Abstract
Mucopolysaccharidosis VII (MPS VII) is a lysosomal storage disease arising from mutations in β-d-glucuronidase (GUSB), which results in glycosaminoglycan (GAG) accumulation and a variety of clinical manifestations including neurological disease. Herein, MPS VII dogs were injected intravenously (i.v.) and/or intrathecally (i.t.) via the cisterna magna with AAV9 or AAVrh10 vectors carrying the canine GUSB cDNA. Although i.v. injection alone at 3 days of age resulted in normal cerebrospinal fluid (CSF) GUSB activity, brain tissue homogenates had only ~1 to 6% normal GUSB activity and continued to have elevated GAG storage. In contrast, i.t. injection at 3 weeks of age resulted in CSF GUSB activity 44-fold normal while brain tissue homogenates had >100% normal GUSB activity and reduced GAGs compared with untreated dogs. Markers for secondary storage and inflammation were eliminated in i.t.-treated dogs and reduced in i.v.-treated dogs compared with untreated dogs. Given that i.t.-treated dogs expressed higher levels of GUSB in the CNS tissues compared to those treated i.v., we conclude that i.t. injection of AAV9 or AAVrh10 vectors is more effective than i.v. injection alone in the large animal model of MPS VII.
Introduction
Mucopolysaccharidosis type VII (MPS VII; Sly syndrome) is a rare lysosomal storage disorder arising from mutations in the β-glucuronidase gene (GUSB) and is inherited in an autosomal-recessive manner.1,2 The GUSB enzyme is involved in the stepwise degradation of dermatan, heparan, and chondroitin sulfates, and its dysfunction results in the accumulation of these glycosaminoglycans (GAGs).3 Clinical manifestations are more severe in MPS VII patients with little or no residual GUSB activity and include intellectual disability, hydrocephalus, hepatosplenomegaly, coarse facial features, dysostosis multiplex, heart valve disorders, and corneal clouding.1,4 Although intellectual disability is a hallmark of central nervous system (CNS) involvement, the underlying cause of cognitive impairment in MPS VII patients is not well understood. Chronic inflammation of the brain is thought to play a substantial role in many lysosomal storage disorders with CNS involvement including the mucopolysaccharidoses.5,6 In MPS VII, impaired autophagy associated with GAG storage and the secondary accumulation of GM2 and GM3 gangliosides generate cellular stress that induces an aggressive inflammatory response leading to a cascade of degenerative processes.5
Few approved clinical therapies exist for the treatment of MPS VII. Hematopoietic stem cell transplantation of allogeneic normal cells or gene-modified autologous cells has reduced neurological symptoms in lysosomal storage disorder patients but still requires a conditioning procedure.7,8 Intravenous enzyme replacement therapy has recently been developed for MPS VII9,10,11 but will probably not prevent neurological disease due to the blood–brain barrier. Although direct CNS delivery with enzyme replacement therapy is currently being pursued in clinical trials for other types of MPS (clinical trials identifier #: NCT02060526, NCT00884949), recombinant enzyme can only provide a short-term effect, and repeated injections into the CNS will most likely be required. In contrast, gene therapy could provide long-term constitutive expression of a therapeutic gene and its protein product after a single dose. However, enzyme levels in brain were only 6% of normal in previous gamma retroviral-vector studies in neonatal MPS VII dogs that resulted in transduction of liver and high serum GUSB activity after intravenous (i.v.) administration.12,13 Similarly, systemic injection of other vectors has not been very efficient at preventing storage in the murine brain (reviewed in Ponder and Haskins14). Therefore, it is likely that delivery to the brain will be necessary to achieve an optimal effect.
Recently, adeno-associated viral (AAV) vectors with capsid proteins from serotype AAV9 and AAVrh10 (rh10) have demonstrated the ability to enter the brains and to transduce neurons and glia of animals following i.v. injection,15,16,17,18,19,20,21,22,23,24,25 intrathecal (i.t. injection into the cerebrospinal fluid (CSF) via the lateral ventricle or the cisterna magna, or direct brain parenchyma injections.15,18,23,24,25,26,27,28 However, a direct comparison of the efficacy of different serotypes given i.v. or i.t. has not been performed in canines, and the effect on a canine model of disease has not been determined. Therefore, our study aimed to discover the distribution of AAV-mediated GUSB expression in the CNS of dogs with naturally occurring MPS VII due to a missense mutation (R166H),29 and the effects on CNS lesions following i.v. and/or i.t. delivery using AAV serotypes AAV9 and rh10. This study demonstrates that i.t. is superior to i.v. delivery in MPS VII dogs, but i.v. injection is still able to reduce some biochemical and histochemical markers of disease.
Results
Intravenous and intrathecal AAV9 and rh10 vectors were well tolerated
MPS VII dogs were treated with AAV vectors as detailed in Table 1 and as described in the Materials and Methods. Animals designated as “i.v.-only” received i.v. injection of vector at 3 days of age, “i.t.-only” dogs received i.t. injection of vector via the cisterna magna at 3 weeks of age, and “i.v.+i.t.” dogs received i.v. injection of one vector at 3 days, and i.t. injection of the alternate vector at 2 months of age. CSF protein and cell counts were determined at 9 days postinjection and again at the end of the study period and were within normal limits for all dogs (data not shown). All dogs were negative for anticapsid antibodies to AAV9 and rh10 in both serum and CSF at the start of the study (Supplementary Tables S1 and S2; Day 0). All treated dogs generated antibodies against the serotype used for treatment, which were much higher in serum than CSF regardless of the route of administration (Supplementary Table S1). Cross-reactive responses were also noted in serum in single-serotype treated dogs. Intriguingly, anticapsid antibodies found in the CSF of all i.t.-treated dogs did not indicate cross-reaction, and higher responses were observed against the rh10 than the AAV9 capsid (Supplementary Table S2).
Table 1. Study design and injection scheme of MPS VII dogs with recombinant AAV vectors.

Supraphysiological GUSB activity was found in CSF of i.t.-injected MPS VII dogs
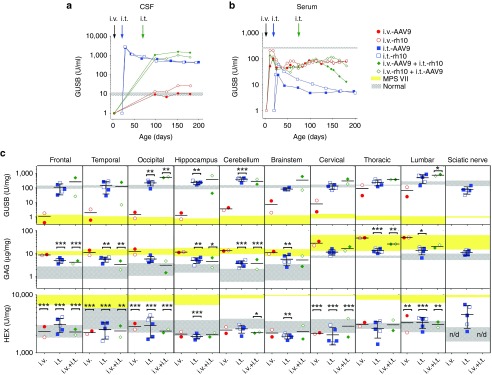
All i.v.-only dogs had CSF GUSB activity ~1.7-fold normal at 6 months postinjection (Figure 1a, red circles), and activity was relatively stable over the study period. In contrast, i.t.-only dogs had >250-fold normal CSF GUSB activity (normal CSF mean ~10.7 U/ml) at peak expression, approximately 7 days postinjection (Figure 1a, blue squares). Activity declined slowly, resulting in average CSF GUSB activity of ~45-fold normal (445 ± 254 U/ml) and ~42-fold normal (419 ± 151 U/ml), for AAV9 and rh10, respectively, at the end of the study. There were no differences between AAV9 and rh10 for i.t.-only dogs in levels of CSF activity or stability of expression (data not shown). i.v.+i.t.-treated dogs exhibited a slightly higher CSF activity at 6 months, with 137- and 81-fold normal levels for i.v.-AAV9+i.t.-rh10 and i.v.-rh10+i.t.-AAV9, respectively (Figure 1a, green diamonds). It is well known that circulating enzyme modified with mannose 6-phosphate (M6P) can be taken up by the M6P receptor on cells.30 The measured percentage of phosphorylated active CSF GUSB enzyme in the i.t.-only dogs was 40.8 ± 8.1%, which was not significantly different from values in normal dogs at 64.9 ± 4.6%.
