Abstract
A major limiting factor retarding the clinical success of dendritic cell (DC)-based genetic immunizations (DNA vaccination) is the scarcity of biologically safe and effective carrier systems for targeting the antigen-encoded DNA vaccines to DCs under in vivo settings. Herein, we report on a potent, mannose receptor selective in vivo DC-targeting liposomes of a novel cationic amphiphile with mannose-mimicking shikimoyl head-group. Flow cytometric experiments with cells isolated from draining lymph nodes of mice s.c. immunized with lipoplexes of pGFP plasmid (model DNA vaccine) using anti-CD11c antibody-labeled magnetic beads revealed in vivo DC-targeting properties of the presently described liposomal DNA vaccine carrier. Importantly, s.c. immunizations of mice with electrostatic complex of the in vivo DC-targeting liposome and melanoma antigen-encoded DNA vaccine (p-CMV-MART1) induced long-lasting antimelanoma immune response (100 days post melanoma tumor challenge) with remarkable memory response (more than 6 months after the second tumor challenge). The presently described direct in vivo DC-targeting liposomal DNA vaccine carrier is expected to find future exploitations toward designing effective vaccines for various infectious diseases and cancers.
Introduction
Dendritic cells (DCs), body's most professional antigen-presenting cells, possess the unique ability of capturing and processing pathogenic antigens in the peripheral blood and tissues. The antigen-loaded DCs migrate through afferent lymphatics to the nearby draining lymph nodes where they present the processed antigen fragments in complexation with both classical major histocompatibility complexes (MHC class I and II) and nonclassical (CD1 family) antigen-presenting molecules to the resting T lymphocytes.1,2,3 Because of such distinguishing antigen-presenting ability of the DCs, DCs pulsed/transduced with tumor-associated or viral antigens are finding increasing applications as vaccines for cancer and infectious diseases. DCs are often ex vivo transfected with tumor/viral antigens-encoded DNA vaccines.4,5,6,7,8,9 Such ex vivo DC transfection-based genetic immunization protocols, although highly efficient in combating cancer, are labor-intensive, time-consuming, and expensive. Autologous DC precursors are painstakingly isolated, the isolated autologous DC precursors are then ex vivo transfected with DNA vaccines, and the ex vivo transfected DCs finally need to be reimplanted in recipient's body for mounting immune response. To this end, both viral and nonviral vectors are now being used for direct in vivo targeting of DNA vaccines to DCs.10,11,12,13,14,15,16 However, achieving long-lasting immunity through use of simple and cost-effective in vivo DC-targeting system remains a formidable challenge.
Previously, we reported that mannose receptor selective liposomes of cationic amphiphiles containing two aliphatic n-hexadecyl nonpolar tails and mannose-mimicking shikimoyl- and quinoyl- head-groups with a lysine spacer in between are efficient DNA vaccine carriers for ex vivo DC transfection-based genetic immunization.9 These priorly reported systems were found to be efficient in inducing long-lasting immune response against melanoma in mice immunized with DCs ex vivo transfected with lipoplexes of melanoma antigen-encoded DNA vaccines.9 However, as described below, the system failed in mounting long-lasting immune response against melanoma when used under direct in vivo DC-targeting mode. We envisaged that the DC transfection efficiency of this new class of mannose receptor selective lipids containing mannose-mimicking shikimoyl- and quinoyl- head-groups need to be further enhanced for making their liposomes effective in targeting DNA vaccines to DCs under in vivo settings. With such rationale in mind, in the present study, we chemically transformed the lysine side chain amino group into the transfection enhancing guanidine group. Herein, we show that liposomes of the cationic amphiphile containing a mannose-mimicking shikimoyl head-group and two n-hexadecyl hydrophobic tails can target DNA vaccines to DCs under in vivo settings when the side chain amino group of the lysine spacer is guanidinylated (lipid 1, Figure 1). We show that direct in vivo immunization (s.c.) of mice with electrostatic complex of the liposome of lipid 1 and melanoma antigen-encoded DNA vaccine (p-CMV-MART1) induces long-lasting antimelanoma immune response (100 days post melanoma tumor challenge) with remarkable memory response (more than 6 months after the second tumor challenge). With the availability of the presently described cationic lipid 1, overcoming the formidable challenge of inducing long-lasting immune response through direct in vivo targeting of tumor antigen-encoded DNA vaccines to DCs will now become feasible. The presently described direct in vivo DC-targeting liposomal DNA vaccine carriers are thus expected to find future applications in effective vaccine developments for various infectious diseases and cancers.
Figure 1.
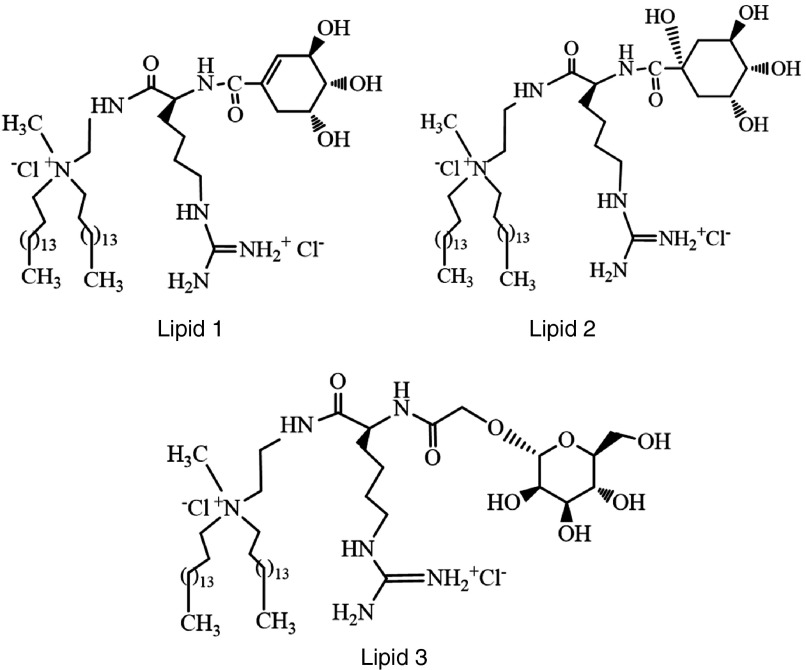
Structures of cationic amphiphiles with mannose-mimicking shikimoyl- (lipid 1) and quinoyl- (lipid 2) head-groups and their mannosyl analog (lipid 3) used in the present study.
Results
Chemistry
The cationic lipids 1 and 2 (Figure 1) containing a guanidinylated lysine spacer between the hydrophobic tails and mannose-mimicking shikimoyl- and quinoyl head-groups as well as their mannosyl analog lipid 3 (Figure 1) were synthesized by conventional peptide coupling of the acetyl protected shikimic, quinic acids and mannose to appropriately derivatized lysinylated amphiphiles followed by quaternization, deprotections, and chloride ion exchange (Supplementary Schemes S1–S3). The details of synthetic schemes, procedures, nuclear magnetic resonance (NMR), and mass spectral data for lipids 1–3 as well as the high-performance liquid chromatography (HPLC) profiles of the purified lipids 1–3 in two different mobile phases are provided in the Supplementary Information (Supplementary Figures S1–S9.
Sizes of the liposomes and lipoplexes
Hydrodynamic diameters (zeta sizes) of the liposomal formulations were measured by dynamic light scattering technique. The sizes of the liposomal formulations of lipids 1–3 were within the range of 134–154 nm (Supplementary Figure S10) and those for the lipoplexes of lipids 1–3 and p-CMV-MART1 (lipid:DNA charge ratios at 4:1) were found to be within the range of 278–292 nm (Supplementary Figure S11).
In vitro DC transfection properties of lipids 1–3
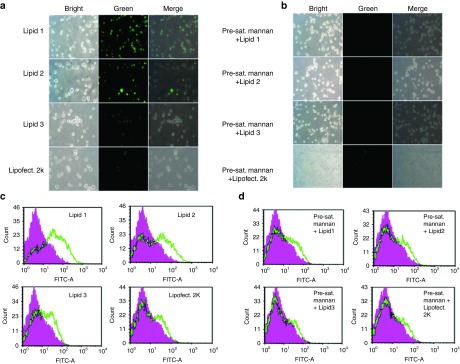
Primary mbmDCs (mouse bone marrow-derived DCs) were isolated from bone marrows in the tibias and fibulas of male C57BL/6J mice as described previously.17 Distinguishing DC surface markers (including MHC II, total MHC II, mannose receptors, CD11c, CD86, H2Kb, and CD40) in the isolated mbmDCs were confirmed by flow cytometry (Supplementary Figure S12). First, we evaluated DC transfection properties of the liposomes of lipids 1–3 by flow cytometry using a GFP plasmid (as a model DNA vaccine). First we transfected mbmDCs with GFP plasmid complexed with liposomes of lipids 1–3 and equimolar 1,2-dioleyol-sn-glycero-3-phosphoethanolamine (DOPE; as co-lipid) at cationic lipid:DNA charge ratio of 4:1 (initial transfection experiments across a range of lipid:DNA charge ratios revealed 4:1 to be the most optimal lipid:DNA charge ratio, data not shown). Transfected cells were visualized by fluorescence microscopy (Figure 2a) and the relative transfection efficiencies of lipids 1–3 were measured by flow cytometry (Figure 2c). In such flow cytometric measurements of transfection efficiency, the autofluorescence intensities of the untreated control cells are subtracted from the fluorescence intensities of the treated cells. The lipoplexes of lipid 1 with mannose-mimicking shikimoyl head-group was found to be the most efficient with ~20% DC transfection efficiency, followed by liposomes of lipids 2 and 3 with ~12% and ~5% DC transfection efficiencies, respectively (Figure 2c). Commercially available LipofectAmine 2000 was found to be very poor in transfecting mbmDCs (Figure 2a,c). Mannose receptor selective DC transfection properties of lipids 1–3 were significantly affected when mbmDCs were preincubated with 1 mg/ml mannan, a natural ligand for mannose receptor (Figure 2b,d), which supports the notion that the DC transfection by lipids 1–3 are mediated via mannose receptor. Furthermore, toward addressing concentration-dependent inhibition by mannan, we evaluated the DC transfection efficiencies of lipids 1–3 using DCs preincubated with increasing mannan concentrations across the 0.25–1.0 mg/ml. The DC transfection efficiencies were adversely affected with increasing concentrations of mannan (Supplementary Figure S13a). Importantly, the DC transfection properties of lipids 1–3 were not significantly affected when DCs were preincubated with laminarin, a commercially available polysaccharide of glucose (Supplementary Figure S13b). Thus, the findings summarized in Figure 2 and the Supplementary Figure S13a,b convincingly demonstrated mannose receptor-mediated DC transfection efficacies of cationic amphiphiles with mannose-mimicking shikimoyl and quinoyl- head-groups.
