Abstract
The transcription factor Pax4 plays a critical role in the determination of α- versus β-cell lineage during endocrine pancreas development. In this study, we explored whether Pax4 gene transfer into α-cells could convert them into functional β-cells and thus provide therapeutic benefits for insulin-deficient diabetes. We found that Pax4 delivered by adenoviral vector, Ad5.Pax4, induced insulin expression and reduced glucagon expression in αTC1.9 cells. More importantly, these cells exhibited glucose-stimulated insulin secretion, a key feature of functional β-cells. When injected into streptozotocin-induced diabetic mice, Pax4-treated αTC1.9 cells significantly reduced blood glucose, and the mice showed better glucose tolerance, supporting that Pax4 gene transfer into αTC1.9 cells resulted in the formation of functional β-cells. Furthermore, treatment of primary human islets with Ad5.Pax4 resulted in significantly improved β-cell function. Detection of glucagon+/Pax4+/Insulin+ cells argued for Pax4-induced α-to-β cell transitioning. This was further supported by quantification of glucagon and insulin bi-hormonal cells, which was significantly higher in Pax4-treated islets than in controls. Finally, direct administration of Ad5.Pax4 into the pancreas of insulin-deficient mice ameliorated hyperglycemia. Taken together, our data demonstrate that manipulating Pax4 gene expression represents a viable therapeutic strategy for the treatment of insulin deficient diabetes.
Introduction
Reprogramming of one specialized cell type into another without reversion to pluripotent cells—that is, transdifferentiation—is a promising and challenging strategy for β-cell replacement therapy in the treatment of insulin-deficient diabetes.1,2 Thus far, the cell types that have been explored for transdifferentiating into β-cells include liver cells and pancreatic exocrine cells.3,4,5,6,7,8,9 In this study, we explored the possibility to convert glucagon-producing α-cells into insulin-producing β-cells. This strategy is particularly attractive because the two pancreatic endocrine cell types are closely related developmentally, spatially, and functionally. Both α-cells and β-cells are pancreatic endocrine cells residing in the islets of Langerhans and sensitive to blood glucose changes. They share a long developmental pathway before the two cell types finally diverge, which is mainly controlled by differential expression of transcription factors.10,11 In addition, it has been commonly observed that in diabetic conditions, there is not only β-cell/insulin deficit but also α-cell/glucagon excess.12,13,14,15 Moreover, it has been shown that following extreme β-cell loss, new β-cells can be formed by transdifferentiation of α-cells,16 suggesting it is possible to convert α-cells into β-cells. Therefore, strategies that induce α-to-β cell transdifferentiation would offer therapeutic benefits for diabetes treatment.
The transcription factor Pax4 consists of a paired domain and a homeodomain, either of which confers its DNA-binding activity.17,18 Predominantly expressed in pancreatic islets, Pax4 plays an essential role in the generation of islet cell progenitors and subsequent differentiation of insulin-producing β-cells and somatostatin-producing δ-cells during embryonic development.19,20,21,22 In addition, Pax4 has been shown to play a critical role in β-cell expansion and survival.19,23 Several Pax4 mutations have been identified to be associated with early onset of type 2 diabetes and maturity-onset diabetes of the young in humans.24,25,26,27,28,29,30 Gene knockout studies have shown that Pax4-deficient mice do not have mature β-cells or δ-cells but have considerably more α-cells.22 Interestingly, studies using conditional Pax4 knock-in mice have shown that ectopic Pax4 expression can restore β-cells, probably by converting progenitor cells into α-cells and then subsequently into β-cells.31,32
Nonetheless, ectopic expression of Pax4 in α-cells via conditional knock-in can cause continuous conversion of the α-cells into β-cells, resulting in an unwanted adverse effect—glucagon deficiency and islet hypertrophy.31,32 For therapeutic purpose, a more manageable strategy is thus needed. In this study, we explored whether adenovirus-mediated Pax4 gene therapy strategy could allow the conversion of α-cells into β-cells using various model systems, and thus providing therapeutic benefits for diabetes treatment.
Results
Ad5-mediated efficient Pax4 gene delivery into αTC1.9 cells
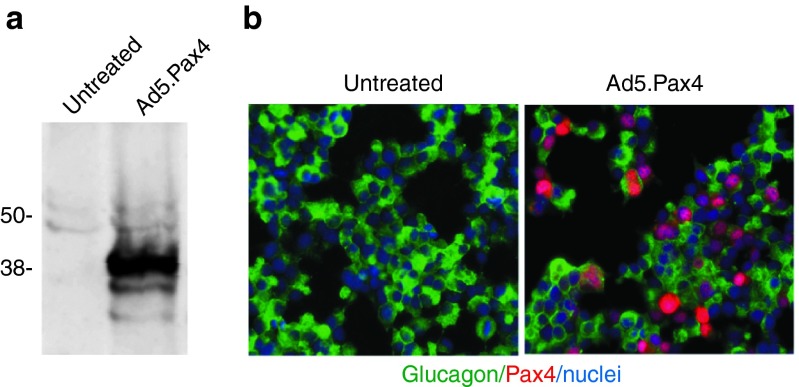
To efficiently deliver Pax4 into α-cells, we developed an adenoviral vector carrying cytomegalovirus (CMV)-promoter-driven human Pax4, namely Ad5.Pax4 vector. Then, we verified Pax4 gene delivery in the α-cell line, αTC1 clone 9 (αTC1.9) cells, using both western blotting and immunohistochemistry assays. As shown in Figure 1, Ad5.Pax4 infection of αTC1.9 cells at the multiplicity of infection of 500 viral particles (VPs) per cell resulted in robust Pax4 gene expression without noticeable toxicity. Western blotting with Pax4 antibody showed a major band corresponding to the molecular weight of Pax4 (~38 KDa) in Ad5.Pax4-treated cells, but not in uninfected αTC1.9 cells (Figure 1a). Immunofluorescence staining confirmed that Pax4 was efficiently delivered into αTC1.9 cells, and it was localized in the nuclei, which is consistent with the distribution pattern of transcription factors (Figure 1b).
Figure 1.
Ad5-mediated Pax4 gene delivery into αTC1.9 cells. The cells were infected with Ad5.Pax4 at MOI of 500 VPs/cell. Two days later, they were collected for either western blotting assay or immunofluorescence staining using a rabbit anti-Pax4 antibody (Sigma-Aldrich, St Louis, MO). (a) Western blotting assay showing Pax4 expression in Ad5.Pax4-infected αTC1.9 cells, not untreated control cells. (b) Immunofluorescence co-staining of glucagon (green) and Pax4 (red). The mouse anti-glucagon antibody from Sigma-Aldrich was used in combination with rabbit anti-Pax4 antibody in the co-staining. Nuclei (blue) staining was included to mark all cells. Pax4 expression was detected in >80% of Ad5.Pax4-infected αTC1.9 cells, suggesting Ad5-mediated efficient Pax4 gene delivery in these α-cells. MOI, multiplicity of infection.
