than M1-CM-treated NS/PCs (Figure 1a,b). Quantitative analysis showed that the neural cell differentiation rates were significantly different between M1- and M2-CM-treated NS/PCs with respect to Tuj1-postive neurons (~10 versus 30%, P < 0.001), Oligo2-positive oligodendrocytes (~7 versus 15%, P < 0.05) and GFAP-positive astrocytes (~80 versus 50%, P < 0.001) (Figure 1c–e).
The M2-CM-differentiated NS/PC-derived cells expressed significantly higher mRNA levels of tuj1 than M1-CM-differentiated cells (eightfold higher, P < 0.001), M0-CM-differentiated cells (1.8-fold higher, P < 0.05) or control cells (~2.25-fold higher, P < 0.05) (Figure 1f). The mRNA expression level of pdgfra, corresponding to platelet-derived growth factor (PDGF) receptor-α (PDGFRα) and another specific marker of oligodendrocytes, was also significantly higher in M2- than in M1-CM-differentiated cells (eightfold higher, P < 0.01), M0-CM-differentiated cells (twofold higher, P < 0.01) or control cells (fourfold higher, P < 0.01) (Figure 1g). However, the mRNA expression level of gfap in M1-CM-differentiated cells was significantly higher than that in M2-CM-differentiated cells (1.8-fold higher, P < 0.01), or that in M0-CM-differentiated cells and control cells (both threefold higher, P < 0.001) (Figure 1h).
The protein expression levels of Tuj1 and Oligo2 were also significantly higher in M2-CM-differentiated cells than in the untreated control or M0-CM-differentiated cells; however, this was not the case for M1-CM-differentiated cells (Figure 1i–k). By contrast, the protein expression levels of GFAP were significantly higher in M1- than in M2-CM-differentiated, M0-CM-differentiated, or untreated control cells (Figure 1i and l). The results of qPCR and western blotting analyses (Figure 1i–l) were consistent with those of immunofluorescence staining (Figure 1a,b), and show that NS/PCs cultured with CM derived from macrophages under any of the polarization states differentiated primarily into astrocytes (penetration rate > 50% for M0, M1, and M2 macrophages). However, NS/PCs cultured with M1-CM were more likely to generate astrocytes in vitro, while NS/PCs cultured with M2-CM were more likely to generate neurons and oligodendrocytes.
Differentiation of engrafted NS/PCs cotransplanted with polarized macrophages into the intact spinal cord
To investigate how polarized macrophages influence the differentiation of NS/PCs in vivo, we first transplanted NS/PCs together with M0, M1, or M2 macrophages into the intact adult murine spinal cord. The NS/PCs were derived from GFP-transgenic mice as described above, and the macrophages were derived from red fluorescent protein (RFP)-transgenic mice generated previously by our research group.22
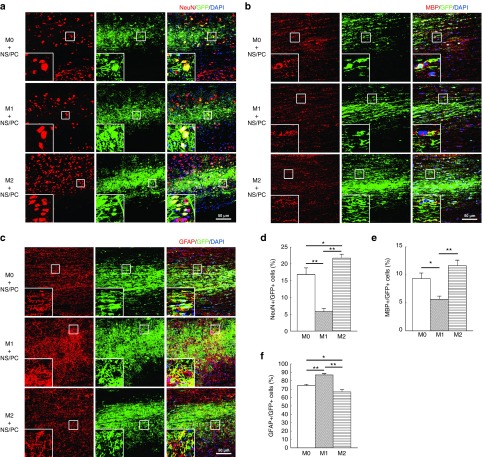
At 12 weeks post-transplantation, a large number of GFP-positive cells was found in the spinal cord tissue (Figure 2a–c), while RFP-positive engrafted M0, M1, and M2 macrophages were too scarce to be detected (data not shown). The percentage of NeuN-positive differentiated neurons generated from the NS/PCs was significantly higher in the engrafted M2 + NS/PC group than in the engrafted M0 + NS/PC group (22 versus 17%, P < 0.05) or the engrafted M1 + NS/PC group (22 versus 5%, P < 0.01) (Figure 2a and d). A comparison of the M0 + NS/PC and M1 + NS/PC groups yielded a smaller number of NeuN-positive neurons in the latter (17 versus 5%, P < 0.01) (Figure 2a and d). These results suggest that M2 macrophages, but not M1 macrophages, encourage NS/PCs to differentiate into neurons following transplantation into the intact spinal cord.
Figure 2.
Differentiation of engrafted neural stem/progenitor cells (NS/PCs) cotransplanted with M0, M1, and M2 macrophages into the intact spinal cord. (a–c) Representative images of NS/PCs derived from GFP-Tg mice and cotransplanted with M0, M1, or M2 macrophages. The NS/PCs were differentiated into (a) NeuN-, (b) MBP-, and (c) glial fibrillary acidic protein (GFAP)-positive cells and detected by IHC. The boxed area in each image is enlarged at the lower left-hand corner of the panel. Space bars = 50 μm. (d–f) The percentages of (d) NeuN-, (e) MBP-, and (f) GFAP-positive cells among the engrafted GFP-expressing NS/PCs are shown. Data in (a–c) are representative of three independent experiments; data in (d–f) were pooled from three independent experiments (*P < 0.05, **P < 0.01).
Oligodendrocytic differentiation was also more prominent in the engrafted M2 + NS/PC and M0 + NS/PC groups than in the engrafted M1 + NS/PC group, as assessed by myelin basic protein (MBP) expression (Figure 2b and e). In the M2 + NS/PC group, 12% of the transplanted NS/PCs differentiated into MBP-positive cells, as opposed to only 9% in the M0 + NS/PC group (P < 0.05) and 5% in the M1 + NS/PC group (P < 0.01) (Figure 2e). Conversely, nearly 90% of the transplanted NS/PCs differentiated into GFAP-positive astrocytes in the M1 + NS/PC group, whereas 73 and 67% of the NS/PCs were GFAP-positive in the M0 + NS/PC and M2 + NS/PC groups, respectively (P < 0.01) (Figure 2f). These results suggest that exogenous polarized M1 macrophages mainly stimulate the differentiation of transplanted NS/PC into astrocytes in the intact spinal cord, while M2 macrophages encourage NS/PC differentiation into neurons and oligodendrocytes.
Polarized macrophages maintain their phenotype after transplantation into the injured spinal cord and induce the differentiation of engrafted NS/PCs
SCI triggers the production of proinflammatory cytokines during the acute injury phase; the proinflammatory cytokines in turn promote the polarization of macrophages into the M1 phenotype.13,14,23,24 Therefore, we asked whether M1 and M2 macrophages polarized in vitro by LPS/IFN-γ or IL-4 could sustain their polarization state after transplantation into the acutely injured spinal cord.
