Abstract
Autologous bone marrow-derived mesenchymal stromal cells (MSCs) for adoptive cell therapy of luminal Crohn's disease (CD) are being tested in clinical trials. However, CD is associated with dysregulation of autophagy and its effect on MSC's immunobiology is unknown. Here, we demonstrate no quantitative difference in phenotype, in vitro growth kinetics and molecular signatures to IFNγ between MSCs derived from CD and healthy individuals. CD MSCs were indistinguishable from those derived from healthy controls at inhibiting T-cell proliferation through an indoleamine 2,3-dioxygenase (IDO)-dependent mechanism. Upon IFNγ prelicensing, both MSC populations inhibit T-cell effector functions. Neither a single-nucleotide polymorphism (SNP) rs7820268 in the IDO gene, nor a widely reported CD predisposing SNP ATG16L1rs2241880 modulated the suppressive function of MSCs carrying these haplotypes. IFNγ stimulation or coculture with activated T cells upregulated the expression of autophagy genes and/or vacuoles on MSCs. Pharmacological blockade of autophagy pathway did not reverse the immunosuppressive properties and IFNγ responsiveness of MSCs confirming the absence of a functional link between these two cell biochemical properties. We conclude that autophagy, but not IDO and IFNγ responsiveness, is dispensable for MSC's immunosuppressive properties. MSCs from CD subjects are functionally analogous to those of healthy individuals.
Introduction
Mesenchymal stromal cells (MSCs) were originally identified as a rare subpopulation of bone marrow cells with osteogenic potential, but have since been found to deploy substantial immune regulatory properties.1,2 Clinical trials have demonstrated promising efficacy of MSC infusion for treating human inflammatory and autoimmune ailments,3 including luminal Crohn's disease (CD). Of note, early phase clinical trials conducted in the Netherlands4and Australia5 have demonstrated the safety and likely utility of marrow-derived MSCs for treating Crohn's disease.6,7 MSCs possess an array of distinctive features rendering them attractive for inflammatory bowel disease (IBD) suppressor adoptive cell therapy. However, issues related to MSC provenance (e.g., marrow or adipose source, autologous or allogeneic random donor), handling and immune plasticity may have a major impact on their utility in on-going clinical trials for luminal and fistular CD (Supplementary Table S1), especially when deployed on an industrial scale. We have also previously demonstrated that allogeneic MSCs are susceptible to immune rejection by hosts with normal immune systems8 and the immunogenicity and rejection of MSCs may be augmented immediately postthaw9 which may well impair the utility of repeated use of unmatched random donor MSCs as would be expected in long-term management of CD.10,11 These variables may provide straightforward biological explanations for the negative outcome of prospective randomized trial of cryobanked industrial random donor MSCs for IBD (NCT01240915). Therefore, the use of metabolically fit, autologous MSCs may provide an optimal therapeutic effect unhindered by host rejection.12
Concerns arise that MSCs derived from subjects with autoimmune and inflammatory disorders such as CD may have defects in phenotype, in vitro growth, and functional immune suppressor characteristics and therefore may not be equivalent to MSCs from healthy random donors. These concerns were raised largely due to the observation of dysfunctionality and attenuated immunosuppressive properties of MSCs derived from patients with autoimmune ailments,13,14,15 although other studies failed to find such defects in related immune disorders.16,17,18 Genome-wide association studies have shown that single-nucleotide polymorphisms (SNPs) predispose individuals to develop autoimmune disorders19 and SNPs in the indoleamine 2,3-dioxygenase (IDO) gene have been shown to be associated with systemic sclerosis.20 In addition, the effects of Crohn's disease-specific autophagy-related genetic risk allele ATG16L1 (Thr300Ala) rs2241880 have been reported to predispose individuals to Crohn's disease.21,22,23 These findings are of particular interest within the field of MSC biology since the veto functions of MSCs are known to be critically dependent on IDO function and the effect of autophagy risk alleles on the phenotype and function of MSCs is unknown. Autophagy is a cellular homeostatic process in which cellular compartments and intracellular pathogens are eliminated under stressful conditions. Impairment of the autophagy pathway has been shown to be associated with altered T- and B-cell responses.24 Importantly, defective autophagy pathway is linked to Crohn's disease susceptibility, which leads to aberrant gastrointestinal immune responses and inflammation in these patients.25 Studies had shown defective autophagy-associated proinflammatory responses in the immune cells of hematopoietic origin derived from Crohn's patients.26 However, it is unknown if autophagy pathway is functionally linked to immunosuppressive properties of MSCs derived from Crohn's patients, which raises the concern of utilizing autologous MSC therapy for Crohn's disease. To address these issues, we here performed a rigorous analysis of phenotype, genotype, and immune function of bone marrow derived MSCs from human subjects with CD and show that these are indistinguishable from that of normal controls.
Results
Phenotype and genetic characteristics of MSCs derived from Crohn's patients
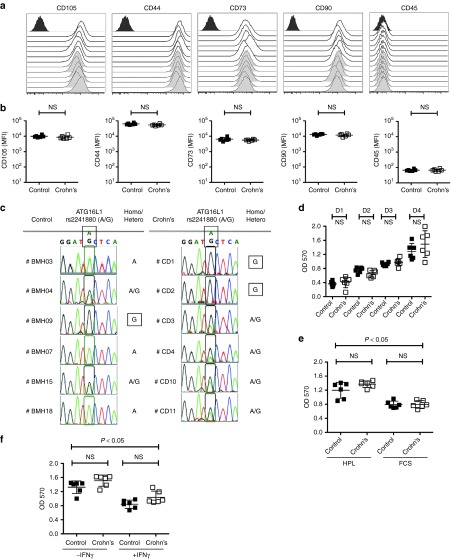
The International Society for Cell Therapy (ISCT) has defined consensus minimal criteria for MSCs30 and we found that there are no significant differences in the phenotypical markers (CD45-CD105+CD44+CD73+CD90+) expressed by MSCs derived Crohn's patients (n = 6) and healthy individuals (n = 6) (Figure 1a,b). The ATG16L1 Thr300Ala (T300A) polymorphism is a risk allele associated with Crohn's disease progression21,22,23 and we tested for its presence in our set of MSCs samples. Among the six MSC samples analyzed from normal volunteers, we observed the following ATG16L1 polymorphisms: wildtype (n = 3), heterozygous T300A (n = 2), and homozygous T300A (n = 1). In the six MSC samples from subjects with Crohn's disease ATG16L1 genotype was: wildtype (n = 0), heterozygous T300A (n = 4), and homozygous T300A (n = 2) (Figure 1c). Comparison of growth kinetics of MSCs between Crohn's patients and healthy individuals were not significantly different (Figure 1d) and we further show that both MSC populations replicate more efficiently in human platelet lysate culture condition compared to fetal calf serum (Figure 1e). Our results also demonstrate that IFNγ leads to a cytostatic response of MSCs from both Crohn's and healthy individuals (Figure 1f).
Figure 1.
Phenotype, growth and genetic characteristics of mesenchymal stromal cells (MSCs) derived from Crohn's patients and healthy individuals. (a) MSCs derived from the bone marrow of healthy individuals and Crohn's patients were subjected to staining for International Society for Cell Therapy-defined surface markers (CD105, CD44, CD73, CD90, CD45) and acquired through flow cytometry. Open and grey histograms represent MSCs from healthy individuals and Crohn's patients respectively. Dark gray histograms at each plot represent the isotype control. (b) Cumulative mean florescence intensity of surface markers is plotted with mean ± SD. (c) Direct sequencing analysis of the PCR product containing ATG16L1 rs2241880 polymorphism of MSCs from Crohn's patients and healthy individuals. Genotype of MSCs for (A/G) polymorphism is shown with A (wildtype), AG(hetero) and G(homo risk) alleles.MSCs from Crohn's and healthy individuals were seeded at (d) similar density in the 96-well plates, (e) with fetal calf serum or human platelet lysate culture medium, (f) with 0 or 50 ng/ml IFNγ in platelet lysate. MTT assay was performed at the (d) indicated time points or (e,f) on fourth day to determine the growth of MSCs. Results are plotted as mean ± SD. Two-tailed T-test was performed to obtain the P values (P < 0.05 significant) in Prism software. Similar results were obtained in a repeat experiment.
