Abstract
Immunotherapy with T cells expressing chimeric antigen receptors (CARs) is an attractive approach to improve outcomes for patients with glioblastoma (GBM). IL13Rα2 is expressed at a high frequency in GBM but not in normal brain, making it a promising CAR T-cell therapy target. IL13Rα2-specific CARs generated up to date contain mutated forms of IL13 as an antigen-binding domain. While these CARs target IL13Rα2, they also recognize IL13Rα1, which is broadly expressed. To overcome this limitation, we constructed a panel of IL13Rα2-specific CARs that contain the IL13Rα2-specific single-chain variable fragment (scFv) 47 as an antigen binding domain, short or long spacer regions, a transmembrane domain, and endodomains derived from costimulatory molecules and CD3.ζ (IL13Rα2-CARs). IL13Rα2-CAR T cells recognized IL13Rα2-positive target cells in coculture and cytotoxicity assays with no cross-reactivity to IL13Rα1. However, only IL13Rα2-CAR T cells with a short spacer region produced IL2 in an antigen-dependent fashion. In vivo, T cells expressing IL13Rα2-CARs with short spacer regions and CD28.ζ, 41BB.ζ, and CD28.OX40.ζ endodomains had potent anti-glioma activity conferring a significant survival advantage in comparison to mice that received control T cells. Thus, IL13Rα2-CAR T cells hold the promise to improve current IL13Rα2-targeted immunotherapy approaches for GBM and other IL13Rα2-positive malignancies.
Introduction
The outcome for glioblastoma (GBM) remains poor and immunotherapy with vaccines or GBM-specific T cells is one attractive strategy to improve survival, since it does not rely on the cytotoxic mechanisms employed by conventional therapies such as chemotherapy and radiation.1,2,3 Genetic modification with chimeric antigen receptors (CARs) allows for the rapid generation of tumor-specific T cells. For GBM-targeted CAR T-cell therapies, several antigens are actively being pursued including interleukin 13 receptor α2 (IL13Rα2), human epidermal growth factor receptor 2 (HER2), epidermal growth factor variant III (EGFRvIII), and erythropoietin-producing hepatocellular carcinoma A2 (EphA2).4,5,6,7,8,9,10,11
IL13Rα2, a cancer testis antigen, is an ideal target for CAR T-cell therapy for GBM since it is expressed in 50–80% of GBM cells and glioma-initiating cells, which are resistant to conventional therapies, but not in normal brain.12,13,14,15 IL13Rα2 expression is associated with poor prognosis,15 and while initial studies indicated that IL13Rα2 is a “decoy receptor”, more recent studies have demonstrated that IL13Rα2 prevents apoptosis and induces TGF-β secretion.16,17,18
CARs consist of an ectodomain, which is most commonly derived from a single-chain variable fragment (scFv), a spacer region, a transmembrane domain, and an endomain.19,20,21 Current IL13Rα2-specific CARs do not contain scFvs, but an IL13-mutein with one or two amino acid substitutions to preferentially redirect T cells to IL13Rα2.7,8,9,10 While one study showed that T cells expressing IL13-mutein CARs did not recognize IL13Rα1-positive cells,22 other studies including ours demonstrated that IL13-mutein CAR T cells readily recognize IL13Rα1-positive targets raising concerns of ‘on target/off cancer toxicity'.7,8
To selectively target IL13Rα2, we previously had generated a monoclonal antibody (MAb) and scFv that specifically recognized IL13Rα2 (clone 47; scFv47) with a high affinity and no cross-reactivity to IL13Rα1.23,24 Here, we report the development of IL13Rα2-specific CARs with a scFv47-based antigen-binding domain (IL13Rα2-CARs). We show that IL13Rα2-CARs require a short spacer region for optimal functionality, and that IL13Rα2-CAR T cells are able to recognize and kill only IL13Rα2-positive and not IL13Rα1-positive target cells in vitro. In addition, IL13Rα2-CAR T cells induce tumor regression in an orthotopic xenograft mouse model of GBM, which was associated with a significant survival advantage.
Results
Generation of IL13Rα2-CAR T cells
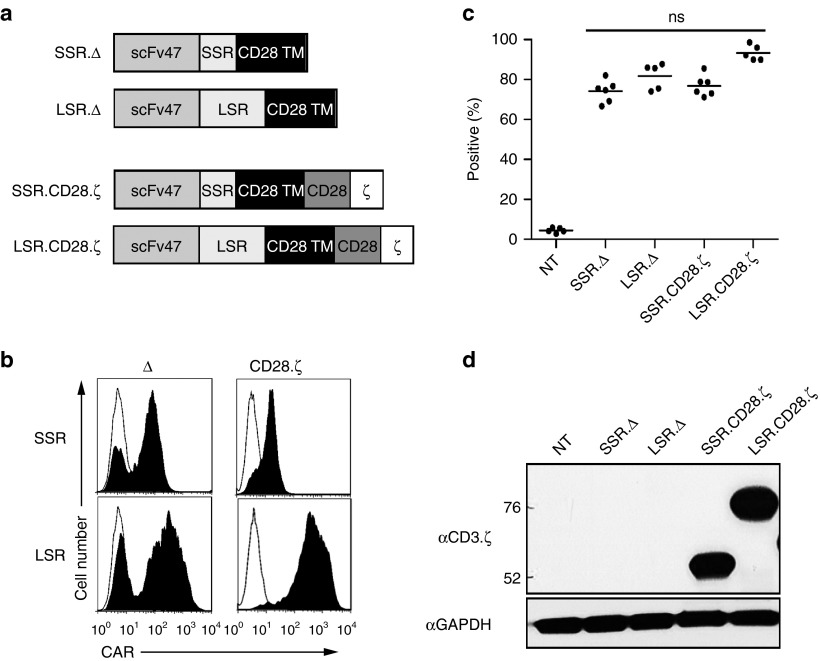
We initially generated two retroviral vectors encoding CARs based on the IL13Rα2-specific MAb clone 47 (Figure 1a).23,24 Both CARs contained a N-terminal leader sequence, a codon-optimized synthetic gene encoding scFv47, a spacer region, a CD28 transmembrane domain, and signaling domains derived from CD28 and CD3.ζ (Figure 1a). As the spacer region, we either used the IgG1 hinge (16 amino acids; short spacer region; IL13Rα2-CAR.SSR.CD28.ζ) or the IgG1-CH2CH3 domain (239 amino acids; long spacer region; IL13Rα2-CAR.LSR.CD28.ζ). As controls, LSR and SSR IL13Rα2-CARs without signaling domains were constructed (IL13Rα2-CAR.SSR.Δ, IL13Rα2-CAR.LSR.Δ; Figure 1a). CD3/CD28-activated T cells from healthy donors were transduced with RD114-pseudotyped retroviral particles, and 4 to 5 days post-transduction T-cell phenotype and CAR expression was determined by fluorescence activated cell sorting (FACS) analysis. CARs were expressed on the cell surface with the transduction efficiency ranged from 74.1 to 93.3% and no significant differences between constructs (Figure 1b,c). Expression of full-length IL13Rα2-CAR.SSR.CD28.ζ and IL13Rα2-CAR.LSR.CD28.ζ was confirmed by western blot using a CD3.ζ antibody for detection (Figure 1d). Western blot analysis under nonreducing conditions revealed no significant differences in dimer/multimer formation between LSR AND SSR CARs (Supplementary Figure S2). Phenotypic analysis revealed a mixture CD4- and CD8-positive T cells. While the ratio of CD8- to CD4-positive T cells was ~3:1 for IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.SSR.Δ, and IL13Rα2-CAR.LSR.Δ T-cell lines, it was ~1.5:1 for IL13Rα2-CAR.LSR.CD28.ζ (Supplementary Figure S1).