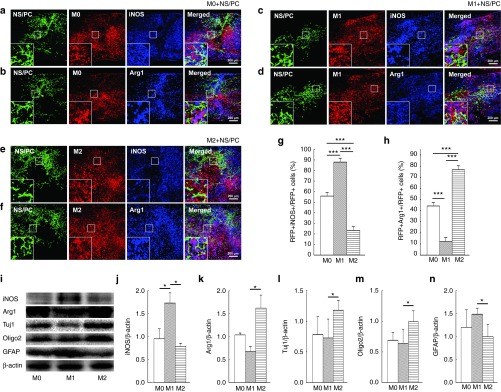
To address this question, we first transplanted RFP-positive M0, M1, or M2 macrophages into the injured spinal cord at 1 day postinjury (dpi). At 3 days post-transplantation, the polarization states of the engrafted macrophages were analyzed by immunohistochemistry (IHC), revealing a large number of transplanted GFP- and RFP-positive cells in the spinal cord tissue (Figure 3a–h). In the M0 + NS/PC group, only a small fraction of the engrafted RFP-positive macrophages expressed iNOS or Arg1 (Figure 3a,b and g,h). The distribution of the engrafted RFP-positive macrophages was similar to that of iNOS-positive cells in the M1 + NS/PC group, where approximately 90% of the RFP staining co-localized with iNOS staining to yield purple-colored cells in the spinal cord sections. However, only about 10% of the RFP-positive cells were also Arg1-positive (Figure 3c,d and g,h). Therefore, iNOS-positive cells clearly predominated over Arg1-positive cells after cotransplantation of M1 macrophages and NS/PCs. By contrast, nearly 80% of Arg1-positive cells were prevalent in sections of the injured spinal cord for the M2 + NS/PC group and colocalized with RFP-positive M2 macrophages, whereas RFP-positive/iNOS-positive cells were less common (Figure 3e,f and g,h).
Figure 3.
Sustainability of engrafted macrophages in the M0, M1, and M2 polarization states. (a–f) Expression of iNOS (a, c, and e) and Arg1 (b, d, and f) in engrafted neural stem/progenitor cells (NS/PCs) and (a and b) M0, (c and d) M1, and (e and f) M2 macrophages at 3 days after transplantation. The boxed area in each image is enlarged at the lower left-hand corner of the panel. Space bars = 200 μm. (g and h) The percentage of (g) RFP+iNOS+ (h) RFP+Arg1+ cells among the engrafted red fluorescent protein (RFP)-positive macrophages are shown. (i) Western blot analysis of polarized macrophage markers (iNOS and Arg1) and neural lineage markers (Tuj1, Oligo2, and glial fibrillary acidic protein (GFAP)) in tissue dissected from the injured spinal cord containing engrafted NS/PCs and M0, M1, or M2 macrophages. (j and k) Densitometric analysis of iNOS and Arg1 protein expression levels in (i). (l–n) Densitometric analysis of Tuj1, Oligo2, and GFAP protein expression levels. Data in (a–i) are representative of three independent experiments; data in (g–h and j–n) are pooled from three independent experiments (*P < 0.05, ***P < 0.001).
In all sections, no engrafted GFP-positive cells expressed iNOS or Arg1, or overlapped with any type of RFP-positive macrophage (Figure 3a–f). Western blotting analysis also confirmed that the protein expression levels of iNOS were higher in the M1 + NS/PC group than in the M0 + NS/PC group (1.8-fold, P < 0.05) and M2 + NS/PC group (3.1-fold, P < 0.05) (Figure 3i and j). However, the protein expression levels of Arg1 were higher in the M2 + NS/PC group than in the M1 + NS/PC group (2.8-fold, P < 0.05) at 3 days after transplantation (Figure 3i and k).
In addition to evaluating the expression of macrophage polarization markers, we also investigated the expression levels of assorted neural cell markers in the spinal cord. Western blot analysis showed significantly higher protein expression levels of Tuj1 and Oligo2, but not GFAP, in the engrafted M2 + NS/PC group than in the other groups. By contrast, the engrafted M1 + NS/PC group exhibited significantly higher levels of GFAP, but not of Tuj1 and Oligo2 (Figure 3i and l–n). Thus, the engrafted, polarized macrophages apparently influenced the differentiation of the cotransplanted NS/PCs as early as 3 days after grafting into the acutely damaged spinal cord. These observations further suggest that the engrafted macrophages are capable of sustaining their polarization state for at least the first 3 days in vivo.
Polarized macrophages differentially affect the generation of neural cells from engrafted NS/PCs after SCI
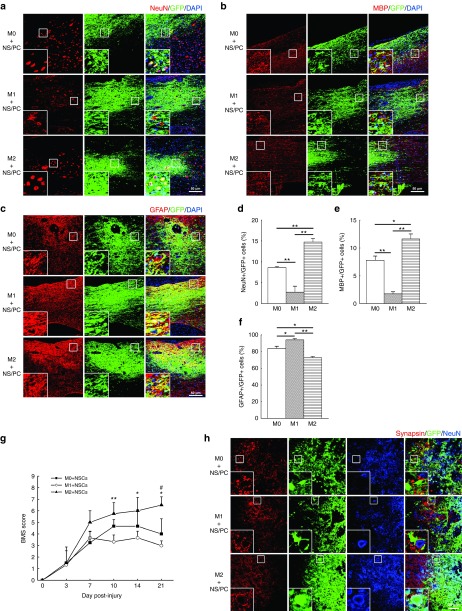
The differentiation of cotransplanted NS/PCs into neurons, oligodendrocytes, and astrocytes was next studied in sections of the injured spinal cord. At 12 weeks after transplantation of the NS/PCs together with M0, M1, or M2 macrophages, almost all of the RFP-positive cells disappeared, leaving behind a preponderance of GFP-positive cells derived from the engrafted NS/PCs (Figure 4 a –c). All three kinds of neural cells were found in the engrafted NS/PC + M0, NS/PC + M1, and NS/PC + M2 groups; however, the number of differentiated neural cells varied from group to group (Figure 4a–f).
Figure 4.
Differentiation of engrafted neural stem/progenitor cells (NS/PCs) cotransplanted with polarized macrophages into the acutely injured spinal cord. (a–c) Representative images of engrafted NS/PCs derived from GFP-Tg mice and cotransplanted with M0, M1, or M2 macrophages. The NS/PCs were differentiated into (a) NeuN-, (b) MBP-, and (c) glial fibrillary acidic protein (GFAP)-positive cells and detected by IHC. The boxed area in each image is enlarged at the lower left-hand corner of the panel. Space bar = 50 μm. (d–f) The percentages of (d) NeuN-, (e) MBP-, and (f) GFAP-positive cells among the engrafted GFP-positive NS/PCs are shown. Data in (a–c) are representative of three independent experiments; data in (d–f) were pooled from three independent experiments (*P < 0.05, **P < 0.01). (g) BMS scores were evaluated in SCI mice engrafted with NS/PCs and M0, M1, or M2 macrophages (n = 6 mice per group). M2 + NS/PC group versus M1 + NS/PC group, *P < 0.05, **P < 0.01; M2 + NS/PC group versus M0 + NS/PC group, #P < 0.05. (h) The expression of synapsin is shown in the injured spinal cord in the three experimental groups. Space bar = 20 μm.
In the M1 + NS/PC-engrafted group, ~3% of the GFP-positive cells were also NeuN-positive. This fraction increased to 9% in the M0 + NS/PC group (P < 0.01) and 15% in the M2 + NS/PC group (P < 0.01) (Figure 4a and d). The M1 + NS/PC groups also showed fewer MBP-positive/GFP-positive cells (2%) than the M0 + NS/PC group (7%, P < 0.01) or the M2 + NS/PC group (12%, P < 0.01) (Figure 4b and e). However, >95% of the GFP-positive cells in the M1 + NS/PC group were also GFAP-positive, as opposed to only 81% (P < 0.05) in the M0 + NS/PC group and 73% (P < 0.01) in the M2 + NS/PC group) (Figure 4c and f). These results confirm that M1 macrophages enhance the differentiation of engrafted NS/PCs into astrocytes, while M2 macrophages enhance their differentiation into neurons and oligodendrocytes.
Locomotor recovery outcomes were examined when NS/PCs were co-transplanted with M0, M1, and M2 macrophages into the injured spinal cord. Mice cotransplanted with M2 macrophages and NS/PCs exhibited significantly higher Basso Mouse Scale (BMS) scores than those cotransplanted with M0 or M1 macrophages and NS/PCs, whereas mice cotransplanted with M1 macrophages and NS/PCs exhibited the worst locomotor recovery and the lowest BMS scores (Figure 4g).
