Abstract
Bacille Calmette–Guérin (BCG), an attenuated strain of Mycobacterium bovis, is the only vaccine available for tuberculosis (TB) control. However, BCG is not an ideal vaccine and has two major limitations: BCG exhibits highly variable effectiveness against the development of TB both in pediatric and adult populations and can cause disseminated BCG disease in immunocompromised individuals. BCG comprises a number of substrains that are genetically distinct. Whether and how these genetic differences affect BCG efficacy remains largely unknown. In this study, we performed comparative analyses of the virulence and efficacy of 13 BCG strains, representing different genetic lineages, in SCID and BALB/c mice. Our results show that BCG strains of the DU2 group IV (BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice) exhibit the highest levels of virulence, and BCG strains of the DU2 group II (BCG-Sweden, BCG-Birkhaug) are among the least virulent group. These distinct levels of virulence may be explained by strain-specific duplications and deletions of genomic DNA. There appears to be a general trend that more virulent BCG strains are also more effective in protection against Mycobacterium tuberculosis challenge. Our findings have important implications for current BCG vaccine programs and for future TB vaccine development.
Introduction
Tuberculosis (TB) remains one of the world's deadliest infectious diseases, causing 1.5 million deaths and 8–10 million new infections annually. Bacille Calmette–Guérin (BCG) is currently the only vaccine used for immunoprophylaxis of TB. BCG was included in the WHO Expanded Program on Immunization in 1974 and more than 4 billion people have been immunized with BCG. Over 90% of children worldwide are vaccinated with BCG and >120 million doses of BCG are administered annually, making it the world's most widely used vaccine. Clinical studies have confirmed that BCG protects against disseminated TB including meningitis and miliary TB in children.1,2 However, in nonendemic countries, BCG vaccination is not performed due to the variable effectiveness (ranging from 0 to 80%) in preventing pulmonary TB in adults and the relatively low incidence of disease in these regions.3 Another issue concerning BCG is its safety. Although BCG is generally considered very safe, there is a substantially higher risk of disseminated BCG disease in children with primary immune deficiencies or HIV infection, which apparently outweighs the potential benefit of TB prevention.4 As a result, the current WHO policy recommends that children who are known to be HIV infected, even if asymptomatic, should not be immunized with BCG.4
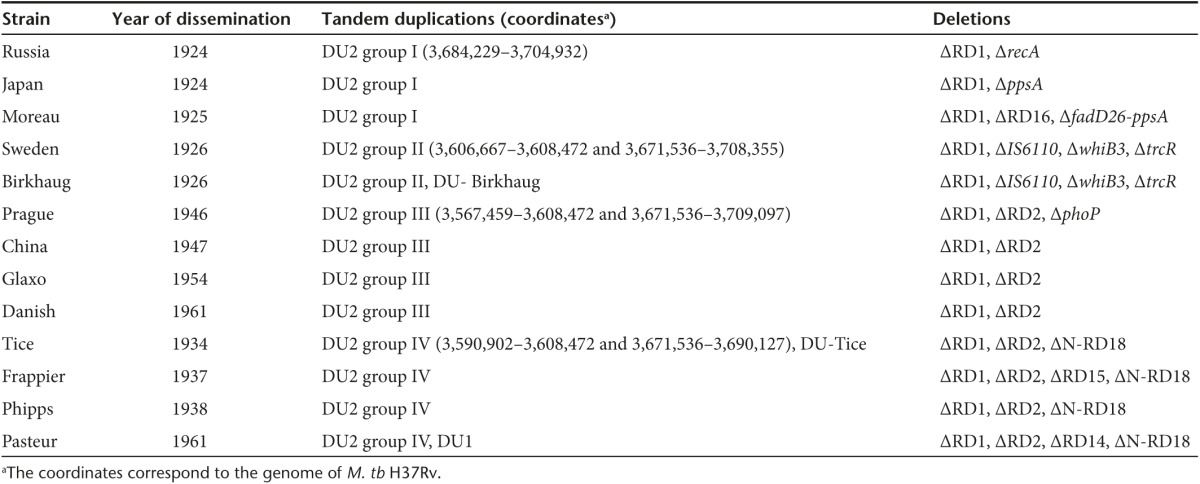