Figure 2.
Lipid 1 with the mannose-mimicking shikimoyl head-group is the most efficient among the lipids 1–3 in transfecting dendritic cells (DCs) in vitro. (a) Fluorescence images of DCs transfected with the lipoplexes of lipids 1–3. (b) Flourescence images of DCs transfected with the lipoplexes of lipids 1–3 in the presence of mannan (1 mg/ml). (c) Efficiencies of the lipoplexes of lipids 1–3 in transfecting mbmDCs measured by flow cytometry. (d) Efficiencies of the lipoplexes of lipids 1–3 in transfecting mbmDCs presaturated with mannan (1 mg/ml) measured by flow cytometry. In each of these transfection experiments, ~5 × 105 cells were used and the cells were transfected with lipoplexes of lipids 1–3 and α5-GFP plasmids containing lipid:DNA charge ratios of 4:1. The degrees of GFP expression in transfected mbmDCs were visualized using epifluorescence microscope and quantified by flow cytometry. Transfection efficiencies in each case were also measured using GFP lipoplexes of the commercially available liposomal transfection kit, LipofectAmine 2000 (extreme right panels). Each experiment was repeated three times and the transfection profiles were found to be similar in each time.
In vivo DC transfection properties of lipids 1–3
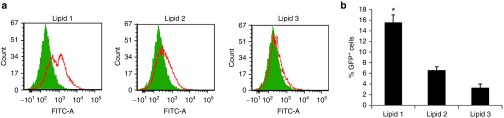
After confirming the mannose receptor-specific in vitro DC transfection efficiencies of lipids 1 and 2, we measured the efficiencies of the liposomal formulations of lipids 1–3 (containing equimolar DOPE) in targeting DNA vaccines to DCs under in vivo conditions using p-CMV-GFP as a model for DNA vaccine. The lipoplexes (liposome:DNA complexes) of lipids 1–3 were injected s.c. into C57BL/6J mice. Twenty-four hours after injection, the percentages of GFP-positive cells in the CD11c+ cells were isolated from the lymph nodes by using anti-CD11c antibody-labeled magnetic beads as described previously.18 Mice injected with only vehicles (5% glucose solution) were used as control. The findings in the flow cytometric study revealed a significantly higher number of GFP-positive cells (~15%) in the draining lymph nodes (CD11c+ cells) of mice injected with lipoplexes of lipid 1 when compared to the corresponding numbers observed in mice injected with lipoplexes of lipids 2 (~6%) and 3 (~3%) (Figure 3a,b). We also evaluated the MART1 expression level in transfected DCs under in vivo conditions using flow cytometric method by injecting mice (n = 2) with lipoplexes of lipids 1–3 and p-CMV-MART1. The findings summarized in Supplementary Figure S14 clearly showed liposomes of lipid 1 to be the most efficient among lipids 1–3 for targeting DNA vaccines to DCs under in vivo conditions.
Figure 3.
Lipid 1 is the most efficient among the lipids 1–3 in transfecting dendritic cells (DCs) in vivo. (a) In vivo DC transfection properties of the lipoplexes of lipids 1–3. Lipoplexes of lipids 1–3 and α5-GFP plasmids were injected (s.c.) into C57BL/6J mice and draining lymph nodes were harvested after 24 hours. Cells were labeled with anti-CD11c magnetic beads and enriched in an Midimax columns and the populations of GFP-positive cells were analyzed by flow cytometry. Cells collected from lymph nodes of mice injected (s.c.) with only vehicle (5% aqueous glucose) were used as negative control. Each experiment was repeated three times and similar FACS profiles were observed in each time. (b) Graphical representation of GFP-positive CD11C+ cells isolated from lymph nodes (*P < 0.05 versus lipoplex of lipid 3 and α5-GFP).
Phenotypic profiling of DCs transfected with lipoplexes of lipid 1 and p-CMV-β-gal
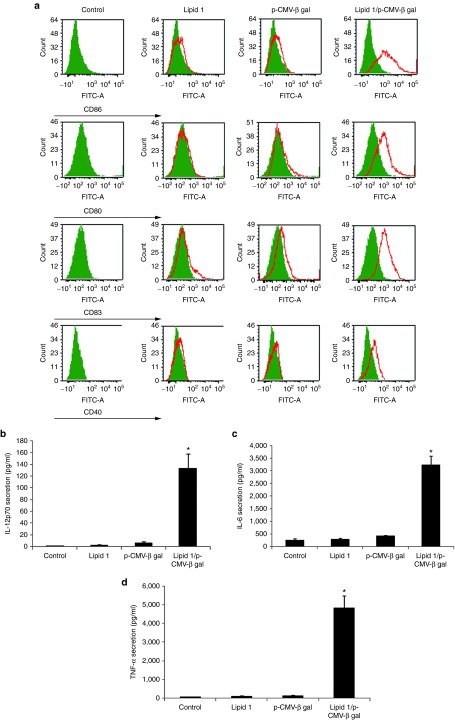
Using nontransfected DCs as control, next we assessed the relative phenotypic changes of DCs transfected with: the most promising lipoplex of lipid 1 and p-CMV-β-gal, only liposomes of lipid 1 and only p-CMV-β-gal. Expression levels of the DC maturation markers CD83, CD40, CD80, and CD86 were most significant in DCs transfected with the lipoplex of lipid 1 and p-CMV-β-gal induced (Figure 4a). Importantly, DCs treated with only liposomes of lipid 1 or with only p-CMV-β-gal plasmid failed to show any such significant phenotypical maturation (Figure 4a).
Figure 4.
Transfection of dendritic cells (DCs) with lipoplex of lipid 1 and p-CMV-β-gal induces phenotypic maturation and enhanced IL-12p70, IL-12, and TNF-α cytokines. (a) Phenotype analysis. DCs treated with only liposomes of lipid 1, only p-CMV-β-gal, and lipoplexes of lipid 1 and p-CMV-β-gal were stained after 24 hours with FITC-labeled antibodies against costimulatory molecules CD40, CD80, CD83, and CD86 and analyzed by flow cytometry. The results are representative of data from three independent experiments. Secretion of (b) IL-12p70 (*P < 0.05 versus untransfected DCs), (c) IL-6 (*P < 0.005 versus untransfected DCs), and (d) TNF-α (*P < 0.05 versus untransfected DCs) from DCs treated with the lipoplexes of lipid 1 and p-CMV-β-gal.
Secreted cytokines from DCs transfected with lipoplex of lipid 1 and p-CMV-β-gal
With a view to gain some insights into the nature of cytokines secreted by transfected DCs, we used ELISA kits for measuring TNF-α, IL-6, and IL-12p70 cytokine levels in the supernatants of DCs treated with: lipoplex of lipid 1 and p-CMV-β-gal plasmid, only liposomes of lipid 1 and only p-CMV-β-gal plasmid. Enhanced secretions of all these three cytokines were observed only in DCs transfected with lipoplexes of lipid 1 and p-CMV-β-gal plasmid compared with lipid 1, p-CMV-β-gal, and untreated control (Figure 4b–d). Enhanced secretions of these immunostimulatory cytokines are consistent with functional maturations of DC after being transfected with lipoplexes of lipid 1 and p-CMV-β-gal.
