In this issue of Molecular Therapy, Tonkin and colleagues provide convincing evidence that insulin-like growth factor-1 (IGF-1) generated by macrophages plays a significant role in coordinating muscle regeneration and inflammation following injury.1 Although IGF-1 is well known for its anabolic effects on skeletal muscle2,3 and its capacity to improve muscle repair following injury,4 the work by Tonkin et al. shows an important function for a relatively brief interval of IGF-1 production by macrophages in promoting muscle regeneration and regulating the inflammatory response.
Muscle regeneration following injuries involves a complex but largely predictable sequence that can restore muscle structure or homeostasis. Much of the regenerative potential of muscle is determined by the capacity of a population of quiescent, myogenic stem cells within muscle to become activated, proliferate, and then differentiate and grow to replace muscle tissue lost to injury or disease. Those cells, called satellite cells, not only replace damaged muscle tissue following their activation, they are also self-renewing, because some daughter cells return to their quiescent niche following activation, to maintain the regenerative capacity of the muscle.5 Thus, for successful muscle regeneration to occur, specific temporal and spatial signals must regulate the magnitude of satellite cell activation, control the extent to which their population expands, and regulate the proportions of the population that restore the stem cell pool or continue to differentiate and grow.
Muscle cell proliferation, differentiation, and growth following injury are controlled by a vast number of soluble factors, including scores of cytokines and growth factors. Although some of these molecules are produced by satellite cells or fully differentiated muscle fibers themselves, factors released by nonmuscle cells can also play key roles in regulating muscle growth.6 Injured muscle is rapidly invaded by tremendous numbers of inflammatory cells, especially macrophages, suggesting they may be a primary source of soluble factors affecting regeneration. Indeed, experimental depletions of invading inflammatory cells disrupt normal patterns of gene expression in injured muscle and slow muscle growth and regeneration following injury.7,8,9,10 Furthermore, specialized subpopulations of macrophages are temporally associated with specific stages of the myogenic program; proinflammatory (M1) macrophages predominate during the proliferative stage of myogenesis, and anti-inflammatory (M2) macrophages are most prevalent during the differentiation stage.6 This temporal linkage between macrophage phenotype and stage of muscle regeneration is essential for normal growth and regeneration. For example, interleukin-10 (IL-10) promotes a shift of muscle macrophages from an M1 to M2 phenotype following injury that coincides with the transition from the proliferation stage to the differentiation stage of myogenesis.11 However, delivery of exogenous IL-10 before endogenous IL-10 is elevated in injured muscle causes a premature shift in macrophage phenotype, leading to defects in muscle regeneration.12 Those observations suggest that there are shared signaling systems that coordinate muscle inflammation and muscle regeneration.
By generating an IGF-1 mutation targeted to myeloid cells, Tonkin et al. found that most IGF-1 expression in injured muscle during the proliferative stage of myogenesis was obliterated in the conditional knockout line. In addition, the numbers of satellite cells at this same stage were greatly reduced in the mutants, showing that macrophage-derived IGF-1 is an important muscle mitogen during regeneration, specifically during early myogenesis. As muscle regeneration proceeded from the proliferative stage of myogenesis toward the stages of terminal differentiation and growth, the contribution of macrophages to the total quantity of IGF-1 expressed in muscle rapidly declined, concomitant with an increased expression of IGF-1 in a nonmyeloid, nonmyogenic cell population, probably fibroblasts. In addition, a particularly interesting and unexpected finding in the new report is that myeloid cell–derived IGF-1 has an autocrine influence on macrophages during muscle regeneration, driving macrophage populations toward a proregenerative, M2 phenotype. Thus, macrophage-derived IGF-1 can improve regeneration by acting directly on muscle cells to expand satellite cell numbers and simultaneously driving macrophages to the M2 phenotype, which has a higher capacity for promoting muscle growth and regeneration.