BCG was derived from a virulent strain of Mycobacterium bovis through in vitro attenuation (230 times passaging) from 1908 to 1921. Beginning in 1924, BCG was distributed to multiple countries worldwide, leading to its diversification into a number of genetically distinct substrains.5 Comparative genome analyses using a variety of techniques (including subtractive hybridization, spotted oligonucleotide arrays, microarray based resequencing, and whole genome sequencing) have uncovered numerous large sequence polymorphisms including deletions and duplications, as well as single nucleotide polymorphisms among BCG strains.6,7,8,9,10,11,12,13,14 Based on these studies, BCG strains can be separated into several different groups. For example, a tandem duplication-2 (DU2) occurs in all BCG strains examined so far, but DU2 exists in four different forms (Table 1). Consequently, BCG strains are divided into four major groups based on the DU2 forms: group I (BCG-Russia, BCG-Japan, and BCG-Moreau), II (BCG-Sweden and BCG-Birkhaug), III (BCG-Danish, BCG-Prague, BCG-Glaxo, and BCG-China), and IV (BCG-Phipps, BCG-Tice, BCG-Frappier, and BCG-Pasteur) (Table 1).8,9,10 The genetic clustering of BCG strains is generally consistent with the historical records of BCG dissemination. For example, BCG strains of DU2 groups I and II were disseminated before 1927 (the early strains) and the strains of groups III and IV were distributed after 1927 (the late strains). Groups III and IV strains also exhibit a deletion in the Region of Difference-2 (RD2) (Table 1).7
Table 1 . Major genetic characteristics of BCG strains included in this study.
It is unequivocal that BCG has evolved over time, but whether this matters in terms of BCG effectiveness against TB is being debated.15,16 Reviews of clinical trials led to a conclusion that the variation in BCG strains is not a significant determinant of overall efficacy.17,18 However, these analyses were based on very limited data available from human studies.17,18 Considering the vast number of publications on BCG, clinical and animal studies directly comparing the effectiveness of different BCG strains have been remarkably scarce (reviewed in ref. 19). Given the recent delineation of genetic differences between BCG strains, a head-to-head comparison of BCG strains of genetic diversity is of great interest. In this study, we compared the virulence and efficacy of 13 BCG strains, representing all genetic lineages, in SCID and BALB/c mice, respectively.
Results
Evaluation of virulence of BCG strains in SCID mice
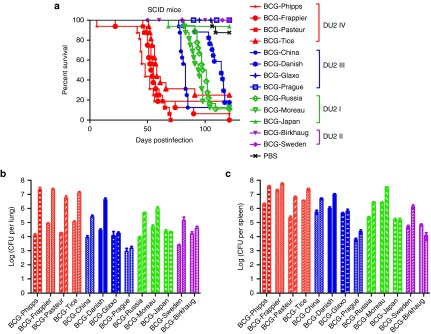
SCID mice, which lack T- and B lymphocytes and are highly immunocompromised, are the reference model for the safety test of live vaccines including recombinant BCG and attenuated M. tb strains in preclinical studies, consented among TB vaccine researchers and regulatory bodies.20,21 The safety of a live vaccine is inferred from its virulence in SCID mice, which is reflected in the ability of the vaccine to replicate in the animal and to cause mortality. To directly compare the virulence of BCG strains, we performed SCID mice infection of 13 BCG strains and monitored the survival of animals over 18 weeks.
