Abstract
Human mesenchymal stem cells (hMSCs) are one of the most widely researched stem cell types with broad applications from basic research to therapeutics, the majority of which require introduction of exogenous DNA. However, safety and scalability issues hinder viral delivery, while poor efficiency hinders nonviral gene delivery, particularly to hMSCs. Here, we present the use of a pharmacologic agent (glucocorticoid) to overcome barriers to hMSC DNA transfer to enhance transfection using three common nonviral vectors. Glucocorticoid priming significantly enhances transfection in hMSCs, demonstrated by a 3-fold increase in efficiency, 4–15-fold increase in transgene expression, and prolonged transgene expression when compared to transfection without glucocorticoids. These effects are dependent on glucocorticoid receptor binding and caused in part by maintenance of normal metabolic function and increased cellular (5-fold) and nuclear (6–10-fold) DNA uptake over hMSCs transfected without glucocorticoids. Results were consistent across five human donors and in cells up to passage five. Glucocorticoid cell priming is a simple and effective technique to significantly enhance nonviral transfection of hMSCs that should enhance their clinical use, accelerate new research, and decrease reliance on early passage cells.
Introduction
Human mesenchymal stem cells (hMSCs) have become one of the most widely researched stem cell types in recent years due to multiple unique properties. hMSCs are capable of in vitro trilineage differentiation (ectoderm, mesoderm, and endoderm)1,2 and can be derived from multiple, abundant sources within the body including bone marrow,3 fat,4 skin,5 muscle,6 and peripheral blood.7 hMSCs also offer advantages over other stem cell types in that they can be ethically derived from adults, are nontumorigenic, and are immunoprivileged.8,9 For these reasons, hMSCs are under much investigation for uses in tissue engineering and regenerative medicine,10 for the targeted delivery and secretion of therapeutic proteins,11,12 and for use in cancer therapy.2 All of these applications either require or would be greatly aided by the introduction of exogenous DNA to encode genes for tissue growth factors, to genetically guide differentiation, or induce production of therapeutic proteins.
Unfortunately, current gene delivery techniques to hMSCs through viral and nonviral methods have shortcomings. Viral gene delivery is highly efficient, yet costly and difficult to produce, with limited genetic cargo capacity, and is prone to safety concerns,13,14 particularly in hMSCs. Furthermore, hMSCs are frequently used in ex vivo therapies, where viral vectors retained within the cells could be released upon implantation into surrounding tissues where those viral vectors may initiate a host immune response, become mutagenic, or even tumorigenic.15,16 Conversely, nonviral gene delivery is considerably safer by comparison to viral delivery, with the added advantages of being inexpensive, simple to produce, and not limited by genetic cargo size; however, nonviral delivery is comparably less efficient,17 particularly to hMSCs. Most nonviral gene delivery methods to hMSCs report transfection efficiencies between 1–10% of cells expressing transgene,18,19,20,21 with transfection efficiencies reported as high as 20% only to cells at passages one or two.19,20,21 For hMSCs to be therapeutically viable while maintaining patient safety, more efficient nonviral gene delivery strategies must be developed. The primary approach to improve nonviral gene delivery is chemical modification existing vectors or de novo synthesis, however this approach has not produced significant increases in the efficient transfection of hMSCs.18,20 An alternative approach to improving gene delivery is to prime cells with a pharmacologic agent to transiently overcome barriers of gene delivery for improved transfection.22,23,24
A potential family of priming agent is glucocorticoids (GC), which are steroid hormones that regulate metabolic activity by binding the GC receptor and translocating to the nucleus, where the receptor acts as a transcription factor to modulate gene expression.25,26 GCs are used widely in the clinic for their potent anti-inflammatory properties. Additionally, dexamethasone (DEX), a synthetic GC, has been shown to dilate nuclear pores of Xenopus laevis oocytes up to 300 nm in diameter27,28 and increase microsomal membrane fluidity in fetal rat livers29; properties that could enhance cellular and nuclear entry of delivered exogenous DNA. GCs such as DEX and the natural GC, cortisol, have also been used to modify polymer- and lipid-based gene delivery systems for nuclear targeting and decreased immune response,30,31 and to prime δ some human and murine immortalized cell lines for transfection.32,33 Additionally, DEX has been shown to have no negative effect on the multipotency of hMSCs, in fact enhancing their trilineage differentiation34 and immunomodulatory properties.35 Due to the promising properties of GCs to potentially overcome some of the barriers to efficient gene delivery, GCs were studied as potential cell priming adjuvants for enhanced transfection to hMSCs.
In this report, we present the effects of GC-priming on bone marrow-derived hMSC transfection outcomes across multiple cell donors, transgenes, and commercially available nonviral reagents. We also demonstrate maintenance of normal hMSC functions, preserving the inherent safety of nonviral gene delivery, and provide a partial mechanistic understanding behind the effects of GC-mediated cell-priming on nonviral gene delivery.
Results
Cell priming enhances transfection outcomes
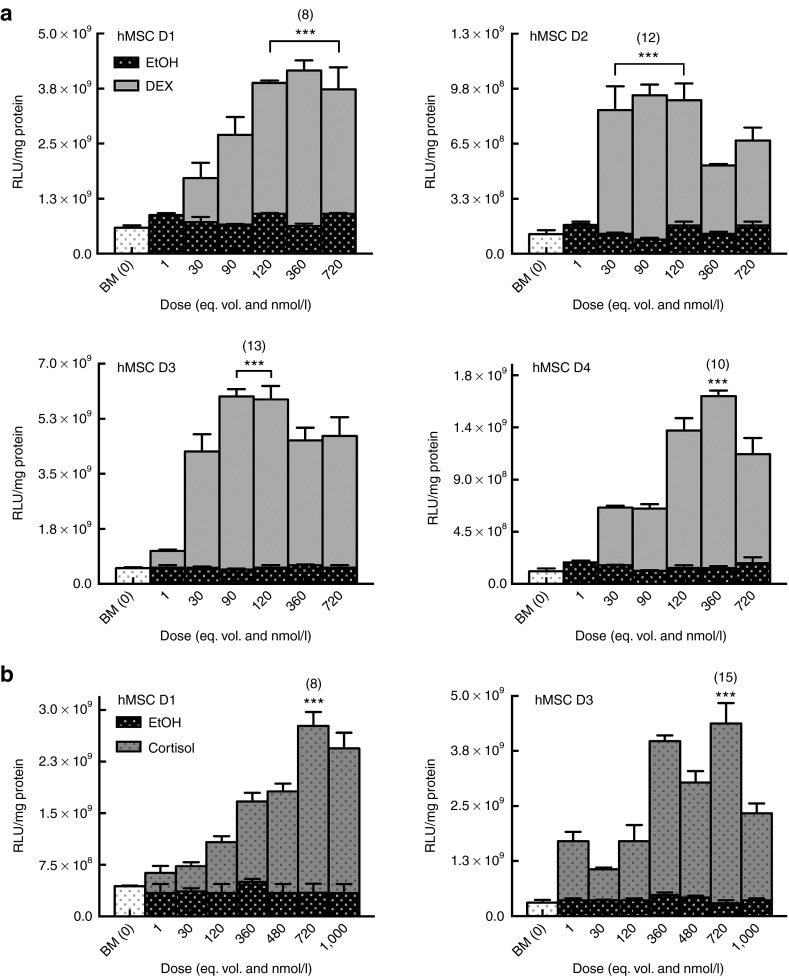
Two GCs were evaluated as cell priming adjuvants for enhancing nonviral transfection in passage four, bone marrow-derived hMSCs. Cells (Table 1) were pretreated with varying doses of DEX or cortisol, over a range of physiological concentrations, 30 minutes prior to delivery of Lipofectamine-LTX (LF-LTX) lipoplexes containing the pEGFP-LUC plasmid. Thirty minutes were determined to be optimal GC pretreatment timing in a separate experiment (Supplementary Figure S1). For all experiments, GC-primed cells were compared to standard transfection in basal media (BM) and transfection in media with an equivalent volume of GC vehicle (EtOH). Transfection outcomes were measured as luciferase (LUC) expression levels, normalized to total protein levels. While transfection in BM and vehicle control (EtOH) were not statistically different from each other, GC-primed cells exhibited statistically increased transfection with increasing GC dose, similar to a classic dose–response curve (Figure 1). Compared to controls, LUC expression in primed cells began to increase at 3E−8 mol/l DEX (Figure 1a) or 3.6E−7 mol/l Cortisol (Figure 1b), with highest transfection enhancement for most donors achieved with 1.2E−7 mol/l DEX or 7.2E−7 mol/l Cortisol, with some donors exhibiting a decrease in transfection levels at higher doses (Figure 1). At optimal GC dose, all cell donors exhibited an 8–15-fold increase in transgene expression (P ≤ 0.001), compared to cells transfected under vehicle control conditions. While fold increases remained relatively similar across all donors, it should be noted that absolute expression levels were different for each donor, ranging across two orders of magnitude. Once GCs were determined to be effective cell-priming adjuvants, additional metrics of transfection were evaluated including transfection efficiency and transgene expression duration.