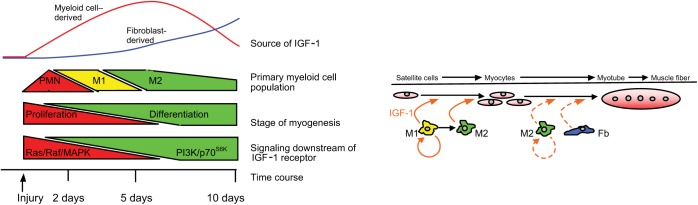
There are some provocative relationships between the kinetics of macrophage invasion into injured muscle, the site of IGF-1 expression, and the signaling pathways activated downstream of the IGF-1 receptor in muscle. IGF-1 effects on muscle are unusual, because IGF-1 can stimulate both proliferation and differentiation, two treatment effects that are typically mutually exclusive. An explanation for this mystery was provided by Coolican et al., who showed that the two opposing outcomes could occur because IGF-1 stimulation of muscle cell proliferation occurred through an IGF-1 receptor/Ras/Raf/MAP kinase pathway but IGF-1 stimulation of muscle differentiation occurred through an IGF-1/PI3-kinase/p70S6K pathway.13 Thus, the opposing effects of IGF-1 stimulation of muscle are attributable to different pathways that mediate those effects. Although untested, these findings collectively support a hypothetical model in which macrophages deliver IGF-1 to injured muscle at early stages of regeneration (Figure 1). The transient upregulation of IGF-1 increases muscle cell proliferation through a Ras/Raf/MAP kinase pathway to promote myogenesis. All the while, IGF-1 works in an autocrine manner to induce a shift in macrophage phenotype, facilitating the transition from the inflammatory to regenerative stages. This transition is complemented by a reduction in inflammatory cytokines that may provide a “switch” in IGF-1 receptor–mediated signaling. For example, tumor necrosis factor-α interferes with the IGF-1/PI3-kinase/p70S6K–mediated muscle differentiation.14 Then, when inflammation declines, fibroblasts deliver a second wave of IGF-1 accompanied by a shift to the PI3-kinase/p70S6K pathway in muscle cells, which promotes muscle differentiation.
Figure 1.
Schematic of temporal relationships between stages of myogenesis, inflammation, and IGF-1 release following muscle injury. Acute injury induces an initial invasion of muscle by neutrophils (PMNs), proinflammatory M1 macrophages, and anti-inflammatory M2 macrophages that coincides with increased expression of insulin-like growth factor-1 (IGF-1) in myeloid cells. Myeloid cell–derived IGF-1 (solid orange arrows) can drive proliferation of activated satellite cells through a pathway mediated by Ras/Raf/MAP kinase signaling in muscle. Myeloid cell–derived IGF-1 also promotes a shift in macrophage phenotype from an M1-biased to an M2-biased population. At approximately 5 days after injury, myeloid cell numbers decline and there is a shift in the primary site of IGF-1 expression from the myeloid compartment to nonmyeloid, nonmuscle cells that are probably fibroblasts (Fb). Muscle cell differentiation to myotubes and subsequent growth to muscle fibers may be increased by IGF-1 produced by fibroblasts or M2 macrophages (dashed orange arrows). IGF-1-mediated muscle differentiation is signaled via a PI3-kinase/p70S6k path during later stages of muscle regeneration.
Does the finding that myeloid cell–derived IGF-1 promotes the M2 macrophage phenotype and thereby contributes to muscle regeneration provide an advance in molecular therapeutics? As noted by the authors, the findings reinforce the current view that the use of nonspecific anti-inflammatory drugs to treat muscle injury or disease has the potential to be detrimental to muscle regeneration15 by disrupting the delivery of proregenerative factors. Do the findings tell us that an increase in myeloid cell–derived IGF-1 will be beneficial for treating muscle injury or disease? This question has been addressed in a general way in studies demonstrating that delivery of supraphysiological IGF-1 to muscle by either transgene expression or direct injection into muscle increases muscle growth and strength.3,4 Unfortunately, prolonged systemic elevation of IGF-1 can also cause hypoglycemia and impaired release of growth hormone,16,17 and may increase the risk of cancer.18 This emphasizes the importance of precise spatial targeting of therapeutic molecules. However, a particularly valuable contribution of the new findings to molecular therapy lies in the clear demonstration of the importance of precise temporal targeting of therapeutic agents to diseased or injured muscle. As the investigators' data show, the period of time in the myogenic program during which myeloid cell–derived IGF-1 mediates its beneficial effects is brief. Once the window is closed, the opportunity may be gone.