Strikingly, there were significant differences in the survival curves of SCID mice infected with different groups of BCG strains (P < 0.0001, log-rank test; Figure 1a). The majority of SCID mice infected with BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice were dead by week 10, and comparison of their survival curves revealed no significant differences among them (log-rank test). The median survival time of SCID mice infected with BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice were 48, 53.5, 55, and 58 days, respectively. In contrast, all SCID mice infected with BCG-Japan, BCG-Birkhaug, BCG-Sweden, BCG-Glaxo, and BCG-Prague survived during the 18-week experiment. The survival rate of SCID mice infected with BCG-Japan, BCG-Birkhaug, BCG-Sweden, BCG-Glaxo, and BCG-Prague were 93.75, 100, 100, 100, and 100%, respectively, which were the same as the phosphate-buffered saline (PBS) control group (87.5%). The virulence levels of the other BCG strains were in between these two groups. The median survival time of SCID mice infected with BCG-China, BCG-Moreau, BCG-Russia, and BCG-Danish were 83, 94.5, 99.5, and 114 days, respectively (Figure 1a). Accordingly, the 13 BCG strains fall into five groups based on their virulence levels: BCG-Phipps, BCG-Pasteur, BCG-Frappier, BCG-Tice > BCG-China > BCG-Russia, BCG-Moreau > BCG-Danish > BCG-Glaxo, BCG-Prague, BCG-Japan, BCG-Sweden, and BCG-Birkhaug. The differences were statistically significant between groups, but not between members of the same group (log-rank analysis).
Figure 1.
Differential virulence of BCG strains in SCID mice. (a) Survival curves of SCID mice infected with 13 different BCG strains. The survival curves were plotted using the Kaplan–Meier method and differences between curves were analyzed using the log-rank test. BCG strains belonging to the same DU2 groups are highlighted in the same color. (b,c) CFUs in the lungs (b) and spleen (c) of SCID mice at 1 and 4 weeks after infection with 13 different BCG strains. The first bar of each strain is the data from week 1 and the second bar is the data from week 4. Data were plotted as mean ± SD (n = 4). BCG, Bacille Calmette–Guérin; CFU, colony-forming unit; PBS, phosphate-buffered saline.
There was a general parallel between the SCID mice survival curves and the ability of individual BCG strains to replicate in the animals. At week 4 postinfection, BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice exhibited the highest counts in the lungs of individual SCID mice, reaching 7.35 log10, 7.34 log10, 6.76 log10, and 7.11 log10 colony-forming units (CFUs), respectively, which were on average 2.5 log10 higher than their counts at the week 1 timepoint (4.11 log10, 4.92 log10, 4.25 log10, and 5.04 log10 CFUs for BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice, respectively) (Figure 1b). BCG-Japan, BCG-Birkhaug, BCG-Prague, and BCG-Glaxo showed little growth in the lungs of SCID mice during the same period, with an average of 4.3 log10 CFUs at week 4 postinfection, which was essentially the same as the average counts (4.1 log10 CFUs) at the week 1 time point. BCG-Russia, BCG-Moreau, BCG-China, BCG-Danish, and BCG-Sweden showed intermediate levels of replication, ranging from 1.2 to 2.1 log10 CFUs higher in the lungs of SCID mice at week 4 than at week 1 postinfection (Figure 1b). A similar trend was observed for BCG counts in the spleen of SCID mice (Figure 1c).
In a separate experiment, three BCG strains (BCG-Pasteur, BCG-Russia, BCG-Japan) representing different virulence levels were selected to repeat the SCID mice infection experiment. Consistent with the results above, all SCID mice infected with BCG-Japan survived the 18-week experiment, while mice infected with BCG-Pasteur and BCG-Russia had a median survival time of 42 and 84 days, respectively.
Evaluation of efficacy of BCG strains in BALB/c mice
Immunocompetent inbred mice (BALB/c and C57BL/6) are widely used for TB vaccine studies because of the low cost and the availability of immunological reagents.22 To compare the protective efficacy of BCG strains, we used a low-dose (100 CFUs of M. tb H37Rv), aerosol infection mouse (BALB/c) model to mimic the natural M. tb infection in humans. As classically demonstrated,23,24,25 the M. tb infection of BALB/c and C57BL/6 mice by aerosol challenge is followed by two phases. The first is the progressive phase in which M. tb grows essentially uninhibitedly for the first 3–4 weeks, resulting in 106~107 CFUs in lungs. The progressive phase ends with the inhibition of further M. tb growth by adaptive immunity. This is followed by a stationary phase, in which the bacterial burden remains largely unchanged for 9–12 months before the mice succumb to TB.