Figure 1.
Generation of IL13Rα2 CAR T cells. (a) Scheme of IL13Rα2 CARs. All CARs contained an N-terminal leader sequence, a codon-optimized synthetic gene encoding for scFv47, a spacer region, a CD28 transmembrane domain, and signaling domains derived from CD28 and CD3.ζ. Spacer region was either the IgG1 hinge (16 amino acids; short spacer region; IL13Rα2-CAR.SSR.CD28.ζ) or the IgG1-CH2CH3 domain (239 amino acids; long spacer region; IL13Rα2-CAR.LSR.CD28.ζ). LSR.Δ and SSR.Δ IL13Rα2-CARs without signaling domains were constructed and served as controls. (b,c) CAR expression was confirmed using FACS analysis. Representative plots (b) and summary data (c) is shown (mean 74.1–93.3%, n = 5–6 per CAR construct). (d) Expression of full-length IL13Rα2-CAR.SSR.CD28.ζ and IL13Rα2-CAR.LSR.CD28.ζ by western blot analysis using a CD3-ζ antibody. CAR, chimeric antigen receptor.
IL13Rα2-CAR T cells recognize IL13Rα2 but not IL13Rα1
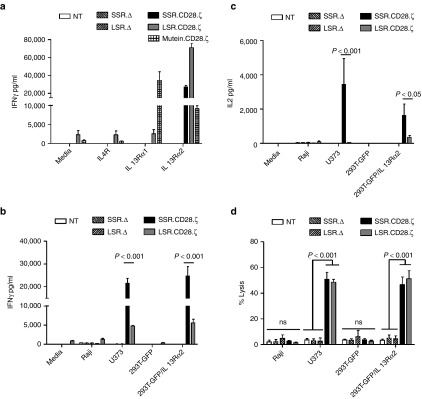
To initially determine the specificity of IL13Rα2-CARs, we cultured T cells expressing IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.LSR.CD28.ζ, IL13Rα2-CAR.SSR.Δ, or IL13Rα2-CAR.LSR.Δ on tissue culture plates that were uncoated or coated with recombinant proteins IL13Rα1, IL13Rα2, or IL4Rα. Nontransduced (NT) T cells and T cells expressing an IL13mutein-CAR.LSR.CD28.ζ10 that recognizes IL13Rα1 and IL13Rα2 served as controls. T cells expressing IL13Rα2-CAR.SSR.CD28.ζ or IL13Rα2-CAR.LSR.CD28.ζ produced significant levels of IFNγ (n = 4, P < 0.001) when stimulated with recombinant IL13Rα2 proteins in comparison to IL13Rα1- or IL4Rα-stimulated T cells (Figure 2a). In contrast, T cells expressing IL13Rα2-CAR.SSR.Δ or IL13Rα2-CAR.LSR.Δ produced no IFNγ in response to all three proteins, indicating that IFNγ production depends on an intact IL13Rα2-CAR signaling domain. IL13Rα2-CAR.LSR.CD28.ζ T cells also produced low levels of IFNγ without activation, indicating “baseline” T-cell activation, which was confirmed by intracellular staining for phosphorylated CD3.ζ (Supplementary Figure S3). IL13mutein-CAR.LSR.CD28.ζ T cells produced significant levels of IFNγ in the presence of IL13Rα1 (n = 4, P < 0.001) and IL13Rα2 (n = 4, P < 0.05) in comparison to NT T cells.
Figure 2.
IL13Rα2-CAR T cells release cytokines after stimulation with recombinant IL13Rα2 protein or IL13Rα2-positive cells. IL13Rα2-CAR or nontransduced (NT) T cells were stimulated with recombinant IL13Rα1, IL13Rα2, or IL4Rα proteins. After 24 hours, IFNγ (a) was measured by ELISA (n = 4). T cells expressing IL13Rα2-CAR constructs, but not controls, expressed significant levels of IFNγ (P < 0.001) when stimulated with recombinant IL13Rα2 protein in comparison to IL13Rα1 and IL4Rα stimulated T cells. IL13Rα2-CAR T cells were cocultured with Raji, U373 cells, 293T-GFP, and 293T-GFP/IL13Rα2 at a 2:1 E:T ratio. NT and CAR.Δ T cells served as controls. (b,c) After 24h cytokines (IFNγ, IL2) were measured by ELISA. (b) U373 and 293T-GFP-IL13Rα2 (IFNγ); SSR.Δ versus SSR.CD28.ζ: n = 6, P < 0.001; LSR.Δ versus LSR.CD28.ζ: n = 6, P < 0.05. (c) U373 and 293T-GFP-IL13Rα2 (IL2); SSR.Δ versus SSR.CD28.ζ: n = 4, P < 0.01; LSR.Δ versus LSR.CD28.ζ: n = 4, NS. (d) Four hours cytotoxicity assay at an E:T ratio of 10:1 (n = 4). CAR, chimeric antigen receptor.
We next confirmed the specificity of IL13Rα2-CAR T cells using cell lines that were negative for IL13Rα1 and IL13Rα2 (Raji), positive for IL13Rα1 (293T-GFP cells), or positive for IL13Rα1 and IL13Rα2 (U373, 293T-GFP/IL13Rα2; Supplementary Figure S4). T cells expressing IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.LSR.CD28.ζ, IL13Rα2-CAR.SSR.Δ, or IL13Rα2-CAR.LSR.Δ were cocultured with Raji, 293T-GFP, or 293T-GFP/IL13Rα2 cells. NT T cells served as controls. After 24 hours, media was collected and the concentration of IFNγ and IL2 was determined by ELISA. IL13Rα2-CAR.SSR.CD28.ζ and IL13Rα2-CAR.LSR.CD28.ζ T cells produced significant amounts of IFNγ only in the presence of U373 or 293T-GFP/IL13Rα2 cells (Figure 2b) with SSR.CAR T cells producing significant more IFNγ than LSR.CAR T cells (n = 6, P < 0.001). IL13Rα2-CAR.SSR.CD28.ζ T cells produced also significant amounts of IL2 in the presence of 293T-GFP/IL13Rα2 and U373 cells, while IL13Rα2-CAR.LSR.CD28.ζ T cells did not (Figure 2c). NT T cells and T cells expressing IL13Rα2-CAR.SSR.Δ or IL13Rα2-CAR.LSR.Δ produced no IFNγ or IL2 in response to any target cells. Finally, we confirmed the specificity of IL13Rα2-CAR T cells in standard cytotoxicity assays using Raji, 293T-GFP, 293T-GFP/IL13Rα2, U373 (Figure 2d). In addition, IL13Rα2-CAR T cells killed the IL13Rα2-positive glioma cell line U87 and primary glioma cells, while IL13Rα2-negative primary glioma cells were not killed (Supplementary Figure S5).