References
- Tonkin, J, Temmerman, L, Sampson, RD, Gallego-Colon, E, Barberi, L, Bilbao, D et al. (2015). Monocyte/macrophage-derived IGF-1 orchestrates murine skeletal muscle regeneration and modulates autocrine polarization. Mol Ther 22: 1189–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell-Braxton, L, Hollingshead, P, Warburton, C, Dowd, M, Pitts-Meek, S, Dalton, D et al. (1993). IGF-I is required for normal embryonic growth in mice. Genes Dev 7: 2609–2617. [DOI] [PubMed] [Google Scholar]
- Barton-Davis, ER, Shoturma, DI, Musaro, A, Rosenthal, N and Sweeney, HL (1998). Viral mediated expression of insulin-like growth factor I blocks the aging-related loss of skeletal muscle function. Proc Natl Acad Sci USA 95: 15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, H, Huang, D, Saederup, N, Charo, IF, Ransohoff, RM and Zhou, L (2011). Macrophages recruited via CCR2 produce insulin-like growth factor-1 to repair acute skeletal muscle injury. FASEB J 25: 358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chargé, SB and Rudnicki, MA (2004). Cellular and molecular regulation of muscle regeneration. Physiol Rev 84: 209–238. [DOI] [PubMed] [Google Scholar]
- Tidball, JG and Villalta, SA (2010). Regulatory interactions between muscle and the immune system during muscle regeneration. Am J Physiol 298: R1173–R1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summan, M, Warren, GL, Mercer, RR, Chapman, R, Hulderman, T, Van Rooijeen, N et al. (2006). Macrophages and skeletal muscle regeneration: a clodronate-containing liposome depletion study. Am J Physiol 290: R1488–R1495. [DOI] [PubMed] [Google Scholar]
- Tidball, JG and Wehling-Henricks, M (2007). Macrophages promote muscle membrane repair and muscle fiber growth and regeneration during modified muscle loading in mice in vivo. J Physiol (Lond) 578: 327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shireman, PK, Contreras-Shannon, V, Ochoa, O, Karia BP, Michalek, JE and McManus, LM (2007). MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol 81: 775–785. [DOI] [PubMed] [Google Scholar]
- Arnold, L, Henry, A, Poron, F, Baba-Amer, Y, van Rooijen, N, Plonquet, A et al. (2007). Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J Exp Med 204: 1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, B, Wehling-Henricks, M, Villalta, SA, Wang, Y and Tidball, JG (2012). Interleukin-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J Immunol 189: 3669–3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perdiguero, E, Sousa-Victor, P, Ruiz-Bonilla, V, Jardi, M, Caelles, C, Serrano, AL et al. (2011). p38/MKP-1-regulated AKT coordinates macrophage transitions and resolution of inflammation during tissue repair. J Cell Biol 195: 307–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolican, SA, Samuel, DS, Ewton, DZ, McWade, FJ and Florini, JR (1997). The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem 272: 6653–6662. [DOI] [PubMed] [Google Scholar]
- Rui, L, Aguirre, V, Kim, JK, Shulman, GI, Lee, A, Corbould, A et al. (2001). Insulin/IGF-1 and TNF-alpha stimulate phosphorylation of IRS-1 at inhibitory Ser307 via distinct pathways. J Clin Invest 107: 181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra, DK, Fridén, J, Schmitz, MC and Lieber RL (1995). Anti-inflammatory medication after muscle injury. A treatment resulting in short-term improvement but subsequent loss of muscle function. J Bone Joint Surg Am 77: 1510–1519. [DOI] [PubMed] [Google Scholar]
- Laron, Z, Klinger, B, Erster, B and Anin, S (1988). Effect of acute administration of insulin-like growth factor I in patients with Laron-type dwarfism. Lancet 2: 1170–1172. [DOI] [PubMed] [Google Scholar]
- Laron, Z, Klinger, B, Silbergeld, A, Lewin, R, Erster, B and Gil-Ad, I (1990). Intravenous administration of recombinant IGF-I lowers serum GHRH and TSH. Acta Endocrinol (Copenh) 123: 378–382. [DOI] [PubMed] [Google Scholar]
- Pollak, MN, Polychronakos, C and Richard, M (1990). Insulin-like growth factor I: a potent mitogen for human osteogenic sarcoma. J Natl Cancer Inst 82: 301–305. [DOI] [PubMed] [Google Scholar]