MSCs from subjects with Crohn's disease deploy an intact response to IFNγ
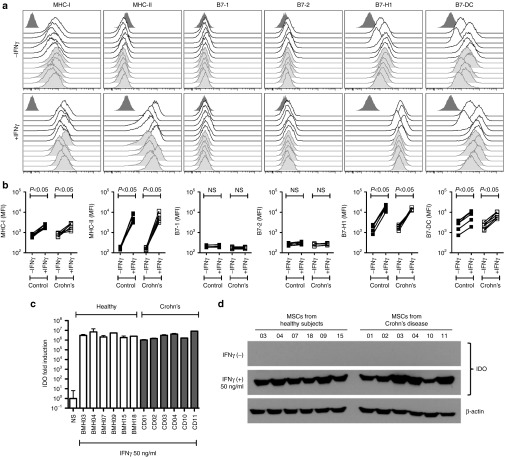
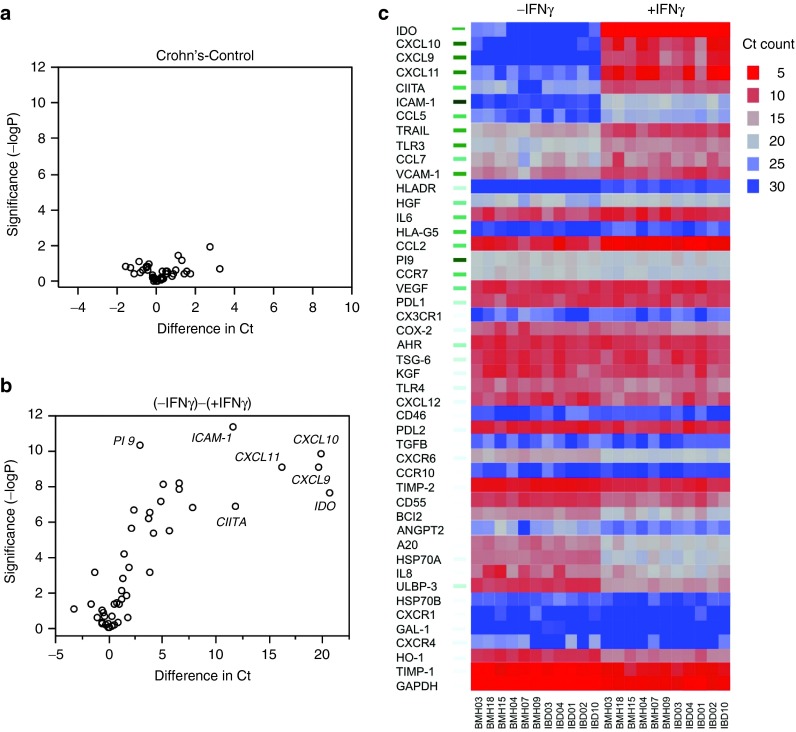
The immunosuppressive potential of MSCs upon transfusion in vivo is dependent upon its response to IFNγ.31 Hence, it is of use to study MSC's responsiveness to IFNγ as a surrogate measure of in vivo suppressive potential.30 In concordance with our published results, IFNγ upregulates MHC class I, class II, B7H1 (PDL1), and B7DC (PDL2) molecules on MSCs derived from healthy individuals and the same feature is observed on MSCs derived from Crohn's patients (Figure 2a,b). Neither costimulatory molecules B7-1 (CD80) nor B7-2 (CD86) were upregulated by IFNγ on either MSC populations (Figure 2a,b). MSCs derived from healthy individuals and Crohn's patients express IDO mRNA and protein at comparable levels post-IFNγ stimulation (Figure 2c,d). We have designed a 48-probe qPCR array platform informed by published reports of MSCs functional responsiveness to IFNγ (Supplementary Table S3). Our results demonstrate that there is no specific difference in the molecular genetic response observed between Crohn's and healthy MSCs (Figure 3a). Volcano plots of significance against difference (measured in Ct counts) show that IFNγ upregulated several genes (IDO, CXCL9, CXCL10, CXCL11, CIITA, ICAM-1, CCL5, TRAIL are highlighted) in both MSC populations (Figure 3b) while no significant differences were observed between the Crohn's and healthy MSCs (Figure 3c).
Figure 2.
IFNγ responsiveness of mesenchymal stromal cells (MSCs) derived from Crohn's patients is similar to that of control individuals. (a) MSCs from Crohn's patients and healthy individuals were stimulated with IFNγ for 48 hours. The cells were stained with the antibodies to the surface markers for HLA-ABC, HLA-DR, B7-1, B7-2, B7H1, and B7DC and subjected to the flow cytometry. Open and grey histograms represent MSCs from healthy individuals and Crohn's patients respectively. Dark gray histograms at each plot represent the isotype control. (b) Cumulative mean florescence intensity of surface markers in the presence and absence of IFNγ is shown. (c) MSCs from Crohn's patients and control individuals were stimulated with IFNγ for 48 hours. Expression level of IDO mRNA relative to GAPDH was evaluated by the quantitative SYBR green real-time PCR. Δ-Δ CT method was applied to calculate the fold induction of IDO over the unstimulated control. (d) IDO expression at protein level is shown by western blot analysis and actin was used as an internal control. Results are plotted as mean ± SD. Two-tailed T-test was performed to obtain the P values (P < 0.05 significant) in Prism software. Similar results were obtained in a repeat experiment.
Figure 3.
Molecular signatures of Crohn's and healthy mesenchymal stromal cells (MSCs) in response to IFNγ. MSCs derived from Crohn's (n = 5) and healthy (n = 6) individuals were stimulated with 20 ng/ml IFNγ for 48 hours and total cDNA were generated from RNA. Transcriptional profile of 48 genes was investigated in Fluidigm nanoscale qPCR 48*48 array plates. Volcano plot were generated using JMP software to determine the CT value difference between MSCs from (a) Crohn's and healthy individuals, (b) without and with IFNγ stimulation. (c) Heat map of MSCs stimulated with IFNγ showing effect of stimulation, with significance included by green bars to left.