Generation of SSR IL13Rα2-CARs with CD28.OX40.ζ, CD28.41BB.ζ, or 41BB.ζ endodomains
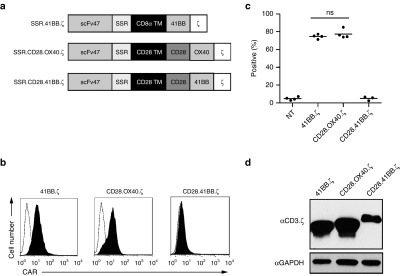
While the results described above demonstrated that IL13Rα2-CAR T cells only recognize IL13Rα2 as judged by cytokine production and cytolytic activity, they also highlighted differences between LSR and SSR IL13Rα2-CARs. Since only IL13Rα2-CAR.SSRs produced IL2 in the presence of IL13Rα2-positive target cells, we focused in the next set of experiments on IL13Rα2-CARs with SSRs, and generated additional CARs with CD28.OX40.ζ, CD28.41BB.ζ, or 41BB.ζ endodomains (Figure 3a). CAR T cells were generated by retroviral transduction and CAR expression on the cell surface was determined by FACS analysis (Figure 3b,c) and western blot (Figure 3d). While all CARs were expressed by T cells as judged by western blot analysis, IL13Rα2-CAR.SSR.CD28.41BB.ζ was not expressed on the cell surface, and was excluded from further analysis.
Figure 3.
Generation of SSR IL13Rα2-CARs with CD28.OX40.ζ, CD28.41BB.ζ or 41BB.ζ endodomains. (a) Scheme of SSR IL13Rα2-CARs. (b,c) CAR expression was confirmed using FACS analysis. Representative plots (b) and summary data (c) is shown. IL13Rα2-CAR.SSR.CD28.OX40.ζ and IL13Rα2-CAR.SSR.CD28.41BB.ζ: mean: 74.6–77.5% (n = 4); IL13Rα2-CAR.SSR.CD28.41BB.ζ: mean: 4.9% (n = 3). (d) Expression of IL13Rα2-CAR.SSR.41BB.ζ, IL13Rα2-CAR.SSR.OX40.CD28.ζ, and IL13Rα2-CAR.SSR.41BB.CD28.ζ by western blot analysis. CAR, chimeric antigen receptor.
Functional comparison of IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.SSR.41BB.ζ, and IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells
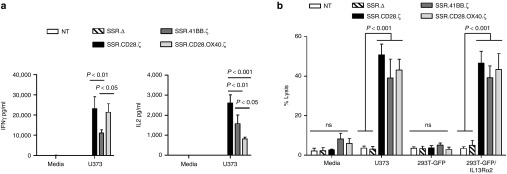
To compare the ability of IL13Rα2-CAR.SSR T cells to produce IFNγ and IL2 in response to antigen exposure, we performed coculture assay with U373 cells. NT and T cells expressing IL13Rα2-CAR.SSR.Δ served as controls. All IL13Rα2-CAR.SSRs with functional endodomains induced IFNγ and IL2 production in the presence of U373 cells, however IL13Rα2-CAR.SSR.41BB.ζ T cells produced significantly less (n = 5, P < 0.05) IFNγ in comparison to IL13Rα2-CAR.SSR.CD28.ζ and IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells (Figure 4a). IL13Rα2-CAR.SSR.CD28.ζ T cells produced the highest amount of IL2, followed by IL13Rα2-CAR.SSR.41BB.ζ and IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells. In cytotoxicity assays, no significant difference was observed between all three constructs using Raji, 293T-GFP, 293T-GFP/IL13Rα2, and U373 as targets (Figure 4b).
Figure 4.
Comparison of IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.SSR.41BB.ζ, and IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells. (a) IL13Rα2-CAR T cells were cocultured with U373 cells at a 2:1 E:T ratio. NT and CAR.Δ T cells served as controls. After 24 hours, IFNγ and IL2 was measured by ELISA (n = 5); SSR.Δ versus SSR.CD28.ζ (U373; IFNγ): P < 0.001; SSR.Δ versus SSR.41BB.ζ (U373; IFNγ): P < 0.05; SSR.Δ versus SSR.CD28.OX40.ζ for (U373; IFNγ): P < 0.001; SSR.Δ versus SSR.CD28.ζ (U373; IL2): P < 0.001; SSR.Δ versus SSR.41BB.ζ (U373; IL2): P < 0.001; SSR.Δ versus SSR.CD28.OX40.ζ (U373; IL2): P < 0.01. (b) Four hours cytotoxicity assay at an E:T ratio of 10:1 (n = 4). CAR, chimeric antigen receptor; ELISA, enzyme-linked immunosorbent assay.
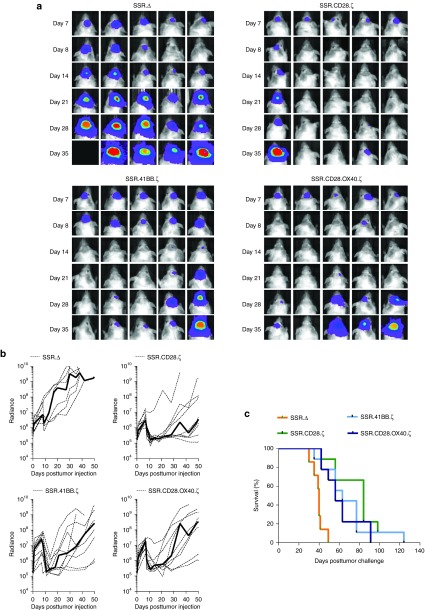
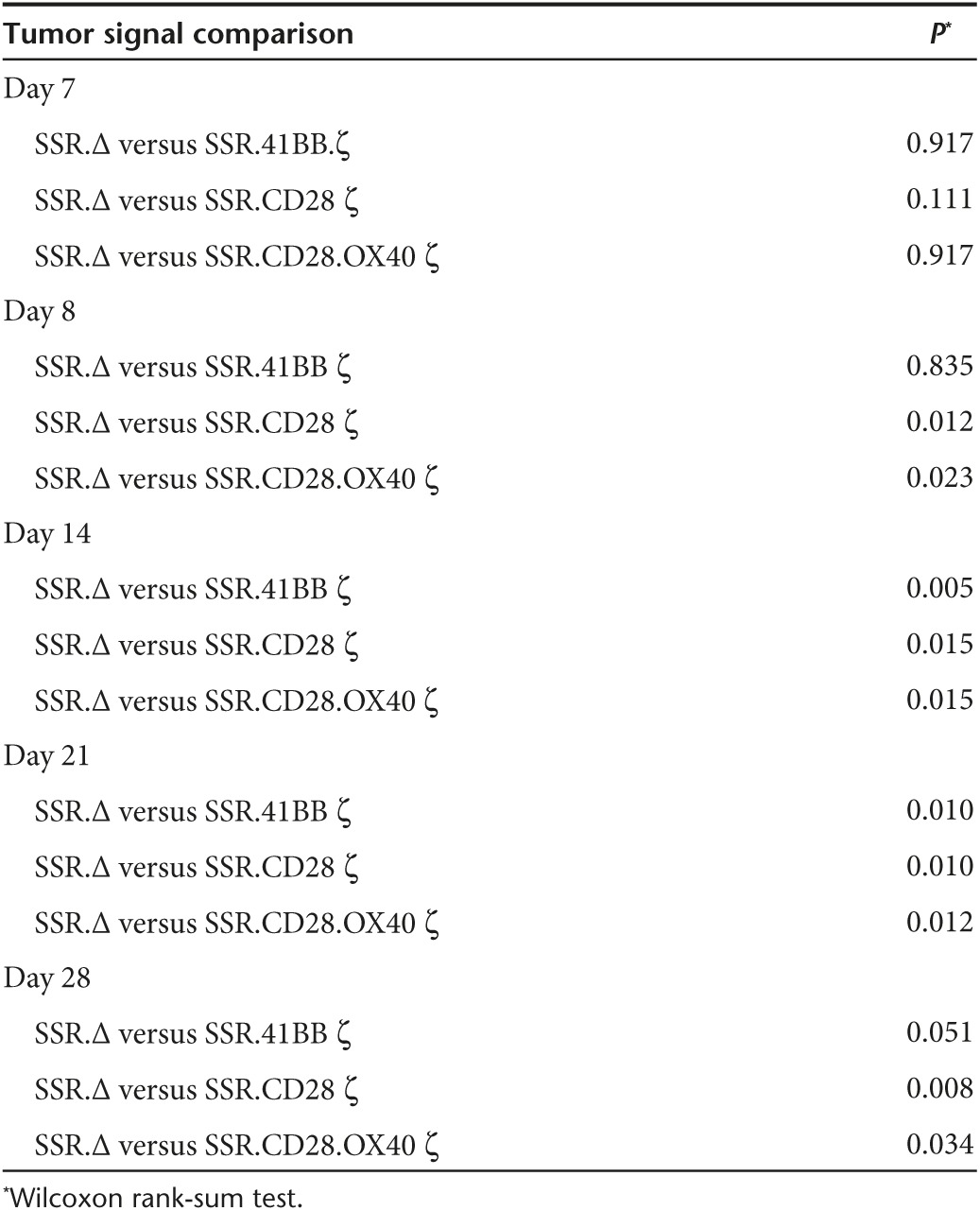
Since all three IL13Rα2-CAR.SSRs T cells with functional endodomains produced IL2, we tested all three constructs in our orthotopic U373 glioma xenograft mouse model in which T cells are directly injected into tumors.6 The model allows for serial bioluminescence imaging since U373 are genetically modified to express an eGFP.ffLuc fusion protein (U373.eGFP.ffLuc). On day 0, U373.eGFP.ffLuc cells were injected stereotactically into brains of SCID mice, and on day 7 T cells expressing IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.SSR.41BB.ζ, IL13Rα2-CAR.SSR.CD28.OX40.ζ, or IL13Rα2-CAR.SSR.Δ were injected intratumorally. While mice treated with IL13Rα2-CAR.SSR.