Table 1. Cell type, donor labels, and demographic data.
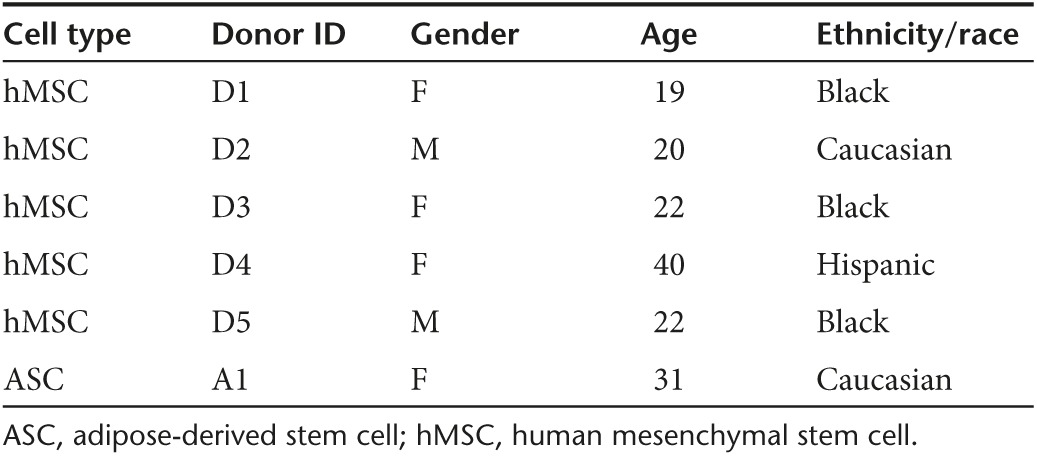
Figure 1.
Glucocorticoid (GC)-priming effect on transgene expression. Human mesenchymal stem cells (hMSCs) exhibit increased luciferase expression when primed with dexamethasone (DEX) or cortisol in a dose-dependent manor. Error bars represent standard error of the mean for triplicate wells collected on duplicate days. (a) hMSCs primed with DEX exhibit the highest transgene expression in response to 1.2E−7 mol/l DEX with an 8- to 13-fold increase in luciferase expression at the optimum dose compared to basal media (BM) alone or with the vehicle control (EtOH). (b) Similarly, hMSCs primed with cortisol exhibit an 8- to 15-fold increase in luciferase expression when compared to unprimed cells (BM and EtOH) with the optimal cortisol dose of 7.2E−7 mol/l.
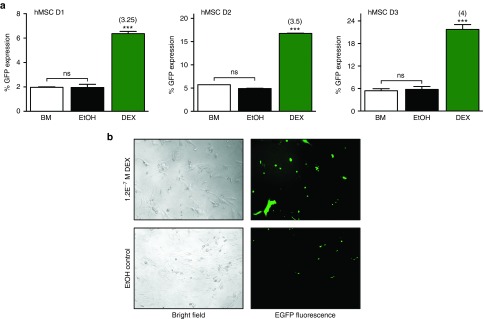
To evaluate the ability of GC-priming to enhance transfection efficiency, the number of cells expressing the delivered transgene out of the total number of treated cells, cells were pretreated as before with the optimal dose of DEX (1.2E−7 mol/l) followed by delivery of LF-LTX/pEGFP-LUC lipoplexes. Flow cytometry analysis revealed a 3- to 4-fold increase in the percentage of hMSCs expressing green fluorescent protein (GFP) across all cell donors, compared to BM and EtOH controls (controls were not statistically different from each other) (Figure 2a). GC-priming resulted in efficiency increases from 2, 5, and 5.5% in unprimed cells to 6.5, 17.5, and 22% GFP expression following GC-priming in passage 4 hMSCs for donors D1, D2, and D3, respectively. Representative fluorescence microscopy images qualitatively showed this increase in transfection efficiency (Figure 2b) in hMSCs primed with DEX prior to transfection with pEGFP-LUC compared to unprimed cells. As with luciferase expression, while the base level of transfection efficiency across donors was different, the fold increase in efficiency as a result of priming was consistent.
Figure 2.
Glucocorticoid (GC)-priming effect on transfection efficiency. (a) Cells primed with 1.2E−7 mol/l dexamethasone (DEX) show a minimum threefold increase in the number of cells expressing green fluorescent protein (GFP) compared to BM alone or the EtOH controls as evaluated by flow cytometry. Error bars represent standard error of the mean for triplicate wells collected on duplicate days. (b) Bright field and fluorescence images of human mesenchymal stem cells expressing GFP, either primed with 1.2E−7 mol/l DEX or an equivalent volume of EtOH.
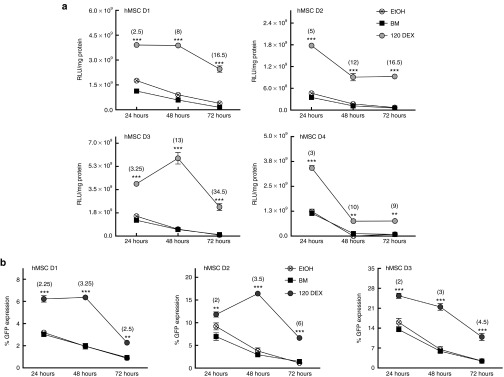
In addition to transgene expression levels and transfection efficiency, the duration of these outcomes was evaluated 24, 48, and 72 hours following DNA delivery in cells primed with 1.2E−7 mol/l DEX compared to cells transfected in BM or media with vehicle control (EtOH). While overall protein expression decreased with time for all treatments, transgene expression was found to remain elevated for at least 72 hours in cells primed with DEX compared to unprimed cells. Absolute LUC expression levels in unprimed cells decreased by 1.5 to 2 orders of magnitude from their highest levels at 24 hours, while GC-primed cells showed a decrease in LUC expression of only 0.25 to 0.75 orders of magnitude from their highest levels at 24 hours for all donors. Additionally, three of four hMSC donors expressed luciferase at statistically elevated levels following GC-priming compared to the 72 hour controls, and also compared to the highest achievable expression in unprimed cells (at 24 hours, P ≤ 0.001) (Figure 3a). Transfection efficiency in cells primed with DEX prior to delivery of complexes also remained elevated in comparison to unprimed cells for at least 72 hours (Figure 3b), with all donors having a 2.5- to 6-fold increase in the percentage of GFP-positive cells 72 hours following DNA delivery, compared to unprimed cells, which exhibited <1.5% GFP at that time
Figure 3.
Glucocorticoid (GC)-priming effect on duration of enhanced transfection outcomes. (a) The increase in transgene expression due to GC-priming remains statistically increased over unprimed cells for at least 72 hours. At 72 hours, all donors express the luciferase transgene at near zero relative light unit/mg total protein (RLU/mg), while GC-primed cells not only express transgene at appreciable levels, but donors 1–3 express transgene at levels greater than the highest levels without GCs (between 72 hours DEX versus 24 hours BM and EtOH, donors 1 and 2, P ≤ 0.001; D3, P ≤ 0.01). Error bars represent standard error (SE) of the mean for triplicate wells collected on duplicate days. (b) In addition to prolonged transgene expression levels, the increase in transfection efficiency is also maintained for at least 72 hours when compared to unprimed cells. Error bars represent SE of the mean for pooled triplicate wells collected on triplicate days.
Next the ability of this method to be used across multiple nonviral delivery vehicles and with multiple transgenes was investigated to ensure protocol robustness and applicability to a wide range of scientific applications and researchers.