The integration of engrafted NS/PCs into the host neuronal circuitry is considered to be of utmost importance for obtaining functional recovery in the injured spinal cord.6,25,26 Therefore, we next assessed synapsin expression levels after SCI and cell transplantation, because high synapsin content is indicative of synapse formation. IHC analysis revealed a few synapsin-positive areas in the M0 + NS/PC and M1 + NSC/PC groups that surrounded GFP-positive cells in a regular array (Figure 4h). The synapsin signals also surrounded host-derived GFP-negative/NeuN-positive cells in the M1 + NC/PC group, but not the NS/PC-derived GFP-positive/NeuN-positive cells (Figure 4h). On the other hand, synapsin expression was greatly increased and found in close contact with NeuN-positive cells derived from both the engrafted GFP-positive NS/PC-derived cells (yellow staining, inset in Figure 4h) and the endogenous GFP-negative host neurons (Figure 4h). Therefore, the NS/PC-derived neurons in the M2 + NS/PC cotransplanted group apparently formed synapses with host neurons.
Taken together, the results in Figure 4 suggest that engrafted M2 macrophages provide an environment in the SCI that is suitable for directing NS/PC differentiation to a neuronal/oligodendrocytic cell fate with improved locomotor recovery, while engrafted M1 macrophages provide an environment directing NS/PC differentiation mainly toward an astrocytic fate with attenuated locomotor recovery. In addition, engrafted M2 macrophages enhanced the integration of NS/PC-derived neurons into the host neuronal circuitry.
Migratory patterns of NS/PCs cotransplanted with polarized macrophages after SCI
In addition to their ability to regulate the differentiation potential of NS/PCs in vivo, we also examined the capacity of engrafted M0, M1, and M2 macrophages to influence the migratory patterns of NS/PC-derived cells in the injured spinal cord. First, NS/PCs were transplanted alone or in combination with M0, M1, or M2 macrophages into acutely damaged spinal cords to generate four experimental groups. The migration of engrafted GFP-positive NS/PC-derived and RFP-positive macrophages were then assessed at 12 weeks post-transplantation. As described above, RFP-positive macrophages were essentially absent from all of the spinal cord sections. However, GFP-positive cells showed different migratory characteristics in the four experimental groups (Figure 5a–d).
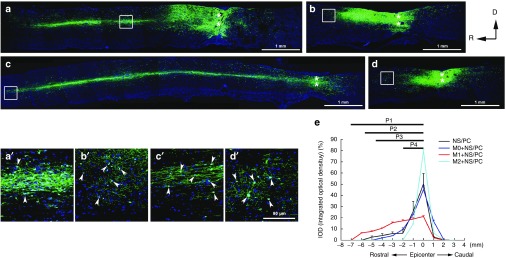
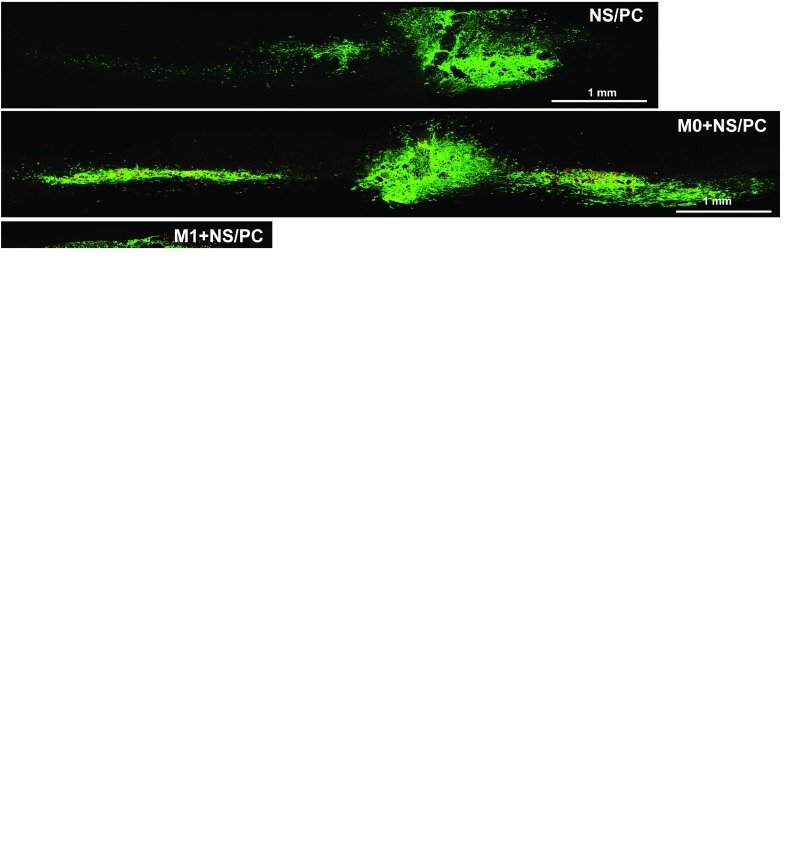
Figure 5.
Migratory patterns of engrafted neural stem/progenitor cell (NS/PC)-derived cells in the injured spinal cord after co-transplantation of NS/PCs and M0, M1, or M2 macrophages. (a–d) Migration of NS/PCs alone without cotransplanted macrophages (a) and with cotransplanted (b) M0, (c) M1, and (d) M2 macrophages. The boxed areas in (a–d) are enlarged in (a'–d'). In a–d, ** denotes the lesion epicenter. Space bars = 1 mm. (a') shows the middle region of the rostral migration stream of the NS/PC-derived cells, while (b'–d') all show end-regions of the rostral migration stream. The arrowheads in (a'–d') indicate GFP-positive NS/PC-derived cells. Space bar = 50 μm. (e) Statistical results showing the integrated optical density (IOD) and the migration distance (P1, P2, P3, and P4) of engrafted NS/PC-derived cells cotransplanted without or with polarized macrophages in injured spinal cord. M1+NS/PCs versus M2+NS/PCs, P1 < 0.001; NS/PCs versus M2+NS/PCs, P2 < 0.001; M0+NS/PCs versus M2+NS/PCs, P3 < 0.001; M0+NS/PCs versus M1+NS/PCs, P4 < 0.01. Statistical data came from three independent experiments. D, dorsal; R, rostral.
In the NS/PC alone group, the GFP-positive cells migrated mainly in the rostral and caudal directions away from the cell-injection site, with a much longer migration distance rostrally than caudally (5 versus 2 mm) (Figure 5a and e and Supplementary Figure S4a). The GFP-positive cells also migrated in the rostral and caudal directions away from the injection site in the M0 + NS/PC group, again with a longer distance rostrallythan caudally (Figure 5b and e and Supplementary Figure S4b). Nevertheless, the total migration distance of the GFP-positive cells in the M0 + NS/PC group was less than that in the NS/PC alone group (4 versus 7 mm) (Figure 5a,b, and e).
The migratory patterns of the NS/PC-derived GFP-positive cells differed widely between the M1 + NS/PC group and the M2 + NS/PC group. In the former, the GFP-positive cells mainly migrated in the rostral direction over a relatively long distance (>7 mm), with a caudal migration distance of only ~2 mm (Figure 5c and e and Supplementary Figure S4c). In the latter case, the GFP-positive cells migrated only a short distance away from the injection site in either the rostral or caudal direction (Figure 5d and e and Supplementary Figure S4d). The enlarged images in Figure 5, A'–D' revealed GFP-positive cells present in the migration stream rather than contributing to long-distance axonal growth, as reported recently.27,28 These results imply that M1 macrophages promote the long-distance migration of engrafted NS/PC-derived cells in the rostral direction, while M2 macrophages restrict the movement of the NS/PC-derived cells and trap them within the injection site.