Δ T cells showed continuous tumor growth within 4 days of T-cell injection, mice treated with IL13Rα2-CAR.SSR T cells that had functional endodomains did not (Figure 5a,b). Comparison of bioluminescence imaging results revealed no significant difference between IL13Rα2-CAR.SSR.Δ T cells and the IL13Rα2-CAR.SSR T cells groups on the day of T-cell injection. However, mice treated with IL13Rα2-CAR.SSR.CD28.ζ or IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells had significantly lower tumor signals as early as 1 day post-treatment in comparison to mice treated with IL13Rα2-CAR.SSR.Δ T cells (day 8, P = 0.012 and P = 0.023; Table 1). This resulted in a significant survival advantage of IL13Rα2-CAR.SSR.CD28.ζ or IL13Rα2-CAR.SSR.CD28.OX40.ζ T-cell-treated mice (P = 0.0002 and P = 0.0092; Figure 5c). While IL13Rα2-CAR.SSR.41BB.ζ T-cell-treated mice responded slower resulting in a significant difference between IL13Rα2-CAR.SSR.Δ T-cell-treated on day 14 (P = 0.005; Table 1), treatment also resulted in a significant survival advantage (P = 0.0039; Figure 5c). IL13Rα2-CAR.SSR.CD28.ζ T-cell-treated mice had the longest median survival (84 days). However, there was no statistical difference in comparison to the median survival of IL13Rα2-CAR.SSR.41BB.ζ (63 days) or IL13Rα2-CAR.SSR.CD28.OX40.ζ (56 days) T-cell-treated mice.
Figure 5.
Treatment of glioma xenograft with T cells expressing IL13Rα2-CARs results in tumor regression and improved overall survival. U373 glioma-bearing mice were treated on day 7 with SSR.CD28.ζ (n = 9), SSR.41BB.ζ (n = 9), or SSR.OX40.CD28.ζ (n = 9) T cells. SSR.Δ CAR T cells (n = 7) served as controls. (a) Representative images for each group and (b) quantitative bioluminescence (radiance = photons/sec/cm2/sr) imaging data for all mice are shown (dotted lines: individual mice; solid lines: median). (c) Kaplan-Meier survival analysis (SSR.Δ versus SSR.CD28.ζ: P = 0.0002; SSR.Δ versus SSR.41BB.ζ: P = 0.0039; SSR.Δ versus SSR.OX40.CD28.ζ: P = 0.0092; SSR.CD28.ζ versus SSR.41BB.ζ: P = 0.4723; SSR.CD28.ζ versus SSR.OX40.CD28.ζ: P = 0.3582; SSR.41BB.ζ versus SSR.OX40.CD28.ζ: P = 0.8374). CAR, chimeric antigen receptor.
Table 1. Tumor signal comparison.
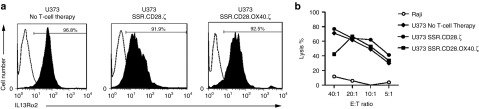
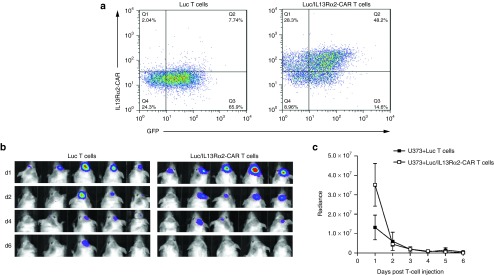
While IL13Rα2-CAR T cells had potent anti-glioma activity, mice eventually developed recurrent gliomas. To investigate the etiology of tumor recurrence, U373 cells were isolated from two tumor-bearing mice that had been treated either with IL13Rα2-CAR.SSR.CD28.ζ or IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells. FACS analysis after short-term culture revealed cell surface expression of IL13Rα2, and these cells were readily killed by IL13Rα2-CAR T cells in cytotoxicity assay (Figure 6). We next determined T-cell persistence by bioluminescent imaging of genetically modified T cells expressing IL13Rα2-CAR.SSR.CD28.ζ and eGFP.ffLuc (Luc/IL13Rα2-CAR T cells) injected into U373 tumors. T cells were detected for about 6 days making limited persistence the most likely explanation for tumor recurrence (Figure 7).
Figure 6.
Analysis of U373 cells isolated from recurrent tumors. U373 cells were isolated from recurrent tumor of mice that were treated with IL13Rα2-CAR T cells. After short-term culture (2–7 days), FACS analysis and cytotoxicity assays were performed. (a) FACS analysis for IL13Rα2. (b) IL13Rα2-CAR T cells killed U373 tumor cells isolated from recurrent tumors in contrast to Raji cells in a standard 4-hour cytotoxicity assay. CAR, chimeric antigen receptor.
Figure 7.
Limited persistence of IL13Rα2-CAR T cells in vivo. SSR.CD28.ζ-CAR T cells were transduced to express eGFP.ffLuc. (a) FACS analysis confirmed the expression of CAR and eGFP.ffLuc transgenes. (b,c) 1 × 105 unmodified U373 cells were injected intracranially into mice. On day 7, mice received 2 × 106 SSR.CD28.ζ.eGFP.ffLuc CAR T cells intracranially using the same tumor coordinates. Bioluminescence imaging was used to monitor T-cell persistence. CAR, chimeric antigen receptor.