GC-mediated cell priming is effective with multiple protocols
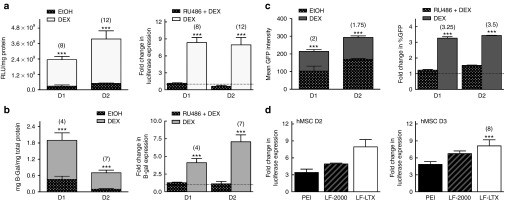
For greater applicability of the GC-priming method, additional nonviral vehicles and transgenes were evaluated in combination with GC-priming. Similar to the effect seen with GC-priming for luciferase expression (Figures 1 and Figure 4a), GC-priming significantly increased the transfection of the plasmid encoding the beta-galactosidase (β-gal) gene, with expression increased 4- to 7-fold over unprimed transfection (Figure 4b). Similarly, GFP protein levels, as measured by GFP intensity, were also significantly increased by 1.75- to 2-fold (Figure 4c) over unprimed transfection.
Figure 4.
Glucocorticoid (GC)-priming effect with multiple reporter genes and delivery vehicles, and in the presence of a GC receptor antagonist. The graphs on the left for (a–c) represent transgene expression magnitude for three different reporter genes (a) Luciferase, (b) B-Galactosidase (B-gal), and (c) green fluorescent protein (GFP) intensity as quantified by flow cytometry. Error bars for luciferase expression (a) and B-gal (b) represent standard error (SE) of the mean for triplicate wells collected on duplicate days, while error bars for GFP expression (c) represent SE of the mean for pooled triplicate wells collected on triplicate days. All reporter plasmids show statistically increased expression in GC-primed cells compared to the EtOH control. The graphs on the right for (a–c) show the complete knockdown of the GC-priming effect on both (a and b) transgene expression and (c) transfection efficiency by the addition of known GC receptor antagonist Mifipristone (RU486), represented as fold change of GC primed cells vs. cells treated with both GC and RU486. (d) GC-priming enhances transgene expression using three of the most common commercially available nonviral vehicles, 25 kD branched polyethylenimine, Lipofectamine-2000, and Lipofectamine-LTX, with significantly increased expression of 3.5- to 5-fold, 5- to 6.75-fold, or 8- to 12-fold, respectively, when compared to cells not primed with GCs.
All experiments reported above were conducted with LF-LTX, a transfection reagent specifically formulated for difficult-to-transfect cells but not as frequently used as Lipofectamine-2000 (LF-2000) or 25 kDa branched polyethylenimine (PEI), which are the two most commonly used vectors for lipid and polymer based transfection, respectively. Therefore the ability of GC-priming to enhance transfection outcomes following gene delivery with multiple commercially available nonviral vectors was evaluated. As before, cells were primed 30 minutes prior to DNA delivery with 1.2E−7 mol/l DEX followed by delivery of DNA complexes prepared with LF-LTX, LF-2000, or PEI containing the pEGFP-LUC plasmid. Identical transfections were also conducted in BM and media containing vehicle control (EtOH) for comparison. The BM and EtOH controls were not statistically different from each other, while GC-primed treatments resulted in transfection levels that were statistically increased relative to transfection levels in unprimed cells for all three nonviral vehicles, with 3.5-to 5-fold increases in LUC expression for PEI treatments, 5- to 6.75-fold increases in LUC expression with LF-2000 treatments, and 8-fold increases in LUC expression following LF-LTX treatments (Figure 4d).
Enhanced transfection outcomes and applicability across a wide range of nonviral delivery protocols illustrate the potential of GC-priming for use across multiple platforms to improve transfection in hMSCs. Next, we investigated potential mechanisms of GC-mediated priming activity and the overall safety of GC-priming to maintain normal cellular function.
Mediators of the GC cell-priming effect
GCs are known to bind the GC receptor prior to its translocation to the cell nucleus.25,26 To determine whether binding of the GC receptor was required for the GC-mediated enhancement to transfection, a GC receptor antagonist was used to competitively bind all GC receptors, prohibiting the delivered DEX from binding to the GC receptor. As a GC receptor antagonist, Mifepristone (RU486) not only binds all receptors preventing GC binding, but also prevents the activation of the GC receptor inhibiting its nuclear translocation.36 Delivery of RU486 to cells prior to GC delivery and subsequent transfection, completely eliminated any increase in transgene expression regardless of reporter gene (Figure 4a–c), with expression levels in RU486+GC delivery equal to transgene expression levels in unprimed cells. These results demonstrate that binding of the GC receptor is required for enhanced transfection outcomes.
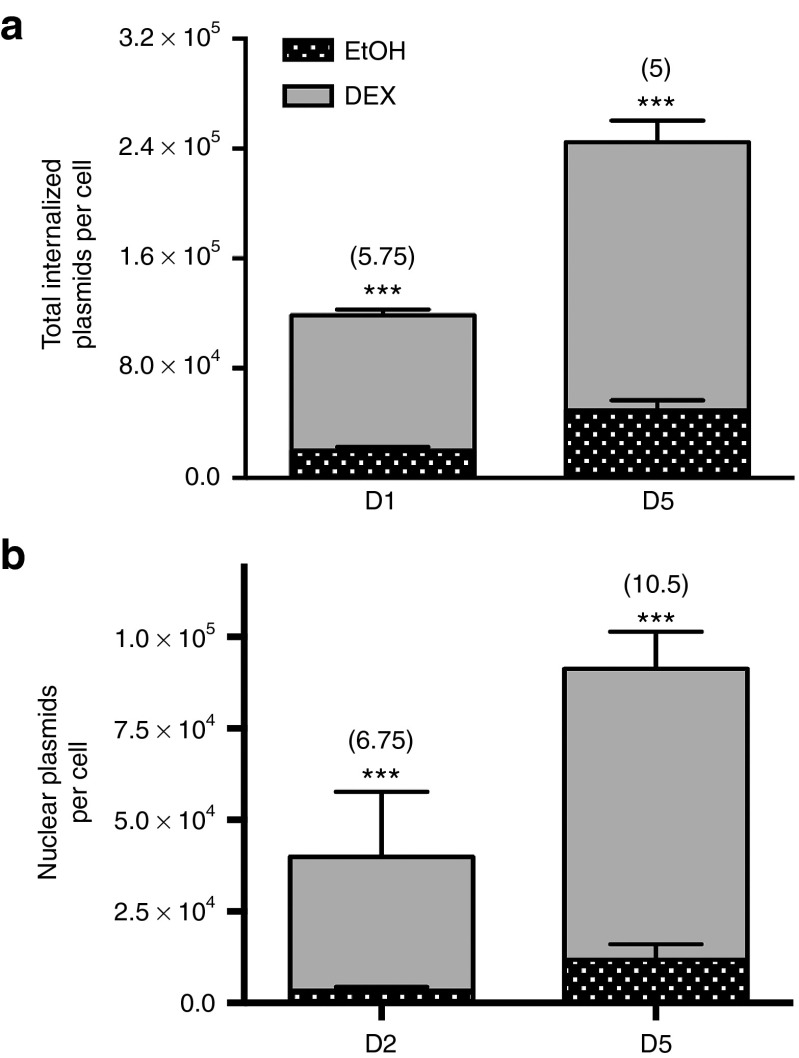
Furthermore, GC-priming may be mediated through enhanced cellular and nuclear entry of plasmid DNA due to GCs effects on membrane fluidity29 and nuclear pore enlargement.27,28 To evaluate internalized cellular plasmids and nuclear localized plasmids, cells were treated with DEX as described previously. Cellular plasmids were quantified via qPCR of DNA isolated from whole cell lysates, with total cell numbers calculated by normalizing Ct values to the ACTA1 gene, which has exactly two copies per cell.37 Nuclear plasmids were quantified similarly, as described previously.38 Quantification of total cellular plasmids and plasmids from isolated cell nuclei revealed a significant increase in both total internalized plasmids and nuclear internalized plasmids (Figure 5) in cells primed with DEX, compared to unprimed cells transfected in BM or EtOH, with a minimum 5-fold increase in total plasmids per cell and a 6.75- to 10.5-fold increase in plasmids internalized into the nucleus per cell (P ≤ 0.001). Together, these studies suggest that GC-mediated priming enhances transfection through binding of the GR receptor and enhancement of cellular and nuclear plasmid internalization.
Figure 5.