Ad5.Pax4 treatment induced insulin expression while reducing glucagon expression in αTC1.9 cells
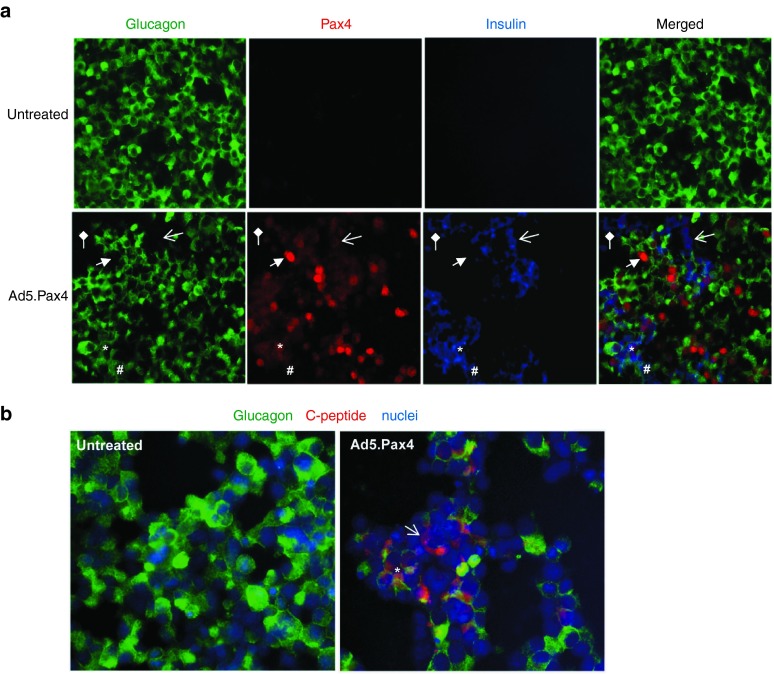
Next, we examined glucagon and insulin expression following Ad5.Pax4 infection. Untreated (naive) αTC1.9 cells expressed high levels of glucagon, with minimal (undetectable) insulin expression (Figure 2a). Following Ad5.Pax4 treatment, insulin became readily detectable in 20–30% of infected cells while glucagon expression reduced, suggesting that Pax4 induced insulin expression and inhibited glucagon expression in αTC1.9 cells (Figure 2a). Several populations of cells on the basis of hormone (glucagon versus insulin) expression were observed due to the forced Pax4 expression. These include: (i) Glucagon−/ Insulin+/ Pax4+ cells, in which glucagon was completely turned off and insulin was turned on; (ii) Glucagon+/ Insulin+/ Pax4+ cells (bi-hormonal cells), in which insulin expression was turned on, but glucagon was not completely inhibited; and (iii) Glucagon–/ Insulin–/ Pax4+ cells, in which glucagon was totally inhibited and insulin was not induced, especially in cells with high levels of Pax4 expression (representing about 10% of infected cells) (Figure 2a). Among the insulin+ cells, most (70–80%) were bi-hormonal cells, and a small percentage (20–30%) expressed insulin only. These populations of cells could represent different stages of α-to-β cell phenotypic conversion. Of note, although very rare, we also observed Pax– but insulin+ or bi-hormonal cells in Ad5.Pax4-treated αTC1.9 cells, which may be due to very low level (below detection) of Pax4 expression.
Figure 2.
Pax4-induced insulin and C-peptide expression in αTC1.9 cells. (a) Expression of glucagon (green), Pax4 (red), and insulin (blue) in untreated or Ad5.Pax4 treated cells. The insulin antibody was generated from guinea pig (Abcam, Cambridge, MA), and Pax4 and glucagon antibodies were the same as described in Figure 1. Shown are representative images from 5 days postinfection. The star (*) marks an example of glucagon+/ Pax4+/ Insulin+ (Pax4-positive bi-hormonal) cells. The open arrow marks an example of glucagon−/ Pax4+/ Insulin+ cells, in which glucagon was completely turned off and insulin expression was turned on in these cells. The block arrow marks an example of glucagon− /Pax4+ /Insulin− cells, in which glucagon expression was turned off, but insulin was not induced. The # marks a bi-hormonal cell with barely detectable Pax4, and the diamond arrow marks an insulin+ cell without detectable Pax4. (b) Expression of glucagon (green) and C-peptide (red). The rabbit anti-C-peptide antibody from Cell Signaling Technology (Danvers, MA) was used. Nuclei staining (blue) was used to mark all cells. The open arrow marks an example of glucagon−/ C-peptide+ cells (glucagon is totally inhibited), and the star (*) marks an example of glucagon+/ C-peptide+ cells (bi-hormonal cells).
Additionally, we examined the presence of C-peptide, a by-product of insulin maturation from proinsulin. As shown in Figure 2b, C-peptide was detected in Pax4-treated αTC1.9 cells, but not in the control cells. Similarly, we could detect many bi-hormonal cells as well as Glucagon–/C-pep+ cells, arguing for α-to-β cell conversion with forced Pax4 expression.
Time course of insulin versus glucagon expression in Ad5.Pax4-treated αTC1.9 cells
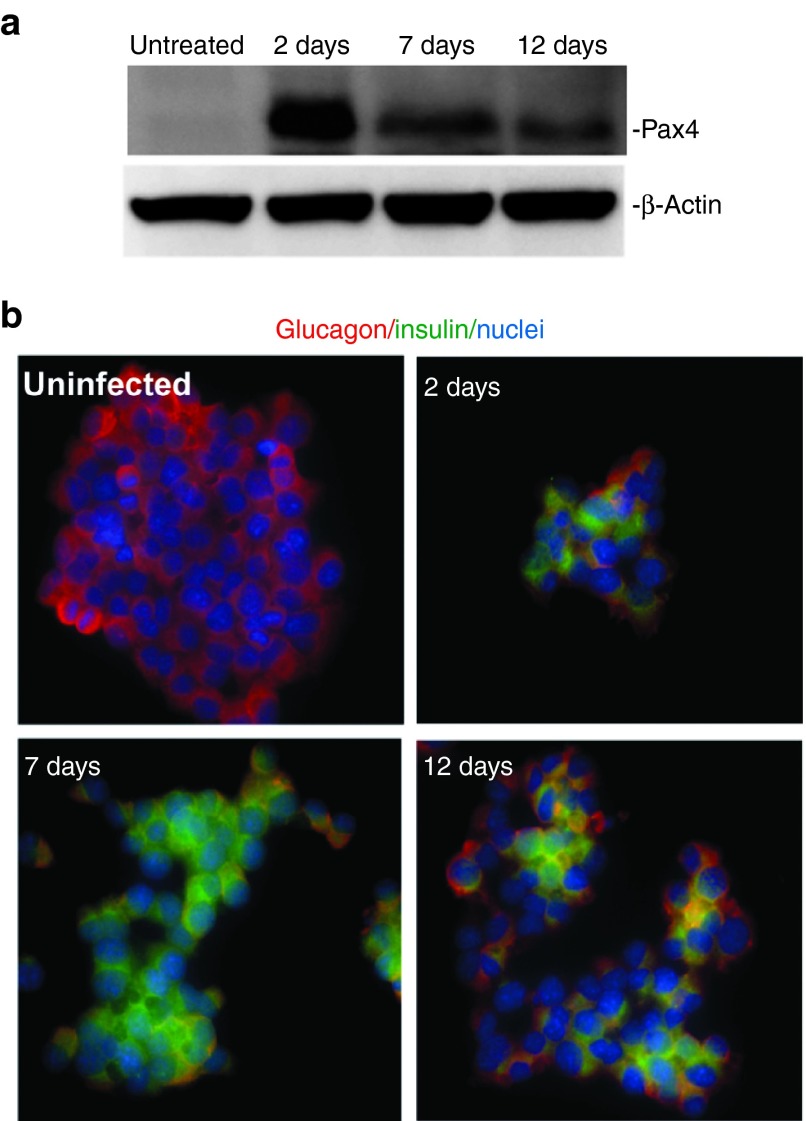
We next examined the time course of insulin versus glucagon expression in αTC1.9 cells following Ad5.Pax4 treatment. Because cultured αTC1.9 cells need to be passaged every 4–5 days to maintain their healthiness, and Ad5-based gene delivery vectors are nonintegrative, Pax4 expression decreased with continued culturing and passaging (Figure 3a). Insulin expression was readily detectable 2 days after Ad5.Pax4 infection, continued to increase until 7 days (first passage) postinfection and started to decline after two passages (equivalent to 12 days postinfection), while in the meantime, glucagon expression showed an opposite pattern (Figure 3b). This time-dependent change confirmed induction of insulin expression and inhibition of glucagon expression by Pax4 gene transfer. The decline of the insulin-producing cells after two passages suggest that either Pax4-induced α-to-β conversion was reversible (with the loss of Pax4) or the converted cells did not survive/proliferate as well as the naive αTC1.9 cells. Of note, early studies indicate that Pax4 acts as a short-term trigger for β-cell lineage determination. Therefore, Pax4-induced α-to-β conversion may be irreversible. Nonetheless, it is possible that the clonal αTC1.9 cells that were not completely converted into β-cells (such as the bi-hormonal cells) might be reversed with the loss of Pax4 expression.