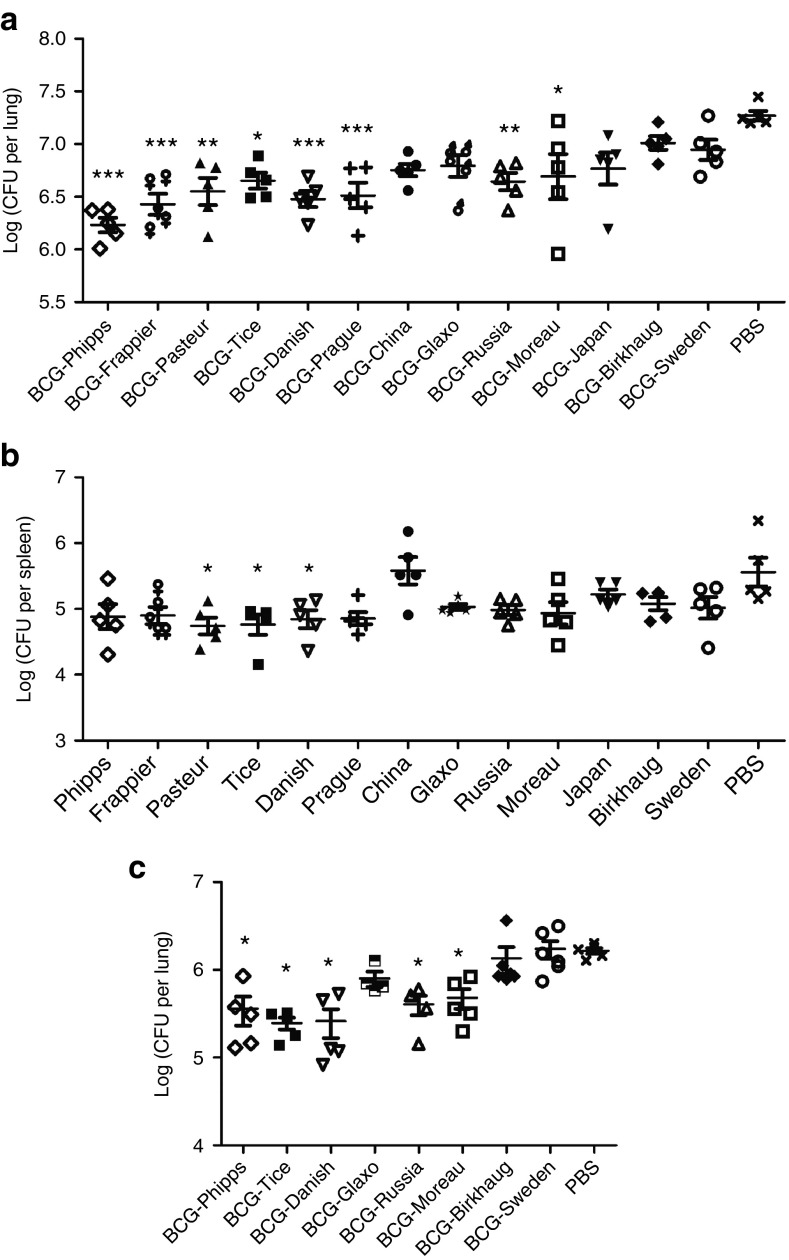
At week 4 post-M. tb challenge, the nonvaccinated group of BALB/c mice had a mean M. tb burden of 7.27 log10 CFUs in the lungs, and there was a significant difference between the mean M. tb burden in the lungs of animal groups vaccinated with various BCG strains and the PBS control group (P < 0.0001, one-way analysis of variance (ANOVA)) (Figure 2a; Table 2). For multiple pair-wise comparisons, one-way ANOVA and Tukey's post hoc tests were performed. It was found that mice vaccinated with BCG-Phipps, BCG-Frappier, BCG-Pasteur, BCG-Tice, BCG-Danish, BCG-Prague, BCG-Russia, and BCG-Moreau had significantly lower M. tb burdens than the nonvaccinated PBS group (Figure 2a), ranging from 0.58 log10 (BCG-Moreau group) to 1.03 log10 CFUs (BCG-Phipps group) lower (Table 2). Mice vaccinated with BCG-China, BCG-Glaxo, BCG-Japan, BCG-Birkhaug, and BCG-Sweden had 0.25–0.52 log10 lower M. tb counts than the PBS group but the differences were not statistically significant (Table 2). In a comparison between the BCG-vaccinated groups, mice vaccinated with BCG-Phipps had significantly lower M. tb counts in the lungs than animals vaccinated with BCG-Birkhaug (ΔCFU = 0.78 log10, P < 0.0001), BCG-Sweden (ΔCFU = 0.71 log10, P < 0.001), BCG-Japan (ΔCFU = 0.54 log10, P < 0.05), and BCG-Glaxo (ΔCFU = 0.56 log10, P < 0.05). BCG-Frappier–vaccinated mice also had significantly lower M. tb burdens than those vaccinated with BCG-Birkhaug (ΔCFU = 0.78 log10, P < 0.05).
