Abstract
The biggest roadblock in development of effective vaccines against human immunodeficiency virus type 1 (HIV-1) is the virus genetic diversity. For T-cell vaccine, this can be tackled by focusing the vaccine-elicited T-cells on the highly functionally conserved regions of HIV-1 proteins, mutations in which typically cause a replicative fitness loss, and by computing multivalent mosaic proteins, which maximize the coverage of potential 9-mer T-cell epitopes of the input viral sequences. Our first conserved region vaccines HIVconsv employed clade alternating consensus sequences and showed promise in the initial clinical trials in terms of magnitude and breadth of elicited CD8+ T-cells. Here, monitoring T-cells restricted by HLA-A*02:01 in transgenic mice, we assessed whether or not the tHIVconsv design (HIVconsv with a tissue plasminogen activator leader sequence) benefits from combining with a complementing conserved mosaic immunogen tHIVcmo, and compared the bivalent immunization to that with trivalent conserved mosaic vaccines. A hierarchy of tHIVconsv ≤ tHIVconsv+tHIVcmo < tCmo1+tCmo2+tCmo3 vaccinations for induction of CD8+ T-cell responses was observed in terms of recognition of tested peptide variants. Thus, our HLA-A*02:01-restricted epitope data concur with previously published mouse and macaque observations and suggest that even conserved region vaccines benefit from oligovalent mosaic design.
Introduction
Although the HIV-1 vaccine field has recently focused almost exclusively on B-cell analysis and induction of broadly neutralizing antibodies (bnAbs), there is now a reawakening of interest in CD8+ T-cell vaccines. This follows from the demonstration that a vaccine vectored by modified cytomegalovirus eradicated SIV from macaques soon after challenge in the absence of nAbs1,2 and that human CD8+ T-cells cleared reactivated provirus in vitro.3 In humans, this is supported indirectly by the incomplete control of acute HIV-1 infection (AHI), which correlates with the first CD8+ T-cell responses, extensive virus escape in targeted epitopes during AHI, which is observed in every patient, and by genome association studies, which show protective effects of certain HLA class I allotypes.4,5,6,7,8,9 Therefore, CD8+ T-cells play an important role in the control of HIV-1 infection10 and their induction is likely to be an important part of any preventive vaccination strategy10,11 and will certainly be crucial to immunotherapy aimed at a functional and/or sterile cure.3,12
The biggest roadblock for effective vaccines is HIV-1 variability and escape. However, vaccine-elicited T-cells can target HIV-1 at its weakest point, which are the functionally conserved regions of HIV-1 proteins that cannot escape without substantial fitness loss3,13,14 and are common to most global variants. Also, at the population level, targeting of particular T-cell epitopes has been correlated with improved viral control, and these beneficial responses tend to be clustered in conserved regions.15,16 In natural HIV-1 infection, conserved regions are typically subdominant, suppressed by dominating, but less protective hypervariable, i.e., easy-to-escape, epitopes. We pioneered the first constant-region vaccine evaluation in HIV-negative adults17,18,19 and proved the concept that CD8+ T-cell responses against conserved regions of mostly Gag and Pol can be induced by vaccination and that these can inhibit replication of several HIV-1 isolates in autologous CD4+ cells.17 An alternative highly effective approach to HIV-1 diversity is the use of so called “mosaic” proteins.20 They are generated in silico from natural sequences by systematically including common and excluding rare 9-mer potential T-cell epitopes (PTEs).21 To maximize the capture of the most common epitope variants in the Los Alamos National Laboratory HIV Sequence Database (LANL-HSD), mosaics are designed as multivalent mutually complementing proteins. Indeed in the nonhuman primate model, full-length protein mosaic immunogens induced CD8+ and CD4+ T-cell responses superior in magnitude, breadth, and depth (number of recognized variants of the same epitope) to either consensus (average amino acid) or natural protein immunogens22,23,24 and mosaic HIV-1 Env immunogens provided a strong protective effect in a SHIV-challenge model.25
Our first-generation vaccine HIVconsv was assembled from 14 protein regions highly conserved among the consensus sequences of four major HIV-1 clades A, B, C, and D.19 The vaccine elicited potent CD8+ T-cell responses in phase 1 human vaccine trials, which were able to inhibit HIV-1 replication in vitro (ref. 17 and unpublished data). It focuses on countering diverse HIV-1 strains at the moment of their entry and reactivation from latent reservoir. Here, we examined whether or not the cognitive breadth and depth of the CD8+ T-cell response induced by the already conserved HIVconsv immunogen further benefits from combining with an HIVconsv-complementing mosaic immunogen, i.e., whether T-cell cross-reactivity with and recognition of different cocirculating strains worldwide increases. In these comparisons, we also included trivalent mosaic immunogens. To make our results more relevant for human studies, we used the transgenic mouse strain HHD, which expresses a chimeric mouse-human class I molecule with a human A*02:01 binding specificity.26