Figure 1.
Circulating GUSB activity and biochemical analysis of nervous tissue. Nine MPS VII dogs were injected i.v.-only, i.t.-only, or i.v.+i.t. with AAV9 or rh10 vectors expressing the canine GUSB cDNA as detailed in Table 1. (a,b) CSF and serum were tested for GUSB activity at the indicated ages. Data are shown for individual animals except for i.t.-only dogs, where the means are plotted for each serotype (i.t.-AAV9, n = 3; i.t.-rh10, n = 2). The average value in normal dogs ± 1 SD is shown as a horizontal gray bar (n = 9), while untreated MPS VII dogs had <1 U/ml of GUSB activity (data not shown). Early CSF time points were not collected for i.v.-treated animals. (c) CNS tissues and peripheral nerve were collected at 6 months of age and tested for GUSB activity, GAG level, and HEX activity. The means of duplicates are recorded for individual animals for all assays, and the means of duplicates ± 1 SD are indicated with error bars for all i.t.-only dogs (n = 5; data for AAV9 and rh10 were pooled together). Values for normal dogs ± 1 SD (gray-thatched horizontal bars, n = 3 to 9) and untreated MPS VII dogs (yellow horizontal bars, n = 3 to 7) are shown for each tissue. Black bars indicate the mean for each group. Arrows above the graph indicate when AAV-injections were given in reference to age and are as follows: i.v.-AAV (black arrow) at 3 days of age for i.v.-only and i.v.+i.t. dogs, i.t.-AAV (blue arrow) at 21 days of age for i.t.-only dogs, and i.t.-AAV (green arrow) at 70 days of age for the i.v.+i.t. dogs. Statistical analysis was performed as indicated in Materials and Methods. Values in other groups were compared with those in untreated MPS VII dogs, and *P = 0.01–0.05; **P = 0.001–0.01; ***P < 0.001.
Serum GUSB activity was also determined. Following i.v. injections, GUSB activity was ~30% of normal at 6 months of age in i.v.-only dogs. In contrast, serum GUSB activity of i.t.-only dogs was only ~1.7% of normal activity (Figure 1b). i.v.+i.t.-injected MPS VII dogs had ~12% of normal serum GUSB activity (Figure 1b).
Normal GUSB activity and reduced GAG storage was found in the nervous tissue of i.t.-treated dogs
CNS tissue was harvested from all treated dogs at 6 months of age and biochemical assays performed on homogenates for GUSB activity, GAG levels, and total HEX activity. These data were compared with untreated MPS VII and normal control tissues. i.v.-only dogs had low GUSB activity in brain with <2% normal activity in the frontal, temporal, and occipital lobes and hippocampus (Figure 1c, top row, red circles). In comparison, i.t.-only dogs had average GUSB activity in cerebrum that was >95% of normal activity (Figure 1c, top row, blue squares). Deeper structures such as the hippocampus (195% normal) and brainstem (125% normal) also had elevated activity levels for i.t.-only dogs, which was not surprising given the close proximity of these structures to the ventricles and surrounding CSF. Results from i.v.+i.t. dogs resembled those found in i.t.-only dogs (Figure 1c, top row, green diamonds).
An increase in GAG concentration is a hallmark of the MPS disorders.12 To assess a therapeutic benefit, total GAG was measured. i.v.-only dogs had elevated GAGs compared to normal dogs (Figure 1c, middle row), which was significant in the frontal lobe (P < 0.001), temporal lobe (P = 0.002), and brainstem (P = 0.006) and was not significantly lower than untreated MPS VII dogs in any region evaluated. In contrast to i.v.-only dogs, all CNS tissue collected from i.t.-only dogs exhibited lower GAG levels than untreated MPS VII dogs, which was significant in the frontal (P < 0.001) and temporal (P = 0.004) lobes, the hippocampus (P = 0.004), and the brainstem (P = 0.007) (Figure 1c). However, GAGs in i.t.-only dogs still remained elevated compared to normal dogs in most brain tissues and was significantly higher compared to normal in the frontal lobe (P = 0.004). Again, results in i.v.+i.t.-treated dogs resembled those of i.t.-only dogs.
Elevation of other lysosomal enzymes such as HEX is another hallmark of MPS. Intriguingly, total HEX activity was reduced in the frontal, temporal, and occipital lobes for the i.v.-only dogs (Figure 1c, bottom row, red circles; P < 0.001 for all regions) and thus appeared to serve as a sensitive correlate for low levels of GUSB activity. HEX activity was also reduced in the i.t.-only and i.v.+i.t. dogs.
GUSB activity is widely distributed in the cerebrum of i.t.-injected MPS VII dogs
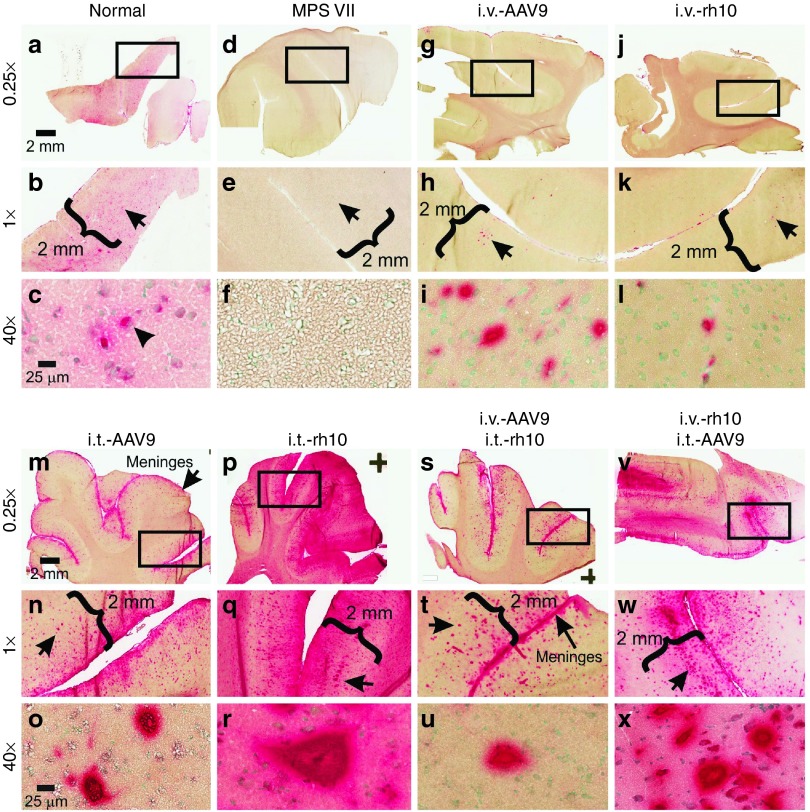
To examine the distribution of enzyme activity, a histochemical GUSB activity stain was used. Normal dogs had diffuse activity throughout the cerebral cortex (Figure 2a,b) with activity found in the soma of cortical neurons (Figure 2c, solid arrowhead), while untreated MPS VII dogs were devoid of GUSB activity (Figure 2d–f). Both i.v.-only dogs had rare cortical neurons with substantial GUSB activity, which likely represented transduced cells (Figure 2g–l), but their frequency was estimated to be only ~0.1%. GUSB activity was also identified in the leptomeninges.