MSCs derived from subjects with Crohn's patients inhibit T-cell proliferation through IDO
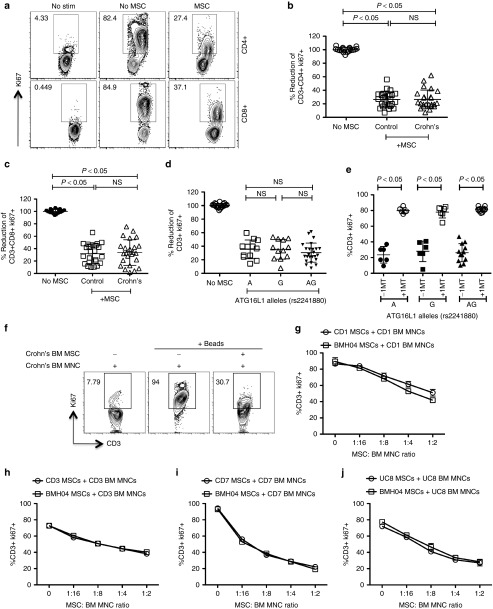
Our results demonstrate that CD MSC populations inhibited the proliferation of CD3+CD4+ and CD3+CD8+ T cells at comparable levels to normals (Figure 4a–c). To delineate the effect of autophagy ATG16L1 (T300A) rs2241880 alleles on the suppressive properties of MSCs, we grouped MSC populations based on their genotype (AA, GG, AG) and assessed their suppressive capabilities. No distinguishable difference was observed in the MSC's suppressive potential with regards to their genetic variation (Figure 4d). Next, we investigated the effect of the ATG16L1 polymorphism on IDO-mediated suppression on MSCs. 1-MT, a pharmacological inhibitor of IDO, abolished the suppressive potential of all MSCs tested independent of ATG16L1 genetic polymorphisms (Figure 4e). Next, we investigated the effect of IBD MSCs (three CD and one Ulcerative Colitis) in inhibiting autologous T cells. Our results demonstrate: (i) T cells derived from Crohn's patients are susceptible to inhibition by autologous MSCs (ii) Crohn's and Healthy MSCs are equally potent in inhibiting T cells derived from Crohn's patients (Figure 4f–j). We compared the suppressive potential of early (1–2) versus late passage (6–7) IBD MSCs (three CD and one Ulcerative Colitis) and observed that MSCs derived from late passage have slightly reduced suppressive potential than early passage (Supplementary Figure S1).
Figure 4.
Crohn's mesenchymal stromal cells (MSCs) inhibit T-cell proliferation through IDO-dependent mechanism. PBMCs cocultured in the presence and absence of MSCs derived from healthy individuals, Crohn's patients were stimulated with SEB. 4 days post-T-cell proliferation was measured by Ki67 intracellular staining. (a) Flow cytometry plot and cumulative % reduction of (b) CD3+CD4+Ki67+ and (c) CD3+CD8+Ki67+ T-cell proliferation is shown. Cumulative is plotted from four independent experiments tested with four unique PBMC donors and n = 6 MSC donors each group. Results are plotted as mean ± SD. (d) Cumulative % reduction of CD3+ Ki67+ T-cell proliferation from four independent PBMC donors in four independent experiments was plotted against unique MSC donor groups based on ATG16L1 rs2241880 A (wild type, n = 3), AG (hetero, n = 6), and G (homo risk, n = 3) alleles. (e) 1-Methyl Tryptophan (1-MT) was added at the concentration of 1 mmol/l to SEB activated PBMCs cocultured in the presence and absence of individual MSC populations and T-cell proliferation was assayed 4 days postcoculture. Cumulative % T-cell proliferation (CD3+Ki67+) was plotted against unique MSC donor groups based on ATG16L1 rs2241880 A (wildtype, n = 3), AG (hetero, n = 3), and G (homo risk, n = 3) alleles from two independent experiments with two unique PBMC Donors. Results are plotted as mean ± SD. Bone marrow mononuclear cells cocultured in the presence and absence of autologous patient MSCs or healthy MSCs were stimulated with anti-CD3, anti-CD28. Four days post stimulation, T cell proliferation was measured by Ki67 intracellular staining. (f) Representative flow cytometry plot and dose-dependent suppressive effect of autologous patient MSCs (g) CD01, (h) CD03, (i) CD07, (j) UC08, and healthy MSCs (BMH04) is shown. Results are plotted as mean ± SD.
IFNγ priming of MSCs inhibit T-cell cytokine secretion
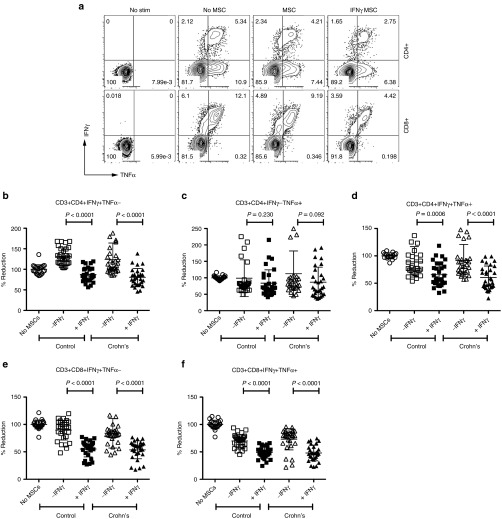
We investigated the effect of Crohn's MSC's ability to suppress CD4+ and CD8+ T-cell effector cytokine secretion upon licensing with IFNγ. Single (IFNγ+TNFα-, IFNγ-TNFα+) and double (IFNγ+TNFα+) cytokine secreting CD4+ and CD8+ T cells were analyzed upon coculture with +/-IFNγ pre-licensed MSCs (Figure 5a). We demonstrate that IFNγ prelicensed healthy and Crohn's MSCs inhibit double cytokine (IFNγ+TNFα+) producing CD4+ and CD8+ T cells at comparable levels (Figure 5d,f). Single cytokine producing (IFNγ+TNFα-), but not IFNγ−TNFα+, CD4+ and CD8+ T cells were also inhibited by both MSC populations (Figure 5b,c,e).
Figure 5.
Superiority of IFNγ prelicensed mesenchymal stromal cells (MSCs) in inhibiting T-cell effector funtion. PBMCs were cocultured in the presence and absence of either MSCs or IFNγ licensed MSCs, derived from healthy individuals or Crohn's patients, with SEB and BFA for 12–14 hours. Cells were subsequently stained with antibodies to CD3, CD4, CD8 and IFNγ and TNFα for flow cytometry. (a) Flow cytometry plot of cytokine expression pattern of IFNγ and TNFα is shown in CD3+CD4+ and CD3+CD8+ T cells. Cumulative CD3+CD4+ Single (b) IFNγ+TNFα-, (c) IFNγ-TNFα+, double (d) IFNγ+TNFα+ and CD3+CD8+ single (e) IFNγ+TNFα-, double (f) IFNγ+ TNFα+ cytokine producing T cells in the presence and absence of either MSCs or IFNγ licensed MSCs is shown. Cumulative (mean ± SD) was plotted from five independent experiments with individual MSC populations (n = 6 healthy or Crohn's) tested against five unique PBMC donors. Results are plotted as mean ± SD. Two-tailed T-test was performed to obtain the P values in Prism software.
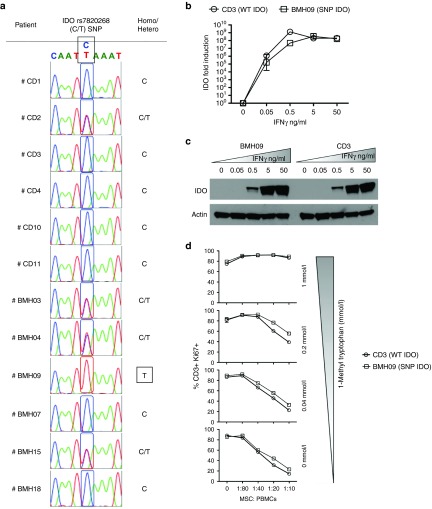
IDO expression and function in MSCs are not modulated by SNP rs7820268 (C6202T)
We investigated whether allelic variations of IDO modulate MSC activity. All MSC populations tested were subjected to IDO SNP analysis. We found that the majority of individuals tested were wildtype (C) or heterozygous (C/T) for the IDO SNP except for one sample derived from a healthy individual who was homozygous rs7820268 (C6202T) (Figure 6a). We compared the IDO mRNA and protein expression levels between wildtype (C6202C) and rs7820268 (C6202T) MSC populations following IFNγ activation. Our results demonstrate that rs7820268 (C6202T) SNP does not modulate its expression at either mRNA or protein levels (Figure 6b,c). In order to determine the effect of rs7820268 (C6202T) SNP on the function of IDO in MSCs, we blocked its activity with 1-MT. A dose-dependent reversal of MSC's suppressive activity with 1-MT was observed independent of rs7820268 (C6202T) SNP (Figure 6d).