Figure 3.
Insulin versus glucagon expression at various time points following Ad5.Pax4 treatment. The αTC1.9 cells plated in multiple dishes were infected with Ad5.Pax4 at MOI of 500 VPs/cell, and cells were either (a) lysed or (b) fixed at 2 days postinfection. In addition, some of the treated cells were passaged, cultured, and then collected as P1 (equivalent to 7 days) and P2 (equivalent to 12 days). (a) Western blotting assays showing Pax4 expression at various time points. β-Actin was used as the loading control. (b) Insulin (green) versus glucagon (red) expression at different time points following Ad5.Pax4 infection as assessed by immunofluorescence staining. Note the insulin signals increased from 2 to 7 days and declined at 12 days. MOI, multiplicity of infection.
Pax4 treatment upregulated the transcription factors essential for β-cell development and β-cell function
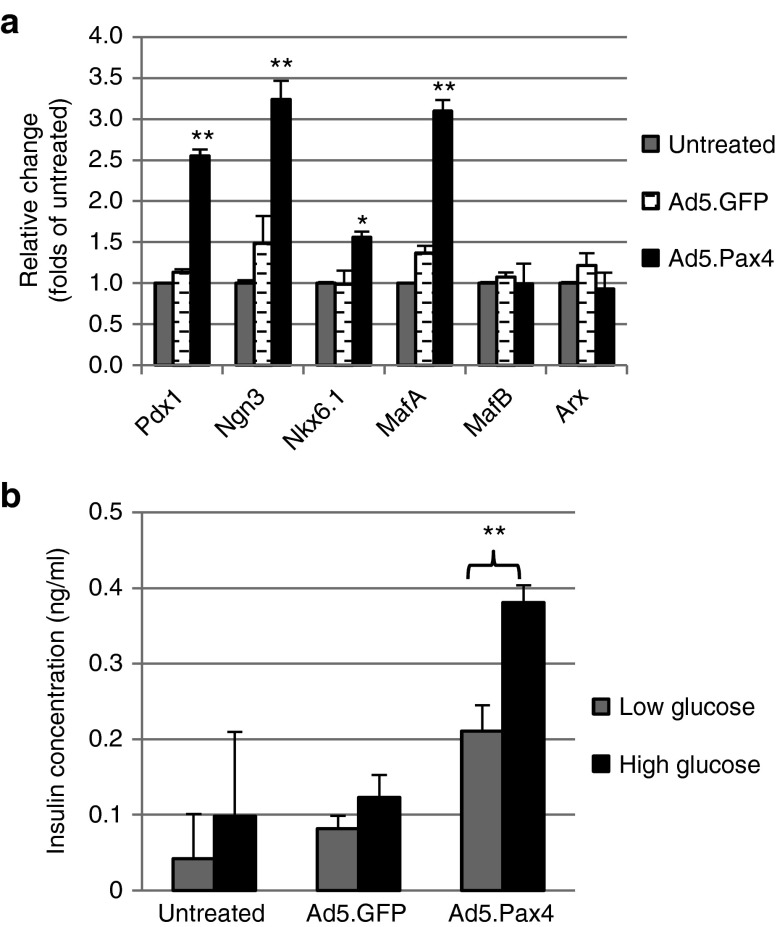
Transcription factors play essential roles in the development and function of islet cells. For example, Pdx1, Ngn3, Nkx6.1, and MafA are essential for the development and functional maintenance of β-cells. MafB and Arx, on the other hand, are essential for α-cell function. To further examine whether Pax4 expression could induce α-to-β cell phenotypic conversion, we examined the expression of these transcription factors. Quantitative real-time PCR (qPCR) assay revealed that, at mRNA level, β-cell–related transcription factors Pdx1, Ngn3, Nkx6.1, and MafA were significantly upregulated by Pax4 treatment (P < 0.05), while MafB and Arx did not appear to be significantly changed (P > 0.05) (Figure 4a). At the protein level, α-cell–relevant transcription factors Arx and MafB showed similar trend—decreasing but not significant—as assessed by both western blotting and immunofluorescence assays (Supplementary Figure S1). The β-cell–specific transcription factors, in contrast, were hardly detectable by either western blotting or immunofluorescence staining (Supplementary Figure S1 and data not shown). The discrepancy may be explained by the extremely low levels of β-cell–specific transcription factors in naive αTC1.9 cells—therefore, even after threefold to fourfold of increase at mRNA level following Pax4 treatment, the proteins were still below the detection level using western blotting and immunofluorescence assays.
Figure 4.
Transcription factors and GSIS in Pax4-treated αTC1.9 cells. (a) qPCR of key β-cell–specific transcription factors in variously treated αTC1.9 cells. Total RNA was extracted from untreated αTC1.9 cells or cells infected with Ad5.GFP or Ad5.Pax4 (500 VPs/cell, 5 days postinfection) and processed for qPCR using primers specific for transcription factors as indicated. Three independent experiments were performed. The data were expressed as mean ± SEM. *(P < 0.05) and **(P < 0.005) indicate significant difference between Ad5.Pax4 group and the two control groups. (b) GSIS assay of Pax4-treated αTC1.9 cells. The cells plated in 24-well plates were either untreated or treated with Ad5.GFP or Ad5.Pax4 at 500 VPs/cell. Five days later, the cells were assessed for GSIS using 5.5 mmol/l as low glucose and 25 mmol/l as high glucose, as described in the Materials and Methods section. The experiments were repeated three times, each time in triplicates. Shown are data from one representative experiment. The data were expressed as mean ± SEM. **P < 0.005. GSIS, glucose-stimulated insulin secretion.
Ad5.Pax4-treated αTC1.9 cells showed functional glucose-stimulated insulin secretion
To investigate whether Pax4-treated αTC1.9 cells became functional β-cells, we performed glucose-stimulated insulin secretion assay in static incubation. The untreated, Ad5.GFP-treated, and Ad5.Pax4-treated αTC1.9 cells were first incubated in 5.5 mmol/l (low) glucose solution for 2 hours, and then in 25 mmol/l (high) glucose solution for another 2 hours, followed by measurement of insulin concentrations in the supernatants. As shown in Figure 4b, in the supernatants of untreated or GFP-treated αTC1.9 cells, insulin concentrations were very low—often hardly detectable. However, insulin was readily detected in the supernatants of Ad5.Pax4-treated cells. More importantly, the data clearly showed that there was a significant increase in insulin secretion in response to high glucose in Ad5.Pax4-treated cells (Figure 4b). These data demonstrated that Pax4 gene transfer into αTC1.9 cells resulted in the formation of functional β-cells.
Therapeutic effect of Ad5.Pax4-treated αTC1.9 cells in diabetic mice
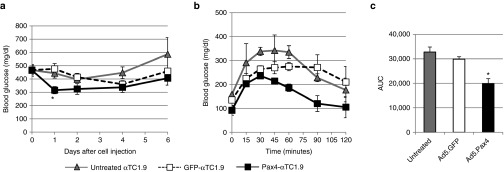
To further confirm that Pax4 gene delivery promoted the formation of functional β-cells from the αTC1.9 cells, we examined whether these cells have therapeutic effects in diabetic mice. The severe compromised immune deficiency (SCID) mice were first rendered diabetic with a single high-dose (130 mg/kg body weight) of streptozotocin (STZ), a toxin that specifically destroys β-cells. Untreated αTC1.9 cells or cells pretreated with Ad5.GFP (GFP-αTC1.9) or Ad5.Pax4 (Pax4-αTC1.9) were injected into the mice intraperitoneally. As shown in Figure 5a, injection of Pax4-αTC1.9 cells significantly reduced blood glucose of the diabetic mice (P < 0.05) compared to either GFP-αTC1.9 (P = 0.0054) or untreated αTC1.9 cells (P = 0.0152) within 24 hours. In the next few days, the average random blood glucose of Pax4-αTC1.9–treated mice remained lower than that of control groups, but the difference became nonsignificant. The diminishing glucose-lowering effect over time may be explained by the fact that the injected cells did not engraft in the peritoneal cavity and gradually died in vivo.