Figure 2.
GUSB stain of cerebral cortex. (a-x) Frozen sections of cerebrum from level R3 (Supplementary Figure S1) were stained for GUSB activity (red color) for samples collected at 6 months of age from animals treated as detailed in Table 1. The boxes in the top rows indicate the region shown at ×1 magnification, and the arrow in the middle rows indicate the region shown at higher power in the lower rows (at ×40). Scale bars are indicated.
In contrast, i.t.-only dogs displayed widespread GUSB activity in cerebral gray matter (Figure 2m,p). Staining extended into all layers of the cerebral cortex (layers I–VI) with intense focal activity in large pyramidal neurons (Figure 2n, o, q, and r). Transduction of neurons, likely in layer V, was more extensive in the i.t.-rh10 dogs (Figure 2n,q, black arrows) than for the i.t.-AAV9 dogs, which is shown for several regions of the cortex in Supplementary Figure S1. GUSB staining in the i.v.+i.t. dogs (Figure 2s–x) resembled that of i.t.-only dogs. Supplementary Figures S2–S7 demonstrate that GUSB activity in the cortex is similar among the lobes evaluated and shows global activity for all of the treated dogs.
Considering the importance of restoring function to all regions of the brain, deeper sections of the brain were also analyzed for GUSB activity, as shown in Supplementary Figures S8–S10. i.v.-only dogs had detectable GUSB activity in the caudate nucleus (Supplementary Figure S8), the midbrain near the aqueduct (Supplementary Figure S9), the crus cerebri (Supplementary Figure S9), and CA3 neurons of the hippocampus (Supplementary Figure S9). In the brainstem (Supplementary Figure S10), neuronal cell bodies positive for GUSB activity were scattered throughout gray matter, although specific nuclei were not identified. In addition, i.v.-only dogs had GUSB activity in the choroid plexus (Supplementary Figure S10), and along axons in the white matter (Supplementary Figure S10). The i.t.-only dogs had similar GUSB activity as the i.v.-only dogs in the caudate, midbrain near the aqueduct, brainstem, choroid plexus, and white matter, but clearly had much higher GUSB activity in the optic tract, crus cerebri, and hippocampus (Supplementary Figures S8–S10).
GUSB activity in the cerebral cortex was associated with reduced storage of secondary compounds and reduced inflammatory cell infiltrate
The accumulation of undegraded metabolites in many of the MPS disorders is associated with persistent activation of the lysosomes, astrogliosis, and inflammation.5 To assess preliminary therapeutic effects of AAV-mediated GUSB expression on storage material, sections of cerebral cortex were analyzed for GM3 ganglioside, lysosomes, astrocytes, and microglia (Figure 3). GM3 ganglioside is a secondary storage material that accumulates in MPS VII brains31 and is apparent at 6 months of age in untreated MPS VII dogs (Figure 3b). Notably, low levels of GUSB enzyme in i.v.-only dogs were associated with a qualitative reduction in GM3 in cortical neurons (Figure 3c,d). In contrast, i.t.-only dogs showed near-complete resolution of GM3 ganglioside accumulation (Figure 3e,f). The intensity of LIMP2-positive vesicles was reduced in both i.v.- and i.t.-only dogs compared to those found in untreated MPS VII tissue, indicating reduced lysosomal distension (Figure 3g–l). Astrogliosis in the cortex was markedly increased in untreated MPS VII dogs as shown by increased staining for GFAP (Figure 3n). i.v.-only dogs showed a reduced intensity in GFAP staining (Figure 3op), while i.t.-only and i.v.+i.t. dogs were similar to normal control dogs (Figure 3m,q,r). Microglia (Iba1-positive cells) are still present in treated dogs (Figure 3u–x) but appear to be reduced compared to untreated MPS VII tissue (Figure 3t). Some Iba1-positive cells are present in normal animals (Figure 3s). Cerebral cortex staining for i.v.+i.t. dogs mirrors those patterns seen in i.t.-only dogs, and no serotype-specific differences were apparent (data not shown).
Figure 3.
Secondary storage, astrogliosis, and inflammatory responses are reduced in treated MPS VII cerebral cortex. Secondary storage was analyzed in cerebral cortex at 6 months of age by immunohistochemical staining in normal, untreated MPS VII (MPS VII), or MPS VII dogs that were treated with AAV vector of the indicated route and serotype. Staining was performed for a secondary storage ganglioside GM3 (a–f) lysosomal compartments were identified with lysosomal integral membrane protein LIMP2 (g–l) astrocytes were identified with glial fibrillary acidic protein (GFAP) (m–r) and microglial cells were identified with ionized calcium-binding adapter molecule 1 (Iba1) (s–x). Not all tissues were stained simultaneously, and many groups only had one dog, but normal and untreated control samples were performed for all batches of stains and appeared similar.
Purkinje cells exhibit GUSB activity in AAV-treated MPS VII dogs
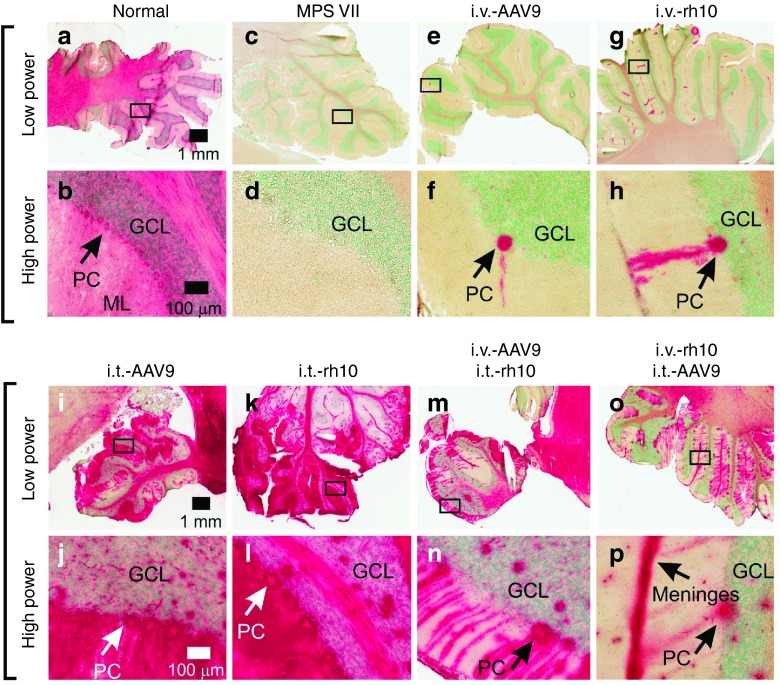
Cerebellar sections of a normal dog reveal diffuse GUSB activity in the molecular layer, Purkinje cells (PC), granular cell layer, and cerebellar white matter (Figure 4a,b). In stark contrast, MPS VII-affected dogs have no visible GUSB activity staining in the cerebellum (Figure 4c,d). Both i.v.-only dogs displayed sparse, focal activity in PCs and limited expression in the white matter (Figure 4e–h). i.t.-only dogs exhibited greatly enhanced expression throughout the cerebellar cortex (Figure 4i–l). The histochemical data correlate well with biochemical data, as GUSB activity was very high in the cerebellum at 3.1-fold normal in i.t.-only dogs, but only 0.03-fold normal in i.v.-only dogs (Figure 1c). Similar patterns were observed for the i.v.+i.t.-injected dogs (Figure 4m–p) as for the i.t.-only dogs.
Figure 4.