Figure 6.
Single-nucleotide polymorphism in IDO (rs7820268) gene does not modulate its expression and function on mesenchymal stromal cells (MSCs). (a) Direct sequencing analysis of the PCR product encompassing rs7820268 in the IDO genes of MSCs from Crohn's patients and healthy individuals. Homozygous and heterozygous polymorphisms are shown. IFNγ-mediated induction of (b) IDO mRNA and (c) protein levels were shown between MSCs possessing wildtype (CD03) and rs7829268 (BMH09) SNP in the IDO gene. (d) SEB-activated PBMCs and MSCs (CD03 and BHM09) were cocultured at the indicated ratios in the presence of variable 1-Methyl Tryptophan concentrations. After 4 days, T-cell proliferation was assayed using Ki67 intracellular staining. % CD3+Ki67+ T cells are plotted as mean ± SD. Similar results were obtained in a repeat experiment.
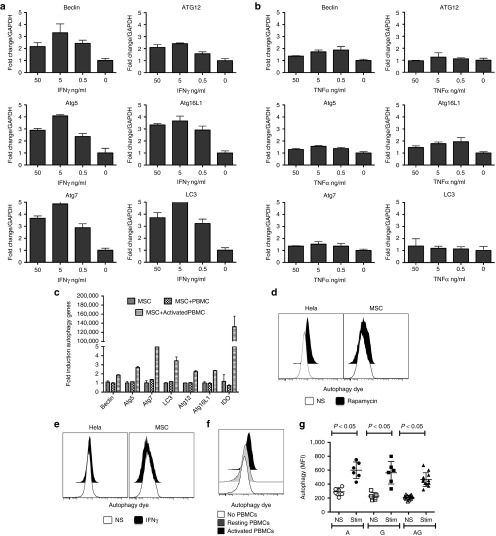
Activated T cells augment autophagic activity by MSCs independent of ATG16L1 polymorphism
In order to identify the role of inflammatory cytokines in modulating expression of genes that are part of the autophagy pathway, we stimulated MSCs with IFNγ and TNFα. Our results demonstrate that IFNγ upregulates the expression of Beclin, ATG12, ATG5, ATG16L1, ATG7, and LC3 less than fivefold, while TNFα does not modulate the expression of any of these genes (Figure 7a,b). To identify the in situ effect of activated T cells in priming MSCs to upregulate the genes of autophagy, we cultured MSCs and activated T cells separated by transwell membranes and observed that activated T cells upregulated MSC autophagy gene expression levels by less than fivefold (Figure 7c). In addition to analyzing the transcription of autophagy-associated genes, we also investigated autophagy influx by staining autophagy vacuoles. Our results demonstrate that rapamycin, a positive regulator of autophagy, induces autophagic activity in HeLa cells, but not in MSCs. Moreover, IFNγ did not induce autophagy in either HeLa cells or MSC (Figure 7d,e). In contrast, activated T cells upregulated HLA-DR and enhanced autophagic activity by MSCs (Figure 7f) (Supplementary Figure S2). Neither Crohn's MSCs nor MSCs harboring the ATG16L1 polymorphism showed defects in autophagic activity (Figure 7g).
Figure 7.
Activated T cells induce autophagy on mesenchymal stromal cells (MSCs). MSCs were stimulated with indicated concentrations of (a) IFNγ or (b) TNFα for 48 hours or 4 days with (c) activated T cells in transwell plate. The RNA was extracted from the cells and the expression levels of Beclin, ATG12, ATG5, ATG16L1, ATG7, and LC3 mRNA was measured by the quantitative SYBR green real-time PCR. GAPDH mRNA levels were used as internal controls. Δ-Δ CT method was used to calculate the fold change. Results are plotted as mean ± SD. Similar results were obtained in a repeat experiment. MSCs and/or Hela cells were subjected to the stimulation with (d) Rapamycin (250 nmol/l for 16 hours) or (e) IFNγ (50 ng/ml for 16 hours) or (f) activated T cells (4 days). Cells were trypsinized and incubated with the autophagy detecting green fluorescent reagent according to the manufacturer instruction. Cells were analyzed by flow cytometry with 488 nm laser excitation and FITC detection filters. Similar results were obtained in a repeat experiment. (g) Cumulative mean fluorescence intensity of autophagy influx in MSCs cultured with activated T cells for 4 days in a transwell plate. MFI was plotted against unique MSC donor groups based on ATG16L1 rs2241880 A (wildtype), AG (hetero), and G (homo risk) alleles from two independent experiments done with two independent PBMC donors. Results are plotted as mean ± SD. Two-tailed T-test was performed to obtain the P values in Prism software.
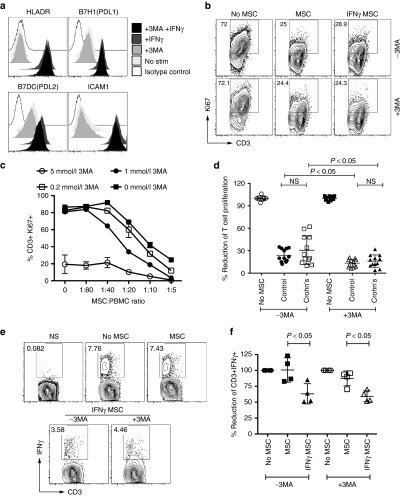
Blocking of autophagic pathway does not alter MSC suppressive potential
To further investigate the role of autophagy in maintaining the veto properties of MSCs, we utilized the pharmacological autophagy inhibitor, 3-methyl adenine (3-MA), to suppress its activity. Addition of 3-MA to HeLa cells stimulated with rapamycin reduced autophagic activity in a dose-dependent manner (Supplementary Figure S3). 3-MA did not modulate the IFNγ-induced expression of HLA-DR, PD-L1, PD-L2, and ICAM-1 suggesting that IFNγ responsiveness is intact in the absence of autophagy in MSCs (Figure 8a). Next, we cultured MSCs and T cells in the presence of variable concentrations of 3-MA. High concentrations of 3-MA blocks T-cell proliferation in the absence of MSCs and lower concentrations of 3-MA do not reverse MSC's suppressive potential on T-cell proliferation (Figure 8b,c). Both Crohn's and healthy MSCs exhibit comparable suppressive effects in the presence 3-MA (Figure 8d). Statistical analysis demonstrates that addition of 3-MA moderately augments MSC's suppressive capacity. At 1 mmol/l concentrations, 1-MT, but not 3-MA, blocks MSC's suppressive activity, suggesting that MSC's suppressive effect on T-cell proliferation is dependent on IDO but not autophagy. We further investigated whether autophagy plays a role in the suppressive mechanism of IFNγ prelicensed MSCs on the cytokine secreting functions of memory T cells. Our results demonstrate that presence of 3-MA does not modulate IFNγ pre-licensed MSC's suppressive effect on T-cell cytokine secretion (Figure 8e,f).
Figure 8.