CXCR4 and CXCL12 participate in the migration of engrafted NS/PC-derived cells
CXCL12 (also known as stromal cell-derived factor 1, or SDF-1) and its receptor, CXCR4, are key regulators of neuronal migration in the developing and injured CNS.29,30,31 For this reason, we hypothesized that CXCL12/CXCR4 signaling might be involved in the migration of the NS/PC-derived cells after cotransplantation of parental NS/PCs with polarized macrophages.
We first evaluated this hypothesis by investigating the expression levels of CXCR4 and CXCL12 in M0, M1, and M2 macrophages. We found that the mRNA levels of cxcr4 were higher in M1 than in M0 or M2 macrophages, and were also higher in M1-CM-treated NS/PCs than in M0- or M2-CM-treated NS/PCs (Figure 6a and b). Western blotting and densitometric analysis confirmed that CXCR4 protein expression levels paralleled cxcr4 mRNA expression levels in NS/PCs stimulated with macrophage-derived CM (Figure 6e and f). The mRNA and protein expression levels of the chemokine C-X-C motif receptor (CXCR7), another receptor for CXCL12, were also higher in M1 macrophages and in NS/PCs cultured with M1-CM (Supplementary Figure S7).
Figure 6.
CXCR4/CXCL12 signaling contributes to the migration of neural stem/progenitor cell (NS/PC)-derived cells in response to polarized macrophages. (a–d) The mRNA expression levels of cxcr4 (a and b) and cxcl12 (c and d) are shown in (a and c) M0-, M1-, and M2-CM-treated NS/PCs and (b and d) M0, M1, and M2 macrophages in vitro. (e) Western blot analysis of CXCR4 expression in M0-, M1-, and M2-CM-treated NS/PCs. Spleen tissue was used as the positive control. (f) Quantitative densitometric analysis indicated that M1-CM-treated NS/PCs expressed elevated levels of CXCR4. (g) CXCL12 expression in NS/PCs cultured with M0, M1-, or M2-CM, as well as in M0, M1, and M2 macrophages. Space bar = 10 μm. (h) IHC images of CXCL12 expression in engrafted NS/PC-derived cells cotransplanted with macrophages into the injured spinal cord. Space bar = 10 μm. (i) NS/PC migration induced by M0, M1-, or M2-CM in a transwell assay with and without AMD3100. Space bar = 50 μm. Data in (a–d) and (f) were pooled from three independent experiments (*P < 0.05, **P < 0.01, ***P < 0.001).
Contrarily, M2 macrophages expressed significantly higher cxcl12 mRNA levels than M0 or M1 macrophages, and NS/PCs expressed significantly higher cxcl12 mRNA levels when cultured with M2-CM than when cultured with M0- or M1-CM (Figure 6c and d). The expression of CXCL12 was also higher in engrafted RFP-positive M2 macrophages, but not in M0 or M1 macrophages, in the injured spinal cord at 3 days after transplantation (Figure 6h). However, only a few NS/PC-derived cells expressed CXCL12 in the injured spinal cord (Figure 6h), which may be due to low level of expression of CXCL12 protein in NS/PC-derived cells. Our results are consistent with those of Jaerve32 and indicate that M2 macrophages (as well as reactive astrocytes) secrete CXCL12 after SCI, after which it can bind to CXCR4 on NS/PC-derived cells.
Lastly, an in vitro transwell experiment showed that the numbers of NS/PC-derived cells stimulated to migrate in response to M0- or M2-CM exceeded those migrating in response to M1-CM (Figure 6i). These findings signify that NS/PCs are less attracted by M1-derived factors than by M0/M2-derived factors (e.g., CXCL12 in M2 macrophages). However, the numbers of cells passing through the filter membrane in the transwell assay were significantly and dose-dependently decreased by treatment of the NS/PCs with AMD3100, a specific antagonist of CXCR4 (Figure 6i). A toxicity assay ruled out the possibility that these results stemmed from cytoxicity of AMD3100 toward NS/PCs (Supplementary Figure S8). Therefore, CXCL12/CXCR4 signaling probably contributes to the migration of NS/PC-derived cells in vivo.
Blockade of CXCR4 influences the migration of engrafted NS/PC-derived cells after SCI
The NS/PCs and polarized macrophages were treated with AMD3100 to interrupt CXCR4 signaling before cotransplantation into the injured spinal cord. At 2 weeks after transplantation, the migratory pattern of NS/PC-derived GFP-positive cells treated withAMD3100 (Figure 7 and Supplementary Figure S9) was quite different from that of untreated NS/PC-derived cells with intact CXCR4 signaling (Figure 5). In the NS/PC alone group, the migration of the progeny cells occurred in both rostral and caudal directions, with increased rostral migration after AMD3100 treatment (Figure 7a). However, rostral and caudal migratory distances were almost identical (~3 mm) in the M0 + NS/PC group after AMD3100 treatment (Figure 7b and Supplementary Figure S9a). By contrast, the GFP-positive cells in the M1 + NS/PC group displayed almost no migration after AMD3100 administration (Figure 7c and Supplementary Figure S9b).
Figure 7.
Migratory patterns of engrafted neural stem/progenitor cell (NS/PC)-derived cells after cotransplantation of AMD3100-treated NS/PCs and macrophages. (a–d) Migration patterns are shown of (a) NS/PCs transplanted alone and (b–d) NS/PCs cotransplanted with (b) M0 macrophages, (c) M1 macrophages and (d) M2 macrophages. In a–d, ** denotes the lesion epicenter. (e) Statistical results showing the integrated optical density (IOD) and the migration distance (P1, P2, P3, and P4) of engrafted NS/PC-derived cells after cotransplantation of AMD3100-treated NS/PCs and macrophages in injured spinal cord. M0+NS/PCs versus M2+NS/PCs, P1 < 0.001; M0+NS/PCs versus M1+NS/PCs, P2 < 0.01; NS/PCs versus M2+NS/PCs, P3 < 0.001; M1+ NS/PCs versus M2+NS/PCs, P4 < 0.001. Space bars = 1 mm.
To our surprise, the NS/PC-derived GFP-positive cells in the M2 + NS/Pc group migrated in the rostral direction by >7 mm after AMD3100 treatment (Figure 7d and Supplementary Figure S9c). Nonetheless, these cells showed almost no migration without CXCR4 blockade (Figure 5d), suggesting that the modulation of CXCR4 signaling is essential for the translocation of NS/PC-derived progeny after co-transplantation of the parental cells with polarized macrophages into the damaged spinal cord.
Discussion
NS/PC transplantation has emerged as one of the most promising therapeutic strategies for SCI because of the potential of NS/PCs to differentiate into neurons. Given the vulnerability of engrafted NS/PCs to pathological environmental forces, many studies have emphasized the importance of modifying the pathological microenvironment of the lesioned spinal cord to improve neuronal differentiation after SCI.