Results
Design of bi- and trivalent conserved-region mosaic immunogens
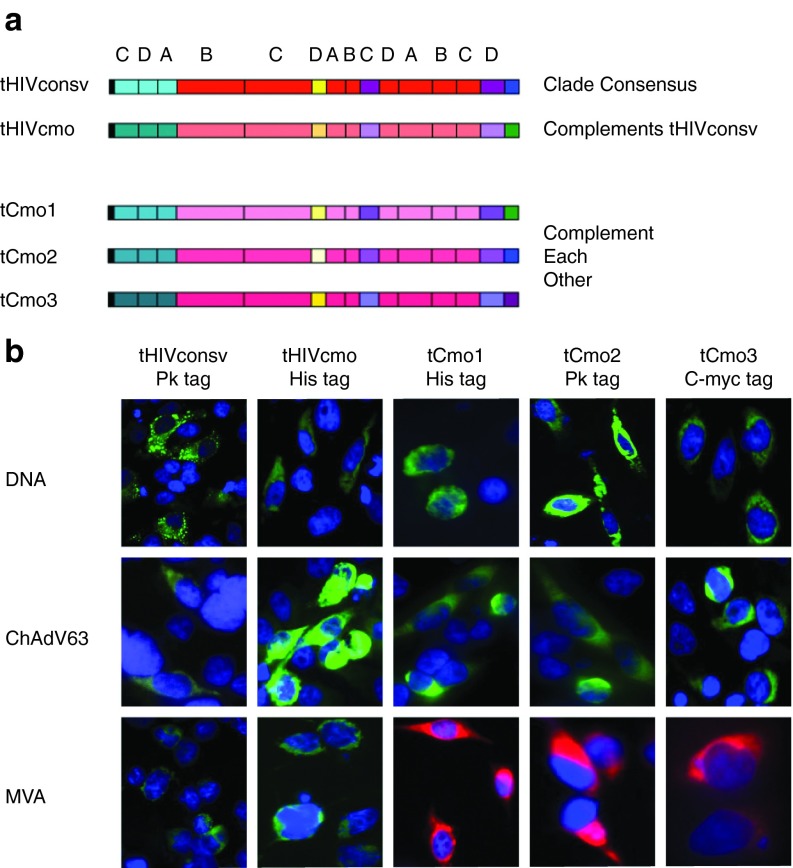
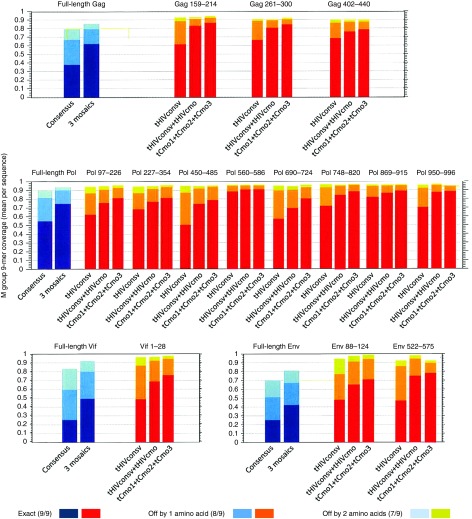
The HIVconsv immunogen19 as used in clinical trials17,18,27 was modified by addition of a human tissue plasminogen activator leader sequence (“t”) and designated tHIVconsv. For the design of bi- and trivalent mosaic immunogens, protein sequences present in the LANL-HSD20 as of October 2010 were used as the baseline data for computing the mosaic sequences of full-length HIV-1 proteins. To compute the HIVcmo immunogen, the 9-mer sequences used in HIVconsv were taken out of the pool of database sequences prior to the iterative process of mosaic design. Only one sequence per patient and sequences spanning full proteins were included, while proteins with frame-shifting mutations or uncertain amino acid positions were excluded. The boundaries of the conserved regions defined by 9-mer coverage were confirmed by per-position Shannon entropy scores.28 Thus, tHIVcmo (conserved mosaic), which complements tHIVconsv to form a bivalent immunogen, and tCmo1, tCmo2, and tCmo3, which together constitute a trivalent immunogen, were designed (Figure 1a). Genes coding for the chimeric proteins assembled from the same 14 regions as tHIVconsv (see Supplementary Figure S1 for the amino acid sequences) were then synthetized and inserted into vaccine vectors plasmid pSG2 DNA (D), nonreplicating chimpanzee adenovirus 63 (C), and nonreplicating poxvirus modified vaccinia virus Ankara (MVA or M). Expression of corresponding proteins in human cells was confirmed by immunofluorescence using C-terminal mAb tags as indicated (Figure 1b). The coverage of all PTEs in the HIV-1 group M for each of the 14 regions comparing tHIVconsv, tHIVconsv+tHIVcmo and tCmo1+tCmo2+tCmo3 is shown in Figure 2. The conserved fragments are compared to the PTE coverage of the full-length protein from which they were derived. Pol is the most conserved HIV-1 polyprotein in HIV-1 overall, followed by Gag,
Figure 1.
The vaccine immunogens and their expression in vitro. (a) Schematic representation of the vaccine immunogens showing 14 highly conserved regions of HIV-1 Gag (cyan), Pol (red), Vif (yellow), and Env (violet) proteins assembled into a chimeric protein with a human tissue plasminogen activator leader sequence (“t”) at the N-terminus (black) and Pk (blue), His (green) and C-myc (purple) tags recognized by mAbs at C-termini of proteins. tHIVconsv (conserved consensus) has HIV-1 sequences of the first-generation conserved region T-cell vaccines described by Létourneau et al. 19 tHIVcmo (conserved mosaic) was designed as a mosaic complementing tHIVconsv for maximum coverage of group M HIV-1 isolates. Immunogens tCmo1, tCmo2, and tCmo3 were designed in silico as a trivalent mosaic20 over the same 14 regions as tHIVconsv and tHIVcmo. (b) All five genes coding for the above immunogens were inserted into a plasmid DNA, nonreplicating simian (chimpanzee) adenovirus ChAdV-63 and nonreplicating poxvirus MVA, and their expression was confirmed by immunofluorescence using fluoroscein isothiocyanate (FITC)-conjugated mAs (green) recognizing the C-terminal tags following transfection of DNA, or rChAdV63 and rMVA infection at multiplicity of infection 10 and 5, respectively, of HeLa cells. rMVAs expressing tHIVconsv and tHIVcmo were markerless for potential human use and the immunogens were detected by FITC-conjugated mAbs, MVA.tCmo1, MVA.tCmo2, and MVA.tCmo3 coexpressed GFP as a rescue marker, and the immunogens were detected by Alexa fluora 594-conjugated mAbs (red). Nuclei are stained using 4‘,6-diamidino-2-phenylindole (DAPI) (blue).
Figure 2.
Coverage of global HIV-1 potential 9-mer T-cell epitopes (PTEs) present in the HIV Sequence Database. The graphs give the coverage of potential 9-mer PTEs of group M HIV-1s. Coverage is defined as the average number of potential epitopes per natural strain that are matched in the full M group sequence database per natural strain, within the bounds of the protein regions considered. Full-length proteins and the tHIVconsv region amino acid positions as in HXB2 are listed above the graphs. For the full-length proteins (Gag, Pol, Vif, and Env), the coverage by consensus and trivalent mosaic are shown in blue. For the conserved vaccine immunogens, the PTE coverages by the consensus as in the tHIVconsv, tHIVconsv+tHIVcmo and trivalent mosaic tCmo1+tCmo2+tCmo3 are shown in red/orange/yellow.