In vivo immunization studies
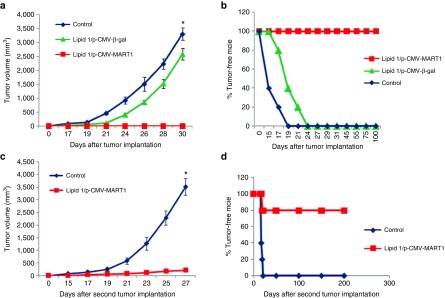
Toward confirming that s.c. immunization with the lipoplexes of lipids 1–3 in mice is capable of inducing immune responses under in vivo settings, first we used p-CMV-β-gal plasmid as a model DNA vaccine. C57BL/6J mice were s.c. immunized with p-CMV-β-gal complexed with liposomes of lipid 1–3 and equimolar DOPE. Two weeks post third immunization, splenocytes and sera were collected and IFN-γ (signature cytokine for cellular immune response) and anti-β-Gal antibodies (humoral immune response) were measured by using anti-IFN-γ-coated and β-Galactosidase-coated ELISA plates, respectively. Mice immunized with only vehicles (5% glucose) were used as control. Importantly, the amount of IFN-γ measured from splenocytes and the amount of anti-β-Gal antibodies measured in sera for the mice group immunized with lipoplexes of lipid 1 were found to be significantly higher than those for the control mice group as well as for the mice groups immunized with lipoplexes of lipids 2 and 3 (Supplementary Figure S15a,b). These initial findings prompted us to conduct in vivo DC-targeted genetic immunization experiment using lipoplexes of lipid 1 (containing shikimoyl- head-group) and p-CMV-MART1 DNA vaccine encoding human MelanA/MART1 which shares 68.6% amino acid sequence identity with its murine counterpart.19 First mice (n = 5) were immunized with the lipoplexes of lipid 1 and p-CMV-MART1 and were subsequently challenged with lethal dose (~1 − 105 cells/mice) of aggressive melanoma tumor. Since the tumor sizes for the control mice group immunized with only vehicle (5% aqueous glucose) became too large on day 30 post tumor challenge and had to be sacrificed, tumor growth was monitored for 30 days. Importantly, all the five mice s.c. immunized with lipoplexes of lipid 1 and p-CMV-MART1 were completely tumor free while tumor sizes in all the five mice s.c. immunized with irrelevant control lipoplexes of lipid 1 and p-CMV-β-gal kept on increasing with time and died within 29 days post tumor challenge (Figure 5a). In sharp contrast, long-lasting (100 days post tumor challenge) antimelanoma protective immunity was observed in mice immunized with lipoplexes of lipid 1 and p-CMV-MART1. All the five immunized mice lived completely tumor-free life for 100 days post tumor challenge (Figure 5b). Collectively, the findings summarized in Figure 5a,b demonstrate that the long-lasting antitumor immune response inducing potential of the in vivo DC-targeting lipoplexes of lipid 1 and p-CMV-MART1. Furthermore, with a view to evaluate memory response, all the five immunized mice which survived 100 days after the first B16F10 challenge were challenged a second time with ~1 × 105 B16F10 cells. Four out of five mice (80%) second time challenged with tumor lived once again completely tumor-free lives for 6 months after the second tumor challenge (Figure 5c,d). We also evaluated the in vivo DC-targeted immunization efficacy of lipoplexes of lipid 2 and p-CMV-MART1 and lipoplexes of lipid 3 and p-CMV-MART1. Importantly, 60% of mice immunized with the lipoplexes of lipid 2 and p-CMV-MART1 lived tumor-free life, whereas only 20% of mice immunized with the lipoplexes of lipid 3 and p-CMV-MART1 lived tumor-free life during the observed time period of 70 days post tumor challenge (Supplementary Figure S16a,b). Stated differently, the findings summarized in Figure 5c,d convincingly demonstrated the efficiency of the presently described in vivo DC-targeting liposomal DNA vaccine carrier of lipid 1 in inducing dramatic memory response in cancer immunotherapy.
Figure 5.
Direct in vivo immunization with lipoplexes of lipid 1 and MART1 (melanoma antigen)-encoded DNA vaccine (p-CMV-MART1) protects syngeneic C57BL/6J mice from lethal melanoma challenge with remarkable memory response. (a) Six- to eight-week-old female syngeneic C57BL/6J mice (each weighing 20–22 g, n = 5) were immunized (s.c) with lipoplexes of lipid 1 and p-CMV-MART1 and lipoplexes of lipid 1 and p-CMV-β-gal (as negative control) using 150 µl 5% glucose solution containing 15 µg DNA, 4:1 lipid:DNA ratio, three times with 7-day intervals. Two weeks post third immunization, mice were challenged with melanoma tumor by s.c. injection of ~1 × 105 B16F10 cells. Tumor volumes (V = ½ ab2 where, a = maximum length of the tumor and b = minimum length of the tumor measured perpendicular to each other) were measured with a slide calipers for up to 30 days. Results represent the means ± SD for n = 5 tumors (*P < 0.005 versus tumor sizes for lipoplexes of lipid 1 and p-CMV- MART1; statistical analysis was performed using students unpaired t-test). (b) Percentage of tumor-free mice immunized (s.c.) with lipoplexes of lipid 1 and p-CMV-MART1 and lipoplexes of lipid 1 and p-CMV-β-gal. (c) All the C57BL/6J mice (n = 5) immunized with lipoplexes of lipid 1 and p-CMV-MART1 which lived a tumor-free life for 100 days post first tumor challenge were challenged a second time with ~1 × 105 B16F10 cells. The short-term antitumor memory responses after this second tumor challenge are shown for 30 days (*P < 0.005 versus tumor sizes for lipoplex of lipid 1 and p-CMV- MART1; statistical analysis was performed using students unpaired t-test). (d) The percentages of tumor-free mice remaining alive up to 180 days after the second melanoma challenge (80%).
Studies on the efficacies of the lipoplexes of lipids 1–3 and p-CMV-MART1 plasmid in regressing established tumor
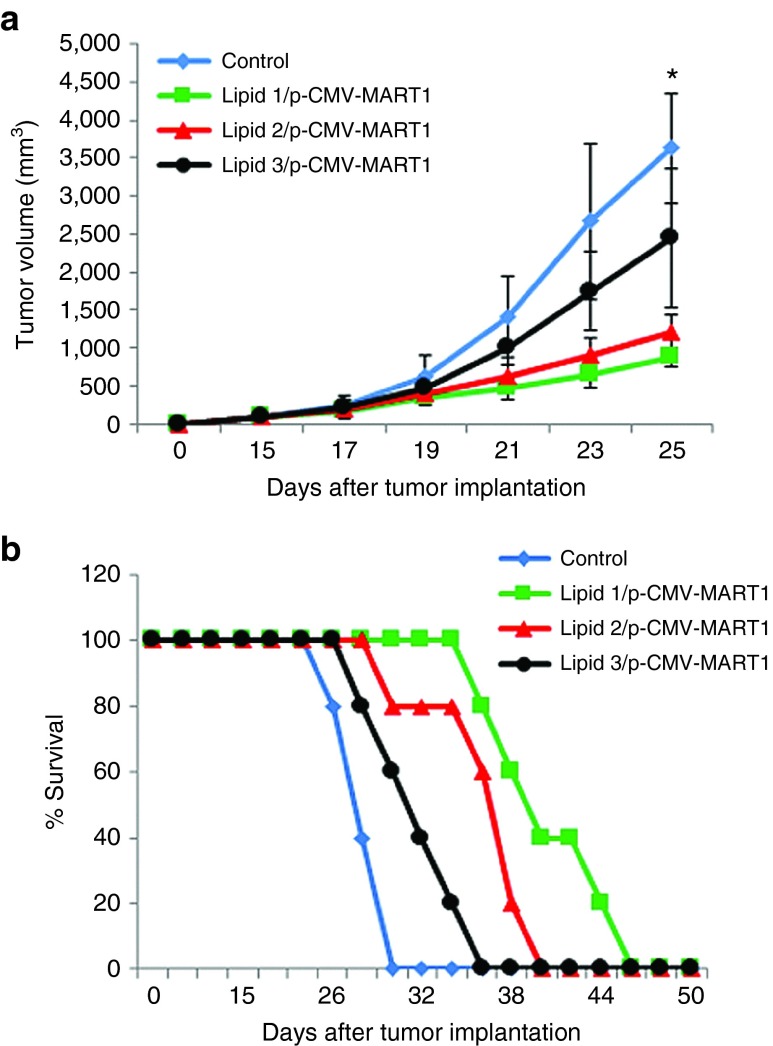
The experiments described above showed that immunization with lipoplex of lipid 1 and p-CMV-MART1 efficiently protected mice from a subsequent tumor challenge. With a view to evaluate the efficacies of these lipoplexes under therapeutic settings, lipoplexes of lipids 1–3 and p-CMV-MART1 were s.c. administered in mice bearing established tumors. Six- to eight-week-old female C57BL/6J mice (each weighing 20–22 g, n = 5) were s.c. injected with ~1.0 × 105 B16F10 cells in their right flanks. On day 15 when tumor became palpable, mice were s.c. immunized with lipoplexes of lipids 1–3 and p-CMV-MART1. This treatment was repeated on days 18 and 21 post tumor inoculation. Findings summarized in Figure 6a,b showed that the mice group immunized with lipoplex of lipid 1 and p-CMV-MART1 were more efficient in inhibiting tumor growth compared to mice groups treated with corresponding lipoplexes of lipids 2 and 3. The overall survivability of tumor-bearing mice group immunized with lipoplexes of lipid 1 and p-CMV-MART1 (45 days) was also higher than that for tumor-bearing mice group immunized with corresponding lipoplexes of lipids 2 and 3 (Figure 6b).
Figure 6.
Antitumor efficacies of the lipoplex of lipid 1 and p-CMV-MART1 under therapeutic settings. (a) Six- to eight-week-old female C57BL/6J mice (each weighing 20–22 g) were injected with ~1 × 105 B16F10 cells s.c. in the right flank. On day 15, mice were randomly sorted into four groups (n = 5) and each group was immunized s.c. with one of the following: lipoplexes of lipid 1 and p-CMV-MART1 (square); lipid 2 and p-CMV-MART1 (triangle); lipid 3 and p-CMV-MART1 (circle). One group was injected with 5% glucose (vehicle control, diamond). This treatment was repeated on days 18 and 21 after tumor cell implantation. Tumor volumes (V = ½ ab2 where, a = maximum length of the tumor and b = minimum length of the tumor measured perpendicular to each other) were measured with a slide caliper for up to 25 days (*P < 0.05 versus lipid 1/p-CMV-MART1). (b) Survival study.