Both detrimental and beneficial actions of neuroinflammation have been reported after SCI,12,13,14 possibly because different types of macrophages/microglia with distinct actions on neuronal regeneration are present at the lesion site.13,14,21,33 M1 macrophages secrete proinflammatory cytokines and upregulate inducible nitric oxide synthase (iNOS) and reactive oxygen species (ROS), both of which are neurotoxic and inhibitory to neurite outgrowth and axonal extension.14,21 By contrast, M2 macrophages release trophic factors and anti-inflammatory cytokines that function in neuroprotection and the promotion of neurite extension.14,21,23 Consistent with the above observations, M2 macrophages are considered to favor the functional recovery in the damaged spinal cord.14 Moreover, previous work has shown that M1 macrophages are more prevalent than M2 macrophages during the acute and chronic phases of SCI, whereas M2 macrophages predominate during the subacute phase.13,14,21 Previous work indicates that SCI triggers the production of proinflammatory cytokines during the acute injury phase which in turn promote the polarization of macrophages into the M1 phenotype.13,14,23,24 Here, we found that RFP-/iNOS+ area was smaller in the M2+NS/PC group (Figure 3e), possibly indicating that M2-polarized macrophage can induce endogenous microglia/macrophage to M2 polarization. On the basis of these results, we propose that functional recovery following the combined transplantation of NS/PCs and M2 macrophages could be due, at least partly, to an increase in the endogenous M2 microglia/macrophage pool.
The survival rate of the transplanted cells is crucial for functional recovery; thus, we collected spinal cord tissues at 1 day, 3 days, 2 weeks, 5 weeks, 9 weeks, and 12 weeks after transplantation to check macrophage polarization and the fate of the NS/PCs in vivo. At 12 weeks post-transplantation, a large number of GFP-positive cells remained in the spinal cord tissue (Figure 2a–c), whereas the number of macrophage cells decreased gradually over time, resulting in the gradual loss of RFP-positive engrafted M0, M1, and M2 macrophages and failure to detect them at 12 weeks post-transplantation (data not shown). However, our results suggest that the engrafted macrophages were capable of maintaining their polarization state for at least the first 3 days in vivo, which maybe long enough for the engrafted macrophages to determine stem cell fate, as suggested by the results of NS/PC differentiation in vitro.
Understanding the mechanisms by which neural stem cells give rise to neurons, astrocytes, and oligodendrocytes is a central question in stem cell biology. The differentiation patterns of the engrafted cells diverge depending on the injury phase-specified microenvironment of the damaged spinal cord,14,25,34 which may be linked to differences in the relative amounts of M1 and M2 macrophages during the different injury phases.9,11,35,36 Extracellular factors that specifically regulate fate determination of stem cells have been identified successfully by multipotent neural stem cells culture.26,27,37 Platelet-derived growth factor (PDGF) and neurotrophin-3(NT3) are potent inducers of neuronal production, and thyroid hormone induces oligodendrocyte differentiation.38,39,40,41,42 Astrocyte differentiation is promoted by both leukemia inhibitory factor (LIF) and ciliary neurotrophic factor (CNTF). Other factors, such as bone morphogenetic protein (BMP), can enhance both neuronal and astrocyte differentiation. The molecular mechanisms that orchestrate these sequential are begin to be elucidated. LIF and CNTF exert their effects primarily via the JaK/ STAT signaling pathway.41,43 BMP-induced astrocyte differentiation appears to be mediated by downstream Smad signaling proteins.44 A new study revealed that neurogenin(Ngn1) represses glial-specific gene transcription, mainly by first sequestering the CBP/p300/Smad1 complex away from glial promoters, and then blocking the JaK/STAT signaling pathway.38,45 Several basic helix-loop-helix (bHLH) transcription factors have been implicated as mediators of neuronal or oligodendrocyte differentiation in the developing CNS. These bHLH factors include oligo1 and oligo2 for oligodendrocyte specification,46,47 and neurogenin1 and 2 (Ngn1 and Ngn2), Mash1, and Math1 for neuronal differentiation.48,49,50 In addition to Olig1/2, it has been reported that Ascl1 also specifies an OPC cell fate in telencephalic NPCs at later developmental stages45,51 and can force an oligodendrocyte fate when overexpressed in NPCs of the adult dentate gyrus.52 In the current study, we found that M2 macrophages polarized from BMDMs in vitro expressed elevated levels of anti-inflammatory cytokines and growth factors (see Supplementary Figure S6). Lu et al. previously showed that growth factors are essential components of a fibrinogen matrix cotransplanted with NS/PCs into the injured spinal cord.27,28 Consistent with the above observations, we propose that M2 macrophages can significantly enhance neuronal and oligodendrocytic differentiation from NS/PCs, even when cotransplanted during the nonoptimal acute injury phase, most likely because they secrete growth factors (i.e., PDGF and NT3) (see Supplementary Figure S6) that enhance the neuronal differentiation of engrafted NS/PCs. Glial-inducing factor leukemia inhibitory factor (LIF) probably allows the NS/PCs transplanted with M1 macrophages to produce more astrocytes. Although our data reveal a distinct difference in the differentiation fate of NS/PC depending on whether they are cotransplanted with M1 or M2 macrophages, further research will be required to obtain greater insight into the mechanisms by which these factors modulate particular cell-fate decisions.
In this study, the cotransplantation of NS/PCs and M2 macrophages clearly promoted functional locomotor recovery. Although NC/PC differentiation was biased toward neurons and oligodendrocytes in the presence of cotransplanted M2 macrophages, >60% of the NS/PC-differentiated cells were astrocytes. Further investigation will be required to determine whether NS/PC differentiation is accompanied by aberrant axonal sprouting and allodynia.
Migration from the injection site toward the injury site is a prerequisite for successful NS/PC-based therapy for SCI.2 Engrafted NS/PC-derived cells translocate both rostrally and caudally during the acute and subacute phases of SCI,6,17,37 but here, we showed a bias toward rostral migration. Although several chemokines/cytokines are responsible for the movement of transplanted NS/PCs in the injured CNS, elevated CXCL12/SDF-1 levels apparently play a central role in recruiting transplanted NS/PCs to the lesion site.32 In the spinal cord, the main sources of CXCL12 are the dorsal corticospinal tract (dCST) and the meninges.53 The expression of CXCL12 does not change rostral to the lesion, but disappears caudally from the degenerating dCST.53 This phenomenon may explain why we observed preferential migration of the transplanted NS/PCs in the rostral direction. Our current results indicate a complete reversal of the migratory patterns of engrafted NS/PC-derived cells following cotransplantation of NS/PCs with M1 versus M2 macrophages. Whereas NS/PC-derived cells exhibited long-distance rostral migration from the site of injection with M1 cotransplantation, almost no migration was observed with M2 cotransplantation. However, the CXCR4 inhibitor, AMD3100, inverted the migration patterns of the engrafted NS/PC-derived cells in response to the cotransplanted macrophages. Although the precise manner by which the polarized macrophages can influence the migration of the engrafted NS/PC-derived cells remains unknown, the CXCR4 pathway is the most likely candidate signaling mechanism. We found that both M2 macrophages and M2-CM-stimulated NS/PCs exhibited high CXCL12 expression, which possibly prevented the engrafted, CXCR4-expressing NS/PCs and their progeny from moving outside the injection site and counteracted the attraction from outside CXCL12 sources.53 By contrast, M1 macrophages and M1-CM-stimulated NS/PCs exhibited low CXCL12 and high CXCR4 expression. Therefore, the progeny of these NS/PCs are expected to be sensitive to areas of high CXCL12 expression, prompting rostral migration as suggested previously.53 Additionally, higher levels of growth factor milieu secreted by M2 macrophages (Supplementary Figure S6) and the tendency toward neuronal differentiation may also have contributed to the limited migration of the engrafted NS/PC-derived cells. However, it is unknown whether the administration of AMD3100 to block CXCL12/CXCR4 signaling alters the differentiation status of M1/M2 macrophages. In summary, although it is unclear whether other chemokines (Supplementary Figure S5) exert biological effects on the migration of the engrafted NS/PC-derived cells, our results suggest strongly that the CXCR4 pathway is the most likely candidate signaling mechanism.