Figure 2.
Variable effectiveness of 13 BCG strains against M. tb challenge in BALB/c mice. (a,b) CFUs in the lungs (a) and spleen (b) at 4 weeks after M. tb challenge in mice immunized with 13 different BCG strains. Each data point represents one mouse and the data are plotted as mean ± SEM (n = 5). Data were analyzed by one-way analysis of variance and Tukey's multiple comparisons. Animal groups exhibiting statistically significant differences with the unvaccinated PBS control group are indicated as follows: *P < 0.05, **P < 0.01, ***P < 0.001. (c) CFUs in the lungs at 4 weeks after M. tb challenge in mice immunized with eight different BCG strains. In the second experiment, eight BCG strains representing four different DU2 groups were selected to repeat the experiment as in (a). Statistically significant differences between BCG groups and the unvaccinated PBS control group are indicated. *P < 0.05. BCG, Bacille Calmette–Guérin; CFU, colony-forming unit; PBS, phosphate-buffered saline.
Table 2. CFUs in lungs and spleens after M. tb challenge in mice immunized with 13 different BCG strains.
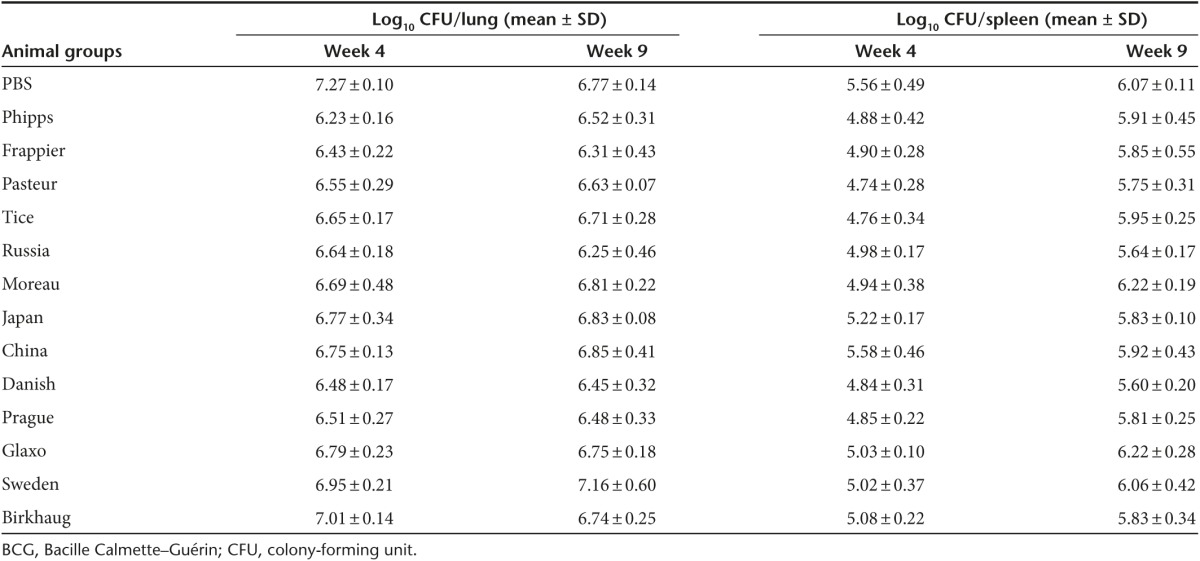
The infection of mice via the respiratory route begins to disseminate to the spleen and liver ~2 weeks after aerosol challenge, and due to the short period of M. tb growth, the bacterial burden in these organs are much lower than in the lungs.22 Consistently, our data showed that the amount of M. tb disseminated to the spleen at week 4 postinfection was lower than that in the lungs by ~1.0–2.0 log10 (Figure 2b; Table 2). The levels of M. tb burden in the spleen generally correlated with that in the lungs of individual animal groups. Mice vaccinated with BCG-Pasteur, BCG-Tice, and BCG-Danish had the lowest M. tb burden in the spleen, on average 0.78 log10 CFUs lower than the PBS control group (P < 0.05, one-way ANOVA and Tukey's test; Figure 2b). Mice vaccinated with BCG-Phipps, BCG-Frappier, BCG-Prague, and BCG-Moreau also had, on average, 0.67 log10 lower M. tb counts than the PBS group, although the differences were