Figure 5.
Pax4-treated αTC1.9 cells ameliorated hyperglycemia of diabetic mice. Untreated, Ad5.GFP or Ad5.Pax4-treated αTC1.9 cells (MOI = 500 VPs/cell) were injected into the peritoneal cavity of STZ-induced diabetic SCID mice (n = 10 mice/group) at the dose of 1 × 107 cells/mouse. (a) Blood glucose (nonfasting) levels of the mice following cell injection. The * indicates the blood glucose of Pax4-αTC1.9 cells injected mice was significantly different from both control groups at that time point (P < 0.05). (b) Glucose tolerance test (GTT) 5 days after cell injection. For the test, glucose was administered at a dosage of 1 g/kg body weight intraperitoneally. (c) Area under the curve (AUC) of the GTT in different treatment groups. *P < 0.05, suggesting the difference between Pax4-αTC1.9 group and the two control groups was statistically significant. MOI, multiplicity of infection.
Additionally, we performed glucose tolerance test (GTT) on these mice 5 days after cell injection. Following overnight fasting, glucose (1 g/kg bodyweight) was administered into each mouse via intraperitoneal injection, and then, blood glucose was monitored for 2 hours (Figure 5b). The dosage of 1 g glucose/kg body weight for GTT was used because the mice had severe diabetes as assessed by their random blood glucose levels (Figure 5a). As shown in Figure 5b, the mice that were treated with Pax4-αTC1.9 cells showed the best tolerance to glucose administration. Analysis of the area under the curve (AUC) of GTT showed that Pax4-αTC1.9 group had significantly lower AUC than untreated αTC1.9 or GFP-αTC1.9 groups, confirming that Pax4-αTC1.9 cells provided significant glucose-lowering benefits in these mice (Figure 5c).
In summary, these data demonstrated that functional β-cells were induced in Ad5.Pax4-treated αTC1.9 cells, which exhibited therapeutic effects in the diabetic mice.
Effects of Pax4 gene delivery in primary human islets
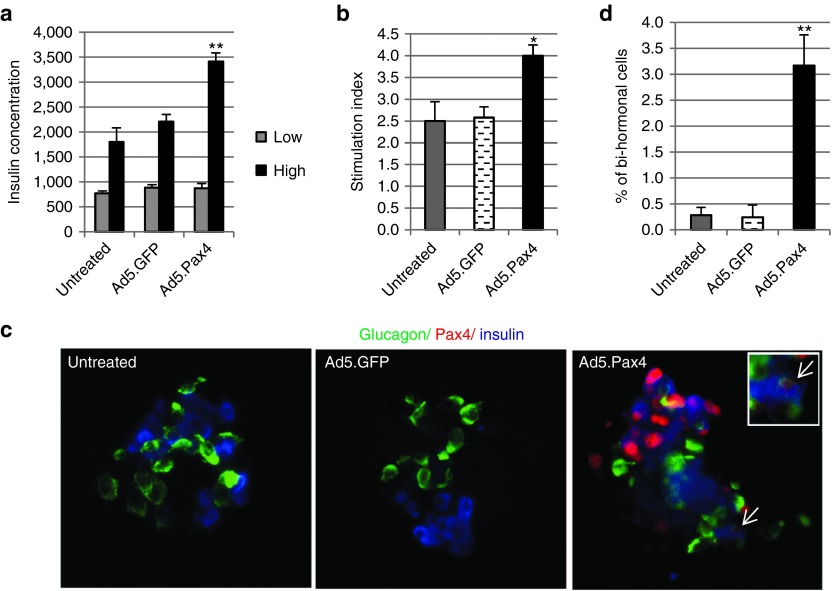
Next, we examined whether Pax4 could induce α-to-β phenotypic conversion in primary human islets, a much more complicated system that can also shed light on the potential effects of Pax4 manipulation in humans. To accomplish that, we treated primary human islets with Ad5.Pax4 or control Ad5.GFP. Immunofluorescence staining revealed abundant Pax4 expression in Ad5.Pax4-treated human islets, but not in untreated or Ad5.GFP-treated islets (Figure 6). Interestingly, Pax4-treated human islets showed significant improvement in β-cell function as assessed by glucose-stimulated insulin secretion (Figure 6a). The stimulation index of Pax4-treated human islets was much higher than that of untreated or Ad5.GFP-treated control groups (Figure 6b). Although in the experimental settings, it was impossible to distinguish whether any of the β-cells were preexisting or converted from α-cells, detection of glucagon+/Insulin+/Pax4+ cells (Pax4+ bi-hormonal cells) indicated that Pax4-induced α-to-β cell transitioning was occurring (Figure 6c). To further confirm this, we quantified glucagon and insulin double positive (bi-hormonal) cells in each treatment group. Our data showed that Ad5.Pax4-treated human islets contained significantly more bi-hormonal cells than control groups (Figure 6d), supporting that Pax4-treatment could induce α-to-β cell transitioning in human iselts.
Figure 6.
Effects of Pax4 gene transfer into human islets. Freshly isolated primary human islets were either untreated or treated with Ad5.Pax4 or Ad5.GFP at MOI of 250 VPs/cell. Three days later, the islets were processed for GSIS assay, in which the islets were first incubated in the low glucose (2.5 mmol/l) solution for 1 hour and then high glucose (25 mmol/l) solution for another hour. (a) Insulin concentration in the supernatants of the human islets following incubation at low and high glucose solution. The ** indicates P < 0.005 when Ad5.Pax4 group was compared with either control group (untreated or Ad5.GFP-treated) at high glucose. (b) Stimulation index (SI) of the human islets treated with Ad5.Pax4 or controls. SI was calculated as the ratio of the insulin concentration at high glucose to that at low glucose. Ad5.Pax4-treated human islets exhibited significantly higher SI than untreated (P = 0.025) or Ad5.GFP-treated islets (P = 0.0068). *P < 0.05 between Ad5.Pax4 and the two control groups. (c) Immunofluorescence analysis of insulin, glucagon, and Pax4 expression in the human islets. The arrow marks a Pax4-expressing bi-hormonal cell (Glucagon+/Insulin+). The enclosed square shows the cell at higher magnification. (d) The percentage of insulin and glucagon double-positive cells over the total α+β cells in each treatment group. The number of total insulin+ cells, glucagon+ cells, and double-positive cells were manually counted from 25 to 30 islets in each group following immunofluorescence staining and microscopy. The percentage of double-positive cells was calculated by dividing the number of double-positive cells by the total α (glucagon+) and β (Insulin+) cells in each islet. The results were expressed as mean ± SEM. Ad5.Pax4-treated islets showed significantly more double-positive (bi-hormonal) cells than untreated (P = 0.0004) and Ad5.GFP-treated (P = 0.0023) human islets. **P < 0.005. GSIS, glucose-stimulated insulin secretion; MOI, multiplicity of infection.
On another note, should Pax4-induced α-to-β cell conversion have occurred in human islets, one would expect fewer α-cells—that is, lower α:β cell ratio—in Pax4-treated islets than controls. We thus analyzed α- and β-cell composition in these islets. Our data showed that α:β ratio varied widely in different donors of human islets, which was consistent with previous studies.33,34,35,36 Nonetheless, Ad5.Pax4-treated human islets tended to have lower α:β cell ratio in each donor (Supplementary Figure S2), although the differences were not statistically significant (P > 0.05). This could be explained by the combination of the relatively low efficiency of α-to-β cell conversion within the experimental duration (4 days postinfection), the wide variation of α:β ratio among islets even within the same donor, and the number of islets analyzed—the sample size of 25–30 islets from each donor might not be sufficient to give rise to statistical significance.