A trend for broader and more cross-reactive responses was observed for bivalent mosaic compared to consensus
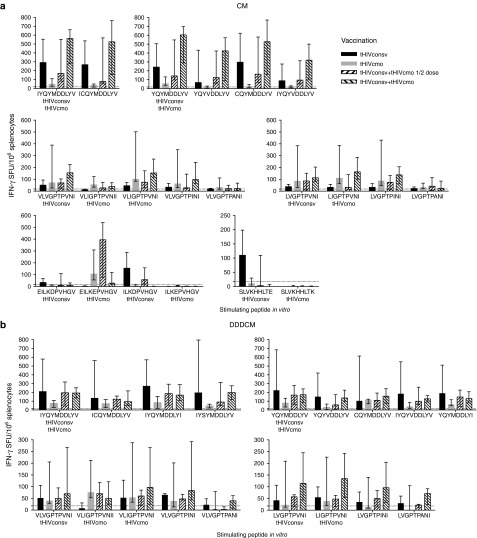
To assess induction of CD8+ T-cell responses restricted by HLA-A*02:01 preclinically, the HHD mouse strain was used. First, to compare the tHIVconsv and tHIVcmo vaccines alone with tHIVconsv+tHIVcmo bivalent vaccines together at ½ and full doses each, HHD mice were immunized using the chimpanzee adenovirus rChAdV63 prime-rMVA boost (CM) regimen. For each known HLA-A*02:01-restricted epitope in the 14 regions, variant peptides with a LANL-HSD frequency of at least 1% of HIV-1 sequences were synthesized supplemented with a few additional peptides to ensure a multiple clade representation (Table 1). Mice were immunized and all peptides in Table 1 were tested, but due to natural immunodominance, a hierarchy of responses was established allowing us to study the depth of recognition only in 4 out of 12 known epitopes (Figure 3a). For the sufficiently immunogenic epitopes, the recognition patterns of variant peptides differed. There was an overall trend for bivalent mosaic to induce broader responses and for the dominant epitopes (I)YQYMDDLYV and (V)LVGPTPVNI, 2× full dose displayed a trend toward higher T-cell frequencies than 2× ½ dose (Figure 3a). Also responses to epitopes ELKEPVHGV and SLVKHHLTE were elicited to detectable levels, with the latter tHIVcmo variant SLVKHHLTK likely showing antagonism29 (Figure 3a). Using the DNA-DNA-DNA-rChAdV63-rMVA (DDDCM) regimen, CD8+ T-cell responses to the immunodominant epitopes were very similar with perhaps a diminished trend in difference between the full and ½ doses of bivalent immunizations for epitope (I)YQYMDDLYV (Figure 3b).
Table 1. List of epitopes present in the 14 highly conserved regions of the tHIVconsv immunogen restricted by HLA-A*02:01.

Figure 3.
The breadth and depth of HLA-A*02:01 T-cell responses elicited by tHIVconsv and tHIVcmo vaccines alone or in a bivalent combination. Groups of 6-week-old female HHD mice were vaccinated intramuscularly with the tHIVconsv (black) or tHIVcmo (gray) vaccines alone, or combined at ½ (bottom-to-top stripes) or full (top-to-bottom stripes) doses each. Full dose were 100 µg of DNA, 108 infectious doses of rChAdV63 and 106 plaque-forming units of rMVA. (a) For all groups, a rChAdV63 prime-rMVA boost (CM) regimen was used, while (b) employed a DNA-DNA-DNA-rChAdV63-rMVA (DDDCM) regimen (Supplementary Table S1). Individual mouse splenocytes were tested for recognition of HLA-A*02:01-restricted epitope variants indicated below the graphs in an IFN-γ ELISPOT assay. Note that other A*02:01-restricted responses were not induced due to the immunodominance of the ones showed and the negative data are not shown. Results are presented as medians with interquartile range (n = 5) after subtracting for each animal the mean no-peptide background (dotted line). Immunogens matching precisely the tested epitopes are indicated on the x-axis below the peptides sequences.
Trivalent mosaic induces superior T-cell responses
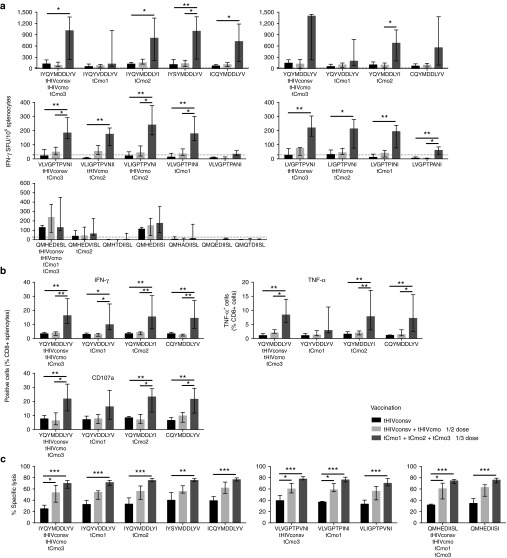
In the initial experiment, trivalent mosaic tCmo1+tCmo2+tCmo3 was delivered by the DDDCM regimen and induced T-cell responses recognizing the same two epitopes (I)YQYMDDLYV and (V)LVGPTPVNI on the top of the hierarchy and also elicited detectable responses to epitopes ALTAICEEM and GMHEDIISL. Statistically inseparable frequencies were induced by 3× full and 3× ⅓ dosing (Supplementary Figure S2).
Next, we directly compared the breadth and depth of HLA-A*02:01-restricted CD8+ T cells induced by a single consensus sequence of tHIVconsv, and bivalent (tHIVconsv+tHIVcmo at ½ dose) and trivalent (tCmo1+tCmo2+tCmo3 at ⅓ dose) mosaics. The magnitude and functionality of responses were determined in IFN-γ ELISPOT assay (Figure 4a), intracellular cytokine staining assay measuring IFN-γ, TNF-α, and CD107a (Figure 4b), and killing assay using variant epitope peptides for target sensitization (Figure 4c). In all three assays and for all peptides with the exception of QMHDIISL (ELISPOT assay), the trivalent mosaic outperformed tHIVconsv alone and tHIVconsv+tHIVcmo. QMHDIISL was restored to being the most potently recognized peptide in the trivalent vaccinations (Figure 4c). Thus for 20 of 21 peptides with a detectable response (Figure 4a), the highest IFN-γ ELlSPOT scores were in the animals that received the trivalent mosaic. This is a highly significant result—with the caveat that the peptides were not completely independent. An exact binomial test comparing this to the null hypothesis that the trivalent mosaic would be highest 1/3 of the time if random, is highly significant, P = 9.8 × 10–9; the probability that the trivalent mosaic will give the is best response of the three vaccines tested was 0.952, with a 95% confidence interval of 0.76–0.999. For the killing assay, there was a consistent trend of bivalent immunization inducing higher percentage lysis relative to tHIVconsv alone. Thus, the bivalent and trivalent combinations not only did not diminish responses to peptide variants that were present in the original monovalent vaccine, as one might expect from diluting the original variant in a polyvalent setting, they enhanced them.