GUSB activity in the cerebellum. Cerebellar sections collected at 6 months of age from normal, untreated MPS VII, or MPS VII dogs that were treated with AAV vectors of the indicated route and serotype were stained for GUSB enzyme activity (red color). The region indicated with a box at low power is shown at high power below it. GUSB-positive Purkinje cells (PC) soma are indicated in some panels. The granular cell layer (GCL) and molecular layer (ML) are indicated. Scale bars are indicated.
High-level GUSB activity found in the spinal cord of all treated dogs and sciatic nerve of i.t.-treated dogs
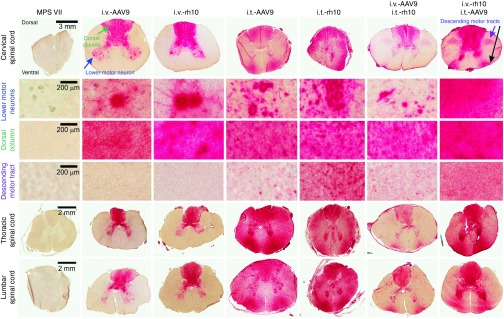
GUSB stain of spinal cord is shown in Figure 5. Untreated MPS VII dogs exhibited no GUSB activity throughout the spinal cord. i.v.-only dogs exhibited high-level GUSB activity throughout the gray matter, including strong focal staining in lower motor neurons of the ventral horn, regardless of the AAV serotype. This correlated with biochemical GUSB activity throughout the spinal cord ranging from 15% at the cervical cord to 59% at the lumbar cord (Figure 1c, top row). GUSB activity was also seen throughout the dorsal columns that house ascending sensory pathways. However, GAGs were elevated in the spinal cord in i.v.-only dogs compared with normal levels (Figure 1c; top versus middle row), which was significant in the thoracic (P < 0.001 versus normal, not significant versus untreated MPS VII) and the lumbar (P = 0.01 versus normal, not significant versus untreated MPS VII) spinal cord. Elevated GAGs may be due to the absence of GUSB activity throughout the rest of the spinal cord (Figure 5). i.t.-only dogs had similar GUSB activity with histochemical staining in the lower motor neurons and dorsal columns as did i.v.-only dogs, but i.t.-only dogs also had positive GUSB staining in the lateral and ventrolateral funiculi. Biochemical GUSB analysis revealed >98% normal activity throughout the spinal cord. This diffuse GUSB activity may explain why GAGs were lower in the spinal cord of i.t.-only dogs compared with untreated MPS VII dogs, which reached significance in the thoracic (P < 0.001) and lumbar (P = 0.04) regions (Figure 1c).
Figure 5.
GUSB activity of the spinal cord. Frozen sections from the cervical, thoracic, and lumbar spinal cord of animals of the indicated groups were stained for GUSB activity (red color). Some regions of the cervical spinal cord were further imaged at higher magnification to show the motor neuron pools (blue arrow), dorsal column (green arrow), and descending motor tracts from the lateral funiculus (purple arrow) as indicated. All cord segments were oriented in the same direction with dorsal at the top and ventral at the bottom. Scale bars are indicated.
i.v.+i.t. dogs had strong staining in the dorsal columns and lower motor neurons, as expected since i.v.-only dogs were positive in these regions. i.v.+i.t. dogs also had strong staining in the lateral corticospinal tract area (Figure 5, descending motor tracts), which was different from i.v.-only dogs. However, i.v.+i.t. dogs had relatively low levels of staining in the other regions of the spinal cord compared with i.t.-only dogs. There were no obvious differences in GUSB activity patterns between the two serotypes tested in this study.
Finally, the sciatic nerve also had enhanced levels of GUSB activity staining in i.t.-only dogs (Supplementary Figure S11), which correlated with the biochemical GUSB data determined at 34% normal (Figure 1c). GUSB staining was noted in myelin sheaths and potentially axons of the sciatic nerve (Supplementary Figure S11). Sciatic nerve samples were not collected from other groups.
Biodistribution identifies higher copy numbers in nervous tissue of i.t.-injected dogs
Vector copy numbers were determined with quantitative PCR on DNA extracted from tissues (Supplementary Figure S12). Low copy numbers were found in brain tissue of the two i.v.-only dogs (mean = 0.01 vector genomes/diploid genome (vg/dg)). Conversely, all i.t.-treated dogs had 100-fold higher average copy numbers in brain tissue (mean = 1 vg/dg), and similar values were identified among the different serotypes. In the spinal cord, vector copy numbers averaged >70-fold higher for i.t.-only compared with i.v.-only dogs. Systemic copy numbers were found at moderate to low levels for most tissues in all treated groups, with liver tissue having the highest copies. Here, average liver copies were 0.43 vg/dg for i.v.-only dogs and 0.13 vg/dg for i.v.+i.t. dogs. Despite the fact that vector was only delivered to the CNS, vector was also detected in liver in i.t.-only dogs at 0.07 vg/dg; vector was also identified in heart, lung, kidney, and spleen for the i.t.-only dogs. These data are consistent with the low serum GUSB activity found in the i.t.-only dogs (Figure 1b).
Intravenous AAV reduces storage in non-CNS tissues
None of the groups had a significant reduction in urine GAGs at 6 months of age compared with values in untreated MPS VII dogs (Figure 6a). However, i.v.-only dogs had a significant reduction in GAGs of the liver and renal medulla, and in HEX activity of liver, spleen, heart, and renal medulla (Figure 6b). In contrast, i.t.-only dogs did not have significant reductions in GAGs or total HEX in any somatic tissue that was analyzed (Figure 6b). Values in i.v.+i.t. dogs resembled those in i.v.-only dogs. GUSB activity staining confirmed the presence of active enzyme in liver and myocardium, confirming that biochemical activity was not due to blood contamination (Supplementary Figure S13). Thin sections indicated an absence of storage in liver and spleen of all i.v.-only and i.v.+i.t. dogs, with a partial reduction of storage lesions in i.t.-only dogs (Supplementary Figure S14).
Figure 6.
Systemic biochemical data. Samples were collected at 6 months of age. (a) Urine GAGs were normalized to the amount of creatinine in the sample using a dye-based binding assay. (b) Samples from liver, spleen, heart, and renal cortex (Renal C) and renal medulla (Renal M) were homogenized and tested for GUSB activity (top row), GAG levels (middle row), and HEX activity (bottom row). The means of duplicates are recorded for all assays for individual i.v.-only or i.v.+i.t.-treated dogs, while averages are shown for i.t.-only dogs ±1 SD (n = 5). Normal values ± 1 SD (gray-thatched horizontal bars, n = 9) and values for untreated MPS VII dogs ± 1 SD (yellow horizontal bars, n = 7) ranges are shown in all graphs. Values in the indicated groups were compared with values in untreated MPS VII dogs as detailed in Materials and Methods. *P = 0.01–0.05; **P = 0.001–0.01; ***P < 0.001.
Clinical outcome in treated dogs
Although behavioral abnormalities have been documented in MPS VII mice, cognitive deficits have yet to be characterized in the canine model due to difficulties inherent in behavioral testing performed in animals with limited mobility due to orthopedic disease.32,33 However, gait was evaluated in the treated dogs. While untreated MPS VII dogs were unable to stand or walk by 6 months of age (Supplementary Video S1), both i.v.-only dogs retained the ability to walk, albeit with mild (M2689) to moderate (M2686) gait abnormalities characterized by a shortened stride length and increased hock (tarsal joint) flexion of the hindlimb. The i.v.-treated dog M2689 could also run. Three of five i.t.-only dogs (M2729, M2760, and M2739) were able to walk, although each showed markedly abnormal hock flexion and none could run, and M2755 and M2757 could not stand. Both i.v.+i.t. dogs (M2685 and M2688) were able to run. Shortened stride length and excessive hock flexion may be due to either joint/orthopedic disease or peripheral nervous system dysfunction. No clear advantage among serotypes was seen.