Discussion
Here, we describe the development and characterization of a new scFv-based CAR, IL13Rα2-CAR that is specific for IL13Rα2. We show that T cells expressing this CAR can effectively target and kill IL13Rα2, but not IL13Rα1-positive target cells and that only IL13Rα2-CARs with a SSR induce IL2 production in a strictly IL13Rα2-dependent manner. Finally, we demonstrate that IL13Rα2-CAR.SSR T cells have potent antitumor activity in vivo.
IL13Rα2, a cancer testis antigen, is aberrantly expressed in GBM and other malignancies such as melanoma, adrenocortical carcinoma, colorectal, pancreatic, ovarian cancers.15,25,26,27 It is a promising immunotherapy target since it is not expressed in most normal tissues and in one study, using nanostring digital RNA counting, belonged to the top 10% of 73 evaluated tumor associated antigens that were differentially expressed between tumors and normal tissues.25 While IL13Rα2 has been targeted with immunotoxins, vaccines, and antigen-specific T cells with encouraging results,10,28,29 few approaches so far have used IL13Rα2-specific mAbs or scFvs as targeting moieties for therapeutics.24,30 We constructed IL13Rα2-specific, scFv-based CARs and determined the influence of long and short spacer regions, as well as endodomains on their function. While IL13Rα2-CAR.SSR.CD28.ζ and IL13Rα2-CAR.LSR.CD28.ζ recognized target cells as judged by IFNγ production, only IL13Rα2-CAR.SSR.CD28.ζ induced IL2 production, indicating better T-cell activation. We confirmed the inability to induce IL2 expression for one additional LSR IL13Rα2-CAR containing a CD28.41BB.ζ endodomain (Supplementary Figure S6). Other groups have also reported that the length of the spacer region contributes to CAR function.31,32 For example, scFvs that bind to an epitope in close proximity to the cancer cell membrane, require long spacer regions for optimal CAR function in contrast to scFvs that bind to epitopes distal to the cell membrane. While the precise epitope within the IL13Rα2 molecule for scFv47 is not defined,23,24 it binds to the same epitope as the parental MAb 47 that competes with IL13 for its binding site.24 Our finding that a SSR confers optimal CAR activity suggests that the epitope is located distal to the cell membrane.
We constructed four SSR IL13Rα2-CARs with different endodomains, CD28.ζ, 41BB.ζ CD28.OX40.ζ, and CD28.41BB.ζ. While all four CARs were expressed as judged by western blot analysis, however no significant cell surface expression was observed for IL13Rα2-CAR.SSR.CD28.41BB.ζ. We explored if changing the transmembrane domain from CD28 to CD8α in IL13Rα2-CAR.SSR.CD28.41BB.ζ would result in better cell surface expression, however no increased expression was observed (Supplementary Figure S7). Since IL13Rα2-CARs.LSR.CD28.41BB.ζ were expressed on the cell surface (Supplementary Figure S6), our results suggests that the SSR interferes with efficient CAR trafficking to the cell surface.
Local or systemic injections of conventional as well as CAR T cells are actively being explored for high-grade glioma.10,33,34 We focused on local injections since in our previous study with human EphA2-CAR T cells in SCID mice we only observed antitumor activity after local and not after tail vein injection,5 most likely reflecting the inability of human T cells to transverse the murine blood brain barrier. IL13Rα2-CAR.SSR.CD28.ζ, IL13Rα2-CAR.SSR.41BB.ζ, and IL13Rα2-CAR.SSR.CD28.OX40.ζ T cells had potent antitumor in vivo resulting in a significant survival advantage. While mice treated with IL13Rα2-CAR.SSR.CD28.ζ T cells had the longest median survival in comparison to IL13Rα2-CAR.SSR.41BB.ζ or IL13Rα2-CAR.SSR.CD28.OX40.ζ T-cell-treated mice, this difference did not reach significance. Our finding that addition of a second costimulatory endodomain does not improve antitumor activity in vivo is in agreement with recent findings by others.35 While we favor one of our second-generation CARs to move forward for further preclinical testing, we are conducting additional experiments to establish the minimal effective dose of each CAR T-cell population, test their efficacy in additional glioma models, and evaluate their ability to kill glioma cells after repeated stimulations.
Our data suggest that tumor recurrence is most likely due to limited T-cell persistence in the tumor milleu in vivo. This limitation may be overcome by modifying the glioma cells to secrete IL-2.22 While effective, this strategy is difficult to translate into the clinical setting. We therefore are exploring additional genetic modifications of IL13Rα2-CAR T cells to enhance their expansions and persistence like transgenic expression of cytokines36,37 and/or silencing negative regulators. For example, gliomas express PD-L1,38,39 and U373 expresses PD-L1, which is upregulated in the presence of IFNγ (Supplementary Figure S8), and could be targeted in future studies.
While we did not observe immune escape, targeting a single antigen is associated with the risk of selecting tumor cells that have down regulated the expression or have deleted the targeted antigen.40,41 In the context of CAR T cells several groups including ours have developed strategies to overcome this limitation by infusing two T-cell products with different specificity, expressing multiple CARs in T cells or expressing a single CAR with dual specificity.42,43,44
We evaluated IL13Rα2-CAR T cells in a immune-deficient mice, which are widely used to study the efficacy of CAR T cells.19 While ideal to study the interaction between human T cells and human tumor cells, immune deficient mice to not recapitulate realistically the complex interactions between adoptively transferred T cells, tumor cells, and the resident immune system, that often contributes to the immunosuppressive tumor microenvironment. To overcome this limitation, several groups have developed immune-competent mouse models to study CAR T cells.11,45 For our IL13Rα2-CAR T cells, such studies would require the genetic modification of glioma cells to express human IL13Rα2, since scFv47-based CAR T cells do not recognize murine IL13Rα2 (Krenciute et al., unpublished data), or the generation of a murine IL13Rα2-specific scFv.
In conclusion, T cells redirected to IL13Rα2 with scFv47-based CARs have potent antitumor activity against glioma cells in vitro, and induce the regression of established GBM xenografts in vivo. Our study adds to the growing literature31,32 that there is an intricate interplay between scFvs, spacer region, transmembrane domain, and endodomain that determines CAR function, and that there is no single optimal configuration that is “one size fits all”. IL13Rα2-CAR T cells may be of value in the treatment of not only IL13Rα2-positive GBMs but also other malignancies in which IL13Rα2 is expressed.
Materials and Methods
Cell lines. U373 (GBM), U87 (GBM), 293T (human embryonic kidney), and Raji (Burkitt's lymphoma) cell line were purchased from the American Type Culture Collection (ATCC; Manassas, VA). GBM4687 and GBMR031 are primary pediatric GBM cell lines,46 and GBM6 and GBM39 are primary adult GBM cell lines.47 The generation of U373 cells expressing enhanced green fluorescent protein and firefly luciferase (U373.eGFP.ffLuc), 293T cells expressing green fluorescent protein (293T.GFP) or IL13Rα2 and GFP (293T.IL13Rα2.GFP) was previously reported.5,7 Cell lines were grown in Roswell Park Memorial Institute or Dulbecco's Modified Eagle Medium (GE Healthcare Life Sciences HyClone Laboratories, Logan, UT) with 10% fetal bovine serum (FBS; GE Healthcare Life Sciences HyClone) and 2 mmol/l GlutaMAX-I (Invitrogen, Carlsbad, CA). The “Characterized Cell Line Core Facility” at MD Anderson Cancer Center, Houston, Texas, performed cell line validation.