Cytotoxic T lymphocyte cytokine assays
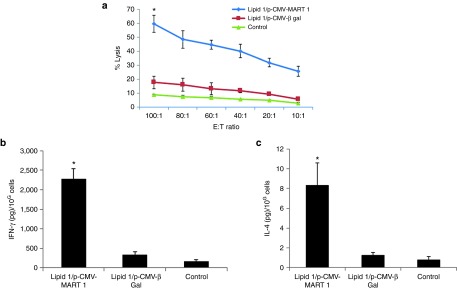
Next, toward probing the relative contributions of humoral and cellular immune responses, we performed the conventional cytotoxic T lymphocyte (CTL) assay and measured the amounts of secreted IFN-γ and IL-4 (signature cytokines for cellular and humoral immune responses, respectively) in the supernatants from the coculture of the effector cells (splenocytes isolated from immunized mice) and the target B16F10 melanoma cells. Lysis of the target melanoma cells by the primed splenocytes was studied across the Effector:Target cell ratio of 10:1–100:1. Importantly, while the effector splenocytes isolated from mice immunized with lipoplexes of lipid 1 and p-CMV-MART1 lysed ~60% target melanoma cells, significantly reduced target cell lysis (~20%) could be effected by splenocytes isolated from mice immunized with lipoplexes of lipid 1 and a control irrelevant p-CMV-β-gal plasmid (Figure 7a). This showed that the mice group immunized with in vivo DC-targeting lipoplexes of lipid 1 and p-CMV-MART1 was more efficient in inducing CTL response against target cell (B16F10). Consistent with such target cell selective lytic activity of the effector splenocytes, results in the cytokine secretion assays also revealed remarkably higher amounts of IFN-γ (compared to secreted IL-4) secreted by the activated T cells (Figure 7b,c). IFN-γ being a distinguishing marker of cellular immune response, the results shown in Figure 7b,c are consistent with the supposition that cell-mediated immunity plays a crucial role behind the presently observed remarkable antitumor immune response.
Figure 7.
Coculture of splenocytes isolated from mice immunized with lipoplexes of lipid 1 and p-CMV-MART1 leads to effective lysis of target B10F10 cells via melanoma specific cytotoxic T lymphocyte (CTL) response, secretion of IFN-γ and IL-4. (a) Six- to eight-week-old female C57BL/6J mice (each weighing 20–22 g, n = 4) were immunized (s.c., three times with 7-day intervals) with lipoplexes of lipid 1 and p-CMV-MART1 and lipoplexes of lipid 1 and p-CMV- β-gal (as negative control). Splenocytes were collected 28 days after the third immunization and the percentages of target melanoma cell lysis (CTL responses) were measured as described under Materials and Methods (*P < 0.005 versus control). (b,c) Two weeks post third immunization, splenocytes were collected, stimulated for 3 days by coculturing with target B16F10 cells. Amounts of IFN-γ (signature cytokine for cellular immune response) and IL-4 (signature cytokine for humoral immune response) released in the coculture supernatants were determined by ELISA (*P < 0.05 versus control).
CD8+ T-cell depletion studies
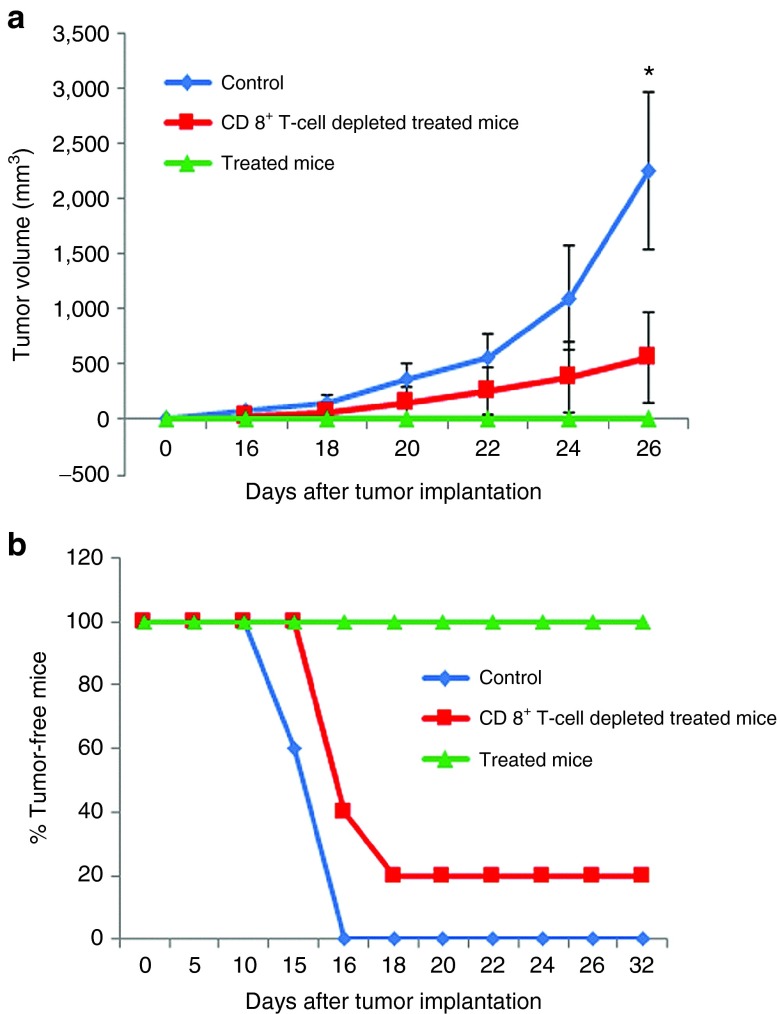
Two groups of C57BL/6J mice (n = 5 for each group) were immunized thrice with the lipoplex of lipid 1 and p-CMV-MART1 on day 0, 7, and 14. The first group received i.p. injections of anti-CD8 mAbs (2.43) on days −4, −1, 2, 6, 13, 17, 21, 25, 29, and the second immunized group did not receive any anti-CD8 mAbs. A third unimmunized group was injected with 5% aqueous glucose (vehicle control group). All the three groups were challenged with B16F10 cells (on day 28 for the first two groups) and tumor growths were measured by slide caliper. The mice group that did not receive anti-CD8 mAbs lived tumor-free lives after being immunized with the lipoplex of lipid 1 and p-CMV-MART1 while all five members of the unimmunized control mice group developed tumors within 15 days (Figure 8a). Importantly, four out of five mice which were immunized and depleted of CD8+ cells developed tumors upon challenge with tumor cells (Figure 8b). These findings convincingly demonstrated that cell-mediated (CD8+ T cells) immunity is playing a critical role in inducing antitumor immune response for the presently described in vivo DC-targeting liposomal DNA vaccine carrier.
Figure 8.
CD8+ T-cell depletion results into significantly compromised antitumor immune response. (a) Two groups of C57BL/6J mice (n = 5 for each group) were immunized thrice with the lipoplex of lipid 1 and p-CMV-MART1 on day 0, 7, and 14. The first group received i.p. injections of anti-CD8 mAbs (2.43) on days −4, −1, 2, 6, 13, 17, 21, 25, and 29 and the second immunized group did not receive any anti-CD8 mAbs. A third unimmunized group was injected with 5% aqueous glucose (vehicle control group). All the three groups were challenged with B16F10 cells (on day 28) and tumor growths were measured by slide caliper. (*P < 0.05 versus treated mice). (b) Percentages of tumor-free mice.
Discussion
DNA vaccination, i.e., use of naked plasmid DNA as a vaccine to prime the immune system, offers a number of practical benefits which are not easily achievable with other existing forms of vaccines such as attenuated viruses, subunit or recombinant protein vaccines, whole tumor cells, etc.20,21,22,23,24 There are a number of distinct advantages in using pDNAs in genetic immunization. They are easy to design and construct and their large-scale production is cost-effective. In contrast to attenuated viral vaccines whose storage and global delivery are complicated by the need of keeping the vaccines cold, plasmid DNAs are fairly stable at room temperature.25 Since the antigens encoded in the DNA vaccines are expressed in situ, their posttranslational modifications happen in similar way as in the case of infection with cognate pathogens. Another distinguishing feature of DNA vaccines is that the immune response induced by such genetic immunization are often primarily cellular in nature which is believed to play crucial roles for effective vaccination against pathogens (e.g., viruses) and cancers.26,27 Despite all these distinguishing advantages, the low in vivo cell transfection efficiencies of naked plasmid DNA, difficulty in preferentially targeting of DNA vaccines to professional antigen-presenting cells (DCs), and the modest intrinsic adjuvant activity of DNA vaccines are impeding their clinical success. To this end, immunization with autologous DCs ex vivo transfected with antigen-encoded DNA vaccines have shown promise in the past.1,4,5,6,7,8,9,28,29 Such ex vivo DC transfection-based immunization protocols are labor-intensive, time-consuming, expensive, and not patient-friendly.