Autophagy pathway is dispensable for mesenchymal stromal cells (MSC's) suppressive effect on T-cell proliferation and cytokine secretion. (a) 1 mmol/l 3-Methyl Adenine (3-MA) was added to MSCs in the presence and absence of 20 ng/ml IFNγ for a period of 48 hours and surface expression of HLADR, PDL1, PDL2, and ICAM-1 was measured through flow cytometry. (b) Representative flow cytometry Plot, (c) dose-dependent effect of 3-MA on T-cell proliferation is shown. 3-MA was added at the indicated concentrations to PBMCs cocultured in the presence and absence of MSCs. T-cell proliferation was measured through intracellular Ki67 staining after 4 days. Similar results were obtained in a repeat experiment. (d) Cumulative % reduction of CD3+Ki67+ T-cell proliferation from two independent experiments with two independent PBMC donors was plotted against (n = 6) Crohn's and (n = 6) Healthy MSC donor groups with ± 1 mmol/l 3-MA. (e) Representative and (f) Cumulative percentage reduction of CD3+IFNγ+ is shown in 12-14hrs intracellular cytokine staining assay. 1 mmol/l 3-MA was added to SEB activated PBMCs cocultured in the presence and absence of ±IFNγ licensed MSCs. Brefeldin A was added to detect CD3+IFNγ+T cells. Cumulative is plotted with two independent MSC donors from healthy and Crohn's in two independent experiments performed with two independent PBMC donors. Results are plotted as mean ± SD. Two-tailed T-test was performed to obtain the P values in Prism software.
Discussion
The immune suppressive fitness of MSC derived from human subjects with autoimmune ailments has been suggested to be impaired which may provide a negative bias in clinical trials examining autologous MSC therapy. Although tissue resident MSCs likely play an important accessory role in regulating the immune response within the intestinal niche, their exact involvement in Crohn's disease pathophysiology is not well-defined.32,33 Similar uncertainty about the intrinsic immune modulatory capacity of marrow-derived MSCs in CD exist and we here interrogated the phenotype, genotype, and link to function which would inform on the intrinsic fitness of autologous MSCs for treatment of CD. Using the International Society of Cell Therapy guidelines,34,35 we have shown that CD MSCs are plastic-adherent when maintained in standard culture conditions, express MHC class I molecules as well as surface CD105, CD90, CD73, and CD44, lack expression of hematopoietic markers akin to normal MSCs. There is no established consensus on how best to ascertain MSC immune plasticity, but analyzing functional responsiveness to inflammatory cytokines such as IFNγ is emerging as an informative approach.30 Indeed, we have shown that MSCs are exquisitely responsive to IFNγ which leads to STAT1-driven upregulation of MHCI expression, CIITA-dependent de novo expression of MHCII36 as well as IDO and PD-LI/L2 upregulation necessary for hMSC suppression of T-cell proliferation and effector functions.28,37 We have here found that CD MSCs recapitulate these functional pathways indistinguishably from normal MSCs whereas CD MSCs robustly upregulate protein expression of MHC I/II, IDO and PD-L1/L2 upon INFγ priming. As with normal MSCs, CD MSCs do not upregulate costimulatory molecules CD80 and CD86, a feature consistent with suppressor function. Indeed, MSCs are anti-inflammatory not only by lacking costimulatory molecules, but also due to their abundant expression of IDO, B7H1 and B7DC, which collectively suppress inflammation. We also show that double cytokine (IFNγ+TNFα+) producing CD4+ and CD8+ T cells are inhibited by MSCs (CD and normal) upon licensing with IFNγ which suggests that CD MSCs could effectively inhibit pathological T cells in IBD patients.38 The absence of an observable effect of IFNγ prelicensed MSCs on single positive IFNγ−TNFα+ T cells is likely a reflection of the low frequency of IFNγ−TNFα+ CD8+ T cells and a wide variation in CD4+ T-cell responses to IFNγ prelicensed MSCs.
MSCs have an ability to promote tissue healing in a variety of injurious settings.39 At one time, it was thought that this was attributable to their stem-progenitor-like character—that is, MSC-derived cells might directly repopulate damaged organs. However, the small number of MSCs recovered at tissue target sites contradicts the hypothesis that MSCs replenish damage tissues by differentiating into gut or other tissue progenitors, and supports the alternative hypothesis that MSCs limit cell death secondary to tissue inflammation.39 Thus, the gut healing effects may be attributable to the MSC secretome, which has broad immunomodulatory activity toward innate and adaptive immune cells.1 MSCs are characterized by a unique transcriptome40 which is significantly altered by IFNγ priming. We performed a nanoscale qRT-PCR Fluidigm array which allows us to interrogate expression levels of 48 transcripts selected on the basis their published association with MSC immune function, homing and regenerative properties (Supplementary Table S3). We compared the quantitative transcriptional response of MSCs (CD and normal), before and after treatment with IFNγ. One half of these genes are differentially expressed at P < 0.001, as indicated by green marks to the left of the heat map which shows hierarchical clustering of genes in samples (red, high expression, blue low). These include prominently IDO, CXCL9, CXCL10, CXCL11, and CIITA. Volcano plots above contrast significance (-logP) against fold-change and show that there is no difference between resting and IFNγ primed MSCs from healthy and CD donors. Furthermore, the response to IFNγ treatment is strongly biased to upregulation for both groups. These data validate our previous observations of that IFNγ induces expression IDO and CIITA but also shows that chemokines CXCL9/10/11 are very substantially increased as well. We have previously published that MSCs can produce N-terminal cleaved antagonistic chemokine variants of CCL2,41 and it has been shown that CXCL9/10/11 are substrates for similar conversion to antagonists by this mechanism.42 Considering that upregulation of CXCR3 expression by lymphoid cells has been implicated in IBD,43 we can therefore speculate that IFNγ induced upregulation of antagonistic isoforms of CXCL9/10/11 by human MSCs would affect the biology of CXCR3+ T-cells in vivo and ameliorate colitis.
Considering the known association between genotype and CD susceptibility, we examined critical germline constitutional SNPs within the genes of IDO and ATG16L1 in MSCs. SNP rs7820268 (C6202T) in the intron of IDO gene has been implicated in increased mRNA instability and altered protein function and it was unclear if an autologous MSC product bearing this critical SNP would negatively impact their suppressor potency.20,44 Our analysis of MSCs derived from subjects bearing homozygous C6202T mutations in IDO gene failed to reveal an effect on IDO induction and function when compared to MSCs with a wild-type genotype. These results demonstrate that the IDO C6202T polymorphism does not translate to MSC suppressor hypofunction and is not a predictor of cell physiological lack of fitness.
The ATG16L1 risk allele T300A has been shown to predispose to Crohn's disease. Mice lacking ATG16L1 in hematopoietic stem cells show enhanced susceptibility to dextran sulphate sodium (DSS)-induced acute colitis due to the excessive production of proinflammatory cytokines IL-1β and IL-18.45 In addition, Crohn's patients with T300A SNPs in ATG16L1 genes show many abnormalities in intestinal Paneth cells, which are the producers of alpha-defensins in the intestine.46 PBMCs from Crohn's patients with T300A SNP in ATG16L1 gene secrete high levels of proinflammatory IL-1β and IL-6 upon in vitro stimulation.26 Recently, this SNP's role in modulating caspase-3 activity leading to the impairment of autophagy was demonstrated in macrophages.47 However, ATG16L1 T300A knock-in mice do not develop spontaneous intestinal inflammation despite possessing morphological defects in Paneth and goblet cells, suggesting that the effect of this polymorphism is cell type specific.48 In addition, it has also been shown that ATG16L1 T300A does not modulate autophagosome formation and autophagy influx in mouse embryonic fibroblasts.49 Consistent with these previous studies, we found that the ATG16L1 T300A SNP does not reverse the immunosuppressive functions nor does impairment of the autophagy pathway in MSCs. Furthermore, pharmacological inhibition of the autophagy pathway does not alter suppressor function. As an aggregate these data indicate that polymorphism of IDO(C6202T) and ATG16L1(T300A) in MSCs (CD or normal) do not appear to affect suppressor function.