In conclusion, this study demonstrated that cotransplantation of NS/PCs with polarized M2 macrophages into localized sites of tissue damage is a promising strategy for SCI repair. M2 macrophages promoted the neuronal differentiation of NS/PCs, restricted the movement of the engrafted cells and their progeny to the injection site, resulting in the replacement of lost cells, and enhanced the interactions of engrafted cells and their progeny with host neurons. These macrophage actions and NS/PC responses were facilitated by CXCL12/CXCR4 signaling. Nevertheless, further study is required to elucidate the cellular mechanisms by which polarized macrophages modify both the microenvironment within the damaged spinal cord and the essential properties of the cotransplanted NS/PCs to improve functional recovery after SCI.
Materials and Methods
Animals. C57BL/6 (B6) and B6/GFP-Tg (transgenic) mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China) and were bred in our animal facilities. B6/RFP-Tg mice with no obvious defects in development were generated by us.22 All housing, surgical, and postoperative care procedures were performed in accordance with The Fourth Military Medical University Animal Care and Use Committee.
SCI model. Male B6 mice received a severe midthoracic (T8–9) cush injury using Dumont type forceps with a spacer of 0.2 mm, as described previously.54
Cell culture. Bone marrow-derived macrophage (BMDMs) were generated as described previously from adult B6 or B6/RFP-Tg mice.55 Briefly, bilateral femurs and tibias of mice were flushed using 26-gauge needles into DMEM/10%FBS (Gibco, Carlabad, CA). Red blood cells were lysed in lysis buffer (0.15 mol/l NH4Cl, 10 mmol/l KHCO3, and 0.1 mmol/l Na2EDTA, pH 7.4). Cells were cultured in DMEM supplemented with 1% penicillin/streptomycin, 1%HEPES, 0.001% b-mercaptoethanol, 10%FBS, and 20% sL929 supernatant from sL929 cells, which secrete macrophage colony-stimulating factor (M-CSF) required for the promotion of bone marrow cell differentiation into macrophages (7–10 days).56
Neural progenitor/stem cells (NS/PCs) were established from the spinal cord of E14-E15 B6/GFP-Tg mice. Briefly, spinal cords were microdissected and stripped of meninges. Then tissues were mechanically dissociated into a single cell suspension. Cells were grown as neurospheres in serum-free neurobasal medium (Gibco, Karlsruhe, Germany) supplemented with 100 μg/ml penicillin/streptomycin (Gibco), 10 mmol/l L-glutamine (Gibco), 20 μl/ml B-27 supplement (Gibco), recombinant epidermal growth factor (EGF) (20 ng/ml, PeproTech, London, UK), and recombinant basic fibroblast growth factor (bFGF) (20 ng/ml, PeproTech).
Macrophage polarization and conditioned medium preparation. To promote polarization into M1 or M2 macrophages, BMDMs were treated with LPS (100 ng/ml; Sigma-Aldrich, L2630-lipopolysaccharides from Escherichia coli 0111:B4) plus IFN-γ (20 ng/ml; Peprotech) or IL-4 (20 ng/ml; Peprotech), respectively, for 24 hours. Sham control (M0 macrophages) received no treatment except for a change in the macrophage medium. Macrophages were stimulated by cultivation for 24 hours in medium containing cytokines, after which the supernatants were removed and the cells were washed twice with phosphate-buffered saline to remove all traces of the tested reagents. Then an equivalent volume of fresh neurobasal medium was added to the culture of treated or untreated macrophages for another 24 hours. Supernatants were collected as basal culture media. The basal media supplemented with 100 μg/ml penicillin/streptomycin (Gibco), 10 mmol/l L-glutamine (Gibco), 20 μl/ml B-27 supplement (Gibco), EGF (5 ng/ml, PeproTech) and FGF (5 ng/ml, PeproTech) constituted the M-CMs including M0-CM, M1-CM, and M2-CM.
NS/PC differentiation in vitro and in vivo. Single cells were harvested from the third passaged neurospheres. Differentiation of NS/PCs in vitro was initiated by resuspending the cells in the collected M-CMs. To analyze the differentiation of NS/PCs in vivo, a mixture of NS/PCs-GFP and macrophage -RFP(1:1, totally 6 × 105) was injected into the injury epicenter 24 hours after injury. The spinal cords were then collected at 1 day, 3 days, 2 weeks, 5 weeks, 9 weeks, and 12 weeks after transplantation to check the polarization of macrophage -RFP and for the differentiation and migration of NS/PCs in vivo.
Cell transplantation. SCI mice described above were randomly divided into five implant groups: (i) SCI-only, (ii) NSPCs-GFP+M0-RFP, (iii) NSPCs-GFP+M1-RFP, (iv) NSPCs-GFP+M2-RFP, (v) NSPCs-GFP only. For transplantation, 6 × 105 mixed cells (NSPCs-GFP: M-RFP 1:1) in 2 μl M-CM were injected once into the crushed site (0.2 mm to the right side of the dorsal midline, depth of 0.5 mm) using a Hamilton syringe (33G, Hamilton).
Immunocytochemistry and immunohistochemistry. Cover slips and sagittal serial sections (10 µm) (a rostral-caudal extent of 1.5 cm centered on the injury site) were fixed with 4% paraformaldehyde for 15 minutes, after which blocking solution (2% BSA/0.3% Triton X-100) was applied for 1 hour at room temperature, followed by the addition of the primary antibody (Supplementary Table S1) diluted in blocking solution and incubation overnight at 4 °C. An appropriate secondary antibody diluted 1:1,000 were then added and incubated (Jackson ImmunoReasearch Laboratories, Dy 488; 594) at room temperature for 2 hours, followed by nuclear staining with 4, 6-diamidino-2-phenylindole. The percent of NS/PCs differentiating into neurons, oligodendrocytes or astrocytes was determined by counting Tuj1/DAPI-, Oligo2/DAPI-, and GFAP/DAPI-positive cells in vitro. The number of transplanted cells becoming glia or neurons was determined by counting cells coexpressing GFP and MBP/NeuN/GFAP, and then dividing it by the total number of GFP cells labeled with DAPI. Fluorescent images were acquired using an Olympus FluoView FV1000 laser scanning confocal microscope (Olympus, Tokyo, Japan).
Boyden chamber migration assay. The Boyden chamber migration assay was used to evaluate NSC migratory capacity toward M0, M1, or M2 macrophages in vitro. Briefly, a single cell suspension of NS/PCs was seeded in upper PLL-coated transwell culture inserts (Nunc) and polarized macrophages were cultured in the lower chamber in NS/PC differentiation medium. Cells were allowed to migrate for 24 hours at 37 °C in a moist 5% CO2 incubator. Cells that did not migrate on the upper side of the transwell were scraped off and cells that migrated to the bottom of the transwell were incubated at room temperature for 30 minutes with hematoxylin and eosin for further analysis.