Generation of retroviral vectors encoding IL13Rα2-scFv-specific CARs. A codon-optimized gene was synthesized by GeneArt (Invitrogen) containing the immunoglobulin heavy-chain leader peptide, and scFv47 flanked by 5' NcoI and 3' BamHI sites. This mini gene was subcloned into SFG retroviral vector containing IL13Rα2-specific CARs (IL13Rα2-CARs) with short or long spacer regions (SSRs, LSRs) and CD28.ζ, CD28.OX40.ζ, CD28.41BB.ζ, or 41BB.ζ endodomains.4,48,49 All CARs contained a CD28 transmenbrane domain except for IL13Rα2-CAR.SSR.41BB.ζ, which had a CD8α transmembrane domain. IL13Rα2-CAR.SSR.Δ and IL13Rα2-CAR.LSR.Δ without an endodomain were generated by PCR cloning. All cloning of the CARs were verified by sequencing (Seqwright, Houston, TX). RD114-pseudotyped retroviral particles were generated by transient transfection of 293T cells as previously described.5
Generation of CAR T cells. Human peripheral blood mononuclear cells from healthy donors were obtained under a Baylor College of Medicine Institutional Review Board-approved protocol, after informed consent was obtained in accordance to the Declaration of Helsinki. To generate IL13Rα2-CAR T cells, peripheral blood mononuclear cells were isolated by Lymphoprep (Greiner Bio-One, Monroe, NC) gradient centrifugation and then stimulated on treated nontissue culture 24-well plates, which were precoated with OKT3 (CRL-8001, ATCC) and CD28 (BD Bioscience, Mountain View, CA) antibodies. Recombinant human interleukin-7 and -15 (IL7, 10 ng/ml; IL15, 5 ng/ml; Proleukin; Chiron, Emeryville, CA) was added to cultures on day 2.50 On day 3, OKT3/CD28-stimulated T cells (2.5 × 105 cells/well) were transduced on RetroNectin (Clontech, Mountainview, CA) coated plates in the presence of IL7 and IL15. On day 5 or 6, T cells were transferred into new wells and subsequently expanded with IL-7 and IL15. Nontransduced (NT) T cells were activated with OKT3/CD28 and expanded in parallel with IL-7 and IL-15. IL13Rα2-CAR expression was determined 4 to 5 days post-transduction.
Flow cytometry. A FACSCalibur (BD Bioscience) or BC Gallios (Beckman Coulter, Brea, CA) instruments were used to acquire immunofluorescence data which were analyzed with CellQuest (BD Bioscience) or BC Gallios (Beckman Coulter) respectively. FlowJo v.7 (FlowJo, LLC Ashland, OR) or Kaluza v1.2 (Beckman Coulter) were used for final data analysis and graphic representation. Isotype controls were immunoglobulin G1–fluorescein isothiocyanate (IgG1-FITC; BD Bioscience), IgG1–phycoerythrin (IgG1-PE; BD Bioscience). SSR IL13Rα2-CAR expression was detected by staining T cells with human IL13Rα2 chimera (R&D Systems, Minneapolis, MN) followed by Fc-FITC (Milipore, Billerica, MA) or Fc-PE (SouthernBiotech, Birmingham, AL). LSR IL13Rα2-CARs were detected using Fc-FITC or Fc-PE. U373 cells were analyzed for PD-L1 expression using a CD271 PE antibody (BD Bioscience). Forward and side scatter gating were used to discriminate live cells from dead cells. Cells were collected and washed once with PBS (Sigma, St. Louis, MO) containing 1% FBS (GE Healthcare Life Sciences HyClone Laboratories; FACS buffer) prior to the addition of antibodies. Cell were incubated for 30 minutes on ice in the dark, washed once, and fixed in FACS buffer with 0.5% paraformaldehyde (BD Bioscience) prior to analysis.
Western blot. Cells were dissociated with PBS + 3 mmol/l ethylenediaminetetraacetic acid and lysed in a buffer containing 50 mmol/l Tris, 150 mmol/l NaCl, 5 mmol/l Ethylenediaminetetraacetic acid, 1% Triton X-100 (all from Sigma), and protease inhibitors (Thermo Scientific, Waltham, MA). Protein concentrations were determined using a Bio-Rad protein assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard. Samples were denatured in Laemmli buffer (Bio-Rad) with βME (2-mercaptoethanol, Bio-Rad; reducing conditions) or without βME (nonreducing condition) at 95 °C for 5 minutes. Cell lysate (5 μg per lane) were run on a 10% SDS polyacrylamide gel and transferred to nitrocellulose membranes (BioRad). Membranes were blocked with 5% milk powder in Tris-buffered saline + 0.1% Tween-20 (all from Sigma) and then probed with anti-CD3.ζ (sc-1239, Santa Cruz Biotechnology, Santa Cruz, CA) or glyceraldehyde-3-phosphate dehydrogenase (sc-47724, Santa Cruz Biotechnology) mouse monoclonal antibodies followed by a horseradish peroxidase-conjugated goat mouse IgG antibody (sc-2005, Santa Cruz Biotechnology). Blots were developed using SuperSignal West Dura Extended Duration Substrate (Thermo Scientific) and exposed to GeneMate Blue Basic Autoradiography Film (BioExpress, Kaysville, UT).
Coculture assays for coculture assays, each CAR was expressed in T cells from the same donor. Biological repeats were done using different donors and data presented in the figures is the average of three to five donors.
Recombinant protein coculture assay. Nontissue culture 24-well plates were precoated with recombinant human IL13Rα1, IL13Rα2, or IL4Rα proteins, (R&D Systems) at a final concentration of 500 ng/well. Plates were washed once using Roswell Park Memorial Institute, and CAR or NT T cells were plated. After 24 hours, supernatants were harvested and IFNγ and IL2 release was measured by ELISA per the manufacturer's instructions (R&D Systems).
Cell culture coculture assay. CAR T cells were cocultured with target cells at a 2:1 effector to target (E:T) ratio in a 24-well plate. NT T cells served as controls. After 24 hours, culture supernatants were harvested, and the presence of IFNγ and IL2 was determined by ELISA as per the manufacturer's instructions (R&D Systems).
Cytotoxicity assay. Standard chromium (51Cr) release assays were performed as previously described.5 Briefly, 1 × 106 target cells were labeled with 0.1 mCi (3.7MBq) 51Cr and mixed with decreasing numbers of effector cells to give effector to target ratios of 40:1, 20:1, 10:1, and 5:1. Target cells incubated in complete medium alone or in 1% Triton X-100 were used to determine spontaneous and maximum 51Cr release, respectively. After 4 hours, supernatants were collected and radioactivity was measured in a gamma counter (Cobra Quantum; PerkinElmer; Wellesley; MA). The mean percentage of specific lysis of triplicate wells was calculated according to the following formula: (test release–spontaneous release)/(maximal release–spontaneous release) × 100.