Figure 4.
Functionality of HLA-A*02:01 T-cell responses induced by mono-, bi-, and trivalent mosaic immunogens. Groups of 6-week-old female HHD mice were vaccinated iintramuscularly with either tHIVconsv vaccines alone (black), ½ doses of tHIVconsv+tHIVcmo (light grey) or ⅓ doses of tCmo1+tCmo2+tCmo3 (dark gray) vaccines using a CM regimen (Supplementary Table S1). The depth and breadth of elicited HLA-A*02:01-restricted T-cell responses were assessed for individual mice in IFN-γ ELISPOT assay (n = 6) (a), intracellular cytokine staining assay (n = 5) detecting IFN-γ, TNF-α, and CD107a (see Supplementary Figure S3 for representative gating strategy) (b) and killing assay (n = 8) (see Supplementary Figure S4 for gating strategy) (c). Results for responding peptides are shown as medians with interquartile range after subtracting for each animal the mean no-peptide background. Non-responding peptides are not shown. Immunogens matching precisely the tested epitopes are indicated on x-axis below the peptide sequences. Analysis of variance test was employed to determine statistically significant differences: * for P < 0.05; ** for P < 0.01; *** for P < 0.001.
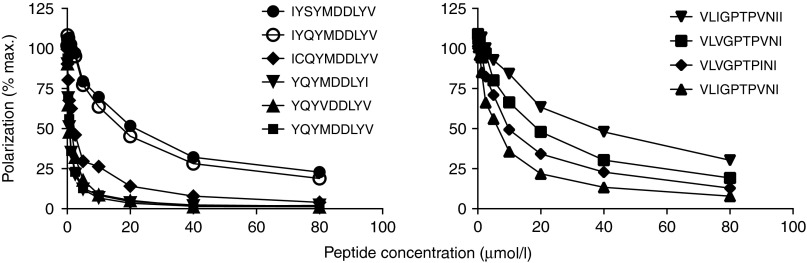
Finally, the ability of selected peptide variants to inhibit the binding of a labeled competing peptide to HLA A*02:01 molecules was used to determined the binding affinities of the variant peptides to HLA-A*02:01 monomers (Figure 5). This assay confirmed the highest HLA-binding affinity for the two index peptides YQYMDDLYV and VLIGPTPIVNI, but a clear-cut relationship between binding affinity, immunogenicity and peptide recognition was not observed.
Figure 5.
Binding affinity of peptide variants to refolded recombinant HLA-A*02:01. For the two immunodominant epitopes, the relative peptide binding affinities to purified recombinant HLA-A*02:01 molecules were determined by a fluorescence polarization completion assay. Binding of TAMRA-labeled peptide FLPSDC*FPSV is reported in millipolarization units (mP) and is plotted as a percentage of the maximum polarization signal that was observed in the absence of competing unlabeled peptide. One of two experiments is shown.
Discussion
Given that circulating HIV-1 strains are extremely variable30 and effective CD8+ T-cell responses are favored by a perfect match between the vaccine and incoming virus epitopes,31 likely an enormous number of PTEs would need to be included in the vaccine cocktails to achieve a global protection against HIV-1 (refs. 21,32). There is a full spectrum of 9-amino acid-region windows in the HIV-1 proteome whereby at one extreme, a window can be well represented by one or two PTEs (conserved regions) and at the other, no highly representative single PTE is observed (highly variable regions). This makes it difficult for full-length proteins to immunize effectively against a whole clade or group M viruses using a single, e.g., consensus sequence.21 For vaccine immunogen design, the global representation of PTE variants greatly improves by focusing on highly functionally conserved regions of proteins.11,19,33,34 As there is a degree of variation even within these regions, the computationally optimized polyvalent mosaic strategy20 further improves the proportion of perfectly matched global variants. For the clinically tested HIVconsv immunogen,17 which consists of alternating clade consensus sequences of conserved regions,19 addition of HIVconsv-complementing conserved mosaic HIVcmo improved the global perfect 9-mer match from an average of 71% to approximately 78% (Figure 2). Here, we demonstrated experimentally that coadministration of the tHIVcmo immunogen with tHIVconsv showed a trend of greater breadth of responses. These improvements might not have reached the full potential of the bivalent mosaic design because of the tHIVconsv consensus sequence, i.e., the bivalent vaccine components tHIVconsv and tHIVcmo were not computed together. Significantly broader and stronger responses were induced by the trivalent mosaic tCmo1+tCmo2+tCmo3.
Our results concur with the previously reported impressive increase in the depth and breadth of T-cell responses induced by mosaic vaccines in mice35,36,37 and nonhuman primates.22,23,24,25,36,38 While the mosaic design is able to theoretically optimize the depth and breadth of vaccine-induced responses in humans, too, to date there are no data in humans that such an approach will actually enhance the same type of response, although full-length HIV-1 Env, Gag, and Pol mosaic vaccines have entered clinical evaluations (NCT02315703). Since HIV-1 evolution is occurring in humans and not in mice or nonhuman primates, these findings may not translate to human studies. As such the current study is the first to demonstrate that a mosaic vaccine approach may actually do what it is intended to do in humans. Thus with the caveats of the transgenic HLA mouse model, i.e., having murine antigen processing and T-cell receptor repertoire, the results obtained here using human HLA-A*02:01 epitopes are a good start and suggestive of future success of this T-cell vaccine strategy in humans.
For each epitope, induction of responses and their recognition of the index and variant forms displayed distinct individual patterns. Typically responses to one variant were stronger that to the other, but between the two mosaics, the induction and recognition of both variants was covered qualitatively and overall on an intermediate (between the two mosaic magnitudes) level. Often, the trivalent mosaic would extend potent T-cell responses to epitopes that were not exactly matched by the vaccine, raising the possibility that exposure to epitope variants will enable responses with greater breadth. The explanation for increased overall frequencies of elicited T-cell responses by trivalent mosaic is less clear and may in part come from the clonality of T-cells recruited into the response; this is currently under investigation. In contrast, the way increased overall magnitude contributes to the greater breadth can be visualized by analogy of multiple uneven peaks being lifted out of water: the higher the lift, the more peaks emerge.