Glucocorticoid (GC)-priming effect on DNA internalization. All error bars represent the standard deviation of the mean for triplicate PCR wells for a single population of cells due to the large number of cells required to isolate nuclei for qRT-PCR. (a) Total plasmids entering unprimed cells is on the order of tens of thousands per cell, while primed cells internalize plasmids an order of magnitude greater, in the hundreds of thousands of plasmids per cell with a minimum 5-fold increase in plasmid copies per cell (P ≤ 0.001). Of the plasmids that internalize, (b) GC-primed cells also internalize more plasmids into the cell nucleus with a 6.75- to 10.5-fold increase in nuclear plasmid copies (P ≤ 0.01 or P ≤ 0.001, respectively) compared to cells treated with vehicle control.
Cell priming helps maintain normal cellular function
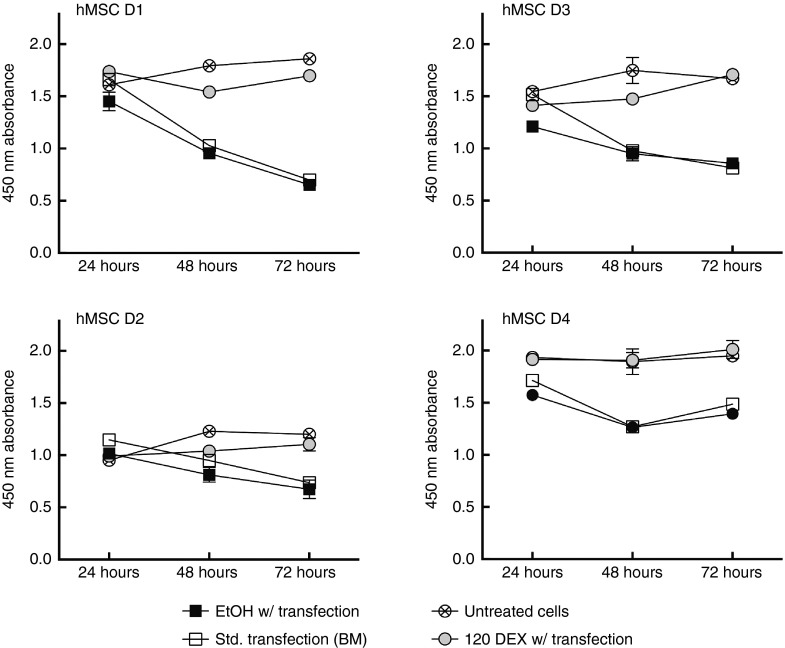
Finally, to corroborate existing literature on the safety of GCs on hMSCs, cell viability and hMSC differentiation potential in the presence of GC-priming were also evaluated. Nonviral gene delivery, while comparably less toxic than viral delivery, can cause mild toxicity in vitro or potential inflammatory reactions in vivo. Here, hMSCs were treated as described above (GC-primed and unprimed) and assayed via WST-1 assay at 24, 48, and 72 hours following treatment to determine metabolic activity for comparison to cells without treatment (Figure 6). After 24 hours, no statistical differences in metabolic activity were found in any of the donors between any of the treatments. However, 48 hours after DNA delivery, all donors except D2 showed δ no significant decrease in metabolic activity between untreated cells and GC-primed treatment, but the unprimed and transfected cells showed a significant decrease (P ≤ 0.001) in metabolic activity when compared to the untreated cells. Similarly, by 72 hours, all unprimed treatments in all donors showed a significant decrease (P ≤ 0.001) in metabolic activity when compared to untreated cells and GC-primed transfection, which again were not statistically different. These data demonstrate that GC-priming reduces toxicity of nonviral vectors in hMSCs by preserving the metabolic activity native to untreated cells.
Figure 6.
Glucocorticoid (GC)-priming effect on cellular metabolism. GC-primed cells and untreated cells showed metabolic activity that was not statistically different (except for D2 at 48 hours, P ≤ 0.01), while cells treated with EtOH and cells transfected in basal media alone (std. Transfection) were also not statistically different. Untreated cells and GC primed cells showed significantly increased metabolic activity when compared to std. transfection and EtOH-treated cells at the 48-hour and 72-hour time points (P ≤ 0.001) (except D2 at 48 hours, P ≤ 0.01). All error bars represent standard error of the mean for triplicate wells.
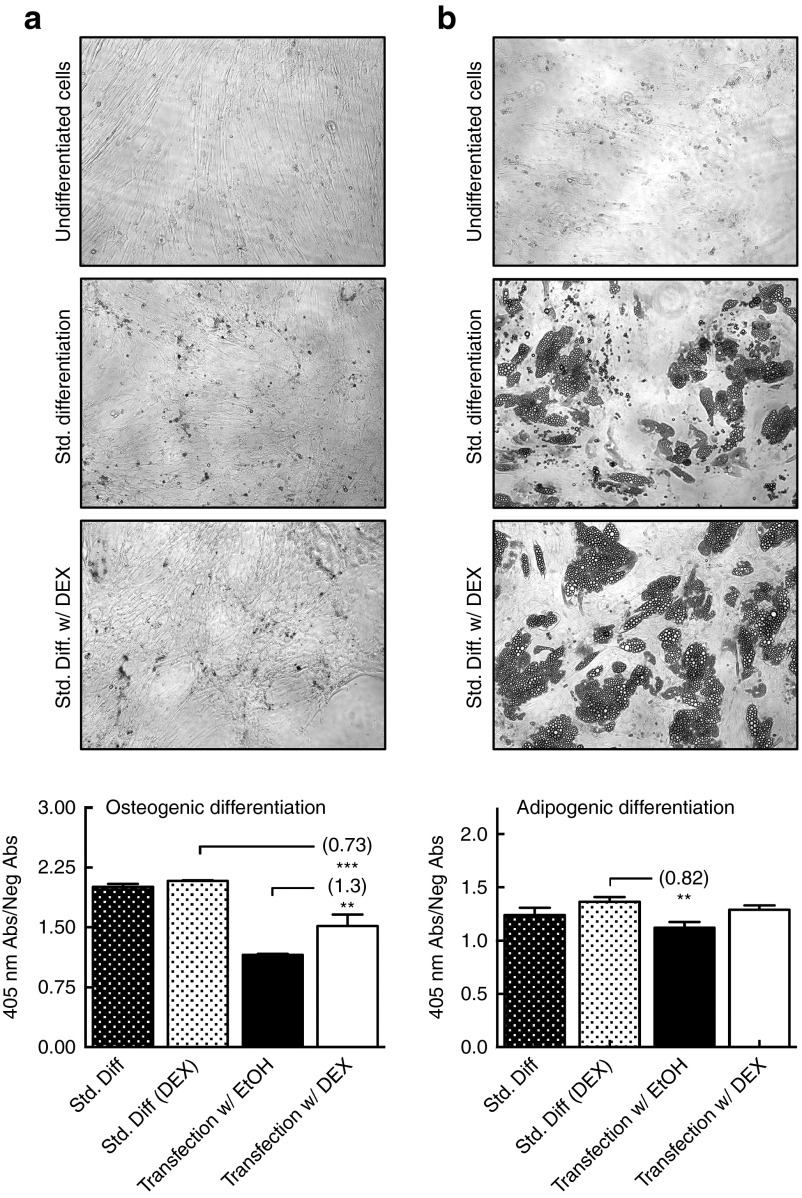
In addition to metabolic activity, stem cells are characterized by their ability to differentiate, a potential that must be preserved with GC-priming. The adipogenic and osteogenic differentiation potential of one cell donor (D2) was evaluated under four conditions: with and without DEX, and with and without transfection. Imaging clearly shows the differentiation of hMSCs into osteoblasts and adipocytes (Figure 7a,b, respectively) with or without GCs, using standard differentiation media. Quantification of osteogenesis by alizarin red absorbance revealed a 0.73-fold reduction in osteoblast content in GC-primed transfected cells compared to standard differentiation, however a 1.3-fold increase in osteoblast differentiation when compared to unprimed transfection in EtOH (Figure 7a). Quantification of adipogenesis by oil red O absorbance revealed no statistical difference in adipocyte content between standard differentiation, standard differentiation with DEX and transfection with GC-priming, but showed a significant 0.82-fold decrease in adipocyte content in transfected but unprimed cells (Figure 7b). These data demonstrate that GC-priming not only maintains the differentiation potential of untransfected hMCSs but also preserves their differentiation potential following transfection.
Figure 7.