Mass produced random donor allogeneic MSCs are being investigated in a number of industry-sponsored clinical trials as a cellular pharmaceutical for treatment of immune disorders, including Crohn's Disease (Prochymal—NCT00294112) and Ulcerative Colitis (Multistem—NCT01240915). Most clinical trials of MSCs for CD involve the use random donor marrow-derived MSCs (Supplementary Table S1). In virtually all clinical studies to date, MSCs are cryopreserved after serial passaging and banked for later use. Human subjects subsequently receive an intravenous transfusion of MSCs which were retrieved from cryostorage no more than a few hours beforehand. It is assumed that post-thaw MSCs deploy the same homing and immune modulatory features as their prefreeze counterparts. We have found this assumption to be flawed. We have discovered that MSCs deploy a heat shock response immediately post thaw which profoundly alters their in vivo distribution and fate post transfusion50 and most importantly, markedly blunts their ability to exert immune modulatory effects.51 This discovery provides a straightforward biological explanation for the negative outcome of a phase II randomized, double-blind, placebo-controlled, parallel group, multi-center study to investigate the safety and efficacy of allogeneic Multistem® in subjects with moderate to severe ulcerative colitis (NCT01240915). We have previously demonstrated that allogeneic MSCs are susceptible to immune rejection by hosts with normal immune systems,8 which may well impair the utility of repeated use of unmatched random donor MSCs as would be expected in long term management of autoimmune ailments.10,11 A conceptual remedy to these potential pitfalls would involve the use of autologous MSC adoptive cell therapy where host rejection is absent and in vivo persistence optimized.12 This approach appears feasible and safe in human clinical trials of marrow-derived MSCs for treatment of luminal CD originating in the Netherlands4 and United States (NCT01659762).
In conclusion, our results provide evidence that CD marrow-derived MSCs are entirely comparable in phenotype and immune plasticity to MSCs from normal volunteers. These data support the use of autologous MSC as a functionally sufficient platform for adoptive cell therapy of CD and these results likely foreshadow similar behavior of adipose-derived MSC from CD subjects popularly examined for treatment of fistular CD. Overall, these results inform the aggregate hypothesis that metabolically fit (e.g., spared the effect of thawing) autologous marrow-derived CD MSCs are safe and may represent a biologically optimal use of this platform to improve human outcomes.
Materials and Methods
Bone marrow MSCs isolation. MSCs were obtained from consenting subjects with Crohn's disease or healthy individuals (n = 6 each). Patients enrolled in this study had active inflammatory bowel disease and were subjected for endoscopic studies to assess disease severity and also to obtain biopsy specimens for clinical histopathology. Histopathological assessment, closest to bone marrow aspiration is given in Supplementary Table S2. Harvested bone marrow was separated by Ficoll density gradient and plated on α-MEM culture medium containing 10% human platelet lysate27,28 and 100 U/ml penicillin/streptomycin (200,000 cell/cm2). Nonadherent cells were removed from culture after 3 days and MSCs are allowed to expand for an additional 7 days (passage 0). Subsequently, MSCs were passaged weekly and replated at a seeding density of 1,000 cells/cm2. All assays were performed using MSC between passage 4 and 7.
MSC growth kinetics. MSCs were seeded on to 96-well plates at a density of 5,000 cells/cm2. Cells were cultured with either medium containing human platelet lysate or fetal calf serum and stimulated with IFNγ 50 ng/ml. MTT assays were performed at the indicated time points by incubating Thiazolyl Blue Tetrazolium Bromide for 5 hours. After 5 hours, formazan crystals were dissolved in DMSO and OD was measured at 570 nm.
Phenotyping of MSCs by flow cytometry. Resting MSCs or IFNγ (50 ng/ml) activated MSCs were subjected to flow cytometry analysis for the expression of HLA-ABCAPC, HLADRPerCP, CD80PE, CD86PE, B7H1PE, B7DCPE, CD105PE, CD44PE, CD73PE, CD90APC CD45PE and appropriate isotype controls (BD Biosciences, St Jose, CA). Mean fluorescent intensity and histogram analysis for the marker expression was performed with Flow Jo software (Tree Star, Ashland, OR).
Genotyping of MSCs. Total genomic DNA was isolated from individual MSC populations using QIAamp DNA Mini Kit according to the manufacturer instructions (Qiagen, Germantown, MD). 100–300 ng of DNA is used for the PCR amplification of the regions containing IDO rs7820268 and ATG16L1 rs2241880 (A/G) with the flanking primers IDO_F: 5′TGTAATGCCTACTGAAGAAAC, IDO_R 5′CTTAAATTATTTTTTGGCTGAATTCAA, ATG_F:CCACAGGTTAGTGTGCAGGA, ATG_R: CACAGCTGACAGAGCCAAAA, respectively. The amplified product was gel purified, sequenced with the amplification primers (Sanger sequencing, Genscript, Piscataway, NJ) and the sequence results were analyzed using 4peaks software Nucleobytes (Amsterdam, The Netherlands).
Expression of autophagy genes. MSCs cultured in the presence and absence of IFNγ or TNFα (for 48 hours) or Staphylococcus Enterotoxin B activated human T cells (in the transwell plate for 4 days) were subjected for mRNA expression analysis. Total RNA was extracted from using RNeasy plus mini kit (Qiagen). Normalized RNA was used to convert cDNA using Quantitect reverse Transcription kit (Qiagen). Sybr green (Perfecta Sybr green fast mix, Quanta biosciences) real-time PCR was performed with primer pairs as described previously (i) Beclin (5′CTTACCACAGCCCAGGCGAAAC, 5′GCCAGAGCATGGAGCAGCAA); (ii) ATG12 (5′ATTGCTGCTGGAGGGGAAGG, 5′GGTTCGTGTTCGCTCTACTGC); (iii) ATG5 (5′AACTGAAAGGGAAGCAGAACCA, 5′CCATTTCAGTGGTGTGCCTTC), (iv) ATG16L1 (5′ACGTACCAAACAGGCACGAG, 5′CAGGTCAGAGATAGTCTGCAAAC), (v) ATG7 (5′ATGCCTGGGCATCCAGTGAACTTC, 5′CATCATTGCAGAAGTAGCAGCCA); (vi) LC3 (5′ATGCCGTCGGACAAGACCTT, 5′TTACACTGACAATTTCATCCCG); (vii) GAPDH (5′CTCTCTGCTCCTCCTGTTCGAC, 5′TGAGCGATGTGGCTCGGCT). ABI 7500 fast real-time PCR system thermal cycler was used for amplification and Δ-Δ CT method was employed to calculate the fold change in expression.
Fluidigm nanoscale 48*48 PCR array. Quantitative RT-PCR was performed using Fluidigm 48 × 48 nanofluidic arrays.29 Briefly, ± IFNγ (20 ng/ml) stimulated cDNA samples from Crohn's and healthy MSCs were pre amplified with 14-cycle PCR reaction for each sample with the combination of 100 ng cDNA with pooled primers as described by TaqMan Pre-Amp Mastermix (Fluidigm BioMark, San Francisco, CA) manufacturer protocols. Two thousand three-hundred four parallel qRT-PCR reactions were performed for each primer pair on each sample on a 48 × 48 array. Amplification was detected in Eva Green detection assay on a Biomark I machine based on standard Fluidigm protocols. PCR data were normalized and analyzed with SAS/JMP Genomics software.
Western blot. IDO protein were detected using primary rabbit anti-human IDO1 (1:1,000; EMD Millipore Corporation, Billerica, MA) or rabbit anti-human β-actin (1:1,000; Cell Signaling Technology, Danvers, MA), and secondary horseradish peroxide-coupled goat anti-rabbit IgG h + l (1:10,000; Bethyl Laboratories, Montgomery, TX). ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ) was used to detect immunoreactive blots.