Discussion
I.V.-only MPS VII dogs have low GUSB expression in the CNS
I.V.-only MPS VII dogs received i.v. injection of 2E13 GC/kg of AAV vectors at day 3 of age, which resulted in limited GUSB expression by histochemical stains in cortical and hippocampal neurons, and Purkinje cells, while average expression levels in other brain tissues were only ~1 to 6% of normal with quantitative assays of homogenates. Enzyme activity for the AAV9-treated dog was similar to that of the rh10-treated dog, although the small number of animals (N = 1) would make it difficult to identify small differences in efficacy. Higher doses were not given due to limited vector quantities. Our results differ from a study that reported very high (30% of normal) expression of N-acetylglucosaminidase-α in nonhuman primate CNS tissue after i.v. administration of an AAV9 vector,34 although quantification in this other study was confounded by the background enzyme activity present in these normal animals. Several other studies in nonhuman primates support our result that i.v. administration is not very efficient in large animals.15,20,28 It is also possible that accumulation of GAGs in the endothelium of blood vessels may inhibit the ability of AAV9 vectors to cross the blood–brain barrier, as has been seen in MPS VII mice.35 However, this phenomenon has yet to be established in the MPS VII dogs. Although CNS GUSB activity was low and CNS GAG levels were not reduced in i.v.-only dogs, lysosomal HEX activity was normalized, and there was a reduction in GM3, LIMP2, GFAP, and Iba1 staining in the cortex. These results demonstrate that relatively low levels of enzyme can indeed reduce markers of disease in the MPS VII brain, which is consistent with previous studies in MPS VII dogs with a gamma retroviral vector.13,36
In the spinal cord, motor neurons were clearly transduced in i.v.-only dogs, which is consistent with the relatively efficient transduction of motor neurons with AAV9 and rh10 vectors after i.v. injection.15,16,21,37 There was a progressive increase in GUSB activity from the cervical to the lumbar region of the spinal cord for unclear reasons, as has been noted in other studies.16,23
i.t.-only dogs have widespread GUSB activity in CNS tissues
i.t.-only dogs received 5E12 GC/kg of vector at 3 weeks of age via the cisterna magna, which resulted in supraphysiological expression throughout the brain and spinal cord. Histochemical staining revealed GUSB activity in neurons as deep as 2 mm from the surface of the cortex and in Purkinje cells, while quantitative enzyme assays reported near-normal GUSB levels in CNS tissues at 6 months of age. Enzyme activity was also high in neurons of the hippocampus and unidentified nuclei of the brainstem, and these transduced neurons may transport enzyme to other parts of the brain via their axons. There was no significant difference in activity between AAV9 and rh10-treated dogs in quantitative GUSB assays. Although it appeared that the rh10 serotype might result in better transduction of neurons in Layer V of the cortex than the AAV9 serotype, too few dogs were evaluated to be conclusive, and both serotypes resulted in transduced cells in the deeper layers. Hinderer et al.27 also found high expression using an i.t.-AAV9 vector, which generated fivefold normal α-l-iduronidase levels in the CNS of a feline model of Hurler syndrome.
Although GUSB activity was near normal in all regions studied and storage was eliminated in the cortex of i.t.-only dogs as assessed by GM3 and LIMP2 stain, total GAG levels were not normalized throughout the nervous system tissues. It is likely that some regions of the brain may fail to achieve sufficient enzyme activity to eliminate GAG accumulation. Indeed, this study showed that the caudate nucleus and some other deep regions had relatively low GUSB activity with histochemical stains. Furthermore, some regions of the cortex and cerebellum were transduced less efficiently than other regions of the same tissue for individual dogs, which may relate to the ability of CSF to freely diffuse to the surface of a particular region. We are currently performing more detailed histochemical evaluations to determine if there are regions that accumulate storage. This study also showed that astrogliosis (GFAP) and activation of microglial cells (Iba1) was eliminated in the cortex of i.t.-only dogs. We are currently testing if reduction of these markers is seen throughout all areas of the brain.
i.t.-only dogs had high GUSB activity throughout the spinal cord, and a reduction in GAG levels compared with untreated MPS VII dogs. Transduction of lower motor neurons was likely responsible for the enzyme activity seen in some axons of the sciatic nerve, while activity in the surrounding myelin sheaths could be due to uptake from neurons that secrete enzyme or to direct transduction of Schwann cells; further studies will need to differentiate between these possibilities. Overall, i.t.-only and i.v.+i.t. dogs had much higher activity in the spinal cord white matter tract regions than did i.v.-only dogs. Although specific areas were not identified, the areas with high enzyme activity likely include the lateral corticospinal tract containing axons from motor neurons of the cortex, and possibly the rubrospinal tract containing axons from neurons of the red nucleus in the midbrain for i.v.+i.t.-only dogs, while i.t.-only dogs had diffuse staining throughout the spinal cord white matter with higher activity at the edges of the tissue, coinciding with ascending tracts.
Effects of i.v.+i.t. treatment
This study showed that a single i.t. injection of either an AAV9 or rh10 vector at 3 weeks of age produced high levels of GUSB activity in the CSF and neuronal tissues of MPS VII dogs, but relatively low levels in serum, and only three of five i.t.-only dogs could walk at 6 months. In contrast, i.v. injections at 2 days of age resulted in greater systemic GUSB expression than i.t. administration, and both i.v.-only treated dogs could run at 6 months. In an attempt to treat both systemic and neurological disease, two dogs received both i.v. (at day 3) and i.t. (at 2 months with the other serotype) injections of AAV. Transduction still occurred in the CNS, and indeed, CSF GUSB in the i.v.+i.t. dogs was slightly higher after the 6-month study period in comparison to the i.t.-only dogs. This may relate to the older age and larger body size when treated at 2.3 months of age, leading to the total vector particles administered that was threefold that used for the i.t.-only group treated at 3 weeks of age. Here, the i.v.+i.t. group benefited from dual treatment, as the CNS values mimicked the i.t.-only dogs' GUSB levels and conversely, peripheral tissue values mirrored those seen in i.v.-only dogs and both dogs maintained mobility throughout the study. Peripheral tissue correction levels were also nearly identical in i.v.-only and i.v.+i.t. dogs, indicating that later i.t.-injections had no adverse effects on peripheral activity after i.v.-AAV injections. Although a slight reduction in urine GAGs was seen in i.v.-treated dogs, overall urine GAG levels were not corrected in any treatment group. Indeed, even high GUSB serum levels in RV-treated dogs failed to completely correct urine GAGs.38 Similar vector copy numbers were also identified in all of the CNS tissues tested among i.t.-only and i.v.+i.t. groups (Supplementary Figure S12) indicating the feasibility of i.t. injections after i.v. administration if a different serotype is used. These data support that whole body correction may be obtained with i.v.+i.t. treatments using different AAV vectors, although more detailed studies on peripheral tissues need to be done to establish the extent of improvement.
Expression is relatively stable in CSF for six months
In this study, i.v.-treated dogs had relatively stable GUSB activity at near-normal levels in CSF for six months, which is likely due to transduction of ependymal cells of the choroid plexus, with a possible contribution from transduced neurons. Similarly, i.t.-only dogs had relatively stable GUSB activity, although the level was much higher at 44-fold normal, expression from leptomeningeal cells probably occurred, and the contribution from neurons was much higher. Since dogs reach maturity at 6 months, and there is little replication in the adult brain, expression from the AAV vectors will likely be maintained long-term after i.t. administration. Indeed, we have now followed one dog for 16 months after i.t. injection and observed stable expression in the CSF (BL Gurda and ME Haskins, unpublished data).