Sometimes, variant versions of epitopes can inhibit responses to the peptide agonist.29,39 This phenomenon of antagonism is rare, but could explain the absence of responses to epitope SLVKHHLTE in the presence of its variant SLVKHHLTK (Figure 3a). Epitope antagonisms can be partially overcome by anatomical separation of immunogen variants during vaccination.40,41,42 So far, this type of immune interference has not been noted as a substantial problem for tested multivalent mosaic designs.22,23,24,25,36,38 Another possible interference may come from adventitious induction of T-cell responses by de novo sequences generated across junctions of adjacent regions, such as those detected in the HIV-CORE 002 trial of the HIVconsv vaccines.17 These are irrelevant for HIV-1 protection, but can dominate relevant protective responses. Here, we did not look for junctional responses in HHD mice and because to date, we have not detected any A*02:01-restricted junctional responses in HIV-CORE 002 volunteers. Thus, we believe that the possibility of junctional responses influencing the results of the present study remains low and would not affect the main finding of this study.
Even a vaccine, which is to protect against a single HIV-1 clade, has to address an extensive genetic variation.43 A study comparing the epitope coverage by 2-, 3-, 4-, and 6-valent mosaics showed that by increasing the number of generated mosaics, the number of variants included in the vaccine grew, and so did the perfect variant coverage, but with diminishing returns with each additional variant. Variant additions incorporate increasingly rare epitopes into the mosaic cocktail, thus potentially diluting the response to the more frequent variants.38 Therefore, a balance must be found between increasing coverage and reducing epitope representation, as well as considering the complexity and cost associated with the number of multiple variants in a vaccine cocktail.20,38,44 Here we have shown a trend for inclusion of two and a definite advantage for three variants, and interestingly, the increased breadth of the response in polyvalent scenarios extends beyond just the variants included in the vaccine, suggesting a vaccine that presents more epitope diversity favors T-cell responses that are better able to recognize more variable forms of the epitope.
Many conserved regions are infrequently recognized in natural infection due to immunodominance of more variable regions. Here we confirm that, when taken out of the full-length protein/whole virus context and presented by a potent delivery method such as heterologous prime-boost regimen of rAdV and rMVA, naturally subdominant epitopes can induce robust CD8+ T-cell responses, which can exert effectively their protective functions.45 A new immunodominant hierarchy is established, but with all epitopes being conserved, i.e., relevant and likely protective.
Mosaic vaccines have been utilized recently for other high-variability pathogens such as hepatitis C virus, Ebola, and influenza viruses.35,37,46,47 Thus, studies in mice showed broad protective responses to several H5N1 influenza viruses and a prolonged protection against challenge with a highly pathogenic avian influenza.46 A mosaic hepatitis C virus genotype-1 vaccine induced stronger responses and was more immunogenic than a natural hepatitis C virus strain vaccine and significantly improved epitope coverage.37,48 The Ebola mosaic conferred protection in a mouse model.35 We predict that focusing on shared conserved protein regions could further improve the number of matches of the mosaic vaccines with diverse pathogen strains and therefore increase the vaccine efficacy.
In conclusion, we have pioneered the use of T-cell vaccines specific for highly conserved HIV-1 regions in the clinic.17,18 Here, we demonstrated that the conserved region strategy benefits from oligovalent mosaic design, as variants in a mosaic cocktail reproducibly enhanced the ability to cross-recognize common, natural peptide variants found in even the conserved regions of HIV-1. These results are applicable to other immunological problems where variability is a major roadblock in developing effective preventive or therapeutic tools.
Materials and Methods
Synthetic genes for tHIVconsvX. Four DNA fragments carrying the open-reading frames coding for the four conserved mosaics (Supplementary Figure S1) were synthesized (Life Technologies) using humanized codons and were preceded by a consensus Kozak sequence at -6 nucleotides to maximize protein expression.
Construction and production of virus-vectored vaccines. For the generation of recombinant ChAdV-63s, synthetic genes with humanized codon coding for the individual conserved-mosaic immunogens were subcloned under the control of the human cytomegalovirus immediate early promoter in plasmid pENTR4_Mono and inserted at the E1 locus of the ChAdV-63 genome by GalK recombineering.49 Recombinant ChAdV-63 vaccines were grown in suspension culture of HEK 293 cells. To generate recombinant MVAs, the genes were subcloned under the control of the modified H5 promoter50 in plasmid MVAgfpTD or MVAgfp and inserted into the thymidine kinase region of the MVA genome to generate either marker-less (tHIVconsv and tHIVcmo) or green fluorescent protein-co-expressing (tCmo1, tCmo2, and tCmo3) vaccines, respectively,51 which were expanded by growth in chicken embryo fibroblasts and titred. For plasmid DNA, synthetic open-reading frames were cloned into plasmid pSG2 under the control of immediate-early human cytomegalovirus promoter and intron A and followed by bovine growth hormone polyadenylation signal. All vaccines were maintained at −80 °C until use.
Immunofluorescence. For DNA vaccines, HeLa cells, in six-well plates, were transfected with each of the plasmid DNA vaccines individually to assess the immunogen expression. Transfection was done using Lipofectamine 3000 Reagent (Invitrogen). For viruses, 70% confluent HeLa cells were infected with the recombinant ChAdV63 and MVA vaccines at a multiplicity of infection of 5 for MVAs for 2 hours or at multiplicity of infection of 10 for rChAdV63 for 1 hour in serum free Dulbecco's Modified Eagle Medium medium at 37 °C, 5% CO2, the medium was replaced and cells were incubated for further 24 hours at 37 °C, 5% CO2. Transfected/infected HeLa cells were fixed with formalin solution, neutral buffered 10% (containing 4% formaldehyde) (Sigma) for 10 minutes on ice and then 20 minutes at room temperature, permeabilized with 0.2% Triton X-100 (TX-100) (Sigma) in phosphate-buffered saline for 5 minutes, washed again with phosphate-buffered saline (×3), blocked with 1% bovine serum albumin in phosphate-buffered saline for 30 minutes and incubated for 3–4 hours at room temperature with a 1:100 dilution of immunofluorescent antibodies targeting specific tag sequences: Pk tag—(IPNPLLGLD); His-6 tag (HHHHHH); or C-myc tag (EQKLISEEDL) (AbD Serotec). Cells were washed, incubated with Vectashield 4',6-diamidino-2-phenylindole (DAPI) nuclear stain mounting medium (Vector laboratories), and examined on a fluorescence microscope (DMI 3000B; Leica). Images were processed using ImageJ software (imagej.nih.gov/ij/).