Glucocorticoid (GC)-priming effect on human mesenchymal stem cell differentiation potential. All error bars represent the standard deviation of the mean for triplicate wells of differentiated cells. (a) Osteogenic differentiation images and spectrophotometric absorbance data of Alizarin Red staining, collected at 405 nm and normalized to the negative control. (b) Adipogenic differentiation images and spectrophotometric absorbance data of Oil Red O staining, collected at 540 nm and normalized to the negative control.
In summary, GC-priming enhances transfection efficiency, transgene expression and duration, and helps to maintain normal metabolic function and differentiation potential; this effect is dependent on GC receptor binding, and mediated in part by increased DNA uptake into the cytosol and nucleus.
Discussion
In this study, GCs were evaluated as cell-priming adjuvants to enhance transfection outcomes in hMSCs; the delivery of GCs was optimized, the effects on transfection were characterized, and some of the basic mechanisms underlying the GC-mediated effects were elucidated. It was shown that pretreatment of hMSCs with GCs (DEX or cortisol) resulted in enhanced transfection efficiency 3- to 3.5-fold, increased transgene expression by 4- to 15-fold depending on reporter gene, increased cellular DNA uptake by 5-fold and nuclear DNA uptake by at least 6-fold, and prolonged transgene expression by 24 additional hours all compared to transfection in unprimed cells. These enhancements are dependent on GC dose, with the optimal dose varying between 90–360 nmol/l of DEX from donor to donor. This variation is hypothesized to be dependent on physiological GC receptor binding affinity. Physiological Kd values are between 1–50 nmol/l for DEX, depending on donor age, disease status, tissue type, and stage of the cell cycle.39,40,41 Due to the increase in DEX concentration required to produce enhanced transfection outcomes beyond physiological Kd, it is hypothesized that the optimal DEX dose (90–360 nmol/l) is the concentration at which receptors become saturated with GC rather than 50% bound. Additionally, this dose variability is an example of inherent differences in donor response similar to the differences in absolute transgene expression levels and the rate at which the transient transgene expression declines over time as observed across cell donors. These differences indicate a base level of transfection ability and longevity inherent to each donor, but also highlight the ability of GC-priming to consistently enhance transfection efficiency and transgene expression at similar fold increases over unprimed transfection regardless of baseline transfection. As such, we did not make comparisons across donors for absolute transgene expression and duration, but rather between GC-primed and unprimed cells within each donor data set. Unlike the variability in optimal dose, there is variability in the fold enhancements of transgene expression with different reporter genes. This variability is thought to be a result of gene size and protein stability, rather than a function of GC-priming, as evidenced by Ribeiro et al.42 who determined smaller genes exhibit up to 2.5-fold greater expression over larger genes. LUC and β-gal expression were evaluated by similar assays, but likely exhibited slightly different fold changes in expression due to their size difference of 6.37 versus 7.5 kb, respectively.
In addition to enhancing transfection outcomes, GC-priming helped transfected hMSCs to maintain the same level of metabolic activity as the untransfected hMSCs and helped preserve differentiation potential in both transfected and untransfected controls, negating the slight toxicity inherent to nonviral gene delivery. In developing novel gene delivery techniques, efficacy and safety are always a balancing act, yet with GC-priming, safety is not sacrificed for efficacy, rather both are enhanced. To further corroborate these findings, future studies on the preservation of hMSC phenotype as evidenced by the presence and absence of traditional hMSC surface markers and an investigation into the effects of GC priming on apoptosis will be evaluated.
Mechanistically, GR binding was determined to be a crucial factor underlying the ability of GCs to enhance nonviral gene delivery. Introduction of a known GC receptor antagonist, Mifepristone, completely negated the enhancements to transfection observed with GC priming (Figure 4), by preventing binding of the delivered GC to the receptor. This mechanism pinpoints cellular pathways regulated by the GR as key targets for further investigation to determine the precise mechanisms, pathways, and genes involved in the ability of GCs to prime cells and enhance transfection. Another potential mechanism underlying the effects of GC-priming on transfection outcomes is enhanced DNA uptake into both the cytosol and cell nucleus. CG-primed hMSCs showed a 5-fold increase in cytosolic plasmids and at least a 6-fold increase in nuclear plasmids compared to unprimed hMSCs. However, more work is needed to understand how the GR mediates increased nuclear uptake.
In addition to being difficult-to-transfect, hMSCs also exhibit a decline in transfection efficiency with increasing passage number.18,19,20,21 While a decline in absolute transgene expression levels was observed with increasing cell passage number, there was no variability in the 3- to 4-fold enhancement in transfection across cell passage number due to GC-priming (Supplementary Figure S2). Due to the known effect of cell passage number on transfection, most other studies have been conducted in passage one and two cells, with the highest achieved efficiencies of up to 20% observed following lipoplex-mediated gene delivery with de novo vehicles.18,19,20,43 Here, all experiments were conducted in passage four cells, yet donor 3 (D3, Table 1) hMSCs exhibited an increase in transfection efficiency from 5.5% without priming to 22% in GC-primed cells. No previous study has achieved such high transfection efficiency in hMSCs, even at low passage number and particularly not with a commercially available delivery vector. Given the known decline in absolute transfection efficiency with increasing passage number, it is hypothesized that GC priming could enhance the transfection efficiency in low passage hMCSs beyond those observed here, a hypothesis that will be evaluated in future studies. Still, the ability of GC-priming to enhance transfection efficiencies consistently even across cell passage number (passages 3–5), gives researchers and clinicians the ability to prolong the therapeutic viability of hMSCs far beyond their previous usage of passage one and two only, reducing cost and cell waste.
Typical studies with primary mammalian cells often rely on a single donor44,45 or do not carry the same donor across experiments.19,21,46,47,48 Here, we have provided additional data breadth by evaluating transfection outcomes across five human donors, further indicating the robustness of GC-priming and uniquely providing a rare data set that allows for comparison of transfection outcomes across multiple donors of varied demographics (Table 1). While this demographic data requires additional donors to draw statistical significance, it provides a good start for potentially drawing conclusions about donor specific baseline transfection ability based on demographic data, which could be used to understand research outcomes, to guide patient-specific care, or even to develop cell-based therapeutics or diagnostics with the highest efficacy.
Along with hMSCs, other cell types were evaluated for this work, with the majority of cell types not showing enhanced transfection outcomes with GC-priming: HEK 293T, D1/ORL/UVA, NIH 3T3, and HeLa cell lines did not show enhanced transfection (data not shown), although HeLa cells were previously shown to be GC responsive following adenoviral DNA delivery.49 However, similar to the GC-mediated transfection enhancement in hMSCs, human adipose-derived stem cells (ASC) showed enhanced transfection outcomes in response to GC-priming (Supplementary Figures S3 and S4), including a 10.75-fold dose–response increase in transfection levels, a 7-fold increase in transfection efficiency, and maintenance of normal metabolic activity following GC-priming when compared to unprimed treatments. While this is preliminary data in just a single cell donor, it may provide insight into the types of cells that may be responsive to GC priming. In addition to hMSCs and ASCs, three previous studies have made note of increased transfection or transduction efficiency in a few immortalized cell lines following treatment with DEX using PEI,33 Lipofectin,32 or adenoviral32,49 delivery vehicles. This study however, is the first to evaluate three transfection outcomes (efficiency, transgene expression, and duration) together, and the first to find efficacy in stem cells (hMSCs) across multiple cell donors, delivery vehicles, and transgenes. To further enhance the utility of GC-priming to enhance nonviral gene delivery, future studies will be conducted with more ASC donors, additional cell types and plasmids, and in vivo analysis.
In summary, GC-mediated cell priming is a simple, effective, and safe technique for enhancing multiple transfection outcomes to levels not previously seen with nonviral delivery in hMSCs. These results demonstrate the ability of GC-mediated cell priming to enhance multiple transfection outcomes in hMSCs across multiple cell donors and passage number with multiple commercially available transfection reagents and transgenes. The enhancements to transfection were found to be dependent on the binding of the GC receptor and are mediated in part by maintenance of normal metabolic activity and increased DNA uptake. GC-priming provides clinicians and researchers a technique to enhance the transfection of hMSCs while maintaining the inherent safety of nonviral gene delivery, that can be implemented immediately in combination with their current transfection protocols to improve the therapeutic potential of these cells in applications such as tissue engineering, the treatment of autoimmune diseases, and cancer therapy.