Autophagy detection. MSCs or HeLa cells (ATCC, Manassas, VA) cultured in the presence and absence of IFNγ or Rapamycin (for 12–16 hours) or Staphylococcus Enterotoxin B activated T cells (in the transwell plate for 4 days) were subjected to autophagy activity assays as described in the manufacturer instructions (Autophagy Detection Kit ab139484, Abcam, Cambridge, MA).
MSC and T-cell coculture. Coculture of MSCs and T cells for proliferation and intracellular cytokine staining assay was well described previously.28 Briefly, MSCs were seeded on to 96-well plates with the density of 5,000/cm2. Forty-eight hours post ±IFNγ licensing, 100,000 random donor human peripheral blood mononuclear cells (PBMCs) are added to each well. 500 ng/ml staphylococcal enterotoxin B (SEB) (Toxin Technology, Sarasota, FL) was used to stimulate the T cells. For intracellular cytokine staining, Brefeldin A was added at the concentration of 10 µg/ml (Sigma) for 12–14 hours and intracellular flow cytometry staining was performed with BD Cytofix and Cytoperm procedure with the antibodies CD3APCCy7, CD4PE, CD8PerCP, TNFαFITC, and IFNγAPC (BD Biosciences, St Jose, CA). Ki67 Proliferation assay was performed after 4 days according to manufacturer instructions (BD Biosciences, San Jose, CA).
Statistical analysis. We randomly selected (n = 6) IBD MSC donors out of 8–10 enrolled in this study. Data were analyzed with the GraphPad Prism 5.0 software. For the comparison of two groups, paired t-test was applied. A two-sided P value <0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. Suppressive potential of IBD MSCs derived from early vs late passage. Figure S2. HLADR induction by activated T cells in Crohn's MSCs. Figure S3. 3MA blocks autophagy activity. Table S1. Clinical trials of MSCs for Luminal and Fistular Crohn's Disease. Table S2. IBD Patient Characteristics. Table S3. Genes significant to MSC immunobiology, biodistribution and Regeneration.
Acknowledgments
We thank Shala Yuan for technical assistance. J.G. is a Georgia Distinguished Cancer scientist. The study was supported by a grant from the Children's Hospital of Atlanta (CHOA) Center for Transplantation and Immune-mediated Disorders.
Supplementary Material
References
- Le Blanc, K and Mougiakakos, D (2012). Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol 12: 383–396. [DOI] [PubMed] [Google Scholar]
- Bernardo, ME and Fibbe, WE (2013). Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell 13: 392–402. [DOI] [PubMed] [Google Scholar]
- Murphy, MB, Moncivais, K and Caplan, AI (2013). Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med 45: e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duijvestein, M, Vos, AC, Roelofs, H, Wildenberg, ME, Wendrich, BB, Verspaget, HW et al. (2010). Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn's disease: results of a phase I study. Gut 59: 1662–1669. [DOI] [PubMed] [Google Scholar]
- Forbes, GM, Sturm, MJ, Leong, RW, Sparrow, MP, Segarajasingam, D, Cummins, AG et al. (2014). A phase 2 study of allogeneic mesenchymal stromal cells for luminal Crohn's disease refractory to biologic therapy. Clin Gastroenterol Hepatol 12: 64–71. [DOI] [PubMed] [Google Scholar]
- Ricart, E, Jauregui-Amezaga, A, Ordás, I, Pinó, S, Ramírez, AM and Panés, J (2013). Cell therapies for IBD: what works? Curr Drug Targets 14: 1453–1459. [DOI] [PubMed] [Google Scholar]
- Voswinkel, J, Francois, S, Simon, JM, Benderitter, M, Gorin, NC, Mohty, M et al. (2013). Use of mesenchymal stem cells (MSC) in chronic inflammatory fistulizing and fibrotic diseases: a comprehensive review. Clin Rev Allergy Immunol 45: 180–192. [DOI] [PubMed] [Google Scholar]
- Eliopoulos, N, Stagg, J, Lejeune, L, Pommey, S and Galipeau, J (2005). Allogeneic marrow stromal cells are immune rejected by MHC class I- and class II-mismatched recipient mice. Blood 106: 4057–4065. [DOI] [PubMed] [Google Scholar]
- Moll, G, Alm, JJ, Davies, LC, von Bahr, L, Heldring, N, Stenbeck-Funke, L et al. (2014). Do cryopreserved mesenchymal stromal cells display impaired immunomodulatory and therapeutic properties? Stem Cells 32: 2430–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François, M and Galipeau, J (2012). New insights on translational development of mesenchymal stromal cells for suppressor therapy. J Cell Physiol 227: 3535–3538. [DOI] [PubMed] [Google Scholar]
- Rameshwar, P (2009). Casting doubt on the safety of “off-the-shelf” mesenchymal stem cells for cell therapy. Mol Ther 17: 216–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galipeau, J (2013). The mesenchymal stromal cells dilemma–does a negative phase III trial of random donor mesenchymal stromal cells in steroid-resistant graft-versus-host disease represent a death knell or a bump in the road? Cytotherapy 15: 2–8. [DOI] [PubMed] [Google Scholar]
- Papadaki, HA, Kritikos, HD, Gemetzi, C, Koutala, H, Marsh, JC, Boumpas, DT et al. (2002). Bone marrow progenitor cell reserve and function and stromal cell function are defective in rheumatoid arthritis: evidence for a tumor necrosis factor alpha-mediated effect. Blood 99: 1610–1619. [DOI] [PubMed] [Google Scholar]
- Ma, J, Ning, YN, Xu, M, Hou, Y, Wang, N, Hou, XY et al. (2013). Thalidomide corrects impaired mesenchymal stem cell function in inducing tolerogenic DCs in patients with immune thrombocytopenia. Blood 122: 2074–2082. [DOI] [PubMed] [Google Scholar]
- Tang, Y, Ma, X, Zhang, H, Gu, Z, Hou, Y, Gilkeson, GS et al. (2012). Gene expression profile reveals abnormalities of multiple signaling pathways in mesenchymal stem cell derived from patients with systemic lupus erythematosus. Clin Dev Immunol 2012: 826182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larghero, J, Farge, D, Braccini, A, Lecourt, S, Scherberich, A, Foïs, E et al. (2008). Phenotypical and functional characteristics of in vitro expanded bone marrow mesenchymal stem cells from patients with systemic sclerosis. Ann Rheum Dis 67: 443–449. [DOI] [PubMed] [Google Scholar]
- Bocelli-Tyndall, C, Bracci, L, Spagnoli, G, Braccini, A, Bouchenaki, M, Ceredig, R et al. (2007). Bone marrow mesenchymal stromal cells (BM-MSCs) from healthy donors and auto-immune disease patients reduce the proliferation of autologous- and allogeneic-stimulated lymphocytes in vitro. Rheumatology (Oxford) 46: 403–408. [DOI] [PubMed] [Google Scholar]
- Bernardo, ME, Avanzini, MA, Ciccocioppo, R, Perotti, C, Cometa, AM, Moretta, A et al. (2009). Phenotypical/functional characterization of in vitro-expanded mesenchymal stromal cells from patients with Crohn's disease. Cytotherapy 11: 825–836. [DOI] [PubMed] [Google Scholar]
- Visscher, PM, Brown, MA, McCarthy, MI and Yang, J (2012). Five years of GWAS discovery. Am J Hum Genet 90: 7–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardito, S, Negrini, S, Conteduca, G, Ferrera, F, Parodi, A, Battaglia, F et al. (2013). Indoleamine 2,3 dioxygenase gene polymorphisms correlate with CD8+ Treg impairment in systemic sclerosis. Hum Immunol 74: 166–169. [DOI] [PubMed] [Google Scholar]