Effect of direct Pax4 gene delivery in pancreas of diabetic mice
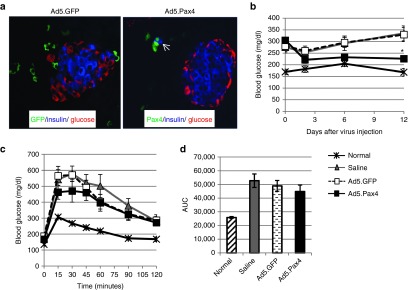
In order to fully explore the therapeutic utility of Pax4, we investigated effects of direct Ad5.Pax4 administration into pancreas of diabetic mice. Gene delivery into pancreatic islets has always been a difficult task in the field. To maximize the accessbility of the vectors to islets in vivo, we employed intra-bile ductal injection, in which the vectors were administered into the common bile duct toward the pancreas so that they could reach all pancreatic areas through the ductal system.37 In this study, we first established mouse diabetes models by multiple low doses of STZ treatment. One week after the last STZ injection, the mice were injected with either basal RPMI media (saline), Ad5.Pax4, or control Ad5.GFP vectors (5 × 109 VPs/mouse). Gene delivery of the control GFP and Pax4 were examined 3 days after bile-ductal injection. As shown in Figure 7a, GFP and Pax4 expression were detected in pancreas, but mostly in the exocrine pancreas (outside of islets) and ductal area. This is not unexpected because GFP and Pax4 were both driven by CMV promoter, and exocrine (acinar) and duct cells are easier to access than islets through ductal system, consistent with previous studies.37,38 Nonetheless, the transgenes were also observed in islets and in β-cells (Figure 7a).
Figure 7.
Direct in vivo examination of the therapeutic effect of Ad5.Pax4 on diabetic mice. Basal RPMI media (saline), Ad5.GFP, or Ad5.Pax4 were injected into the pancreas of diabetic mice via intra-bile ductal injection, at the dosage of 5 × 109 VPs/250 µl saline/mouse (n = 5 per group). (a) Immunofluorescence analysis of transgene expression in pancreas 3 days following injection. The transgenes GFP and Pax4 were detected in pancreas, but mostly outside of islets. The open arrow in Ad5.Pax4 group marks a Pax4+/Insulin+ cell. (b) Random blood glucose (nonfasting) levels of the mice. The mice that received Ad5.Pax4 had lower blood glucose than control-treated diabetic mice, but higher than that of normal mice. *P < 0.05, which was statistically significant, compared to saline or Ad5.GFP-treated diabetic mice at the indicated time point. (c) GTT of the mice 15 days after bile ductal injection. Similarly, Ad5.Pax4-treated mice had better glucose tolerance than the other control groups, although not as good as normal mice. (d) AUC of GTT for different treatment groups. AUC, area under the curve.
To investigate whether Pax4 in vivo gene delivery offered any therapeutic benefits, we monitored blood glucose of the mice. The random blood glucose of Ad5.Pax4-treated mice was found to be consistently lower than Ad5.GFP or saline-treated mice, and the difference became statistically significant at 12 days after vector injection (P < 0.05 versus either control group) (Figure 7b). Furthermore, GTT assay showed that Ad5.Pax4 treated mice had slightly better tolerance to glucose administration than Ad5.GFP or saline-treated mice (Figure 7c), although the AUC of the GTT curves did not appear to be statistically significant (Figure 7d).
Taken together, these results suggest that Pax4 gene delivery into pancreas was beneficial for β-cell function in insulin-deficient diabetes models. Nonetheless, it should be noted that, without lineage tracing, it is impossible to determine whether any of the β cells were converted from α-cells in the diabetic mice. Due to the very few Pax4+ cells inside the pancreatic islets, we felt that it was not reliable to use the number of Glucagon+/Insulin+ bi-hormonal cells as an indicate of Pax4-induced α-to-β cell transition in vivo. On the other hand, because previous studies have demonstrated that Pax4 can promote β-cell survival and α-to-β cell transdifferentiation,19,23,31,32 it is reasonable to conclude that the protective effect of Ad5.Pax4 in these diabetic mice was a result of the dual actions of Pax4.
Discussion
In this study, we investigated whether Pax4 could convert α-cells into functional β-cells, and thus conferring therapeutic benefits for the treatment of insulin-deficient diabetes. Our data demonstrated that Ad5-mediated Pax4 gene delivery could induce the α-cells to adopt β-cell phenotype in both αTC1.9 cells and in primary human islets. Furthermore, Pax4-treated human islets showed significant improvement in β-cell function, and direct injection of Ad5.Pax4 into pancreas of diabetic mice provided therapeutic benefits. Although the improvement of β-cell functions in human islets and in vivo may be attributable to Pax4-mediated β-cell protection as previous studies have shown Pax4 promotes β-cell survival and expansion,19,23 Pax4-induced β-cell regeneration from α-cells could have contributed in this matter as well.
Transdifferentiating other cell types into β-cells has been under exploration for the treatment of insulin-deficient diabetes for over a decade. The most notable success has been achieved with the use of liver cells and pancreatic exocrine cells, both using a series of transcription factors.3,5,6,7 It has been shown that a combination of three transcription factors, Ngn3, Pdx1, and MafA, can reprogram the pancreatic exocrine cells into functional β-cells in vivo.5 Even though the induced β-cells are not organized into islet structure, which may have limited their effectiveness, they are able to ameliorate hyperglycemia of STZ-induced diabetic mice.5 In mouse liver, the β-cell–specific transcription factor Pdx1 has been found to induce insulin expression, which can also ameliorate hyperglycemia of diabetic mice.3 In human liver cells, cotreatment with other transcription factors including Nkx6.1, Pax4, and MafA appears to promote Pdx1-induced liver-to-pancreatic β-cell reprogramming.6,7,8,9 Sequential treatment of the primary human liver cells with transcription factors in the order of Pdx1, Pax4, and MafA has resulted in significantly more mature transdifferentiated β-cells, suggesting that liver-to-pancreas transdifferentiation is a progressive and hierarchical process.6 Our current study of inducing α-to-β cell conversion with Pax4 represents a major alternative to the aforementioned transdifferentiation strategies. Although not as abundant as liver cells or pancreatic exocrine cells, α-cells intrinsically resemble β-cells more closely and are located within the islets. Plus, it is well known that α-cell composition increases in diabetic condition in both animal models and human.12,13,14,15 Therefore, inducing α-to-β cell transdifferentiation has its own advantage and clinical relevance.
Pax4 was chosen as the target for our strategy because earlier studies have demonstrated that it plays a key role in the determination of β- versus α-cell lineage: its deletion results in the lack of β-cells with excessive α-cells, and its conditional knock-in converts α-cells into β-cells.20,22,23,31,32 Our gene therapy strategy using Ad5.Pax4 confirmed these discoveries and validated its therapeutic potential for the treatment of diabetes. Our results align well with the role of Pax4 in progressive conversion of liver cells to pancreatic β-cells mentioned above6 and in the differentiation of unihormonal insulin-positive cells from human embryonic stem cells as Gage et al.39 have shown recently.