Materials and Methods
Cell culture. Bone marrow-derived human mesenchymal stem cells (hMSCs) and adipose derived human stem cells (ASCs) (Table 1) were purchased at passage 2 from Lonza (Lonza, Walkersville, MD). hMSCs are positive for CD29, CD44, CD105, and CD166 cell surface markers and negative for CD14, CD34, and CD45 (Lonza). ASCs are positive for CD13, CD29, CD44, CD73, CD90, CD105, CD166, and negative for the CD14, CD31, CD45 cell surface markers (Lonza). Cells were expanded and cultured in complete MSCGM media (Lonza) for hMSCs or complete ADSC media (Lonza) for ASCs and incubated at 37 °C with 5% CO2 until 80% confluent. Cells were then washed with 1× phosphate-buffered saline (PBS) and dissociated with 0.05% trypsin-ethylenediamine tetraacetic acid (EDTA) (Gibco, Life Technologies, Grand Island, NY), an equal volume of complete media was then added. Cells were counted with trypan blue and a hemocytometer, pelleted, and resuspended in 1 ml of media per 6 × 104 cells and either frozen (in 5% dimethyl sulfoxide) in liquid nitrogen for future passages, or seeded for experimentation. Cells were seeded into 48 or 24 well plates (Thermo Scientific, Rockford, IL) at a density of 6,000 cells/cm2 for hMSCs or 8,000 cells/cm2 for ASCs, per manufacturer's protocols. Cells were allowed to adhere for 48 hours at 37 °C with 5% CO2 prior to DNA complex delivery.
Priming reagents. DEX, cortisol, and mifepristone (RU486) were purchased from Sigma (Sigma-Aldrich, St. Louis, MO). All reagents were dissolved in absolute ethanol (EtOH), stored at −20 °C, then thawed prior to delivery in volumes not exceeding 1% total media volume. RU486 was delivered 1 hour prior to GCs at a concentration of 1.2 μmol/l. DEX or cortisol was delivered to cells 30 minutes prior to DNA delivery at concentrations between 1–720 or 1–1,000 nmol/l, respectively. Optimal GC delivery timing was determined prior to larger scale studies (Supplementary Figure S1) As a vehicle control, EtOH was delivered to cells in place of GCs at the same time points and volumes as GCs. See Supplementary Figure S5 for treatment timeline.
Transfection. Complexes were formed as follows and added drop-wise to cell media 48 hours after seeding. All complexes were formed with either pEGFP-LUC plasmid DNA (Clonetech, Mountain View, CA), which encodes both the enhanced GFP and firefly luciferase protein (LUC), or pCMV-LacZ plasmid (Clonetech), which encodes the β-Galactosidase (β-gal) protein; both plasmids nonintegrating, produce transient transfection, and are under the direction of a CMV promoter. Plasmids were purified from Escherichia coli bacteria using Qiagen (Valencia, CA) regents and stored in Tris-EDTA buffer (10 mmol/l Tris, 1 mmol/l EDTA, PH 7.4) at −20 °C. Lipoplexes were formed with Lipofectamine LTX (LF-LTX) or Lipofectamine 2000 (LF-2000) (both Life Technologies, Grand Island, NY), following manufacturer's instructions. For LF-LTX, complexes were formed in serum-free, Opti-MEM media (Life Technologies) by first incubating plasmid DNA and “Plus” reagent diluted in media for 10 minutes at room temperature (RT), then adding transfection reagent diluted in media to the DNA and “Plus” reagent and incubating an additional 30 minutes at RT. For LF-2000, complexes were formed in serum-free, Opti-MEM media by adding transfection reagent diluted in media drop-wise to DNA in media and incubating for 20 minutes at RT. Lipoplex transfection conditions were optimized by varying lipid to DNA ratio and amount of DNA and optimized for high transfection and low cytotoxicity at 1:2 (LF-LTX) and 1:1.5 (LF-2000) lipid:DNA ratio and 0.28 µg DNA/cm2 (data not shown). For polyplexes, branched 25 kDa PEI (Sigma-Aldrich) was dissolved in Tris-buffered saline at 1 mg/ml and stored at −20 °C. Polyplexes were formed in 1× Tris-buffered saline solution by dropwise addition of PEI solution to DNA solution, briefly vortexed for 10 seconds, and incubated for 15 minutes at RT. Polyplex transfection conditions were optimized by varying nitrogen to phosphate ratio (N:P) and amount of DNA. Optimal conditions, for high transfection and low cytotoxicity at N:P of 20 and 0.32 µg DNA/cm2 of pDNA were used (data not shown).
Fluorescence microscopy. Microscopy was conducted at 24, 48, and 72 hours following DNA delivery to confirm eGFP protein expression and evaluate cell morphology using a Lecia DMI 3000B fluorescence microscope (Lecia Microsystems GmbH, Wetzlar, Germany).
Protein assays. Cells were washed with 1× PBS, then lysed with 200 µl of 1× reporter lysis buffer (Promega, Madison, WI) and gentle rocking for 15 minutes at RT. Lysates were stored at −80 °C if not analyzed immediately. Luciferase expression levels were quantified by luminescence in relative light units with a Luciferase Assay kit (Promega) and luminometer (Turner Designs, Sunnyvale, CA). β-gal activity was quantified by colorimetric assay using the β-Galactosidase Enzyme Assay System (Promega) and spectrophotometer (Beckman Coulter, Indianapolis, IN); absorbances collected at 420 nm. Both Luciferase and B-gal transfection levels were normalized to total protein levels determined with a Pierce BCA protein colorimetric assay (Pierce, Rockford, IL); absorbances collected at 562 nm.
Flow cytometry. Flow cytometry was used to quantify EGFP expression. Cells were washed twice with 1× PBS, and dissociated with 0.05% trypsin-EDTA, an equal volume of growth media was then added. Three wells of identical treatments were pooled into 5 ml round bottomed tubes (BD Falcon, Bedford, MA) and placed on ice. Flow cytometry was conducted on a FACS Calibur (Beckton Dickinson Biosciences, San Jose, CA) with an excitation at 488 nm. GFP signal was collected on the FL1 detector using a 530/30 band-pass filter. Analysis was performed with a live cell gate in forward and side scatter to remove cell debris and clumped cells. A minimum of 5,000 events per treatment was collected for reporting of transfection efficiency (% GFP). Acquisition and analysis were performed using CellQuest Pro (BD Biosciences). Flow cytometry data was collected in duplicate (three pooled wells/n) on duplicate days for an n = 4 per donor.
Metabolic activity. Metabolic activity was assessed using a Water Soluble Tetrazolium (WST-1) salt cell proliferation assay kit (Roche, Indianapolis, IN), according to manufacturer's protocol. Briefly, cells were washed with 1× PBS and incubated at 37 °C in WST-1 solution (10 vol% WST-1 reagent in phenol-free Dulbecco's Modified Eagle Medium) for 5 hours. After incubation, absorbance values were measured on an Epoch Microplate spectrophotometer (BioTek, Winooski, VT) at 450 nm.
Plasmid quantification. To quantify plasmids internalized into the cytoplasm and cell nucleus, hMSCs were seeded into eight T-75 flasks (Falcon): 4 DEX treatment, and 4 EtOH control, then transfected as described previously. After 48 hours, cells were dissociated and four flasks per treatment were combined and resuspended in 2 ml of nuclei buffer (10 mmol/l PIPES buffer at ph 7.4, 1 mmol/l dithiothreitol, 2 mmol/l MgCl2, 10 mmol/l KCl). Cells were incubated at RT in nuclei buffer for 30 minutes, then lysed via dounce homogenizer (10 strokes). Treatment lysates were split in half: 1 ml for cellular plasmid quantification and 1 ml for nuclear plasmid quantification. Nuclei were isolated via iodixanol gradient, as previously described.38 Nuclei isolation was confirmed by Hoechst stain and fluorescence microscopy (data not shown). Both nuclei and whole cell suspension were treated with 0.5% sodium dodecyl sulfate and DNA was collected in the aqueous phase by phenol extraction and further purified by performing a second extraction of the aqueous phase with 25:24:1 phenol/chloroform/isoamyl alcohol (Thermo-Fisher). After an ethanol precipitation, the DNA was suspended in ddH2O. DNA quality was assessed by spectrophotometric absorbance ratio of 260/280 nm with a NanoDrop (Thermo Scientific) (data not shown). Quantification of nuclear plasmids was performed as previously described38 using quantitative real-time reverse-transcription polymerase chain reaction (qRT-PCR) to determine the number of plasmid copies. qRT-PCR data was normalized as previously described37 to cell number determined by using the slope obtained for eGFP curve to convert CT value obtained for ACTA1 to copy number. ACTA1 was assumed to be a single copy gene and to have the same qRT-PCR efficiencies as eGFP; therefore every two copies of ACTA1 represent a single cell. ACTA1 primers (IDT, Skokie, IL) were designed: Forward 5′-TCAGAAAGATTCCTACGTGGGCGA-3′, ACTA1 Reverse 5′-TGTGGTGCCAGATCTTCTCCATGT-3′ and eGFP primer sequences were used from.50 Due to the high volume of cells required to perform this assay, data points were collected at pooled n = 1 (from four flasks of treated cells) for each donor and treatment.