Western blotting. Cells or spinal cord segments (1 cm centered at the injury site) were homogenized in lysis buffer (pH 8.0; 50 mmol/l Tris-HCl containing 150 mmol/l NaCl, 5 mmol/l ethylenediaminetetraacetic acid, 1 mmol/l dithiothreitol, 0.5% deoxysodium cholate, 0.1% SDS, 20 μg/ml protease inhibitors aprotinin, 1 mmol/l sodium orthovanadate, 1 mmol/l mercaptoethanol, and 5 mmol/l sodium fluoride), centrifuged, and the supernatants were collected. Protein amounts were determined using the Bradford method. Equal amounts of protein from the supernatants were denatured at 100 °C for 5 minutes, resolved by 12% SDS-PAGE and transferred onto a nitrocellulose membrane for 1 hour at 300 mA. The membrane was blocked in 5% nonfat milk for 1 hour and incubated with one of the primary antibodies listed in Table 1 overnight at 4 °C. Antibody labelling was detected by incubating cultures for 1 hour at room temperature with appropriate HRP-conjugated secondary antibodies (1:8,000; Jackson ImmunoResearch, West Rove, PA) and visualized with the enhanced chemiluminescence kit and the Bio-Rad Image Lab system. Detection of β-actin was done to assess equal loading. Densitometric analysis was done using Image J software (NIH, Bethesda, MD).
RNA extraction, reverse transcription, and quantitative real-time PCR array (qRT-PCR). RNA was isolated via Trizol (Invitrogen, Carlsbad, CA) extraction and reverse transcribed using the SYBR Premix Ex TaaTMII kit (DRR036A;TaKaRa, Otsu, Japan), both according to the manufacturer's instructions. We assayed the expression of specific mRNAs using Bio-Rad CFX 96 real-time PCR analysis (qRT-PCR; Bio-Rad Laboratories, Hercules, CA) with selected gene-specific primer pairs (Supplementary Table S2) and SYBR Green master mix (Applied Biosystems) in 20 μl reactions. Expression was normalized to β-actin for each sample.
Behavioral assessment. Behavioral testing (n ≥ 6 for each group) was conducted using a standardized open-field locomotor rating scale (the Basso Mouse Scale (BMS)), as previously described.57 Testing was done at 0, 3, 7, 10, 14, and 21 dpi after cell transplantation.
Statistical analysis. The results are presented as means ± the standard deviation (SD). Multiple comparisons were done by a one-way analysis of variance, followed by Bonferroni's post hoc test. Results were considered statistically significant at P < 0.05. All statistical analyses were conducted using SPSS 16.0 software program.
SUPPLEMENTARY MATERIAL Figure S1. Polarization states of BMDM-derived macrophages in vitro. Figure S2. Gene-specific expression patterns in M1 and M2 macrophages. Figure S3. Confirmation of NS/PC identity. Figure S4. Migratory patterns of engrafted NS/PC-derived cells after injection of NS/PCs with and without co-transplanted macrophages into the injured spinal cord. Figure S5. Chemokine expression patterns in macrophages and NS/PCs. Figure S6. Expression patterns of growth factors and VCAM in cultured macrophages (*p < 0.05, **p < 0.01, ***p < 0.001). Figure S7. In vitro expression patterns of CXCR7 in NS/PCs and macrophages. Figure S8. Cytotoxicity analysis of AMD3100. Figure S9. Migratory patterns of engrafted NS/PC-derived cells after co-transplantation of AMD3100-treated NS/PCs and macrophages. Table S1. Antibodies for IHC. Table S2. The primer sequences for QRT-PCR analysis.
Acknowledgments
We thank Ms Jianyong Qiu and Lingling Fei for their technical help. This work has been funded by The National Natural Science Foundation of China(81100899, 31271127, 81371364, and 31201094). The authors have no conflicts of interest to declare.
Supplementary Material
References
- Han, SS and Fischer, I (2000). Neural stem cells and gene therapy: prospects for repairing the injured spinal cord. JAMA 283: 2300–2301. [PubMed] [Google Scholar]
- Mothe, AJ and Tator, CH (2012). Advances in stem cell therapy for spinal cord injury. J Clin Invest 122: 3824–3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothe, AJ and Tator, CH (2013). Review of transplantation of neural stem/progenitor cells for spinal cord injury. Int J Dev Neurosci 31: 701–713. [DOI] [PubMed] [Google Scholar]
- Weiss, S, Dunne, C, Hewson, J, Wohl, C, Wheatley, M, Peterson, AC et al. (1996). Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci 16: 7599–7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, QL, Zhang, YP, Howard, RM, Walters, WM, Tsoulfas, P and Whittemore, SR (2001). Pluripotent stem cells engrafted into the normal or lesioned adult rat spinal cord are restricted to a glial lineage. Exp Neurol 167: 48–58. [DOI] [PubMed] [Google Scholar]
- Abematsu, M, Tsujimura, K, Yamano, M, Saito, M, Kohno, K, Kohyama, J et al. (2010). Neurons derived from transplanted neural stem cells restore disrupted neuronal circuitry in a mouse model of spinal cord injury. J Clin Invest 120: 3255–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan, CC (2008). Inflammation: beneficial or detrimental after spinal cord injury? Recent Pat CNS Drug Discov 3: 189–199. [DOI] [PubMed] [Google Scholar]
- Donnelly, DJ and Popovich, PG (2008). Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209: 378–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David, S and Kroner, A (2011). Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12: 388–399. [DOI] [PubMed] [Google Scholar]
- Lawrence, T and Natoli, G (2011). Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11: 750–761. [DOI] [PubMed] [Google Scholar]
- Sica, A and Mantovani, A (2012). Macrophage plasticity and polarization: in vivo veritas. J Clin Invest 122: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel, JC, Nakamura, S, Guan, Z, van Rooijen, N, Ankeny, DP and Popovich, PG (2009). Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci 29: 3956–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter, R, Miller, O, Yovel, G, Rosenzweig, N, London, A, Ruckh, J et al. (2013). Recruitment of beneficial M2 macrophages to injured spinal cord is orchestrated by remote brain choroid plexus. Immunity 38: 555–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura, S, Yasuda, A, Iwai, H, Takano, M, Kobayashi, Y, Nori, S et al. (2013). Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol Brain 6: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butovsky, O, Ziv, Y, Schwartz, A, Landa, G, Talpalar, AE, Pluchino, S et al. (2006). Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci 31: 149–160. [DOI] [PubMed] [Google Scholar]
- McDonald, JW, Liu, XZ, Qu, Y, Liu, S, Mickey, SK, Turetsky, D et al. (1999). Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med 5: 1410–1412. [DOI] [PubMed] [Google Scholar]
- Lepore, AC and Fischer, I (2005). Lineage-restricted neural precursors survive, migrate, and differentiate following transplantation into the injured adult spinal cord. Exp Neurol 194: 230–242. [DOI] [PubMed] [Google Scholar]
- Belmadani, A, Tran, PB, Ren, D and Miller, RJ (2006). Chemokines regulate the migration of neural progenitors to sites of neuroinflammation. J Neurosci 26: 3182–3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong, SY and Turnley, AM (2011). Regulation of adult neural precursor cell migration. Neurochem Int 59: 382–393. [DOI] [PubMed] [Google Scholar]
- McWhorter, FY, Wang, T, Nguyen, P, Chung, T and Liu, WF (2013). Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci USA 110: 17253–17258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kigerl, KA, Gensel, JC, Ankeny, DP, Alexander, JK, Donnelly, DJ and Popovich, PG (2009). Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J Neurosci 29: 13435–13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caiyong Yu, FLJG (2009). Establishment of red and green flurescene protein transgenic mouse model. J Fourth Med Univ 8: 679–682. [Google Scholar]