Mice and immunization regimens. For all experiments, mice of the HHD strain were used, which were bred and housed at the Functional Genomics Facility, University of Oxford, and express a chimeric HLA class I molecule with human A*02:01 α-1 and α-2 domains for peptide binding and mouse α-3 domain linked covalently to the human β2m.26 Groups of 6- to 8-week-old female HHD mice were immunized intramuscularly under general anesthesia with standard doses of 108 infectious units (IU) of recombinant ChAdV63, 5 × 106 plaque-forming units of recombinant MVA or 100 μg of plasmid DNA alone or in combination. The intervals after DNA and rChAdV63 administrations were 3 and 6 weeks, respectively. All procedures and care were approved by the local Research Ethics Committee, University of Oxford and conformed strictly to the United Kingdom Home Office Guidelines under the Animals (Scientific Procedures) Act 1986. Experiments were conducted under Project License 30/2833 held by T.H.
Peptides. Peptides for this study were selected based on being known for HLA-A*02:01 presentation, and the frequencies of the variants of each peptide in the M group was resolved using QuickAlign, (www.hiv.lanl.gov/content/sequence/QUICK_ALIGNv2/QuickAlign.html). ChinaPeptides manufactured HLA-A*02:01-binding peptides and their variants to purity >95%. Peptides were dissolved in DMSO at a concentration of 20 mg/ml and stored at −80 °C. Working stocks of 4 mg/ml were prepared by diluting 20 mg/ml stocks with phosphate-buffered saline. Peptides were used in assays at a final concentration of 3 μg/ml unless otherwise stated.
Murine IFN-γ ELISPOT assay. The ELISPOT assay was performed using the Mouse IFN-γ ELISpot kit (Mabtech). Immune splenocytes were collected and tested separately from individual mice. Spots were visualized using sequential applications of a biotin-conjugated secondary anti-IFN-γ mAb (R4-6A2, Rat IgG1), an alkaline phosphatase and a chromogenic substrate (Bio-Rad) and counted using the AID ELISpot Reader System (Autoimmun Diagnostika) and the results were expressed at spot-forming units (SFU)/106 splenocytes.
Intracellular cytokine staining. Cytokine production by splenocytes from immunized mice was assessed by intracellular cytokine staining as described previously.52 Briefly, splenocytes were stimulated individual peptides for 90 minutes at 37 °C, and then for further 5 hours in the presence of brefeldin A (Golgiplug, BD Biosciences) to prevent cytokine secretion. Cells were surface stained with anti-CD4-Pacific blue, anti-CD8-PE-Texas red (both eBioscience) antibodies and LIVE/DEAD fixable aqua dead cell stain (Invitrogen), and then permeabilized and incubated with various combinations of anti-IL-2-fluoroscein isothiocyanate, anti-IL-4-PE, anti-TNF-α-PE and anti-IFN-γ-APC monoclonal antibodies (Biolegend). Samples were acquired on an LSR II flow cytometer (BD Biosciences) and data analyzed using FlowJo version 9.5.2 (Tree Star).
Ex vivo killing assay. Killing assay was performed as described previously.52 Equal numbers of naive HHD splenocytes were differentially labeled with either 800 or 32 nmol/l of CFSE according to the manufacturer's specifications. Splenocytes from immunized mice were prepared as described above, mixed with the differentially CFSE-labeled target cells at an effector-to-target (ET) ratio of 10:1 or 5:1 and incubated overnight at 37 °C. The cells were washed, stained with a live/dead marker and analyzed using flow cytometry. Cytotoxicity was calculated as follows: % specific lysis = 100 × (number of unpulsed control cells – number of peptide-pulsed cells)/number of unpulsed control cells.
HLA binding assay. HLA binding assays were performed as described previously53 but with the following modifications. Briefly, recombinant HLA-A*02:01 molecules were refolded with human β2-microglobulin and the UV-conditional peptide KILGFVF-ANP-V (ANP = 3-amino-3-(2-nitro) phenyl-propionic acid, Peptide Synthetics).54 çTAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. Next, 0.15 µmol/l of refolded monomeric HLA-A*02fos was mixed with 0.15 µmol/l human β2-microglobulin, and exposed to 360 nm UV light for 20 minutes at 4 °C, before being added to a 96-well plate containing 0.1 µmol/l fluorescent peptide FLPSDC*FPSV (C* denotes TAMRA-labeled cysteine) and variable concentrations (0–80 μmol/l) of the candidate peptides. After an overnight incubation at room temperature, the polarization of the fluorophore-labeled peptide was measured and was obtained from the equation, mP = 1,000 × (S − G × P)/(S + G × P), where S and P are background-subtracted fluorescence count rates (S = polarized emission filter is parallel to the excitation filter; P = polarized emission filter is perpendicular to the excitation filter), and G (grating) is an instrument- and assay-dependent factor.
Statistical analysis. Statistical analyses were performed using GraphPad Prism version 5 (La Jolla, CA). Simple comparisons were performed using two-way Student's t-test. Multiple comparisons were performed using the Kruskal-Wallis test with Dunn's multiple comparison post-test for nonparametric data. A P value <0.05 was considered significant after adjustment for multiple comparisons. The binomial test was performed with the R package (www.r-project.org).
SUPPLEMENTARY MATERIAL Table S1. Vaccination regimens. Figure S1. Amino acid sequences of the immunogens. Figure S2. The breadth and depth of HLA-A*02:01 T-cell responses elicited by trivalent conserved mosaic vaccines. Figure S3. Representative gating for an intracellular cytokine staining (ICS) assay. Figure S4. Representative FACS plots showing gating strategy for ex-vivo killing.
Acknowledgments
The work is jointly funded by the UK Medical Research Council (MRC G1001757) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreements. S.A.-J. is supported by the King Abdullah scholarship by the Ministry of Higher Education, Kingdom of Saudi Arabia. B.O. was funded in part by the International AIDS Vaccine Initiative and made possible by the support of the United States Agency for International Development and other donors. The full list of IAVI donors is available at http://www.iavi.org. B.K. was funded through: the Center for HIV/AIDS Vaccine Immunology and Immunogen Discovery (CHAVI-ID; UM1-AI100645) of the National Institute of Allergy and Infectious Diseases USA. T.H. is the Jenner Institute Investigator. The authors have no competing interests other than T.H. and B.K. are the inventors on PCT Application No. PCT/US2014/058422.