Orthotopic xenograft SCID mouse model. All animal experiments followed a protocol approved by the Baylor College of Medicine Institutional Animal Care and Use Committee. Experiments were performed as described previously5 with a few modifications. ICR-SCID mice were purchased from Taconic (IcrTac:ICR-Prkdcscid; Fox Chase C.B-17 SCID ICR; Taconic, Hudson, NY). Male 7- to 9-week-old mice were anesthetized, the head was shaved and the mice were immobilized in a Cunningham Mouse/Neonatal Rat Adaptor (Stoelting, Wood Dale, IL) stereotactic apparatus fitted into an E15600 Lab Standard Stereotaxic Instrument (Stoelting), and then scrubbed with 1% povidone-iodine. A 10-mm skin incision was made along the midline. The tip of a 30G ½ inch needle mounted on a Hamilton syringe (Hamilton, Reno, NV) served as the reference point. A 1 mm burr-hole was drilled into the skull 1 mm anterior and 2 mm to the right of the bregma. 1 × 105 U373.eGFP.ffLuc cells in 2.0 µl were injected 3-mm deep to the bregma, corresponding to the center of the right caudate nucleus over 5 minutes. The needle was left in place for 3 minutes, to avoid tumor cell extrusion, and then withdrawn over 5 minutes. Seven days after tumor cell injection, animals were treated with 2 × 106 effector cells in 2 µl to the same tumor coordinates. The incision was closed with 2–3 interrupted 7.0 Ethilon sutures (Ethicon, Somerville, NJ). A subcutaneous injection of 0.03–0.1 mg/kg buprenorphine (Buprenex RBH, Hull, England) was given for pain control.
Bioluminescence imaging. Isofluorane anesthetized animals were imaged using the IVIS system (IVIS, Xenogen, Alameda, CA) 10–15 minutes after 150 mg/kg D-luciferin (Xenogen) was injected per mouse intraperitoneally. The photons emitted from the luciferase-expressing tumor cells were quantified using Living Image software (Caliper Life Sciences, Hopkinton, MA). A pseudo-color image representing light intensity (blue least intense and red most intense) was generated and superimposed over the grayscale reference image. Mice were euthanized when the tumor radiance was greater than 1 × 109 on two occasions or when they met euthanasia criteria (neurological deficits, weight loss, signs of distress) in accordance with the Center for Comparative Medicine at Baylor College of Medicine.
Statistical analysis. All in vitro experiments were performed at least in triplicate, GraphPad Prism 5 software (GraphPad software, La Jolla, CA) was used for statistical analysis. Measurement data were presented as mean ± standard deviation. The differences between means were tested by appropriate tests. The significance level used was P < 0.05. For the mouse experiments, changes in tumor radiance from baseline at each time point were calculated and compared between groups using t-test or Wilcoxon rank-sum test, whichever appropriate. Survival determined from the time of tumor cell injection was analyzed by the Kaplan-Meier method and differences in survival between groups were compared by the Wilcoxon test.
SUPPLEMENTARY MATERIAL Figure S1. Phenotypic analysis of IL13Rα2-CAR T-cell lines. Figure S2. Western blot of IL13Rα2-CAR T cells. Figure S3. IL13Rα2-CAR.LSR.CD28.ζ induces constitutive CD3.ζ phosphorylation. Figure S4. Cell surface expression of IL13Rα1 and IL13Rα2. Figure S5. Cell surface expression of IL13Rα2 in brain tumor cell lines. Figure S6. Generation and characterization of LSR.CD28.41BB.ζ CAR T cells. Figure S7. Generation of SSR.CD28.41BB.ζ CAR T cells. Figure S8. FACS analysis of PD-L1 expression on U373 cell surface with and without IFNγ stimulation.
Acknowledgments
This work was supported by NIH Grants 1R01CA173750-01 and 1R21NS089802-01, Cookies for Kids Cancer, and the James S McDonnell Foundation. The Center for Cell and Gene Therapy has a research collaboration with Celgene and Bluebird Bio. GD, MSL, IVB, and SG have patent applications in the field of T-cell and gene-modified T-cell therapy for cancer and/or IL13Rα2-targeted therapies.
Supplementary Material
References
- Suryadevara, CM, Verla, T, Sanchez-Perez, L, Reap, EA, Choi, BD, Fecci, PE et al. (2015). Immunotherapy for malignant glioma. Surg Neurol Int 6(Suppl 1): S68–S77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Gool, SW (2015). Brain Tumor Immunotherapy: What have We Learned so Far? Front Oncol 5: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reardon, DA, Freeman, G, Wu, C, Chiocca, EA, Wucherpfennig, KW, Wen, PY et al. (2014). Immunotherapy advances for glioblastoma. Neuro Oncol 16: 1441–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, N, Salsman, VS, Kew, Y, Shaffer, D, Powell, S, Zhang, YJ et al. (2010). HER2-specific T cells target primary glioblastoma stem cells and induce regression of autologous experimental tumors. Clin Cancer Res 16: 474–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, KK, Naik, S, Kakarla, S, Brawley, VS, Shaffer, DR, Yi, Z et al. (2013). T cells redirected to EphA2 for the immunotherapy of glioblastoma. Mol Ther 21: 629–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, LA, Scholler, J, Ohkuri, T, Kosaka, A, Patel, PR, McGettigan, SE et al. (2015). Rational development and characterization of humanized anti-EGFR variant III chimeric antigen receptor T cells for glioblastoma. Sci Transl Med 7: 275ra22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krebs, S, Chow, KK, Yi, Z, Rodriguez-Cruz, T, Hegde, M, Gerken, C et al. (2014). T cells redirected to interleukin-13Rα2 with interleukin-13 mutein–chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1. Cytotherapy 16: 1121–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, S, Sengupta, S, Tyler, B, Bais, AJ, Ma, Q, Doucette, S et al. (2012). Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells. Clin Cancer Res 18: 5949–5960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, CE, Starr, R, Aguilar, B, Shami, AF, Martinez, C, D'Apuzzo, M et al. (2012). Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin Cancer Res 18: 2199–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, CE, Badie, B, Barish, ME, Weng, L, Ostberg, JR, Chang, WC et al. (2015). Bioactivity and Safety of IL13Rα2-Redirected Chimeric Antigen Receptor CD8+ T Cells in Patients with Recurrent Glioblastoma. Clin Cancer Res 21: 4062–4072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson, JH, Choi, BD, Sanchez-Perez, L, Suryadevara, CM, Snyder, DJ, Flores, CT et al. (2014). EGFRvIII mCAR-modified T-cell therapy cures mice with established intracerebral glioma and generates host immunity against tumor-antigen loss. Clin Cancer Res 20: 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada, H, Low, KL, Kohanbash, G, McDonald, HA, Hamilton, RL and Pollack, IF (2008). Expression of glioma-associated antigens in pediatric brain stem and non-brain stem gliomas. J Neurooncol 88: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi, BH, Puri, RA, Leland, P, Varricchio, F, Gupta, G, Kocak, M et al.; US Pediatric Brain Tumor Consortium. (2008). Identification of interleukin-13 receptor alpha2 chain overexpression in situ in high-grade diffusely infiltrative pediatric brainstem glioma. Neuro Oncol 10: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami, M, Kawakami, K, Takahashi, S, Abe, M and Puri, RK (2004). Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer 101: 1036–1042. [DOI] [PubMed] [Google Scholar]
- Brown, CE, Warden, CD, Starr, R, Deng, X, Badie, B, Yuan, YC et al. (2013). Glioma IL13Rα2 is associated with mesenchymal signature gene expression and poor patient prognosis. PLoS One 8: e77769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsi, LC, Kundu, S, Palomo, J, Xu, B, Ficco, R, Vogelbaum, MA et al. (2011). Silencing IL-13Rα2 promotes glioblastoma cell death via endogenous signaling. Mol Cancer Ther 10: 1149–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner-Feigl, S, Strober, W, Kawakami, K, Puri, RK and Kitani, A (2006). IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med 12: 99–106. [DOI] [PubMed] [Google Scholar]