hMSC differentiation. Five differentiation treatments were evaluated for adipogenic and osteogenic differentiation: undifferentiated cells, standard differentiation, 1.2E−7 M DEX pretreatment followed by standard differentiation, 1.2E−7 M DEX pretreatment and lipofection followed by standard differentiation, and EtOH pretreatment and lipofection followed by standard differentiation, all following manufacturer's protocols. Briefly for adipogenesis, hMSCs were seeded at 21,000 cells/cm2 for untransfected treatments and 6,000 cells/cm2 for transfected treatments. Following GC-priming and lipofection as described above, cells were grown to 100% confluence followed by three weeks of media changes: three rounds of two days in adipogenic induction media then three days in adipogenic maintenance media, ending in seven days in maintenance media. Briefly for osteogenic differentiation, hMSCs were seeded at 3,100 cells/cm2 for untransfected treatments and 6,000 cells/cm2 for transfected treatments. Following GC-priming and lipofection as described above, cells were immediately differentiated by maintenance in osteogenic cell media for 3 weeks with media changes every 3 days.
Differentiation was evaluated via cell staining and quantified by absorbance. After differentiation, cells were washed in 2 ml of 1× PBS before fixation in 4% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA) for 20 minutes. Cells were then washed with 2 ml of ddH2O per well. For adipose cells, cells were then incubated at RT with 2 ml of 60% Isopropanol for five minutes. After incubation, isopropanol was replaced with 2 ml of Oil Red O (three parts Oil Red O in two parts 1× PBS) (Sigma-Aldrich) and incubated at RT for 5 minutes. After incubation, Oil Red O was removed, and cells were washed three times with 2 ml of RT ddH2O. Bound Oil Red O was then eluted with 1 ml 99% isopropanol, and the elution absorbance was read at 540 nm by spectrophotometer (BioTek, Winooski, VT). For osteoblast cells, cells were incubated at RT for 45 minutes in 2 ml Alizarin Red (pH 4.2) (Santa Cruz Biotechnology, Dallas, TX). Alizarin Red was removed and bound stain was eluted with 800 μl of 50 °C, 10% acetic acid (v/v) and incubated at RT for 30 minutes. Elution absorbance was read at 405 nm by spectrophotometer (BioTek). All absorbance data was normalized to the undifferentiated controls.
Statistical analysis. All experiments were performed in triplicate wells evaluated on the same day or as pooled triplicate wells evaluated on triplicate days (n = 3), unless otherwise noted. Due to the influence of cell passage number on stem cell behavior and limited cell availability for each donor, no statistical methods were used to predetermine sample size. Experiments were conducted with as many donors as possible, based on cell availability. All data for independent data points are reported as mean ± standard error. Where only one experiment could be conducted, data for replicate values are reported as the mean ± standard deviation, as noted. Comparative analyses were completed using one- or two-way analysis of variance with Bonferroni post-hoc analysis depending on the comparisons being drawn. All data collected and analyzed were assumed to be normally distributed. Statistical difference was considered at P ≤ 0.01 (**) and P ≤ 0.001 (***). Statistics and fold changes highlighted within figures are between primed treatments versus unprimed (EtOH) treatments, unless noted. Statistics were calculated using Prism GraphPad software (GraphPad Software, La Jolla, CA).
SUPPLEMENTARY MATERIAL Figure S1. GC delivery time effect on transfection outcomes. Figure S2. Effect of cell passage number on transfection outcomes following GC-priming. Figure S3. Preliminary data for GC-priming of ASCs. Figure S4. Fluorescence images of ASCs primed with GCs vs. EtOH controls. Figure S5. Experimental design timeline.
Acknowledgments
Support for this research was provided in part by funds from the National Science Foundation Graduate Research Fellowship (DGE-10410000) and CAREER (CBET-1254415), American Heart Association (10SDG2640217), University of Nebraska-Lincoln Tobaccos Settlement Funds, and USDA CSREES-Nebraska (NEB-21–146 and NEB-26–211). This study was supported in part by the Flow Cytometry core facility funded by NIGMS grant number P30 GM103509. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. The authors have no potential conflicts of interest.
Supplementary Material
References
- Satija, NK, Singh, VK, Verma, YK, Gupta, P, Sharma, S, Afrin, F et al. (2009). Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med 13: 4385–4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uccelli, A, Pistoia, V and Moretta, L (2007). Mesenchymal stem cells: a new strategy for immunosuppression? Trends Immunol 28: 219–226. [DOI] [PubMed] [Google Scholar]
- Friedenstein, AJ, Chailakhjan, RK and Lalykina, KS (1970). The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet 3: 393–403. [DOI] [PubMed] [Google Scholar]
- Fraser, JK, Wulur, I, Alfonso, Z and Hedrick, MH (2006). Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol 24: 150–154. [DOI] [PubMed] [Google Scholar]
- Orciani, M and Di Primio, R (2013). Skin-derived mesenchymal stem cells: isolation, culture, and characterization. Methods Mol Biol 989: 275–283. [DOI] [PubMed] [Google Scholar]
- Jankowski, RJ, Deasy, BM and Huard, J (2002). Muscle-derived stem cells. Gene Ther 9: 642–647. [DOI] [PubMed] [Google Scholar]
- Chong, PP, Selvaratnam, L, Abbas, AA and Kamarul, T (2012). Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res 30: 634–642. [DOI] [PubMed] [Google Scholar]
- Hass, R, Kasper, C, Böhm, S and Jacobs, R (2011). Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun Signal 9: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada, N, Gronthos, S and Bartold, PM (2013). Immunomodulatory effects of stem cells. Periodontol 2000 63: 198–216. [DOI] [PubMed] [Google Scholar]
- Caplan, AI (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213: 341–347. [DOI] [PubMed] [Google Scholar]
- Nauta, AJ and Fibbe, WE (2007). Immunomodulatory properties of mesenchymal stromal cells. Blood 110: 3499–3506. [DOI] [PubMed] [Google Scholar]
- Jorgensen, C, Djouad, F, Apparailly, F and Noël, D (2003). Engineering mesenchymal stem cells for immunotherapy. Gene Ther 10: 928–931. [DOI] [PubMed] [Google Scholar]
- Naldini, L (2011). Ex vivo gene transfer and correction for cell-based therapies. Nat Rev Genet 12: 301–315. [DOI] [PubMed] [Google Scholar]
- Heilbronn, R, Weger, S. Viral vectors for Gene Transfer: Current Status of Gene Therapeutics. Handb Exp Pharmacol 197, 143–170 (2010). [DOI] [PubMed] [Google Scholar]
- Chen, CY, Wu, HH, Chen, CP, Chern, SR, Hwang, SM, Huang, SF et al. (2011). Biosafety assessment of human mesenchymal stem cells engineered by hybrid baculovirus vectors. Mol Pharm 8: 1505–1514. [DOI] [PubMed] [Google Scholar]
- Ojala, DS, Amara, DP and Schaffer, DV (2015). Adeno-associated virus vectors and neurological gene therapy. Neuroscientist 21: 84–98. [DOI] [PubMed] [Google Scholar]
- Jones, CH, Chen, CK, Ravikrishnan, A, Rane, S and Pfeifer, BA (2013). Overcoming nonviral gene delivery barriers: perspective and future. Mol Pharm 10: 4082–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L, Gao, Y, Xue, YN, Huang, SW and Zhuo, RX (2013). The effectiveness, cytotoxicity, and intracellular trafficking of nonviral vectors for gene delivery to bone mesenchymal stem cells. J Bioact Polym 28: 204–217. [Google Scholar]