Aimed at resolving the above-mentioned problems associated with ex vivo DC transfection-based protocols for genetic immunization, global efforts have begun toward developing protocols for direct in vivo targeting of antigen-encoded DNA vaccines to DCs. Promising recently developed strategies for direct in vivo targeting of DNA vaccines to DCs include use of antibody to target DC-specific receptors including DEC-205, DNGR-1, CD11c, etc. for delivering encoded antigens or use of DC-specific promoter (e.g., CD11c) for expressing cytokines required for enhancing vaccine efficacies.30,31,32,33,34,35 Daftarian et al. showed the use of fifth-generation polyamidoamine (G5-PAMAM) dendrimers possessing a DNA-loading surface and covalently grafted MHC class II-targeting peptides as a Universal DNA platform.12 In vivo DC-targeting of a genetic vaccine encoding both cytomegalovirus (CMV)-driven vaccine Aghsp70 and DC-specific CD11c-driven active transcription factor XBP1 has been shown to induce both prophylactive and therapeutic antitumor immunity.13 Raghuwanshi et al. recently succeeded in developing an in vivo DC-targeted chitosan nanoparticles for nasal DNA immunization against nucleocapsid (N) protein of severe acute respiratory syndrome coronavirus (SARS-CoV) as antigen.14 Cao et al. constructed in vivo DC-targeting DNA vaccine by fusing tumor-associated antigen HER2/neu ectodomain to single chain antibody fragment (scFv) from NLDC-145 antibody specific for DC-restricted surface molecule DEC-205 and demonstrated its potent antitumor cellular and humoral immune response inducing efficacies.15 Raviv et al. demonstrated that mannosylated polyion complexes are safe and effective systems for in vivo targeting of genes to CD11c+ DCs.16
It is important to emphasize that our previously reported liposomes of cationic lipids containing the mannose-mimicking shikimoyl- and quinoyl- head-groups with pure lysine spacer (i.e., without the lysine side chain amine group being guanidinylated as is the case for the presently described lipids 1–3, Figure 1) were capable of mounting long-lasting immune response only under ex vivo DC transfection-based DNA vaccination.9 DCs are hard-to-transfect. Although the DC transfection efficacies of these previously reported lipids containing pure lysine spacer between the mannose-mimicking head-group and hydrophobic tails9 were ~12%, the lipids failed in mounting long-lasting immune response against melanoma challenge when used on the direct in vivo immunization mode (Supplementary Figure S17). Since prior studies demonstrated efficient gene transfer properties of guanidinylated cationic amphiphiles,36,37 we decided to convert the amine group in the lysine side chain of our previously reported lipids9 with guanidine group using conventional guanidinylation reagent HgCl2/N,N-di-Boc-thiourea. A number of distinguishing chemical characteristics contribute to the enhanced gene transfer efficiencies of guanidinylated cationic amphiphiles. The guanidinium groups, because of their high pKa values (~12–13), remain protonated across a much wider range of pH compared to other basic groups and thereby exhibit strong electrostatic binding properties with the phosphates group of the macromolecular DNA molecules under the physiological pH. In addition, they form characteristic parallel zwitterionic N-H+…O− hydrogen bonds with the phosphate ions of the DNA molecules and form hydrogen bonds with nucleic acid bases.37 In vitro and in vivo DC transfection studies clearly demonstrated that lipid 1 was most efficient compared to lipids 2 and 3. Hydrodynamic diameters of the liposomes of lipids 1–3 were found to be within the range 134–154 nm (Supplementary Figure S10) and the corresponding ranges for the lipoplexes of lipids 1–3 were within 278–292 nm (Supplementary Figure S11). Such similar hydrodynamic diameters of the lipoplexes of lipids 1–3 are consistent with the supposition that the sizes of lipoplexes are unlikely to play major role in modulating the DC transfection efficiencies of lipids 1–3. We do not understand why increase in ex vivo DC transfection efficiency from ~12% (as was the case for our previous reported lipid9) to just ~20% (through conversion of the lysine amino side chain to guanidine in the present study) imparts in vivo DC-targeting ability to the lipid. Perhaps the minimum (threshold) ex vivo DC transfection efficiency for imparting in vivo DC-targeting ability to cationic lipid lies near 20%. However, deciphering such threshold ex vivo DC transfection of cationic lipids, if any, would be impossible to find out without in-depth future structure–activity studies using more number of structural analogs such as use of multiple shikimoyl- and quinoyl- head-groups, use of multiple guanidine groups in the head-group regions, etc.
An important issue is worth mentioning at this point of discussion. Although in the present study we have demonstrated the in vivo DC-targeting ability of the mannose receptor-specific lipoplex of lipid 1, the present findings do not preclude the possibility of macrophage and possibly other myeloid cells (many of which express mannose receptors on their cell surface) being transfected by the lipoplexes of lipid 1. Since DCs are the most potent antigen-presenting cells in mounting adaptive immune response, herein our primary focus is on the in vivo DC-transfecting ability of the presently described lipids. We have no clue yet as to the origin of more DC-targeting efficacy of lipoplex of lipid 1 compared to the lipoplexes of lipids 2 and 3. The issue remains elusive at this stage of investigation. Clearly further mechanistic studies such as binding studies using purified mannose receptor need to be undertaken in future toward gaining insights into the relative in vivo DC-targeting efficacies of the lipoplexes of lipids 1–3.
Despite significant recent progresses in the emerging field of in vivo DC-targeted genetic immunization, the challenge of designing a simple, safe, and effective system for in vivo targeting of DNA vaccines to DCs remains formidable. To this end, using a syngeneic mouse melanoma model, we have shown that immunization with tumor antigen (MART1)-encoded DNA vaccines in complexation with the presently described next-generation liposomal DNA vaccine carrier is capable of mounting long-lasting immune response against lethal dose of melanoma tumor challenge (all five immunized mice lived completely tumor-free lives during 100 days post first tumor challenge). p-CMV-MART1 DNA vaccine encodes 18 kDa Human MelanA/MART1 antigen which shares 68.6% amino acid sequence identity with its murine counterpart.19 MelanA/MART1 is a human melanocyte lineage-specific antigen expressed by majority of human malignant melanoma. Effective antigen-specific immunity ensues when DCs present the processed antigen fragments of the MART1 to both CD4+ and CD8+ T cells. DCs present processed antigen fragments to CD4+ cells in complexation with MHC II molecules which causes differentiation of CD4+ T cells into T-helper 1 (Th1) and T-helper 2 (Th2) cells (inducers of cellular and humoral immune responses, respectively). When DCs present the processed MART1 protein fragments to CD8+ T cells in complexation with MHC I molecules, the activated CD8+ T cells differentiate into CTLs (the effector T cells) which kill the affected cells with help from CD4+ T cells.38 Importantly, in the present study, 80% of mice (which lived completely tumor-free lives for 100 days after the first tumor challenge) lived again completely tumor-free lives for 200 days post second tumor challenge. Precise mechanism as to how such long-lasting immune responses against melanoma antigen is induced by the presently described in vivo DC-targeting lipoplex of lipid 1 remains an open question at this stage of investigation. CD4 Th1 cells secret IFN-γ which induces further proliferation of CTLs. Presumably, effective in vivo DC-targeting of the p-CMV-MART1 DNA vaccine in complexation with the liposomes of lipid 1 ensures secretion of large quantity of IFN-γ (as was observed in the present case) by CD4 Th1 cells which in turn leads to proliferation of CTLs including strong induction of memory T cells (Supplementary Figure S18a,b). Another important issue deserves mention at this point. Prior studies showed that lipoplexes, in general, are effective in blocking tumor growth in mice due to their capabilities in inducing acute immune responses.39 Findings in the CTL assay summarized in Figure 7a clearly show that target cell (B16F10) lysis happens only to the extent of ~20% by splenocytes (at E:T ratio of 100:1) of mice immunized with control lipoplex of lipid 1 and p-CMV-β-Gal plasmid (control DNA vaccine with no encoded tumor antigen). Contrastingly, the degree of target cell lysis by splenocytes of mice immunized with lipoplexes of lipid 1 and p-CMV-MART1 DNA vaccine was found to be ~60% as shown in Figure 7a. Consistent with these findings in the CTL assay, both the amounts of secreted IFN-γ (markers of cellular immune response) and IL-4 (markers of humoral immune response) for mice immunized with lipid 1:p-CMV MART1 lipoplexes were found to be about seven times higher than the amounts of secreted IFN-γ and IL-4 for mice immunized with control lipoplex of lipid 1 and p-CMV-β-Gal plasmid (Figure 7b,c based on assay performed 4 weeks after immunization). Thus, nonspecific immune response inducing properties of lipoplexes are unlikely to play major role in the presently described long-lasting immune response. Furthermore, the findings in the newly conducted experiments using CD8+ depleted mice (Figure 8) clearly support the notion that CD8+ T cells play an important in mounting long-lasting immune response described herein. Stated differently, the results summarized in Supplementary Figure S13a,b and Figure 7a–c convincingly demonstrated that heightened cellular immune responses play crucial roles behind the remarkably sustained protective immunity against melanoma tumor described herein.
We have shown that liposomes of cationic amphiphiles containing a mannose-mimicking shikimoyl- head-group, two n-hexadecyl hydrophobic tails, and a guanidinylated lysine spacer in between the mannose-mimicking head-groups and nonpolar tails hold great promise for direct in vivo targeting of tumor antigen-encoded DNA vaccines to DCs in designing DC-based cancer vaccines. With the availability of the presently described simple liposomal in vivo DC-targeting system, avoiding potentially unsafe viral vectors for targeting DCs under in vivo settings in genetic immunization will now be feasible. The dramatically long-lasting primary immune responses against melanoma in all immunized mice and the remarkable memory responses delineated herein support the future systemic promises of the presently described direct in vivo DC-targeting liposomal DNA vaccine carrier in combating cancer and potentially many other challenging infectious diseases with known pathogenic antigens through direct DNA vaccination in animal and human body.