Supplementary Material
References
- Hansen, SG, Ford, JC, Lewis, MS, Ventura, AB, Hughes, CM, Coyne-Johnson, L et al. (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, SG, Piatak, M Jr, Ventura, AB, Hughes, CM, Gilbride, RM, Ford, JC et al. (2013). Immune clearance of highly pathogenic SIV infection. Nature 502: 100–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, K, Pertea, M, Rongvaux, A, Wang, L, Durand, CM, Ghiaur, G et al. (2015). Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature 517: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow, P, Lewicki, H, Hahn, BH, Shaw, GM and Oldstone, MB (1994). Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol 68: 6103–6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellay, J, Shianna, KV, Ge, D, Colombo, S, Ledergerber, B, Weale, M et al. (2007). A whole-genome association study of major determinants for host control of HIV-1. Science 317: 944–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goonetilleke, N, Liu, MK, Salazar-Gonzalez, JF, Ferrari, G, Giorgi, E, Ganusov, VV et al.; CHAVI Clinical Core B. (2009). The first T cell response to transmitted/founder virus contributes to the control of acute viremia in HIV-1 infection. J Exp Med 206: 1253–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulder, PJ, Brander, C, Tang, Y, Tremblay, C, Colbert, RA, Addo, MM et al. (2001). Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412: 334–338. [DOI] [PubMed] [Google Scholar]
- Koup, RA, Safrit, JT, Cao, Y, Andrews, CA, McLeod, G, Borkowsky, W et al. (1994). Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol 68: 4650–4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogg, GS, Jin, X, Bonhoeffer, S, Dunbar, PR, Nowak, MA, Monard, S et al. (1998). Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279: 2103–2106. [DOI] [PubMed] [Google Scholar]
- Walker, B and McMichael, A (2012). The T-cell response to HIV. Cold Spring Harb Perspect Med 2:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke, T (2014). Conserved immunogens in prime-boost strategies for the next-generation HIV-1 vaccines. Expert Opin Biol Ther 14: 601–616. [DOI] [PubMed] [Google Scholar]
- Margolis, DM and Hazuda, DJ (2013). Combined approaches for HIV cure. Curr Opin HIV AIDS 8: 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, AL, Mann, JK, Omarjee, S, Ndung'u, T, Walker, BD and Chakraborty, AK (2013). Translating HIV sequences into quantitative fitness landscapes predicts viral vulnerabilities for rational immunogen design. Immunity 38: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie, AJ, Pfafferott, KJ, Chetty, P, Draenert, R, Addo, MM, Feeney, M et al. (2004). HIV evolution: CTL escape mutation and reversion after transmission. Nat Med 10: 282–289. [DOI] [PubMed] [Google Scholar]
- Mothe, B, Llano, A, Ibarrondo, J, Daniels, M, Miranda, C, Zamarreño, J et al. (2011). Definition of the viral targets of protective HIV-1-specific T cell responses. J Transl Med 9: 208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi, H, Akahoshi, T, Koyanagi, M, Chikata, T, Naruto, T, Maruyama, R et al. (2015). Clinical Control of HIV-1 by Cytotoxic T Cells Specific for Multiple Conserved Epitopes. J Virol 89: 5330–5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borthwick, N, Ahmed, T, Ondondo, B, Hayes, P, Rose, A, Ebrahimsa, U et al. (2014). Vaccine-elicited human T cells recognizing conserved protein regions inhibit HIV-1. Mol Ther 22: 464–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayton, EJ, Rose, A, Ibrahimsa, U, Del Sorbo, M, Capone, S, Crook, A et al. (2014). Safety and tolerability of conserved region vaccines vectored by plasmid DNA, simian adenovirus and modified vaccinia virus ankara administered to human immunodeficiency virus type 1-uninfected adults in a randomized, single-blind phase I trial. PLoS One 9: e101591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Létourneau, S, Im, EJ, Mashishi, T, Brereton, C, Bridgeman, A, Yang, H et al. (2007). Design and pre-clinical evaluation of a universal HIV-1 vaccine. PLoS One 2: e984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer, W, Perkins, S, Theiler, J, Bhattacharya, T, Yusim, K, Funkhouser, R et al. (2007). Polyvalent vaccines for optimal coverage of potential T-cell epitopes in global HIV-1 variants. Nat Med 13: 100–106. [DOI] [PubMed] [Google Scholar]
- Li, F, Malhotra, U, Gilbert, PB, Hawkins, NR, Duerr, AC, McElrath, JM et al. (2006). Peptide selection for human immunodeficiency virus type 1 CTL-based vaccine evaluation. Vaccine 24: 6893–6904. [DOI] [PubMed] [Google Scholar]
- Barouch, DH, O'Brien, KL, Simmons, NL, King, SL, Abbink, P, Maxfield, LF et al. (2010). Mosaic HIV-1 vaccines expand the breadth and depth of cellular immune responses in rhesus monkeys. Nat Med 16: 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulot, SL, Korber, B, Giorgi, EE, Vandergrift, N, Saunders, KO, Balachandran, H et al. (2015). Comparison of Immunogenicity in Rhesus Macaques of Transmitted-Founder, HIV-1 Group M Consensus, and Trivalent Mosaic Envelope Vaccines Formulated as a DNA Prime, NYVAC, and Envelope Protein Boost. J Virol 89: 6462–6480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra, S, Liao, HX, Zhang, R, Muldoon, M, Watson, S, Fischer, W et al. (2010). Mosaic vaccines elicit CD8+ T lymphocyte responses that confer enhanced immune coverage of diverse HIV strains in monkeys. Nat Med 16: 324–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barouch, DH, Stephenson, KE, Borducchi, EN, Smith, K, Stanley, K, McNally, AG et al. (2013). Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell 155: 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascolo, S, Bervas, N, Ure, JM, Smith, AG, Lemonnier, FA and Pérarnau, B (1997). HLA-A2.1-restricted education and cytolytic activity of CD8(+) T lymphocytes from beta2 microglobulin (beta2m) HLA-A2.1 monochain transgenic H-2Db beta2m double knockout mice. J Exp Med 185: 2043–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, T et al. Control of HIV-1 replication in vitro by vaccine-induced human CD8+ T cells through conserved subdominant Pol epitopes. Vaccine (in press). [DOI] [PMC free article] [PubMed]
- Korber, BT, Farber, RM, Wolpert, DH and Lapedes, AS (1993). Covariation of mutations in the V3 loop of human immunodeficiency virus type 1 envelope protein: an information theoretic analysis. Proc Natl Acad Sci USA 90: 7176–7180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerman, P, Rowland-Jones, S, McAdam, S, Edwards, J, Daenke, S, Lalloo, D et al. (1994). Cytotoxic T-cell activity antagonized by naturally occurring HIV-1 Gag variants. Nature 369: 403–407. [DOI] [PubMed] [Google Scholar]