- Bartolomé, RA, García-Palmero, I, Torres, S, López-Lucendo, M, Balyasnikova, IV and Casal, JI (2015). IL13 Receptor α2 Signaling Requires a Scaffold Protein, FAM120A, to Activate the FAK and PI3K Pathways in Colon Cancer Metastasis. Cancer Res 75: 2434–2444. [DOI] [PubMed] [Google Scholar]
- Dotti, G, Gottschalk, S, Savoldo, B and Brenner, MK (2014). Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 257: 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen, MC and Riddell, SR (2015). Designing chimeric antigen receptors to effectively and safely target tumors. Curr Opin Immunol 33: 9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadelain, M, Brentjens, R and Rivière, I (2013). The basic principles of chimeric antigen receptor design. Cancer Discov 3: 388–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlon, KS, Brown, C, Cooper, LJ, Raubitschek, A, Forman, SJ and Jensen, MC (2004). Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res 64: 9160–9166. [DOI] [PubMed] [Google Scholar]
- Balyasnikova, IV, Wainwright, DA, Solomaha, E, Lee, G, Han, Y, Thaci, B et al. (2012). Characterization and immunotherapeutic implications for a novel antibody targeting interleukin (IL)-13 receptor α2. J Biol Chem 287: 30215–30227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J, Young, J, Solomaha, E, Kanojia, D, Lesniak, MS, Balyasnikova, IV (2015). A novel single-chain antibody redirects adenovirus to IL13Ra2-expressing brain tumors. Sci Rep (accepted for publication) [DOI] [PMC free article] [PubMed]
- Beard, RE, Abate-Daga, D, Rosati, SF, Zheng, Z, Wunderlich, JR, Rosenberg, SA et al. (2013). Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clin Cancer Res 19: 4941–4950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujisawa, T, Nakashima, H, Nakajima, A, Joshi, BH and Puri, RK (2011). Targeting IL-13Rα2 in human pancreatic ductal adenocarcinoma with combination therapy of IL-13-PE and gemcitabine. Int J Cancer 128: 1221–1231. [DOI] [PubMed] [Google Scholar]
- Barderas, R, Bartolomé, RA, Fernandez-Aceñero, MJ, Torres, S and Casal, JI (2012). High expression of IL-13 receptor α2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res 72: 2780–2790. [DOI] [PubMed] [Google Scholar]
- Kunwar, S, Prados, MD, Chang, SM, Berger, MS, Lang, FF, Piepmeier, JM et al.; Cintredekin Besudotox Intraparenchymal Study Group. (2007). Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol 25: 837–844. [DOI] [PubMed] [Google Scholar]
- Okada, H, Kalinski, P, Ueda, R, Hoji, A, Kohanbash, G, Donegan, TE et al. (2011). Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol 29: 330–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioi, M, Seetharam, S and Puri, RK (2008). Targeting IL-13Ralpha2-positive cancer with a novel recombinant immunotoxin composed of a single-chain antibody and mutated Pseudomonas exotoxin. Mol Cancer Ther 7: 1579–1587. [DOI] [PubMed] [Google Scholar]
- Hudecek, M, Lupo-Stanghellini, MT, Kosasih, PL, Sommermeyer, D, Jensen, MC, Rader, C et al. (2013). Receptor affinity and extracellular domain modifications affect tumor recognition by ROR1-specific chimeric antigen receptor T cells. Clin Cancer Res 19: 3153–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haso, W, Lee, DW, Shah, NN, Stetler-Stevenson, M, Yuan, CM, Pastan, IH et al. (2013). Anti-CD22-chimeric antigen receptors targeting B-cell precursor acute lymphoblastic leukemia. Blood 121: 1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow, KH and Gottschalk, S (2011). Cellular immunotherapy for high-grade glioma. Immunotherapy 3: 423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuessler, A, Smith, C, Beagley, L, Boyle, GM, Rehan, S, Matthews, K et al. (2014). Autologous T-cell therapy for cytomegalovirus as a consolidative treatment for recurrent glioblastoma. Cancer Res 74: 3466–3476. [DOI] [PubMed] [Google Scholar]
- Künkele, A, Johnson, AJ, Rolczynski, LS, Chang, CA, Hoglund, V, Kelly-Spratt, KS et al. (2015). Functional Tuning of CARs Reveals Signaling Threshold above Which CD8+ CTL Antitumor Potency Is Attenuated due to Cell Fas-FasL-Dependent AICD. Cancer Immunol Res 3: 368–379. [DOI] [PubMed] [Google Scholar]
- Hoyos, V, Savoldo, B, Quintarelli, C, Mahendravada, A, Zhang, M, Vera, J et al. (2010). Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 24: 1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegram, HJ, Lee, JC, Hayman, EG, Imperato, GH, Tedder, TF, Sadelain, M et al. (2012). Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119: 4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avril, T, Saikali, S, Vauleon, E, Jary, A, Hamlat, A, De Tayrac, M et al. (2010). Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol 225: 22–33. [DOI] [PubMed] [Google Scholar]
- Parsa, AT, Waldron, JS, Panner, A, Crane, CA, Parney, IF, Barry, JJ et al. (2007). Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med 13: 84–88. [DOI] [PubMed] [Google Scholar]
- Gottschalk, S, Ng, CY, Perez, M, Smith, CA, Sample, C, Brenner, MK et al. (2001). An Epstein-Barr virus deletion mutant associated with fatal lymphoproliferative disease unresponsive to therapy with virus-specific CTLs. Blood 97: 835–843. [DOI] [PubMed] [Google Scholar]
- Sampson, JH, Heimberger, AB, Archer, GE, Aldape, KD, Friedman, AH, Friedman, HS et al. (2010). Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol 28: 4722–4729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grada, Z, Hegde, M, Byrd, T, Shaffer, DR, Ghazi, A, Brawley, VS et al. (2013). TanCAR: A Novel Bispecific Chimeric Antigen Receptor for Cancer Immunotherapy. Mol Ther Nucleic Acids 2: e105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegde, M, Corder, A, Chow, KK, Mukherjee, M, Ashoori, A, Kew, Y et al. (2013). Combinational targeting offsets antigen escape and enhances effector functions of adoptively transferred T cells in glioblastoma. Mol Ther 21: 2087–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anurathapan, U, Chan, RC, Hindi, HF, Mucharla, R, Bajgain, P, Hayes, BC et al. (2014). Kinetics of tumor destruction by chimeric antigen receptor-modified T cells. Mol Ther 22: 623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochenderfer, JN, Yu, Z, Frasheri, D, Restifo, NP and Rosenberg, SA (2010). Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116: 3875–3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z, Zhao, X, Mao, H, Baxter, PA, Huang, Y, Yu, L et al. (2013). Intravenous injection of oncolytic picornavirus SVV-001 prolongs animal survival in a panel of primary tumor-based orthotopic xenograft mouse models of pediatric glioma. Neuro Oncol 15: 1173–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkaria, JN, Yang, L, Grogan, PT, Kitange, GJ, Carlson, BL, Schroeder, MA et al. (2007). Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther 6: 1167–1174. [DOI] [PubMed] [Google Scholar]
- Pulè, MA, Straathof, KC, Dotti, G, Heslop, HE, Rooney, CM and Brenner, MK (2005). A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol Ther 12: 933–941. [DOI] [PubMed] [Google Scholar]
- Vera, J, Savoldo, B, Vigouroux, S, Biagi, E, Pule, M, Rossig, C et al. (2006). T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 108: 3890–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, Y, Zhang, M, Ramos, CA, Durett, A, Liu, E, Dakhova, O et al. (2014). Closely related T-memory stem cells correlate with in vivo expansion of CAR.CD19-T cells and are preserved by IL-7 and IL-15. Blood 123: 3750–3759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.