Materials and Methods
General procedures, materials, and reagents. LCQ ion trap mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with an ESI source or Micromass Quattro LC triple quadrapole mass spectrometer was used for ESI mass spectral analysis of the target lipids 1–3. Varian FT 300, 400 MHz, and 600 MHz NMR Spectrometers were used in running 1H NMR spectra of target lipids 1–3 and their synthetic intermediates. The quinic and shikimic acids used for synthesis of lipids 1 and 2 were from Fluka (Switzerland) and the d-Mannose used for synthesis of lipid 3 was from SD Fine Chemicals, Hyderabad, India. The EDCI and HOBT used for coupling qunic and shikimic acids to tertiary amine intermediate were procured from Sigma-Aldrich (St. Louis, MO), and the silica gel (60–120 mesh) used for performing column chromatography was purchased from Acme Synthetic Chemicals (Mumbai, India). Reversed phase analytical HPLC analysis using two different mobile phases (A: pure methanol and B: 95:5, v/v, methanol/water) were used in conforming more than 95% purities of the target lipids 1–3. p-CMV-MART1 plasmid DNA used in the present study was a kind gift from Dr. Van den Eynde, Ludwig Institute for Cancer Research, Brussels, Belgium. Dr. Nalam Madhusudhana Rao kindly provided us with sample of p-CMV-SPORT-β-gal plasmid. The CTL assay kit and the ELISA kits for cytokine assays were procured from Thermo Scientific (Waltham, MA), and Promega (Madison, CA). Mouse FITC-conjugated CD11c, H2Kb, CD40, CD86, CD83, CD80, and mannose receptor antibodies were purchased from Bio-Legend (San Diego, CA). Mouse phycoerythrin-conjugated CD4+ and CD11c and FITC-conjugated CD8+ antibodies were purchased from Cell Signaling (Danvers, MA). Mouse anti-MHC II monoclonal antibody used was from Chemicon (Billerica, MA). Anti-MART1 mAb was purchased from Pierce Biotechnology (Rockford, MA). IL-12p70, IL-6, and TNF-α ELISA kits were procured from R&D Systems (Minneapolis, MN). Anti-CD11c antibody-labeled magnetic beads and Midimax columns were purchased from Miltenyi Biotec (Bergisch Gladbach, Germany). Cholesterol, cell culture media, fetal bovine serum, and the FITC-conjugated rat anti-mouse secondary antibody were purchased from Sigma-Aldrich. NP-40, antibiotics, and agarose were obtained from Hi-media, India. B16F10 (murine melanoma cells) was procured from the National Centre for Cell Sciences (NCCS), Pune, India. Dulbecco's modified Eagle's medium with 10% fetal bovine serum was used in culturing B16F10 cells at 37 °C in a humidified atmosphere containing 5% CO2/95% air. C57BL/6 mice (each weighing 20–22 g and 6–8 weeks old) were obtained from National Institute of Nutrition, Hyderabad, India and all the in vivo experiments were done following Institutional Bio-Safety and Ethical Committee Guidelines using approved animal protocols.
Preparation of liposomes. The co-lipid DOPE and lipids 1–3 (at a mole ratio 1:1) were dissolved in chloroform and the solvent from the resulting homogeneous solution was removed with a thin stream of nitrogen gas. The residue was dried under high vacuum for 8 hours and the vacuum dried residue upon hydration in deionized water for overnight afforded the in vivo DC-targeting liposomes. The appropriate weights of lipids 1–3 and DOPE was used such that the resulting liposomes contained 1 mmol/l lipids 1–3 and 1 mmol/l DOPE for all in vitro experiments and the corresponding concentrations were 5 mmol/l for all in vivo experiments. The hydrated lipid film was then briefly vortexed (30 seconds) and thereafter sonicated to clarity using a Branson 450 sonifier at 100% duty cycle and 25 W output power. The resulting clear aqueous liposomes were then complexed with plasmid DNAs for preparing lipoplexes.
Hydrodynamic size measurements by dynamic light scattering. The hydrodynamic diameters of the liposomes and the lipoplexes of lipids 1–3 (using p-CMV-MART1 as DNA) were analyzed by dynamic light scattering performed on a DynaPro Nano dynamic light scattering system (Wyatt Technologies, Santa Barbara, CA). All measurements were performed at a fixed angle of 90° at room temperature (25 °C).
Isolation of primary mbmDCs. mbmDCs were isolated from the tibias and fibulas of C57/BL6J mice as described previously.17 Briefly, bone marrow was collected from tibias and fibulas of male C57BL/6J mice. Bone and debris from the collected bone marrow was removed by passing through a nylon mesh and the pass through cells were resuspended in complete DC medium (RPMI-1640) containing 10% fetal bovine serum, 50 µmol/l β-mercaptoethanol, 2 mmol/l glutamine, 1% nonessential amino acids, 20 ng/ml granulocyte-macrophage colony-stimulating factor, 10 ng/ml IL-4, and 1% antibiotic solution. Cell media were changed every 2 days with fresh DC medium. The aggregated cells were dislodged by gently pipetting with RPMI after 6 days, the dislodged cells pulled together, and centrifuged at 280g for 10 minutes at room temperature. The supernatant was discarded and the pellets resuspended in complete DC medium at 1 × 106 cells/ml. The resuspended cells were then placed in 100-mm cell culture Petri dishes (at ~1 × 107 cells/dish) using 10 ml medium per dish. The nonadherent cells were collected after 24 hours through gently swirling the dish and were used for both transfection and flow cytometry experiments. Cultures of the collected nonadherent DCs were maintained in a humidified atmosphere with 5% CO2 at 37 °C.
FACS protocol. Cells (~4 × 106) were fixed in 2% formaldehyde solution for 2 hours, centrifuged, and the supernatant was discarded. The pelleted cells were then suspended in 3 ml wash buffer (phosphate-buffered saline (PBS) containing 2% fetal bovine serum and 0.1% sodium azide) and incubated for 30 min at 37 °C. The cell suspension was divided equally into Eppendorf tubes containing ~5 × 105 cells in each tube, centrifuged, the supernatants discarded, and finally, to the cell pellets in each tube 100 µl commercially available phycoerythrin-labeled mouse monoclonal antibodies at a dilution of 1:100 (in wash buffer) was added. About 100 µl wash buffer was added to one tube as a control. The cell suspensions were then cultured at room temperature for 2.5 hours, centrifuged, and the supernatants were discarded. The cell pellets were washed with wash buffer (2 × 1 ml). The washed cells were then incubated for 1 hour after sequential additions of commercially available unlabeled anti-MHC II monoclonal antibody at a dilution of 1:100 and 100 µl of FITC-conjugated secondary antibody at 1:200 dilution (for measuring MHC II marker profiles). The sequence of such centrifugation and washing were repeated for three times and the final cell pellets were suspended in 1 ml wash buffer. The flow cytometry histograms were then recorded in a FACS-caliber instrument (Becton Dickinson, Franklin Lakes, NJ). Cells were permeabilized with 90% methanol for 30 minutes before incubating with antibodies for measuring total MHC II expression in DCs. The GFP expression in transfected mbmDCs were measured by flow cytometry after harvesting and washing the transfected cells with wash buffer (2 × 500 µl) and the washed pellets were suspended in 1 ml wash buffer. The flow cytometry histograms were then recorded using untreated mbmDCs as controls. Phenotype analysis of transfected DCs were performed using FITC-labeled monoclonal antibodies as described above.
Flow cytometric method for studying in vivo DC-targeting. Liposomes of lipids 1–3 were complexed with α5-GFP plasmid and the resulting lipoplexes were injected (s.c.) into C57BL/6J mice. Two draining lymph nodes were harvested after 24 hours and digested in complete RPMI medium containing 400 U/ml of collagenase at 37 °C for 30 minutes. The resulting single-cell suspension was washed with PBS containing 1% bovine serum albumin. Cells were labeled with anti-CD11c magnetic beads (Miltenyi Biotec), incubated at 4 °C for 15 minutes and enriched in Midimax columns (Miltenyi Biotec) following the manufacturer's instructions. The purity of isolated enriched DCs after affinity purification over the anti-CD11c magnetic beads was measured by flow cytometry using FITC-labeled CD11c antibody. The enriched CD11c+ cells DCs were found to be ~98% pure by flow cytometry. Finally the percentage of GFP+ DCs in this enriched DCs were measured by flow cytometry. DCs collected from lymph nodes of mice immunized with only vehicle were used as control.
Cytokine secretion. DC culture supernatants were harvested 24 hours after transfection and stored at −20 °C until use. Levels of IL-12 p70, IL-6, and TNF-α concentrations were assessed using commercially available ELISA kits (R&D Systems) by following Manufacturer's instructions.
ELISA for anti-β-gal antibody. Anti-β-gal antibodies were measured using ELISA as described previously.40 Briefly, 96-well ELISA plates were coated with β-gal protein (0.3 µg/well). After overnight incubation at 4 °C, the plates were washed with PBS (3 × 200 µl) and blocked with 1% bovine serum albumin in PBS for 2 hours at room temperature. The plates were then washed with PBS containing 0.05% Tween-20 (3 × 200 µl), incubated with serially diluted mouse sera (100 µl) at room temperature for 2 hours, and washed again with PBS containing 0.05% Tween-20 (3 × 200 µl). About 100 µl of prediluted (1:1,000) horse radish peroxidase-conjugated anti-mouse secondary antibody was added to each well of the plate, incubated at room temperature for 2 hours, and the unbound secondary antibody was removed by washing with PBS containing 0.05% Tween-20 (3 × 200 µl). About 100 µl of ABTS (Calbiochem, St. Louis, MO) was added to each well and the mixture incubated in dark at room temperature for 10 minutes. The absorbance was finally measured at 405 nm by ELISA reader (Bio-Tek instruments, UK).