Conclusions
In this study, we demonstrate that i.t. injection of recombinant AAV9 and rh10-serotyped vectors results in ~50-fold higher expression and a more profound effect on markers of disease than does treatment with the same vector given i.v.. Although i.v. administration was less efficient, it still reduced a secondary lysosomal enzyme and markers of astrogliosis and neuroinflammation and may exert a beneficial effect on neurological function, an aspect that was not studied here. In disease models with low therapeutic target levels, i.v.-only injections could potentially provide sufficient delivery to the CNS to achieve a therapeutic effect. However, in the case of MPS VII where high tissue activity is necessary, it appears that i.t. injections will be necessary for efficient treatment in the CNS. Overall, the therapeutic outcome and preliminary safety profiles generated here support further studies to advance AAV gene therapy for MPS VII and other CNS disorders into a clinical setting.
Materials and Methods
Animals, AAV vector injections, and CSF collections. The MPS VII dog colony is maintained at the National Referral Center for Animal Models of Human Genetic Disease at the School of Veterinary Medicine of the University of Pennsylvania. All protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pennsylvania. For i.t. injections via the cisterna magna, the suboccipital region of propofol-anesthetized animals was punctured with a 22 gauge spinal needle. Following collection of 1–2 ml of CSF by gravity, 1–2 ml of vector in 0.9% sterile saline was injected over 1–2 minutes. Intravenous injections (1–2 ml volumes over 1–2 minutes) were performed via the cephalic vein. Treated dogs were sampled weekly for the first month and then monthly for the remainder of the study. For repeated CSF collections, dogs were anesthetized with propofol and 0.5–1 ml of CSF was collected by gravity flow from the cisterna magna as detailed above. After all procedures, the animals were visually monitored closely over the next 48 hours. At the end of the study (6 months of age) euthanasia was performed with an i.v. overdose of pentobarbital (>80 mg/kg).
Treatment groups and study design. Animals designated “i.v.-only” received an i.v. injection of AAV9 (dog M2686) or rh10 (dog M2689) at 3 days of age. Animals designated “i.t.-only” received an i.t. injection of AAV9 (dogs M2739, M2757, and M2760) or rh10 (dogs M2729 and M2755) at 3 weeks of age. Animals designated “i.v.+i.t.” received i.v. injection of one vector at 3 days of age and i.t. injection of the alternate vector at 2.3 months of age as detailed in Table 1. No adverse events were identified after i.v. or i.t. injection. Animals were euthanized at 6 months of age.
Vector production. The vector genome encoded AAV2 i.t.Rs flanking a transcription unit containing the canine cDNA of GUSB29 under the control of a chicken β-actin promoter. The promoter used contained the cytomegalovirus enhancer with the β-actin promoter and a synthetic intron, which are 100% identical with nucleotide 721–2454 of Genbank39 accession number GI:388594935. The i.t.Rs were from AAV2 and are 100% identical with nucleotide 4660 to 4493 of GI:209616 (ref. 40). The plasmid was cross-packaged by the Institute of Human Gene Therapy at the University of Pennsylvania into AAV9 (ref. 41) and rh10 (ref. 42) capsids resulting in two recombinant viral vectors each containing the same single-stranded AAV2-genome, and large-scale vector preparations were generated as previously described.41
Neutralizing antibody titers. Neutralizing antibody assays were performed on Huh7 cells as previously described.43 Assays on CSF samples used sterile PBS as a transduction control. The lowest dilution for the assay was usually 1:5 for serum and CSF, although some samples began with a 1:10 dilution due to low sample volume.
DNA extraction and biodistribution studies. Total DNA from tissues was extracted using a QIAamp DNA Mini Kit (Qiagen, Valencia, CA) and from ethylenediaminetetraacetic acid–treated blood samples using the QIAamp DNA Blood Mini Kit (Qiagen) following the manufacturer's recommended protocol. Real-time PCR (TaqMan Universal Master Mix; Applied Biosystems, Foster City, CA) was used to detect and quantify vector genomes in total DNA extracted from frozen samples as previously described,44 using primer and probe sets targeted to sequences within the nRBG polyadenylation signal (forward primer 5′-GCCAAAAATTATGGGGACAT-3′, reverse primer 5′-ATTCCAACACACTATTGCAATG-3′, probe 6FAM-ATGAAGCCCCTTGAGCATCTGACTTCT-TAMRA).
Tissue processing and protein determination. Tissue were homogenized as detailed in the Supplementary Materials and Methods section in sample buffer (0.9% NaCl, 0.2% Triton), and the supernatant of the lysate was used for protein, enzyme, and GAG assays.
Enzyme assay. Five to ten microliters of tissue homogenate, serum, or CSF was incubated in 200 μl of total volume with the appropriate substrate for 1 to 3 hours at 37 °F, and the reactions stopped with 2 ml of stop buffer (0.32 mol/l of glycine, 0.2 mol/l of sodium carbonate, pH 10.5) as detailed in the supplemental methods. The fluorescence of the product (4-methylumbelliferyl (4-MU)) was measured with the VersaFluor Fluorometer (Bio-Rad) at an excitation wavelength of 365 nm and an emission wavelength of 450 nm. The enzyme activity was calculated from the standard curve of the 4-MU product of the reaction, with 1 unit defined as 1 nmole of substrate converted to product in 1 hour at 37 °F.
Glycosaminoglcyan assays. Tissue GAGs were assessed using the Blyscan sulfated GAG assay (Biocolor, Carrickfergus, Antrim, UK) using the manufacturer's suggested protocol with chondroitin-4-sulfate as the standard (Sigma, St Louis, MO). Depending on the tissue origin and genotype, 10 to 50 µl of homogenate were assayed. The final value was calculated using the standard curve and normalized to protein concentration.
Mannose phosphorylation levels on GUSB. Soluble cation-independent mannose 6-phosphate receptor was coupled to affinity column media as previously described,45 and samples were applied to an equilibrated column, washed, and eluted with M6P as detailed in the Supplementary Materials and Methods. GUSB activity in unbound and bound fractions was determined using 4MU-β-glucuronide fluorescent substrate. These values were then used to calculate the percentage of mannose phosphorylation on GUSB enzyme.
Tissue preparation and histology. Whole brain and spinal cord were extracted immediately following euthanasia. The brain was sectioned in the coronal plane into left and right hemispheres. Five transverse sections were evaluated from the frontal lobe (R0; Supplementary Figure S1) to the occipital lobe (R5). Pieces from the right hemisphere were frozen in optimal cutting temperature embedding media for cryosectioning, and sections were fixed and stained for GUSB activity as previously described46 using an overnight incubation followed by a nuclear counterstain with methyl green from Vector Labs (Burlingame, CA). Sections from the left hemisphere were used for biochemistry and DNA copy analysis after flash freezing in liquid nitrogen as detailed above. Pieces from the cerebral lobes and the cerebellum contained some underlying white matter. Other pieces from the left hemisphere were used for immunohistochemistry.
For GM3 immunohistochemistry, brain slices were fixed overnight in 4% paraformaldehyde/PBS, equilibrated sequentially in 15 and 30% sucrose, and frozen in optimal cutting temperature embedding medium. GM3 immunostaining was performed as previously described27 using mouse monoclonal antibody DH2 (Glycotech, Gaithersburg, MD) followed by a biotinylated secondary antimouse antibody (Jackson Immunoresearch, West Grove, PA) and detection with a Vectastain Elite ABC kit (Vector Labs).