- King, WJ, Kouris, NA, Choi, S, Ogle, BM and Murphy, WL (2012). Environmental parameters influence non-viral transfection of human mesenchymal stem cells for tissue engineering applications. Cell Tissue Res 347: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madeira, C, Mendes, RD, Ribeiro, SC, Boura, JS, Aires-Barros, MR, da Silva, CL et al. (2010). Nonviral gene delivery to mesenchymal stem cells using cationic liposomes for gene and cell therapy. J Biomed Biotechnol 2010: 735349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W, Li, W, Ou, L, Flick, E, Mark, P, Nesselmann, C et al. (2011). Polyethylenimine-mediated gene delivery into human bone marrow mesenchymal stem cells from patients. J Cell Mol Med 15: 1989–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plautz, SA, Boanca, G, Riethoven, JJ and Pannier, AK (2011). Microarray analysis of gene expression profiles in cells transfected with nonviral vectors. Mol Ther 19: 2144–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, TM, Plautz, SA and Pannier, AK (2013). Network analysis of endogenous gene expression profiles after polyethyleneimine-mediated DNA delivery. J Gene Med 15: 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercauteren, D, Rejman, J, Martens, TF, Demeester, J, De Smedt, SC and Braeckmans, K (2012). On the cellular processing of non-viral nanomedicines for nucleic acid delivery: mechanisms and methods. J Control Release 161: 566–581. [DOI] [PubMed] [Google Scholar]
- Coutinho, AE and Chapman, KE (2011). The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol Cell Endocrinol 335: 2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwenberg, M, Stahn, C, Hommes, DW and Buttgereit, F (2008). Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids 73: 1025–1029. [DOI] [PubMed] [Google Scholar]
- Kastrup, L, Oberleithner, H, Ludwig, Y, Schafer, C and Shahin, V (2006). Nuclear envelope barrier leak induced by dexamethasone. J Cell Physiol 206: 428–434. [DOI] [PubMed] [Google Scholar]
- Shahin, V (2006). The nuclear barrier is structurally and functionally highly responsive to glucocorticoids. Bioessays 28: 935–942. [DOI] [PubMed] [Google Scholar]
- Kapitulnik, J, Weil, E and Rabinowitz, R (1986). Glucocorticoids increase the fluidity of the fetal-rat liver microsomal membrane in the perinatal period. Biochem J 239: 41–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruneich, JA, Price, A, Zhu, J and Diamond, SL (2004). Cationic corticosteroid for nonviral gene delivery. Gene Ther 11: 668–674. [DOI] [PubMed] [Google Scholar]
- Kim, HA, Yi, N, Ryu, JW, Oh, B, Lee, M. Development of Dexamethasone Conjugated Low Molecular Weight Polyethylenimine as a Gene Delivery Carrier for Gene Therapy of Glioblastoma. Mol Ther 20, S157–S158 (2012). [Google Scholar]
- Braun, S, Jenny, C, Thioudellet, C, Perraud, F, Claudepierre, MC, Längle-Rouault, F et al. (1999). In vitro and in vivo effects of glucocorticoids on gene transfer to skeletal muscle. FEBS Lett 454: 277–282. [DOI] [PubMed] [Google Scholar]
- Bernasconi, AG, Rebuffat, AG, Lovati, E, Frey, BM, Frey, FJ and Galli, I (1997). Cortisol increases transfection efficiency of cells. FEBS Lett 419: 103–106. [DOI] [PubMed] [Google Scholar]
- Xiao, Y, Peperzak, V, van Rijn, L, Borst, J and de Bruijn, JD (2010). Dexamethasone treatment during the expansion phase maintains stemness of bone marrow mesenchymal stem cells. J Tissue Eng Regen Med 4: 374–386. [DOI] [PubMed] [Google Scholar]
- Ankrum, JA, Dastidar, RG, Ong, JF, Levy, O and Karp, JM (2014). Performance-enhanced mesenchymal stem cells via intracellular delivery of steroids. Sci Rep 4: 4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewson, CA, Campwala, H, Liu, WL, Yeadon, M and Ballard, SA (2009). Use of the glucocorticoid receptor antagonist, RU486 (Mifepristone) in an in vitro competition assay to explore the functional duration of action of corticosteroids. Am J Respir Crit Care Med 179: 1.19098155 [Google Scholar]
- Martin, TM, Wysocki, BJ, Beyersdorf, JP, Wysocki, TA and Pannier, AK (2014). Integrating mitosis, toxicity, and transgene expression in a telecommunications packet-switched network model of lipoplex-mediated gene delivery. Biotechnol Bioeng 111: 1659–1671. [DOI] [PubMed] [Google Scholar]
- Cohen, RN, van der Aa, MA, Macaraeg, N, Lee, AP, Szoka Jr, FC (2009). Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J Control Release 135, 166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa, AR, Lane, SJ, Cidlowski, JA, Staynov, DZ and Lee, TH (2000). Glucocorticoid resistance in asthma is associated with elevated in vivo expression of the glucocorticoid receptor beta-isoform. J Allergy Clin Immunol 105: 943–950. [DOI] [PubMed] [Google Scholar]
- Sun, X, Fischer, DR, Pritts, TA, Wray, CJ and Hasselgren, PO (2002). Expression and binding activity of the glucocorticoid receptor are upregulated in septic muscle. Am J Physiol Regul Integr Comp Physiol 282: R509–R518. [DOI] [PubMed] [Google Scholar]
- Esmailpour, N, Högger, P and Rohdewald, P (1998). Binding kinetics of budesonide to the human glucocorticoid receptor. Eur J Pharm Sci 6: 219–223. [DOI] [PubMed] [Google Scholar]
- Ribeiro, S, Mairhofer, J, Madeira, C, Diogo, MM, Lobato da Silva, C, Monteiro, G et al. (2012). Plasmid DNA size does affect nonviral gene delivery efficiency in stem cells. Cell Reprogram 14: 130–137. [DOI] [PubMed] [Google Scholar]
- Crisostomo, PR, Wang, M, Wairiuko, GM, Morrell, ED, Terrell, AM, Seshadri, P et al. (2006). High passage number of stem cells adversely affects stem cell activation and myocardial protection. Shock 26: 575–580. [DOI] [PubMed] [Google Scholar]
- Deng, W, Fu, M, Cao, Y, Cao, X, Wang, M, Yang, Y et al. (2013). Angelica sinensis polysaccharide nanoparticles as novel non-viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine 9: 1181–1191. [DOI] [PubMed] [Google Scholar]
- Park, JS, Na, K, Woo, DG, Yang, HN, Kim, JM, Kim, JH et al. (2010). Non-viral gene delivery of DNA polyplexed with nanoparticles transfected into human mesenchymal stem cells. Biomaterials 31: 124–132. [DOI] [PubMed] [Google Scholar]
- Studeny, M, Marini, FC, Champlin, RE, Zompetta, C, Fidler, IJ and Andreeff, M (2002). Bone marrow-derived mesenchymal stem cells as vehicles for interferon-beta delivery into tumors. Cancer Res 62: 3603–3608. [PubMed] [Google Scholar]
- Hoare, M, Greiser, U, Schu, S, Mashayekhi, K, Aydogan, E, Murphy, M et al. (2010). Enhanced lipoplex-mediated gene expression in mesenchymal stem cells using reiterated nuclear localization sequence peptides. J Gene Med 12: 207–218. [DOI] [PubMed] [Google Scholar]
- Haleem-Smith, H, Derfoul, A, Okafor, C, Tuli, R, Olsen, D, Hall, DJ et al. (2005). Optimization of high-efficiency transfection of adult human mesenchymal stem cells in vitro. Mol Biotechnol 30: 9–20. [DOI] [PubMed] [Google Scholar]
- Lin, T, Gu, J, Zhang, L, Davis, JJ, Huang, X, Cabbini, G et al. (2003). Enhancing adenovirus-mediated gene transfer in vitro and in vivo by addition of protamine and hydrocortisone. J Gene Med 5: 868–875. [DOI] [PubMed] [Google Scholar]
- Hama, S, Akita, H, Iida, S, Mizuguchi, H and Harashima, H (2007). Quantitative and mechanism-based investigation of post-nuclear delivery events between adenovirus and lipoplex. Nucleic Acids Res 35: 1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.