It is well established that Pax4 inhibits glucagon gene expression, acting through inhibition of Pax6-mediated transactivation to repress glucagon promoter.40,41,42 Our current study and others have confirmed that forced Pax4 expression reduces glucagon expression.39 Effect of Pax4 on insulin expression, however, has been quite intriguing. Although it is clear that Pax4 is essential for β-cell development and function as assessed using various knockout and transgenic mice,20,31,43 biochemical studies have shown that Pax4 is also a repressor of insulin promoter.17,40,42,44 The reconciliation of this discrepancy is proposed to lie in the timing of Pax4 expression: Pax4 expression peaks at E13.5, gradually diminishes, and becomes undetectable in adult islets, which allows Pax4 to suppress α-cell differentiation in early pancreatic development and to permit β-cell differentiation later.40,42 In agreement, we did not detect endogenous Pax4 expression in β- or α-cells of untreated human islets or mouse pancreas. Interestingly, we also noted that, following Pax4 gene transfer into αTC1.9 cells, insulin expression was often not detected in the cells expressing high levels of Pax4, although glucagon expression was totally inhibited (Figure 2a). In contrast, most bi-hormonal αTC1.9 cells expressed moderate levels of Pax4, and many insulin uni-hormonal cells had very weak Pax4 expression (Figure 2a). These results indicate that the expression level and/or timing of Pax4 need to be optimized in order to fully realize its potential in inducing α-to-β cell conversion.
We would like to note that, while we were revising our manuscript, Chen et al.45 published their study examining Pax4 effects on pancreatic α-cells using an adeno-associated virus vector. In contrast to our study, Chen et al. did not observe pax4-induced insulin expression, glucagon suppression, or protection of STZ-induced diabetes. Several factors may explain the discrepancies. First is the Pax4-expression cassette. In order to avoid any interference with the functionality of Pax4 protein, we did not include any tags in either end of Pax4 protein. The adeno-associated virus-Pax4 vector generated by Chen et al., on the other hand, carries mouse Pax4 that is linked to downstream GFP via 2A peptide.45 Theoretically, the 2A peptide can lead to the cleavage between Pax4 and GFP proteins, leaving the majority of 2A sequences (>20 amino acid residues) at the C-terminal end of Pax4. It is also known the efficiency of 2A-mediated cleavage varies significantly for different proteins,46,47 and it is unclear how efficient Pax4 was cleaved from the fusion protein in this study. Uncleaved GFP or even the remaining 2A sequences might have interfered with Pax4 functionality. Another reason for the discrepancies may be the diabetes models that were used in examining the therapeutic effects of Pax4 via bile ductal injection. Chen et al. used a single high dose of STZ model, which is a more severe diabetes model than the multiple low doses of STZ model that we used and thus was more difficult to be overcome by Pax4 treatment. In fact, their quantification data revealed over 20% increase in pancreatic insulin content in Pax4-treated mice compared to controls, although the data were not statistically significant.45 Taken together, it is likely that the negative data obtained by Chen et al. are due to a nonoptimal study design.
In summary, our data demonstrated that Pax4 gene transfer into α-cells was a viable strategy to regenerate functional β-cells. In combination with previous discoveries on Pax4-mediated β-cell protection and Pax4-induced β-cell regeneration from α-cells,19,23,31,32 our study suggests manipulating Pax4 expression is a promising avenue toward the development of β-cell replacement therapy for insulin deficient diabetes.
Materials and Methods
Animals and diabetes models. Female C57BL/6 (Charles River Laboratory, Wilmington, MA) and SCID (Frederick Cancer Research, Hartford, CT) mice were used in the study to establish diabetes models using STZ. Both single high dose (130 mg/kg body weight) and multiple low doses of STZ (50 mg/kg body weight for 5 consecutive days) models were used in this study, and they were established in the same way as we described previously.37 All animal procedures were approved by the Institutional Animal Care and Use Committee in Tulane University.
Cell culture and viral infection. The mouse pancreatic α-cell line, αTC1 clone 9 (αTC1.9), was purchased from ATCC (Manassas, VA). The cells were cultured in Dulbecco's Modified Eagle's Medium containing 16.5 mmol/l glucose supplemented with 10% dialyzed fetal bovine serum in a humidified incubator with 5% CO2, with media refreshing every other day. For adenoviral vector infection, 2 days after plating, the αTC1.9 cells were infected with the vectors at a multiplicity of infection of 500 VPs per cell. The cells were cultured for various days until subsequent assays.
Generation of Ad5.Pax4 vector. Human Pax4 cDNA was purchased from OriGene (Rockville, MD). The full-length Pax4 coding region was first cloned into pShuttle-CMV vector and then transferred into human adenovirus serotype 5 (Ad5)-based pAdeasy-1 vector by homologous recombination as described by the manufacturer's protocol (Agilent Technologies, Santa Clara, CA). The resultant plasmid, namely pAd5.Pax4, thus contained CMV promoter-driven human Pax4 in the E1 region of Ad5 genome. The adenoviral vector Ad5.Pax4 was rescued, amplified, purified, and titrated as we have described previously.37,48
Immunofluorescence staining. Immunofluorescence staining was performed essentially as we have described previously.15,37 Briefly, the cultured αTC1.9 cells were first fixed with 4% paraformaldehyde in phosphate-buffered saline, permeabilized with 0.25% Triton-X, and blocked in blocking solution (2% glycine, 2% bovine serum albumin, and 5% fetal bovine serum, 50 mmol/l NH4Cl in phosphate-buffered saline) for 1 hour. Then, the cells were incubated with primary antibodies for 2 hours, and then with corresponding secondary antibodies conjugated to various fluorescent agents (all obtained from Jackson ImmunoResearch Laboratories, West Grove, PA). For nuclear staining, 4′,6-diamidino-2-phenylindole (from Fisher Scientific, Waltham, MA) was added to the cells and incubated for 10 minutes at room temperature. Following extensive washing, the coverslips were air-dried, mounted, and processed for fluorescence microscopy. Images were taken with a Nikon Eclipse Ti-S inverted microscope that was equipped with NIS-BR Microscope Imaging Software (Nikon Instruments, Melville, NY).
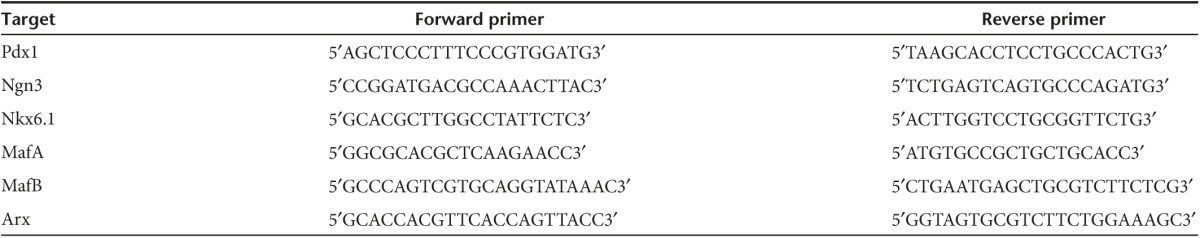
Quantitative RT-PCR. Total RNA was extracted from variously treated αTC1.9 cells using Qiagen RNeasy mini kit (Valencia, CA). The concentrations and purities of the extracted total RNA were determined by A260 and A260/A280 measurement, respectively, on a NanoDrop 2000 Spectrophotometers (Thermo Scientific, Wilmington, DE). Real-time quantitative RT-PCR for various genes, in triplicates, were performed with corresponding PCR primers (Table 1) using the Mx3000p qPCR system (Agilent Technologies). The assays were repeated multiple times to validate the results. The data were expressed as the mean of the normalized fold expression ± SEM, in which β-actin was used as the reference gene.
Table 1. Primers used in qPCR analysis of transcription factors.
Glucose-stimulated insulin secretion. Human islets cultured in 24-well plates, 4 wells per group, were preincubated in Krebs–Ringer bicarbonate (KRB) solution containing 2.5 mmol/l glucose (KRB-low) for 30 minutes at 37 °C. Then, the supernatant was replaced with 400 µl of fresh KRB-low solution and incubated for 1 hour at 37 °C. Following plate centrifugation (1,000 rpm, 5 minutes), the supernatants were collected (marked as low glucose). Next, fresh KRB solution containing 25 mmol/l glucose (KRB-high) was added at 400 µl per well. Following another hour of incubation at 37 °C, the supernatants were collected (marked as high glucose). Insulin concentrations in the supernatants were measured using human insulin ELISA kits following the manufacturer's instruction (ALPCO Diagnostics, Salem, NH). The data were expressed as mean ± SEM.