For immunostaining with GFAP, LIMP2, and Iba1, sections were fixed in formalin and embedded in paraffin, and 6-µm samples were treated for antigen retrieval as detailed.27 Primary antibodies used were rabbit antibodies against GFAP (glial fibrillary acidic protein; used at 1:1,000; Abcam Ab7260, Cambridge, MA) and LIMP2 (lysosomal integral membrane protein-2 used at 1:200; Novus Biologicals NB400-129, Littleton, CO), and goat antibody against Iba1 (ionized calcium-binding adaptor molecule 1; used at 1:100; Abcam Ab5076). Fi.t.C- or TRi.t.C-labeled donkey antirabbit or antigoat (Jackson Immunoresearch) served as secondary antibody. Samples used for thin sections were fixed and embedded in plastic as detailed27 and 1-µm sections were stained with toluidine blue.
Statistics. Values in different groups were compared using ANOVA with Holm–Sidak post hoc analysis if normality and equal variance tests passed, and with ANOVA on ranks with Dunn's post-hoc analysis if those tests failed. *P = 0.01–0.05, **P = 0.001–0.01, ***P < 0.001. A P value of <0.05 was considered significant.
SUPPLEMENTARY MATERIAL Figure S1. Diagram of brain sectioning technique and examples of GUSB staining from different regions of the cerebral cortex for i.t.-treated MPS VII dogs. Figures S2. GUSB stain at 6 months of age at level R0 (cranial). Figures S3. GUSB stain at 6 months of age at level R1 (just cranial to temporal lobe). Figures S4. GUSB stain at 6 months of age at level R2. Figures S5. GUSB stain at 6 months of age at level R3. Figures S6. GUSB stain at 6 months of age at level R4. Figures S7. GUSB staining at 6 months of age at level R5 (occipital lobe). Figure S8. GUSB stain of the caudate nucleus. Figure S9. GUSB stain of the midbrain and hippocampus. Figure S10. GUSB stain of the brainstem, choroid plexus, and white matter. Figure S11. GUSB stain of sciatic nerve in i.t.-only treated dogs. Figure S12. Biodistribution in AAV-treated MPS VII tissues. Figure S13. GUSB stain of liver and left myocardial free wall (heart). Figure S14. Thin sections of liver and spleen. Table S1. Neutralizing antibody titers against AAV9 and AAVrh10 capsids in serum. Table S2. Neutralizing antibody titers against AAV9 and AAVrh10 capsids in CSF. Video S1. Subjective mobility observations in AAV-treated MPS VII dogs. Materials and Methods
Acknowledgments
Support for this study was provided by NIH grants DK54481, OD P40 -010939, and P30DK047757. We thank the cadre of veterinary students who provide care for the animals and Kate Berger, a medical genetics resident at the University of Pennsylvania School of Veterinary Medicine, for medical oversight. M.E.H. and C.H.V. are stockholders of BioMarin Pharmaceuticals. J.M.W. is an advisor to REGENXBIO, Dimension Therapeutics, Solid Gene Therapy, and Alexion and is a founder of, holds equity in, and has a sponsored research agreement with REGENXBIO and Dimension Therapeutics; in addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. Other authors declare no conflict of interest.
Supplementary Material
References
- Muenzer, J (2011). Overview of the mucopolysaccharidoses. Rheumatology (Oxford) 50 (suppl. 5): v4–12. [DOI] [PubMed] [Google Scholar]
- Sly, WS, Brot, FE, Glaser, J, Stahl, PD, Quinton, BA, Rimoin, DL et al. (1974). Beta-glucuronidase deficiency mucopolysaccharidosis. Birth Defects Orig Artic Ser 10: 239–245. [PubMed] [Google Scholar]
- Tomatsu, S, Shimada, T, Mason, RW, Montaño, AM, Kelly, J, LaMarr, WA et al. (2014). Establishment of glycosaminoglycan assays for mucopolysaccharidoses. Metabolites 4: 655–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sly, WS, Quinton, BA, McAlister, WH and Rimoin, DL (1973). Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr 82: 249–257. [DOI] [PubMed] [Google Scholar]
- Archer, LD, Langford-Smith, KJ, Bigger, BW and Fildes, JE (2014). Mucopolysaccharide diseases: a complex interplay between neuroinflammation, microglial activation and adaptive immunity. J Inherit Metab Dis 37: 1–12. [DOI] [PubMed] [Google Scholar]
- Ausseil, J, Desmaris, N, Bigou, S, Attali, R, Corbineau, S, Vitry, S et al. (2008). Early neurodegeneration progresses independently of microglial activation by heparan sulfate in the brain of mucopolysaccharidosis IIIB mice. PLoS One 3: e2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto, T, Kono, K, Morimoto, K, Inoue, Y, Shintaku, H, Hattori, H et al. (2001). Brain magnetic resonance imaging in 23 patients with mucopolysaccharidoses and the effect of bone marrow transplantation. Ann Neurol 50: 79–92. [DOI] [PubMed] [Google Scholar]
- Yamada, Y, Kato, K, Sukegawa, K, Tomatsu, S, Fukuda, S, Emura, S et al. (1998). Treatment of MPS VII (Sly disease) by allogeneic BMT in a female with homozygous A619V mutation. Bone Marrow Transplant 21: 629–634. [DOI] [PubMed] [Google Scholar]
- Fox, JE, Volpe, L, Bullaro, J, Kakkis, ED and Sly, WS (2015). First human treatment with investigational rhGUS enzyme replacement therapy in an advanced stage MPS VII patient. Mol Genet Metab 114: 203–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh, HT, Grubb, JH, Vogler, C and Sly, WS (2012). Biochemical evidence for superior correction of neuronal storage by chemically modified enzyme in murine mucopolysaccharidosis VII. Proc Natl Acad Sci USA 109: 17022–17027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, LH, Erway, LC, Vogler, CA, Sly, WS, Nicholes, A, Grubb, J et al. (1998). Enzyme replacement therapy for murine mucopolysaccharidosis type VII leads to improvements in behavior and auditory function. J Clin Invest 101: 1394–1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder, KP, Melniczek, JR, Xu, L, Weil, MA, O'Malley, TM, O'Donnell, PA et al. (2002). Therapeutic neonatal hepatic gene therapy in mucopolysaccharidosis VII dogs. Proc Natl Acad Sci USA 99: 13102–13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, B, O'Malley, TM, Xu, L, Vite, C, Wang, P, O'Donnell, PA et al. (2006). Expression in blood cells may contribute to biochemical and pathological improvements after neonatal intravenous gene therapy for mucopolysaccharidosis VII in dogs. Mol Genet Metab 87: 8–21. [DOI] [PubMed] [Google Scholar]
- Ponder, KP and Haskins, ME (2007). Gene therapy for mucopolysaccharidosis. Expert Opin Biol Ther 7: 1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan, AK, Duque, S, Foust, KD, Morales, PR, Braun, L, Schmelzer, L et al. (2011). Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther 19: 1971–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher, T, Dubreil, L, Colle, MA, Maquigneau, M, Deniaud, J, Ledevin, M et al. (2014). Intracisternal delivery of AAV9 results in oligodendrocyte and motor neuron transduction in the whole central nervous system of cats. Gene Ther 21: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque, S, Joussemet, B, Riviere, C, Marais, T, Dubreil, L, Douar, AM et al. (2009). Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther 17: 1187–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federici, T, Taub, JS, Baum, GR, Gray, SJ, Grieger, JC, Matthews, KA et al. (2012). Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther 19: 852–859. [DOI] [PubMed] [Google Scholar]