- Shechter, R, London, A, Varol, C, Raposo, C, Cusimano, M, Yovel, G et al. (2009). Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med 6: e1000113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensel, JC, Nakamura, S, Guan, Z, van Rooijen, N, Ankeny, DP and Popovich, PG (2009). Macrophages promote axon regeneration with concurrent neurotoxicity. J Neurosci 29: 3956–3968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, Y, Sawamoto, K, Miyata, T, Miyao, S, Watanabe, M, Nakamura, M et al. (2002). Transplantation of in vitro-expanded fetal neural progenitor cells results in neurogenesis and functional recovery after spinal cord contusion injury in adult rats. J Neurosci Res 69: 925–933. [DOI] [PubMed] [Google Scholar]
- Bonner, JF, Connors, TM, Silverman, WF, Kowalski, DP, Lemay, MA and Fischer, I (2011). Grafted neural progenitors integrate and restore synaptic connectivity across the injured spinal cord. J Neurosci 31: 4675–4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P, Wang, Y, Graham, L, McHale, K, Gao, M, Wu, D et al. (2012). Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 150: 1264–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, P, Woodruff, G, Wang, Y, Graham, L, Hunt, M, Wu, D et al. (2014). Long-distance axonal growth from human induced pluripotent stem cells after spinal cord injury. Neuron 83: 789–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imitola, J, Raddassi, K, Park, KI, Mueller, FJ, Nieto, M, Teng, YD et al. (2004). Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA 101: 18117–18122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiveron, MC and Cremer, H (2008). CXCL12/CXCR4 signalling in neuronal cell migration. Curr Opin Neurobiol 18: 237–244. [DOI] [PubMed] [Google Scholar]
- Guyon, A (2014). CXCL12 chemokine and its receptors as major players in the interactions between immune and nervous systems. Front Cell Neurosci 8: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaerve, A, Bosse, F and Müller, HW (2012). SDF-1/CXCL12: its role in spinal cord injury. Int J Biochem Cell Biol 44: 452–456. [DOI] [PubMed] [Google Scholar]
- Yao, A, Liu, F, Chen, K, Tang, L, Liu, L, Zhang, K et al. (2014). Programmed death 1 deficiency induces the polarization of macrophages/microglia to the M1 phenotype after spinal cord injury in mice. Neurotherapeutics 11: 636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, S, Ishii, K, Yamane, J, Iwanami, A, Ikegami, T, Katoh, H et al. (2005). In vivo imaging of engrafted neural stem cells: its application in evaluating the optimal timing of transplantation for spinal cord injury. FASEB J 19: 1839–1841. [DOI] [PubMed] [Google Scholar]
- Ben-Hur, T, Ben-Menachem, O, Furer, V, Einstein, O, Mizrachi-Kol, R and Grigoriadis, N (2003). Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol Cell Neurosci 24: 623–631. [DOI] [PubMed] [Google Scholar]
- Ricci-Vitiani, L, Casalbore, P, Petrucci, G, Lauretti, L, Montano, N, Larocca, LM et al. (2006). Influence of local environment on the differentiation of neural stem cells engrafted onto the injured spinal cord. Neurol Res 28: 488–492. [DOI] [PubMed] [Google Scholar]
- Karimi-Abdolrezaee, S, Eftekharpour, E, Wang, J, Morshead, CM and Fehlings, MG (2006). Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26: 3377–3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, Y, Nadal-Vicens, M, Misono, S, Lin, MZ, Zubiaga, A, Hua, X et al. (2001). Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell 104: 365–376. [DOI] [PubMed] [Google Scholar]
- Williams, BP, Park, JK, Alberta, JA, Muhlebach, SG, Hwang, GY, Roberts, TM et al. (1997). A PDGF-regulated immediate early gene response initiates neuronal differentiation in ventricular zone progenitor cells. Neuron 18: 553–562. [DOI] [PubMed] [Google Scholar]
- Ghosh, A and Greenberg, ME (1995). Distinct roles for bFGF and NT-3 in the regulation of cortical neurogenesis. Neuron 15: 89–103. [DOI] [PubMed] [Google Scholar]
- Bonni, A, Sun, Y, Nadal-Vicens, M, Bhatt, A, Frank, DA, Rozovsky, I et al. (1997). Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science 278: 477–483. [DOI] [PubMed] [Google Scholar]
- Johe, KK, Hazel, TG, Muller, T, Dugich-Djordjevic, MM and McKay, RD (1996). Single factors direct the differentiation of stem cells from the fetal and adult central nervous system. Genes Dev 10: 3129–3140. [DOI] [PubMed] [Google Scholar]
- Rajan, P and McKay, RD (1998). Multiple routes to astrocytic differentiation in the CNS. J Neurosci 18: 3620–3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima, K, Yanagisawa, M, Arakawa, H, Kimura, N, Hisatsune, T, Kawabata, M et al. (1999). Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science 284: 479–482. [DOI] [PubMed] [Google Scholar]
- Nakatani, H, Martin, E, Hassani, H, Clavairoly, A, Maire, CL, Viadieu, A et al. (2013). Ascl1/Mash1 promotes brain oligodendrogenesis during myelination and remyelination. J Neurosci 33: 9752–9768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, QR, Cai, L, Rowitch, D, Cepko, CL and Stiles, CD (2001). Ectopic expression of Olig1 promotes oligodendrocyte formation and reduces neuronal survival in developing mouse cortex. Nat Neurosci 4: 973–974. [DOI] [PubMed] [Google Scholar]
- Zhou, Q and Anderson, DJ (2002). The bHLH transcription factors OLIG2 and OLIG1 couple neuronal and glial subtype specification. Cell 109: 61–73. [DOI] [PubMed] [Google Scholar]
- Ma, Q, Kintner, C and Anderson, DJ (1996). Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87: 43–52. [DOI] [PubMed] [Google Scholar]
- Guillemot, F, Lo, LC, Johnson, JE, Auerbach, A, Anderson, DJ and Joyner, AL (1993). Mammalian achaete-scute homolog 1 is required for the early development of olfactory and autonomic neurons. Cell 75: 463–476. [DOI] [PubMed] [Google Scholar]
- Fode, C, Gradwohl, G, Morin, X, Dierich, A, LeMeur, M, Goridis, C et al. (1998). The bHLH protein NEUROGENIN 2 is a determination factor for epibranchial placode-derived sensory neurons. Neuron 20: 483–494. [DOI] [PubMed] [Google Scholar]
- Parras, CM, Galli, R, Britz, O, Soares, S, Galichet, C, Battiste, J et al. (2004). Mash1 specifies neurons and oligodendrocytes in the postnatal brain. EMBO J 23: 4495–4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessberger, S, Toni, N, Clemenson, GD Jr, Ray, J and Gage, FH (2008). Directed differentiation of hippocampal stem/progenitor cells in the adult brain. Nat Neurosci 11: 888–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tysseling, VM, Mithal, D, Sahni, V, Birch, D, Jung, H, Belmadani, A et al. (2011). SDF1 in the dorsal corticospinal tract promotes CXCR4+ cell migration after spinal cord injury. J Neuroinflammation 8: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plemel, JR, Duncan, G, Chen, KW, Shannon, C, Park, S, Sparling, JS et al. (2008). A graded forceps crush spinal cord injury model in mice. J Neurotrauma 25: 350–370. [DOI] [PubMed] [Google Scholar]
- Longbrake, EE, Lai, W, Ankeny, DP and Popovich, PG (2007). Characterization and modeling of monocyte-derived macrophages after spinal cord injury. J Neurochem 102: 1083–1094. [DOI] [PubMed] [Google Scholar]
- Burgess, AW, Metcalf, D, Kozka, IJ, Simpson, RJ, Vairo, G, Hamilton, JA et al. (1985). Purification of two forms of colony-stimulating factor from mouse L-cell-conditioned medium. J Biol Chem 260: 16004–16011. [PubMed] [Google Scholar]
- Basso, DM, Fisher, LC, Anderson, AJ, Jakeman, LB, McTigue, DM and Popovich, PG (2006). Basso Mouse Scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J Neurotrauma 23: 635–659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.