- Gaschen, B, Taylor, J, Yusim, K, Foley, B, Gao, F, Lang, D et al. (2002). Diversity considerations in HIV-1 vaccine selection. Science 296: 2354–2360. [DOI] [PubMed] [Google Scholar]
- Lee, JK, Stewart-Jones, G, Dong, T, Harlos, K, Di Gleria, K, Dorrell, L et al. (2004). T cell cross-reactivity and conformational changes during TCR engagement. J Exp Med 200: 1455–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber, B, Gaschen, B, Yusim, K, Thakallapally, R, Kesmir, C and Detours, V (2001). Evolutionary and immunological implications of contemporary HIV-1 variation. Br Med Bull 58: 19–42. [DOI] [PubMed] [Google Scholar]
- Rolland, M, Nickle, DC and Mullins, JI (2007). HIV-1 group M conserved elements vaccine. PLoS Pathog 3: e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, OO, Ali, A, Kasahara, N, Faure-Kumar, E, Bae, JY, Picker, LJ et al. (2015). Short conserved sequences of HIV-1 are highly immunogenic and shift immunodominance. J Virol 89: 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenimore, PW, Muhammad, MA, Fischer, WM, Foley, BT, Bakken, RR, Thurmond, JR et al. (2012). Designing and testing broadly-protective filoviral vaccines optimized for cytotoxic T-lymphocyte epitope coverage. PLoS One 7: e44769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong, WP, Wu, L, Wallstrom, TC, Fischer, W, Yang, ZY, Ko, SY et al. (2009). Expanded breadth of the T-cell response to mosaic human immunodeficiency virus type 1 envelope DNA vaccination. J Virol 83: 2201–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusim, K, Dilan, R, Borducchi, E, Stanley, K, Giorgi, E, Fischer, W et al. (2013). Hepatitis C genotype 1 mosaic vaccines are immunogenic in mice and induce stronger T-cell responses than natural strains. Clin Vaccine Immunol 20: 302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santra, S, Muldoon, M, Watson, S, Buzby, A, Balachandran, H, Carlson, KR et al. (2012). Breadth of cellular and humoral immune responses elicited in rhesus monkeys by multi-valent mosaic and consensus immunogens. Virology 428: 121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larke, N, Im, EJ, Wagner, R, Williamson, C, Williamson, AL, McMichael, AJ et al. (2007). Combined single-clade candidate HIV-1 vaccines induce T cell responses limited by multiple forms of in vivo immune interference. Eur J Immunol 37: 566–577. [DOI] [PubMed] [Google Scholar]
- Liu, J, Ewald, BA, Lynch, DM, Nanda, A, Sumida, SM and Barouch, DH (2006). Modulation of DNA vaccine-elicited CD8+ T-lymphocyte epitope immunodominance hierarchies. J Virol 80: 11991–11997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez, F, Harkins, S, Slifka, MK and Whitton, JL (2002). Immunodominance in virus-induced CD8(+) T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J Virol 76: 4251–4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario, M, Bridgeman, A, Quakkelaar, ED, Quigley, MF, Hill, BJ, Knudsen, ML et al. (2010). Long peptides induce polyfunctional T cells against conserved regions of HIV-1 with superior breadth to single-gene vaccines in macaques. Eur J Immunol 40: 1973–1984. [DOI] [PubMed] [Google Scholar]
- Williamson, C, Morris, L, Maughan, MF, Ping, LH, Dryga, SA, Thomas, R et al. (2003). Characterization and selection of HIV-1 subtype C isolates for use in vaccine development. AIDS Res Hum Retroviruses 19: 133–144. [DOI] [PubMed] [Google Scholar]
- Korber, BT, Letvin, NL and Haynes, BF (2009). T-cell vaccine strategies for human immunodeficiency virus, the virus with a thousand faces. J Virol 83: 8300–8314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Im, EJ, Hong, JP, Roshorm, Y, Bridgeman, A, Létourneau, S, Liljeström, P et al. (2011). Protective efficacy of serially up-ranked subdominant CD8+ T cell epitopes against virus challenges. PLoS Pathog 7: e1002041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamlangdee, A, Kingstad-Bakke, B, Anderson, TK, Goldberg, TL and Osorio, JE (2014). Broad protection against avian influenza virus by using a modified vaccinia Ankara virus expressing a mosaic hemagglutinin gene. J Virol 88: 13300–13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusim, K, Kesmir, C, Gaschen, B, Addo, MM, Altfeld, M, Brunak, S et al. (2002). Clustering patterns of cytotoxic T-lymphocyte epitopes in human immunodeficiency virus type 1 (HIV-1) proteins reveal imprints of immune evasion on HIV-1 global variation. J Virol 76: 8757–8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusim, K, Fischer, W, Yoon, H, Thurmond, J, Fenimore, PW, Lauer, G et al. (2010). Genotype 1 and global hepatitis C T-cell vaccines designed to optimize coverage of genetic diversity. J Gen Virol 91(Pt 5): 1194–1206. [DOI] [PubMed] [Google Scholar]
- Warming, S, Costantino, N, Court, DL, Jenkins, NA and Copeland, NG (2005). Simple and highly efficient BAC recombineering using galK selection. Nucleic Acids Res 33: e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Z, Martinez, J, Zhou, W, La Rosa, C, Srivastava, T, Dasgupta, A et al. (2010). Modified H5 promoter improves stability of insert genes while maintaining immunogenicity during extended passage of genetically engineered MVA vaccines. Vaccine 28: 1547–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins, R, Bridgeman, A, Bourne, C, Mbewe-Mvula, A, Sadoff, JC, Both, GW et al. (2011). Optimizing HIV-1-specific CD8+ T-cell induction by recombinant BCG in prime-boost regimens with heterologous viral vectors. Eur J Immunol 41: 3542–3552. [DOI] [PubMed] [Google Scholar]
- Ondondo, B, Abdul-Jawad, S, Bridgeman, A and Hanke, T (2014). Characterization of T-cell responses to conserved regions of the HIV-1 proteome in BALB/c mice. Clin Vaccine Immunol 21: 1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hateren, A, Bailey, A, Werner, JM and Elliott, T (2015). Plasticity of empty major histocompatibility complex class I molecules determines peptide-selector function. Mol Immunol 68(2 Pt A): 98–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann, C, van Hateren, A, Trautwein, N, Neerincx, A, Duriez, PJ, Stevanović, S et al. (2015). TAPBPR alters MHC class I peptide presentation by functioning as a peptide exchange catalyst. Elife 4. doi: 10.7554/eLife.09617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.