In vivo immunization. Six- to eight-week-old female syngeneic C57BL/6J mice (each weighing 20–22 g, n = 5) were immunized (s.c.) thrice (with 7-day intervals) separately with lipoplexes (lipoplexes of lipid 1 and p-CMV-MART1 in one group and the lipoplexes of lipids 1–3 and p-CMV-β-gal in other groups) in 150 µl 5% glucose solution containing 15 µg plasmid DNA with lipid:DNA charge ratio of 4:1. The group immunized with lipoplexes of lipids 1–3 and p-CMV-β-gal were used to measure humoral (β-gal antibody) and cellular (IFN-gamma) immune responses. The group immunized with lipoplexes of lipid 1 and p-CMV-MART1 as well as lipoplexes of lipid 1 and p-CMV-β-gal (as control) were used in CTL as well as cytokine (IL-4 and IFN-gamma) assays.
Tumor challenge experiment. About 1 ml cell dissociation solution (Sigma-Aldrich) was used to harvest B16F10 melanoma cells from T25 culture flasks and the harvested cells were washed with PBS (2 × 500 µl) and suspended in Hanks' balanced salt solution (5 × 105 cells/ml). Two weeks after the third immunization, each of the 6–8-week-old female C57BL/6J mice (n = 5) were challenged (s.c.) with 1 × 105 B16F10 melanoma cells in 100 µl Hanks' balanced salt solution. Palpable tumors were detected 2 weeks post tumor inoculation. Thereafter, daily measurements of perpendicular tumor diameters were taken and whenever any measurements exceeded 14 mm, mice were euthanized. The second s.c. tumor challenge (with ~1 × 105 B16F10 cells in 100 µl Hanks' balanced salt solution) were performed in all the five mice which lived a tumor-free live 100 days after the first tumor challenge.
Survivability studies under therapeutic settings. Six- to eight-week-old female C57BL/6J mice (each weighing 20–22 g) were s.c. injected in the right flanks with ~1 × 105 B16F10 cells. On day 15, mice were randomly sorted into four groups (n = 5 in each group). On day 15, 18, and 21 post tumor inoculation, mice were s.c. immunized with lipoplex of lipid 1 and p-CMV-MART1 (Group 1); lipoplex of lipid 2 and p-CMV-MART1 (Group 2); lipoplex of lipid 3 and p-CMV-MART1 (Group 3); and 5% aqueous glucose alone (Group 4, the vehicle control group). Tumor volumes (V = ½ ab2, where a = maximum length of the tumor and b = minimum length of the tumor measured perpendicular to each other) were measured with a slide caliper for up to 25 days.
ELISA for in situ IFN-γ and IL-4. The assays for measuring these cytokines were performed as described previously.41 Two weeks after the third immunization, mice were sacrificed and their spleens were isolated by mincing the spleens with a syringe plunger. The erythrocytes were lysed with 1 ml of lysis buffer (0.14 mol/l ammonium chloride in 0.02 mol/l Tris–HCl, pH 7.2). The viable cells were counted by hemocytometer and used for IFN-γ and IL-4 assay by ELISA after 3 days stimulation with the target B16F10 cells following manufacturer's protocol (Endogen Mouse IFN-γ Elisa kit, and mouse IL-4 Elisa kit, Pierce Biotechnology). Briefly, splenocytes in 50 µl complete medium were added to 96-well plates precoated with anti-mouse IFN-γ or anti-mouse IL-4 antibodies (at 1 × 106 cells/well), incubated for 12 hours at 37 °C in presence of 5% CO2. The incubated cells were then washed with wash buffer (3 × 200 µl) and 50 µl biotinylated secondary antibody was added to each well. The mixtures were incubated for 1 hour at room temperature and washed with wash buffer (3 × 200 µl). The washed cells were next incubated with 100 µl of streptavidin–horse radish peroxidase solution for 30 minutes, washed with wash buffer (3 × 200 µl), and treated with 100 µl 3,3′,5,5′-tetramethylbenzidine substrate solution and the mixture incubated for 30 minutes in dark. The reaction was stopped by adding 100 µl of stop solution to each well and the absorbance read on a microplate reader at 450 nm.
Phenotype analysis of T cells. For phenotype analysis of CD4+ and CD8+ T cells in splenocytes and lymph nodes of immunized mice, single-cell suspensions of spleen cells and lymph nodes were prepared from mice 14 days after the third immunization. Cells were stained with FITC-conjugated monoclonal anti-CD8 antibody and phycoerythrin-conjugated monoclonal anti-CD4 antibody and analyzed by flow cytometry. Cells collected from spleen and lymph nodes of mice immunized with only vehicle were used as control.
CTL assays. CTL assays were performed as described previously.42 Briefly, single-cell suspensions of splenocytes were prepared from mice 28 days after the third immunization. Cells were seeded onto 6-well plates (~1 × 107 cells/well) and cocultured with B16F10 cells (~1 × 106 cells/well) in RPMI complete medium containing 100 U/ml antibiotic solution (Sigma-Aldrich) and 50 U/ml IL-2 (Thermo Scientific) for 72 hours. The appropriate numbers of resulting effector (E) cells were incubated with 10,000 fresh target (T) B16F10 cells such that the E:T ratios varied within 10:1 to 100:1 in each well of U-bottomed 96-well plates. The mixtures were then incubated for 6 hours at 37 °C in 5% CO2 and finally the lactate dehydrogenase levels in cell culture supernatants were measured by CTL assay kit following the manufacturer's (Promega) instructions.
In vivo T-cell depletion studies. Two groups of C57BL/6J mice (n = 5 for each group) were immunized thrice with the lipoplex of lipid 1 and p-CMV-MART1 on day 0, 7, and 14. The first group received i.p. injections of 100 µg anti-CD8 mAbs (2.43) on days −4, −1, 2, 6, 13, 17, 21, 25, and 29 and the second immunized group did not receive any anti-CD8 mAbs. A third unimmunized group was injected with 5% aqueous glucose (vehicle control group) on days −4, −1, 2, 6, 13, 17, 21, 25, and 29. All the three groups were challenged with B16F10 cells (on day 28) and tumor growths were measured by slide caliper.
Statistical analysis. Data are represented as mean ± SD. Each individual treatment group was compared with the control untreated group using the Student's t-test. P <0.05 was considered as significant.
SUPPLEMENTARY MATERIAL Scheme S1. Synthesis of lipid 1. Scheme S2. Synthesis of lipid 2. Scheme S3. Synthesis of control mannosylated lipid 3. Figure S1. 1H NMR (400 MHz, CDCl3 + CD3OD) Spectrum for lipid 1. Figure S2. ESI Mass Spectrum for lipid 1. Figure S3. 1H NMR (400 MHz, CDCl3 + CD3OD) Spectrum for lipid 2. Figure S4. ESI Mass Spectrum for lipid 2. Figure S5. 1H NMR (600 MHz, CD3OD) Spectrum for lipid 3. Figure S6. ESI Mass Spectra for lipid 3. Figure S7. Representative HPLC Chromatograms for lipid 1 using pure methanol as mobile phase (A) and using 95:5 methanol:water, v/v, as the mobile phase (B). Figure S8. Representative HPLC Chromatograms for lipid 2 using pure methanol as mobile phase (A) and using 95:5 methanol:water, v/v, as the mobile phase (B). Figure S9. Representative HPLC Chromatograms for lipid 3 using pure methanol as mobile phase (A) and using 95:5 methanol:water, v/v, as the mobile phase (B). Figure S10. Hydrodynamic diameters of the liposomal formulations of lipids 1–3. Figure S11. Hydrodynamic diameters of the lipoplexes of lipids 1–3 and p-CMV-MART1 (lipid:DNA charge ratios at 4:1) in presence of Serum free RPMI medium. Figure S12. Confirmation of the presence of DCs surface markers by flow cytometry. Figure S13. Lipids 1–3 transfect DCs via mannose receptors. Figure S14. MART1 expression in transfected DCs in vivo with lipoplexes of lipids 1–3 and p-CMV-MART1. Figure S15. Immunization with lipoplexes of lipid 1–3 and p-CMV-β-gal produces both cellular and humoral immune responses in mice. Figure S16. Immunization with lipoplexes of lipid 2 and 3 and p-CMV-MART1 are less efficient in protecting syngeneic C57BL/6J mice from lethal melanoma challenge. Figure S17. Liposomes of our previously described cationic lipid containing the mannose-mimicking head-group with pure lysine spacer (Srinivas, R. et al. Biomaterials 2012, 33, 6220–6229) was capable of mounting long-lasting immune response only in ex vivo DC-transfection based DNA vaccination mode and not under direct in vivo immunization with lipoplexes. Figure S18. Immunization (s.c.) of mice with lipoplex of lipid 1 and p-CMV-MART1 and lipid 1 and p-CMV-β-gal significantly enhances the populations of both CD4+ and CD8+ T cells in mouse splenocytes and lymph nodes.
Acknowledgments
We thank Van den Eynde, Nalam Madhusudhana Rao, and Gopal Pande for providing us with p-CMV-MART1, p-CMV-SPORT-β-Gal, and pα5GFP plasmids, respectively. We sincerely acknowledge the insightful comments and constructive criticisms of all the reviewers on the previously submitted version of our manuscript. This work was supported by the Council of Scientific and Industrial Research, Government of India, New Delhi (CSC-0302) and the CSIR-Mayo Clinic Collaborative Project (MLP-0018). A.G., G.M., and S. K. G. thank the Council of Scientific and Industrial Research, Government of India, New Delhi for their doctoral research fellowships.