For αTC1.9 cells, glucose-stimulated insulin secretion was performed using the same procedure but with several modifications because of the very low levels of insulin in the culture. These include: the KRB-low solution contained 5.5 mmol/l glucose instead of 2.5 mmol/l; the incubation time was 2 hours at both KRB-low and KRB-high conditions. Finally, the insulin concentrations were measured using ultrasensitive mouse, not human, insulin ELISA kit (ALPCO Diagnostics, Salem, NH).
Glucose tolerance test. This was performed essentially as described before.49 Briefly, following overnight fasting (>15 hours), the mice were first weighed and then injected intraperitoneally with sterile glucose solution at the dose of 1 g/kg body weight. Their blood glucose was measured with the AlphaTrak glucose meter (Abbott Laboratories, Chicago, IL) at different time points, including 0 (prior to injection), 15, 30, 45, 60, 90, and 120 minutes postinjection. AUC was calculated using GraphPad Prism statistical software (GraphPad Software, La Jolla, CA).
Intra-bile ductal injection. C57BL/6 mice (n = 5 for each group) were first subjected to multiple low doses of STZ treatment (50 mg/kg body weight for 5 consecutive days). One week later, they were injected with Ad5.Pax4 or Ad5.GFP at the dosage of 5 × 109 VPs (diluted in 250 µl of basal RPMI media) per mouse, essentially the same as we described previously.37
Treatment of diabetic mice with Ad5.Pax4-treated αTC1.9 cells. The αTC1.9 cells (sufficient to treat 10 mice per group) were treated with Ad5.GFP or Ad5.Pax4 at multiplicity of infection of 500 VPs/cell. Untreated cells were included as control. Two days later, the immune compromised SCID mice were injected with STZ at a dose of 130 mg/kg body weight. After 3 days, most mice became diabetic (random blood glucose >350 mg/dl). Then, the treated or untreated αTC1.9 cells were collected, resuspended in basal culture media, and injected into each mouse via intraperitoneal injection, at the dosage of 1 × 107 cells in 500 µl basal media. The mice were monitored for their random blood glucose for the next 2 weeks, and GTT was performed 5 days after cell injection.
SUPPLEMENTARY MATERIAL Figure S1. Expression of α- or β-cell specific transcription factors following Ad5.Pax4 infection of αTC1.9 cells. Figure S2. Analysis of α:β cell ratio in human islets treated with Ad5.Pax4.
Acknowledgments
We thank Robert N. Bone in the University of Alabama at Birmingham, Beibei Xu and Meifen Wu in Tulane University for their technical assistance. We are also thankful for the COBRE core facility in Tulane Hypertension and Renal Center of Excellence, and Tulane Pathology Core Laboratory for their excellent services. This work was supported by the National Institutes of Health (NIH) grant DK081463 (H. Wu) and a Boehringer Ingelheim grant IIS2012-10170 (H. Wu). H. Wang was supported by NIH grants EB015744 and DK097544. F.M.-J. was supported by NIH grant DK074970 and American Diabetes Association grant 7-13-BS-101. V.A.F. was supported by NIH grants HHSN268201100027C, DK094006, GM103629, and DK098246. The authors declare no conflict of interest related to this work.
Supplementary Material
Expression of α- or β-cell specific transcription factors following Ad5.Pax4 infection of αTC1.9 cells.
Analysis of α:β cell ratio in human islets treated with Ad5.Pax4.
References
- Graf, T and Enver, T (2009). Forcing cells to change lineages. Nature 462: 587–594. [DOI] [PubMed] [Google Scholar]
- Zhou, Q and Melton, DA (2008). Extreme makeover: converting one cell into another. Cell Stem Cell 3: 382–388. [DOI] [PubMed] [Google Scholar]
- Ferber, S, Halkin, A, Cohen, H, Ber, I, Einav, Y, Goldberg, I et al. (2000). Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med 6: 568–572. [DOI] [PubMed] [Google Scholar]
- Ogihara, T, Fujitani, Y, Uchida, T, Kanno, R, Choi, JB, Hirose, T et al. (2008). Combined expression of transcription factors induces AR42J-B13 cells to differentiate into insulin-producing cells. Endocr J 55: 691–698. [DOI] [PubMed] [Google Scholar]
- Zhou, Q, Brown, J, Kanarek, A, Rajagopal, J and Melton, DA (2008). In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 455: 627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berneman-Zeitouni, D, Molakandov, K, Elgart, M, Mor, E, Fornoni, A, Domínguez, MR et al. (2014). The temporal and hierarchical control of transcription factors-induced liver to pancreas transdifferentiation. PLoS One 9: e87812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gefen-Halevi, S, Rachmut, IH, Molakandov, K, Berneman, D, Mor, E, Meivar-Levy, I et al. (2010). NKX6.1 promotes PDX-1-induced liver to pancreatic β-cells reprogramming. Cell Reprogram 12: 655–664. [DOI] [PubMed] [Google Scholar]
- Tang, DQ, Cao, LZ, Chou, W, Shun, L, Farag, C, Atkinson, MA et al. (2006). Role of Pax4 in Pdx1-VP16-mediated liver-to-endocrine pancreas transdifferentiation. Lab Invest 86: 829–841. [DOI] [PubMed] [Google Scholar]
- Tang, DQ, Lu, S, Sun, YP, Rodrigues, E, Chou, W, Yang, C et al. (2006). Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors. Lab Invest 86: 83–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver-Krasinski, JM and Stoffers, DA (2008). On the origin of the beta cell. Genes Dev 22: 1998–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardo, AS, Hay, CW and Docherty, K (2008). Pancreatic transcription factors and their role in the birth, life and survival of the pancreatic beta cell. Mol Cell Endocrinol 294: 1–9. [DOI] [PubMed] [Google Scholar]
- Liu, Z, Kim, W, Chen, Z, Shin, YK, Carlson, OD, Fiori, JL et al. (2011). Insulin and glucagon regulate pancreatic α-cell proliferation. PLoS One 6: e16096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahier, J, Goebbels, RM and Henquin, JC (1983). Cellular composition of the human diabetic pancreas. Diabetologia 24: 366–371. [DOI] [PubMed] [Google Scholar]
- Yoon, KH, Ko, SH, Cho, JH, Lee, JM, Ahn, YB, Song, KH et al. (2003). Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J Clin Endocrinol Metab 88: 2300–2308. [DOI] [PubMed] [Google Scholar]
- Zhang, Y, Zhang, Y, Bone, RN, Cui, W, Peng, JB, Siegal, GP et al. (2012). Regeneration of pancreatic non-β endocrine cells in adult mice following a single diabetes-inducing dose of streptozotocin. PLoS One 7: e36675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorel, F, Népote, V, Avril, I, Kohno, K, Desgraz, R, Chera, S et al. (2010). Conversion of adult pancreatic alpha-cells to beta-cells after extreme beta-cell loss. Nature 464: 1149–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujitani, Y, Kajimoto, Y, Yasuda, T, Matsuoka, TA, Kaneto, H, Umayahara, Y et al. (1999). Identification of a portable repression domain and an E1A-responsive activation domain in Pax4: a possible role of Pax4 as a transcriptional repressor in the pancreas. Mol Cell Biol 19: 8281–8291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang, D, Powell, SK, Plummer, RS, Young, KP and Ruggeri, BA (2007). PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol 73: 1–14. [DOI] [PubMed] [Google Scholar]
- Brun, T and Gauthier, BR (2008). A focus on the role of Pax4 in mature pancreatic islet beta-cell expansion and survival in health and disease. J Mol Endocrinol 40: 37–45. [DOI] [PubMed] [Google Scholar]