- Fu, H, Dirosario, J, Killedar, S, Zaraspe, K and McCarty, DM (2011). Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther 19: 1025–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, SJ, Matagne, V, Bachaboina, L, Yadav, S, Ojeda, SR and Samulski, RJ (2011). Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther 19: 1058–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hordeaux, J, Dubreil, L, Deniaud, J, Iacobelli, F, Moreau, S, Ledevin, M et al. (2015). Efficient central nervous system AAVrh10-mediated intrathecal gene transfer in adult and neonate rats. Gene Ther 22: 316–324. [DOI] [PubMed] [Google Scholar]
- Rafi, MA, Rao, HZ, Luzi, P, Luddi, A, Curtis, MT and Wenger, DA (2015). Intravenous injection of AAVrh10-GALC after the neonatal period in twitcher mice results in significant expression in the central and peripheral nervous systems and improvement of clinical features. Mol Genet Metab 114: 459–466. [DOI] [PubMed] [Google Scholar]
- Schuster, DJ, Dykstra, JA, Riedl, MS, Kitto, KF, Belur, LR, McIvor, RS et al. (2015). Biodistribution of adeno-associated virus serotype 9 (AAV9) vector after intrathecal and intravenous delivery in mouse. Front Neuroanat 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sondhi, D, Hackett, NR, Peterson, DA, Stratton, J, Baad, M, Travis, KM et al. (2007). Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh.10 rhesus macaque-derived adeno-associated virus vector. Mol Ther 15: 481–491. [DOI] [PubMed] [Google Scholar]
- Swain, GP, Prociuk, M, Bagel, JH, O'Donnell, P, Berger, K, Drobatz, K et al. (2014). Adeno-associated virus serotypes 9 and rh10 mediate strong neuronal transduction of the dog brain. Gene Ther 21: 28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, SJ, Nagabhushan Kalburgi, S, McCown, TJ and Jude Samulski, R (2013). Global CNS gene delivery and evasion of anti-AAV-neutralizing antibodies by intrathecal AAV administration in non-human primates. Gene Ther 20: 450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinderer, C, Bell, P, Gurda, BL, Wang, Q, Louboutin, JP, Zhu, Y et al. (2014). Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther 22: 2018–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaranch, L, Salegio, EA, San Sebastian, W, Kells, AP, Foust, KD, Bringas, JR et al. (2012). Adeno-associated virus serotype 9 transduction in the central nervous system of nonhuman primates. Hum Gene Ther 23: 382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, J, Bouvet, A, DeSanto, C, Fyfe, JC, Xu, D, Wolfe, JH et al. (1998). Cloning of the canine beta-glucuronidase cDNA, mutation identification in canine MPS VII, and retroviral vector-mediated correction of MPS VII cells. Genomics 48: 248–253. [DOI] [PubMed] [Google Scholar]
- Sands, MS, Vogler, CA, Ohlemiller, KK, Roberts, MS, Grubb, JH, Levy, B et al. (2001). Biodistribution, kinetics, and efficacy of highly phosphorylated and non-phosphorylated beta-glucuronidase in the murine model of mucopolysaccharidosis VII. J Biol Chem 276: 43160–43165. [DOI] [PubMed] [Google Scholar]
- McGlynn, R, Dobrenis, K and Walkley, SU (2004). Differential subcellular localization of cholesterol, gangliosides, and glycosaminoglycans in murine models of mucopolysaccharide storage disorders. J Comp Neurol 480: 415–426. [DOI] [PubMed] [Google Scholar]
- Chang, PL, Lambert, DT and Pisa, MA (1993). Behavioural abnormalities in a murine model of a human lysosomal storage disease. Neuroreport 4: 507–510. [DOI] [PubMed] [Google Scholar]
- Parente, MK, Rozen, R, Cearley, CN and Wolfe, JH (2012). Dysregulation of gene expression in a lysosomal storage disease varies between brain regions implicating unexpected mechanisms of neuropathology. PLoS One 7: e32419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murrey, DA, Naughton, BJ, Duncan, FJ, Meadows, AS, Ware, TA, Campbell, KJ et al. (2014). Feasibility and safety of systemic rAAV9-hNAGLU delivery for treating mucopolysaccharidosis IIIB: toxicology, biodistribution, and immunological assessments in primates. Hum Gene Ther Clin Dev 25: 72–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, YH, Claflin, K, Geoghegan, JC and Davidson, BL (2012). Sialic acid deposition impairs the utility of AAV9, but not peptide-modified AAVs for brain gene therapy in a mouse model of lysosomal storage disease. Mol Ther 20: 1393–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe, JH, Sands, MS, Harel, N, Weil, MA, Parente, MK, Polesky, AC et al. (2000). Gene transfer of low levels of beta-glucuronidase corrects hepatic lysosomal storage in a large animal model of mucopolysaccharidosis VII. Mol Ther 2: 552–561. [DOI] [PubMed] [Google Scholar]
- Snyder, BR, Gray, SJ, Quach, ET, Huang, JW, Leung, CH, Samulski, RJ et al. (2011). Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Gene Ther 22: 1129–1135. [DOI] [PubMed] [Google Scholar]
- Smith, LJ, Martin, JT, O'Donnell, P, Wang, P, Elliott, DM, Haskins, ME et al. (2012). Effect of neonatal gene therapy on lumbar spine disease in mucopolysaccharidosis VII dogs. Mol Genet Metab 107: 145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson, DA, Karsch-Mizrachi, I, Lipman, DJ, Ostell, J and Wheeler, DL (2005). GenBank. Nucleic Acids Res 33(Database issue): D34–D38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samulski, RJ, Srivastava, A, Berns, KI and Muzyczka, N (1983). Rescue of adeno-associated virus from recombinant plasmids: gene correction within the terminal repeats of AAV. Cell 33: 135–143. [DOI] [PubMed] [Google Scholar]
- Lock, M, Alvira, M, Vandenberghe, LH, Samanta, A, Toelen, J, Debyser, Z et al. (2010). Rapid, simple, and versatile manufacturing of recombinant adeno-associated viral vectors at scale. Hum Gene Ther 21: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, G, Vandenberghe, LH, Alvira, MR, Lu, Y, Calcedo, R, Zhou, X et al. (2004). Clades of adeno-associated viruses are widely disseminated in human tissues. J Virol 78: 6381–6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcedo, R, Vandenberghe, LH, Gao, G, Lin, J and Wilson, JM (2009). Worldwide epidemiology of neutralizing antibodies to adeno-associated viruses. J Infect Dis 199: 381–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, P, Moscioni, AD, McCarter, RJ, Wu, D, Gao, G, Hoang, A et al. (2006). Analysis of tumors arising in male B6C3F1 mice with and without AAV vector delivery to liver. Mol Ther 14: 34–44. [DOI] [PubMed] [Google Scholar]
- Sleat, DE, Sohar, I, Lackland, H, Majercak, J and Lobel, P (1996). Rat brain contains high levels of mannose-6-phosphorylated glycoproteins including lysosomal enzymes and palmitoyl-protein thioesterase, an enzyme implicated in infantile neuronal lipofuscinosis. J Biol Chem 271: 19191–19198. [DOI] [PubMed] [Google Scholar]
- Wolfe, J and Sands, M. (1996). Protocols for Gene Therapy in Neuroscience: Towards Gene Therapy of Neurological Disorders. John Wiley & Sons: New York, pp. 263–274. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.