Supplementary Material
References
- Banchereau, J and Steinman, RM (1998). Dendritic cells and the control of immunity. Nature 392: 245–252. [DOI] [PubMed] [Google Scholar]
- Banchereau, J, Briere, F, Caux, C, Davoust, J, Lebecque, S, Liu, YJ et al. (2000). Immunobiology of dendritic cells. Annu Rev Immunol 18: 767–811. [DOI] [PubMed] [Google Scholar]
- Reis, CS (2001). Dendritic cells as sensors of infection. Immunity 14: 495–498. [DOI] [PubMed] [Google Scholar]
- Hsu, FJ, Benike, C, Fagnoni, F, Liles, TM, Czerwinski, D, Taidi, B et al. (1996). Vaccination of patients with B-cell lymphoma using autologous antigen-pulsed dendritic cells. Nat Med 2: 52–58. [DOI] [PubMed] [Google Scholar]
- Nestle, FO, Alijagic, S, Gilliet, M, Sun, Y, Grabbe, S, Dummer, R et al. (1998). Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med 4: 328–332. [DOI] [PubMed] [Google Scholar]
- Irvine, AS, Trinder, PK, Laughton, DL, Ketteringham, H, McDermott, RH, Reid, SC et al. (2000). Efficient nonviral transfection of dendritic cells and their use for in vivo immunization. Nat Biotechnol 18: 1273–1278. [DOI] [PubMed] [Google Scholar]
- Lu, W, Arraes, LC, Ferreira, WT and Andrieu, JM (2004). Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat Med 10: 1359–1365. [DOI] [PubMed] [Google Scholar]
- Srinivas, R, Karmali, PP, Pramanik, D, Garu, A, Mahidhar, YV, Majeti, BK et al. (2010). Cationic amphiphile with shikimic acid headgroup shows more systemic promise than its mannosyl analogue as DNA vaccine carrier in dendritic cell based genetic immunization. J Med Chem 53: 1387–1391. [DOI] [PubMed] [Google Scholar]
- Srinivas, R, Garu, A, Moku, G, Agawane, SB and Chaudhuri, A (2012). A long-lasting dendritic cell DNA vaccination system using lysinylated amphiphiles with mannose-mimicking head-groups. Biomaterials 33: 6220–6229. [DOI] [PubMed] [Google Scholar]
- Paulis, LE, Mandal, S, Kreutz, M and Figdor, CG (2013). Dendritic cell-based nanovaccines for cancer immunotherapy. Curr Opin Immunol 25: 389–395. [DOI] [PubMed] [Google Scholar]
- Dewitte, H, Verbeke, R, Breckpot, K, De Smedt, SC and Lentacker, I (2014). Nanoparticle design to induce tumor immunity and challenge the suppressive tumor microenvironment. Nano Today 9: 743–758. [Google Scholar]
- Daftarian, P, Kaifer, AE, Li, W, Blomberg, BB, Frasca, D, Roth, F et al. (2011). Peptide-conjugated PAMAM dendrimer as a universal DNA vaccine platform to target antigen-presenting cells. Cancer Res 71: 7452–7462. [DOI] [PubMed] [Google Scholar]
- Tian, S, Liu, Z, Donahue, C, Falo, LD Jr and You, Z (2012). Genetic targeting of the active transcription factor XBP1s to dendritic cells potentiates vaccine-induced prophylactic and therapeutic antitumor immunity. Mol Ther 20: 432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuwanshi, D, Mishra, V, Das, D, Kaur, K and Suresh, MR (2012). Dendritic cell targeted chitosan nanoparticles for nasal DNA immunization against SARS CoV nucleocapsid protein. Mol Pharm 9: 946–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, J, Jin, Y, Li, W, Zhang, B, He, Y, Liu, H et al. (2013). DNA vaccines targeting the encoded antigens to dendritic cells induce potent antitumor immunity in mice. BMC Immunol 14: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raviv, L, Jaron-Mendelson, M and David, A (2015). Mannosylated polyion complexes for in vivo gene delivery into CD11c(+) dendritic cells. Mol Pharm 12: 453–462. [DOI] [PubMed] [Google Scholar]
- Inaba, K, Inaba, M, Romani, N, Aya, H, Deguchi, M, Ikehara, S et al. (1992). Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nchinda, G, Kuroiwa, J, Oks, M, Trumpfheller, C, Park, CG, Huang, Y et al. (2008). The efficacy of DNA vaccination is enhanced in mice by targeting the encoded protein to dendritic cells. J Clin Invest 118: 1427–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai, Y, Yang, JC, Spiess, P, Nishimura, MI, Overwijk, WW, Roberts, B et al. (1997). Cloning and characterization of the genes encoding the murine homologues of the human melanoma antigens MART1 and gp100. J Immunother 20: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutzler, MA and Weiner, DB (2008). DNA vaccines: ready for prime time? Nat Rev Genet 9: 776–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, MA (2011). DNA vaccines: an historical perspective and view to the future. Immunol Rev 239: 62–84. [DOI] [PubMed] [Google Scholar]
- Mennuni, C, Calvaruso, F, Facciabene, A, Aurisicchio, L, Storto, M, Scarselli, E et al. (2005). Efficient induction of T-cell responses to carcinoembryonic antigen by a heterologous prime-boost regimen using DNA and adenovirus vectors carrying a codon usage optimized cDNA. Int J Cancer 117: 444–455. [DOI] [PubMed] [Google Scholar]
- Rice, J, Dossett, ML, Ohlén, C, Buchan, SL, Kendall, TJ, Dunn, SN et al. (2008). DNA fusion gene vaccination mobilizes effective anti-leukemic cytotoxic T lymphocytes from a tolerized repertoire. Eur J Immunol 38: 2118–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian, J, Zheng, Y, Zheng, C, Wang, L, Qin, H, Hong, S et al. (2012). Active vaccination with Dickkopf-1 induces protective and therapeutic antitumor immunity in murine multiple myeloma. Blood 119: 161–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaak, SG, Haanen, JB, Beijnen, JH, Nuijen, B (2010). Naked plasmid DNA formulation: effect of different disaccharides on stability after lyophilisation AAPS Pharm Sci Tech 11: 344–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley, ME, Wunderlich, JR, Robbins, PF, Yang, JC, Hwu, P, Schwartzentruber, DJ et al. (2002). Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science 298: 850–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed, R and Akondy, RS (2011). Insights into human CD8(+) T-cell memory using the yellow fever and smallpox vaccines. Immunol Cell Biol 89: 340–345. [DOI] [PubMed] [Google Scholar]
- Melief, CJ (2008). Cancer immunotherapy by dendritic cells. Immunity 29: 372–383. [DOI] [PubMed] [Google Scholar]
- Klebanoff, CA, Acquavella, N, Yu, Z and Restifo, NP (2011). Therapeutic cancer vaccines: are we there yet? Immunol Rev 239: 27–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilboa, E (2007). DC-based cancer vaccines. J Clin Invest 117: 1195–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman, RM (2008). Dendritic cells in vivo: a key target for a new vaccine science. Immunity 29: 319–324. [DOI] [PubMed] [Google Scholar]
- Palucka, K, Ueno, H and Banchereau, J (2011). Recent developments in cancer vaccines. J Immunol 186: 1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancho, D, Mourão-Sá, D, Joffre, OP, Schulz, O, Rogers, NC, Pennington, DJ et al. (2008). Tumor therapy in mice via antigen targeting to a novel, DC-restricted C-type lectin. J Clin Invest 118: 2098–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminschi, I, Lahoud, MH and Shortman, K (2009). Enhancing immune responses by targeting antigen to DC. Eur J Immunol 39: 931–938. [DOI] [PubMed] [Google Scholar]
- Tian, S, Liu, Z, Donahue, C, Noh, HS, Falo, LD Jr. and You, Z (2009). Transcriptional IL-15-directed in vivo DC targeting DNA vaccine. Gene Ther 16: 1260–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, J and Chaudhuri, A (2005). Design, syntheses, and transfection biology of novel non-cholesterol-based guanidinylated cationic lipids. J Med Chem 48: 812–820. [DOI] [PubMed] [Google Scholar]
- Vigneron, JP, Oudrhiri, N, Fauquet, M, Vergely, L, Bradley, JC, Basseville, M et al. (1996). Guanidinium-cholesterol cationic lipids: efficient vectors for the transfection of eukaryotic cells. Proc Natl Acad Sci USA 93: 9682–9686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaech, SM and Ahmed, R (2003). Immunology. CD8 T cells remember with a little help. Science 300: 263–265. [DOI] [PubMed] [Google Scholar]
- Whitmore, M, Li, S and Huang, L (1999). LPD lipopolyplex initiates a potent cytokine response and inhibits tumor growth. Gene Ther 6: 1867–1875. [DOI] [PubMed] [Google Scholar]
- McKeever, U, Barman, S, Hao, T, Chambers, P, Song, S, Lunsford, L et al. (2002). Protective immune responses elicited in mice by immunization with formulations of poly(lactide-co-glycolide) microparticles. Vaccine 20: 1524–1531. [DOI] [PubMed] [Google Scholar]
- McKinney, DM, Skvoretz, R, Qin, M, Ishioka, G and Sette, A (2000). Characterization of an in situ IFN-gamma ELISA assay which is able to detect specific peptide responses from freshly isolated splenocytes induced by DNA minigene immunization. J Immunol Methods 237: 105–117. [DOI] [PubMed] [Google Scholar]
- Mockey, M, Bourseau, E, Chandrashekhar, V, Chaudhuri, A, Lafosse, S, Le Cam, E et al. (2007). mRNA-based cancer vaccine: prevention of B16 melanoma progression and metastasis by systemic injection of MART1 mRNA histidylated lipopolyplexes. Cancer Gene Ther 14: 802–814. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.