- Hampe, J, Franke, A, Rosenstiel, P, Till, A, Teuber, M, Huse, K et al. (2007). A genome-wide association scan of nonsynonymous SNPs identifies a susceptibility variant for Crohn disease in ATG16L1. Nat Genet 39: 207–211. [DOI] [PubMed] [Google Scholar]
- Rioux, JD, Xavier, RJ, Taylor, KD, Silverberg, MS, Goyette, P, Huett, A et al. (2007). Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat Genet 39: 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapaquette, P, Brest, P, Hofman, P and Darfeuille-Michaud, A (2012). Etiology of Crohn's disease: many roads lead to autophagy. J Mol Med (Berl) 90: 987–996. [DOI] [PubMed] [Google Scholar]
- Gianchecchi, E, Delfino, DV and Fierabracci, A (2014). Recent insights on the putative role of autophagy in autoimmune diseases. Autoimmun Rev 13: 231–241. [DOI] [PubMed] [Google Scholar]
- Nys, K, Agostinis, P and Vermeire, S (2013). Autophagy: a new target or an old strategy for the treatment of Crohn's disease? Nat Rev Gastroenterol Hepatol 10: 395–401. [DOI] [PubMed] [Google Scholar]
- Plantinga, TS, Crisan, TO, Oosting, M, van de Veerdonk, FL, de Jong, DJ, Philpott, DJ et al. (2011). Crohn's disease-associated ATG16L1 polymorphism modulates pro-inflammatory cytokine responses selectively upon activation of NOD2. Gut 60: 1229–1235. [DOI] [PubMed] [Google Scholar]
- Copland, IB, Garcia, MA, Waller, EK, Roback, JD and Galipeau, J (2013). The effect of platelet lysate fibrinogen on the functionality of MSCs in immunotherapy. Biomaterials 34: 7840–7850. [DOI] [PubMed] [Google Scholar]
- Chinnadurai, R, Copland, IB, Patel, SR and Galipeau, J (2014). IDO-independent suppression of T cell effector function by IFN-γ-licensed human mesenchymal stromal cells. J Immunol 192: 1491–1501. [DOI] [PubMed] [Google Scholar]
- Nath, AP, Arafat, D and Gibson, G (2012). Using blood informative transcripts in geographical genomics: impact of lifestyle on gene expression in fijians. Front Genet 3: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krampera, M, Galipeau, J, Shi, Y, Tarte, K and Sensebe, L; MSC Committee of the International Society for Cellular Therapy (ISCT) (2013). Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy 15: 1054–1061. [DOI] [PubMed] [Google Scholar]
- Ren, G, Zhang, L, Zhao, X, Xu, G, Zhang, Y, Roberts, AI et al. (2008). Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2: 141–150. [DOI] [PubMed] [Google Scholar]
- Powell, DW, Pinchuk, IV, Saada, JI, Chen, X and Mifflin, RC (2011). Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73: 213–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armaka, M, Apostolaki, M, Jacques, P, Kontoyiannis, DL, Elewaut, D and Kollias, G (2008). Mesenchymal cell targeting by TNF as a common pathogenic principle in chronic inflammatory joint and intestinal diseases. J Exp Med 205: 331–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici, M, Le Blanc, K, Mueller, I, Slaper-Cortenbach, I, Marini, F, Krause, D et al. (2006). Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317. [DOI] [PubMed] [Google Scholar]
- Horwitz, EM, Le Blanc, K, Dominici, M, Mueller, I, Slaper-Cortenbach, I, Marini, FC et al.; International Society for Cellular Therapy. (2005). Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7: 393–395. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez, R, François, M, Boivin, MN, Stagg, J and Galipeau, J (2007). Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-gamma, TGF-beta, and cell density. J Immunol 179: 1549–1558. [DOI] [PubMed] [Google Scholar]
- François, M, Romieu-Mourez, R, Li, M and Galipeau, J (2012). Human MSC suppression correlates with cytokine induction of indoleamine 2,3-dioxygenase and bystander M2 macrophage differentiation. Mol Ther 20: 187–195. [DOI] [PubMed] [Google Scholar]
- Zenewicz, LA, Antov, A and Flavell, RA (2009). CD4 T-cell differentiation and inflammatory bowel disease. Trends Mol Med 15: 199–207. [DOI] [PubMed] [Google Scholar]
- Romieu-Mourez, R, Coutu, DL and Galipeau, J (2012). The immune plasticity of mesenchymal stromal cells from mice and men: concordances and discrepancies. Front Biosci (Elite Ed) 4: 824–837. [DOI] [PubMed] [Google Scholar]
- Ren, J, Jin, P, Sabatino, M, Balakumaran, A, Feng, J, Kuznetsov, SA et al. (2011). Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy 13: 661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafei, M, Hsieh, J, Fortier, S, Li, M, Yuan, S, Birman, E et al. (2008). Mesenchymal stromal cell-derived CCL2 suppresses plasma cell immunoglobulin production via STAT3 inactivation and PAX5 induction. Blood 112: 4991–4998. [DOI] [PubMed] [Google Scholar]
- Cox, JH, Dean, RA, Roberts, CR and Overall, CM (2008). Matrix metalloproteinase processing of CXCL11/I-TAC results in loss of chemoattractant activity and altered glycosaminoglycan binding. J Biol Chem 283: 19389–19399. [DOI] [PubMed] [Google Scholar]
- Schroepf, S, Kappler, R, Brand, S, Prell, C, Lohse, P, Glas, J et al. (2010). Strong overexpression of CXCR3 axis components in childhood inflammatory bowel disease. Inflamm Bowel Dis 16: 1882–1890. [DOI] [PubMed] [Google Scholar]
- Chinnadurai, R, Waller, EK, Galipeau, J and Nooka, AK (2013). From single nucleotide polymorphisms to constant immunosuppression: mesenchymal stem cell therapy for autoimmune diseases. Biomed Res Int 2013: 929842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh, T, Fujita, N, Jang, MH, Uematsu, S, Yang, BG, Satoh, T et al. (2008). Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature 456: 264–268. [DOI] [PubMed] [Google Scholar]
- Cadwell, K, Liu, JY, Brown, SL, Miyoshi, H, Loh, J, Lennerz, JK et al. (2008). A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature 456: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy, A, Li, Y, Peng, I, Reichelt, M, Katakam, AK, Noubade, R et al. (2014). A Crohn's disease variant in Atg16l1 enhances its degradation by caspase 3. Nature 506: 456–462. [DOI] [PubMed] [Google Scholar]
- Lassen, KG, Kuballa, P, Conway, KL, Patel, KK, Becker, CE, Peloquin, JM et al. (2014). Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A 111: 7741–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, N, Saitoh, T, Kageyama, S, Akira, S, Noda, T and Yoshimori, T (2009). Differential involvement of Atg16L1 in Crohn disease and canonical autophagy: analysis of the organization of the Atg16L1 complex in fibroblasts. J Biol Chem 284: 32602–32609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnadurai, R, Garcia, MA, Sakurai, Y, Lam, WA, Kirk, AD, Galipeau, J et al. (2014). Actin cytoskeletal disruption following cryopreservation alters the biodistribution of human mesenchymal stromal cells in vivo. Stem Cell Reports 3: 60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François, M, Copland, IB, Yuan, S, Romieu-Mourez, R, Waller, EK and Galipeau, J (2012). Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-γ licensing. Cytotherapy 14: 147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.