- Collombat, P, Mansouri, A, Hecksher-Sorensen, J, Serup, P, Krull, J, Gradwohl, G et al. (2003). Opposing actions of Arx and Pax4 in endocrine pancreas development. Genes Dev 17: 2591–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood, AL, Li, S, Jones, K and Melton, DA (2007). Notch signaling reveals developmental plasticity of Pax4(+) pancreatic endocrine progenitors and shunts them to a duct fate. Mech Dev 124: 97–107. [DOI] [PubMed] [Google Scholar]
- Sosa-Pineda, B, Chowdhury, K, Torres, M, Oliver, G and Gruss, P (1997). The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature 386: 399–402. [DOI] [PubMed] [Google Scholar]
- Brun, T, Franklin, I, St-Onge, L, Biason-Lauber, A, Schoenle, EJ, Wollheim, CB et al. (2004). The diabetes-linked transcription factor PAX4 promotes {beta}-cell proliferation and survival in rat and human islets. J Cell Biol 167: 1123–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo, W, Endo, M, Ishizu, K, Nakamura, A and Tajima, T (2011). A novel PAX4 mutation in a Japanese patient with maturity-onset diabetes of the young. Tohoku J Exp Med 223: 113–118. [DOI] [PubMed] [Google Scholar]
- Kooptiwut, S, Plengvidhya, N, Chukijrungroat, T, Sujjitjoon, J, Semprasert, N, Furuta, H et al. (2012). Defective PAX4 R192H transcriptional repressor activities associated with maturity onset diabetes of the young and early onset-age of type 2 diabetes. J Diabetes Complications 26: 343–347. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis, F, Smith, SB, Le May, C, Leal, SM, Gautier, JF, Molokhia, M et al. (2004). PAX4 gene variations predispose to ketosis-prone diabetes. Hum Mol Genet 13: 3151–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plengvidhya, N, Kooptiwut, S, Songtawee, N, Doi, A, Furuta, H, Nishi, M et al. (2007). PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab 92: 2821–2826. [DOI] [PubMed] [Google Scholar]
- Shimajiri, Y, Sanke, T, Furuta, H, Hanabusa, T, Nakagawa, T, Fujitani, Y et al. (2001). A missense mutation of Pax4 gene (R121W) is associated with type 2 diabetes in Japanese. Diabetes 50: 2864–2869. [DOI] [PubMed] [Google Scholar]
- Sujjitjoon, J, Kooptiwut, S, Chongjaroen, N, Tangjittipokin, W, Plengvidhya, N and Yenchitsomanus, PT (2015). Aberrant mRNA splicing of paired box 4 (PAX4) IVS7-1G>A mutation causing maturity-onset diabetes of the young, type 9. Acta Diabetol (epub ahead of print). [DOI] [PubMed]
- Chapla, A, Mruthyunjaya, MD, Asha, HS, Varghese, D, Varshney, M, Vasan, SK et al. (2015). Maturity onset diabetes of the young in India - a distinctive mutation pattern identified through targeted next-generation sequencing. Clin Endocrinol (Oxf) 82: 533–542. [DOI] [PubMed] [Google Scholar]
- Collombat, P, Xu, X, Ravassard, P, Sosa-Pineda, B, Dussaud, S, Billestrup, N et al. (2009). The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into alpha and subsequently beta cells. Cell 138: 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hasani, K, Pfeifer, A, Courtney, M, Ben-Othman, N, Gjernes, E, Vieira, A et al. (2013). Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell 26: 86–100. [DOI] [PubMed] [Google Scholar]
- Bosco, D, Armanet, M, Morel, P, Niclauss, N, Sgroi, A, Muller, YD et al. (2010). Unique arrangement of alpha- and beta-cells in human islets of Langerhans. Diabetes 59: 1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissova, M, Fowler, MJ, Nicholson, WE, Chu, A, Hirshberg, B, Harlan, DM et al. (2005). Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem 53: 1087–1097. [DOI] [PubMed] [Google Scholar]
- Henquin, JC and Rahier, J (2011). Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia 54: 1720–1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan, Y, Orci, L, Malaisse-Lagae, F, Perrelet, A, Patel, Y and Unger, RH (1982). Quantitation of endocrine cell content in the pancreas of nondiabetic and diabetic humans. Diabetes 31: 694–700. [DOI] [PubMed] [Google Scholar]
- Bone, RN, Icyuz, M, Zhang, Y, Zhang, Y, Cui, W, Wang, H et al. (2012). Gene transfer of active Akt1 by an infectivity-enhanced adenovirus impacts β-cell survival and proliferation differentially in vitro and in vivo. Islets 4: 366–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi, H, Yamato, E, Tashiro, F, Ikegami, H, Ogihara, T and Miyazaki, J (2003). beta-cell neogenesis induced by adenovirus-mediated gene delivery of transcription factor pdx-1 into mouse pancreas. Gene Ther 10: 15–23. [DOI] [PubMed] [Google Scholar]
- Gage, BK, Baker, RK and Kieffer, TJ (2014). Overexpression of PAX4 reduces glucagon expression in differentiating hESCs. Islets 6: e29236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, HV, Jørgensen, MC, Andersen, FG, Jensen, J, F-Nielsen, T, Jørgensen, R et al. (2000). Pax4 represses pancreatic glucagon gene expression. Mol Cell Biol Res Commun 3: 249–254. [DOI] [PubMed] [Google Scholar]
- Ritz-Laser, B, Estreicher, A, Gauthier, BR, Mamin, A, Edlund, H and Philippe, J (2002). The pancreatic beta-cell-specific transcription factor Pax-4 inhibits glucagon gene expression through Pax-6. Diabetologia 45: 97–107. [DOI] [PubMed] [Google Scholar]
- Smith, SB, Ee, HC, Conners, JR and German, MS (1999). Paired-homeodomain transcription factor PAX4 acts as a transcriptional repressor in early pancreatic development. Mol Cell Biol 19: 8272–8280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collombat, P, Hecksher-Sørensen, J, Broccoli, V, Krull, J, Ponte, I, Mundiger, T et al. (2005). The simultaneous loss of Arx and Pax4 genes promotes a somatostatin-producing cell fate specification at the expense of the alpha- and beta-cell lineages in the mouse endocrine pancreas. Development 132: 2969–2980. [DOI] [PubMed] [Google Scholar]
- Campbell, SC, Cragg, H, Elrick, LJ, Macfarlane, WM, Shennan, KI and Docherty, K (1999). Inhibitory effect of pax4 on the human insulin and islet amyloid polypeptide (IAPP) promoters. FEBS Lett 463: 53–57. [DOI] [PubMed] [Google Scholar]
- Chen, L, Zhang, J, Zhang, Z, Chu, Y, Song, B and Cai, W (2015). Pax4 Expression does not transduce pancreatic alpha cells to beta cells. Cell Physiol Biochem 36: 1735–1742. [DOI] [PubMed] [Google Scholar]
- Funston, GM, Kallioinen, SE, de Felipe, P, Ryan, MD and Iggo, RD (2008). Expression of heterologous genes in oncolytic adenoviruses using picornaviral 2A sequences that trigger ribosome skipping. J Gen Virol 89: 389–396. [DOI] [PubMed] [Google Scholar]
- Luke, GA, de Felipe, P, Lukashev, A, Kallioinen, SE, Bruno, EA and Ryan, MD (2008). Occurrence, function and evolutionary origins of ‘2A-like' sequences in virus genomes. J Gen Virol 89: 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, T, Tang, Y, Ugai, H, Perry, LE, Siegal, GP, Contreras, JL et al. (2007). Genetic incorporation of the protein transduction domain of Tat into Ad5 fiber enhances gene transfer efficacy. Virol J 4: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Malley, TJ, Fava, GE, Zhang, Y, Fonseca, VA and Wu, H (2014). Progressive change of intra-islet GLP-1 production during diabetes development. Diabetes Metab Res Rev 30: 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expression of α- or β-cell specific transcription factors following Ad5.Pax4 infection of αTC1.9 cells.
Analysis of α:β cell ratio in human islets treated with Ad5.Pax4.