not statistically significant. The M. tb burden in animals vaccinated with the remaining BCG strains, except BCG-China, were lower than the PBS control group, ranging from 0.34 log10 (BCG-Japan) to 0.58 log10 CFUs (BCG-Russia), but these differences were not statistically significant (one-way ANOVA and Tukey's post hoc test).
At week 9 postchallenge, the infection has entered into the stationary phase and the M. tb burden in the PBS group was stabilized at 6.77 log10 and 6.07 log10 CFUs in the lungs and spleen, respectively (Table 2). The differences in M. tb burden between the BCG-vaccinated groups and the PBS group diminished. BCG-vaccinated animals had on average 6.65 log10 and 5.89 log10 CFUs in the lungs and spleen, respectively, and none of the BCG-vaccinated animal groups were significantly different than the PBS control group (Table 2).
To determine if the results above were reproducible, two BCG strains of each genetic lineage (DU2 groups I–IV) were selected and the experiment was performed at a different laboratory under similar conditions (i.e., s.c. vaccination with BCG strains followed by aerosol infection of M. tb). The M. tb burden in the lungs of animals at week 4 postinfection were determined and analyzed. Consistently, mice vaccinated with BCG-Phipps, BCG-Tice, BCG-Danish, BCG-Russia, and BCG-Moreau had significantly lower M. tb burden in the lungs, ranging from 0.54 to 0.83 log10 CFUs lower than the PBS group (P < 0.05, one-way ANOVA and Tukey's post hoc test; Figure 2c). No significant differences were found between the bacterial burden in mice vaccinated with BCG-Sweden, BCG-Birkhaug, and BCG-Glaxo compared to the PBS group.
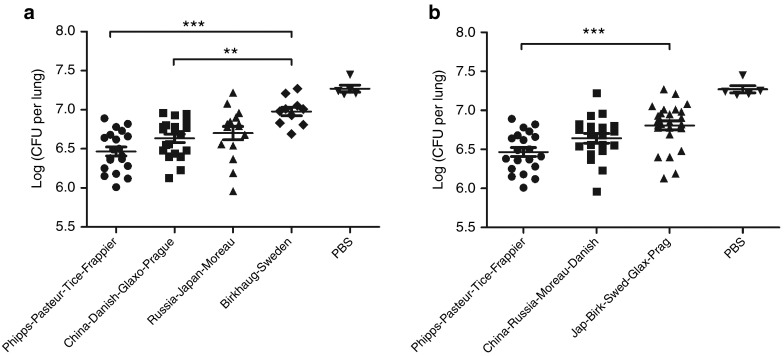
The protective efficacy of BCG strains appeared to correlate with their genetic clustering based on tandem duplications. We replotted the lung M. tb burdens of Figure 2a by combining data of BCG strains of the same DU2 group (Figure 3a). The mean M. tb burden of animals vaccinated with groups I–IV BCG strains were 6.70 log10, 6.98 log10, 6.63 log10, and 6.47 log10 CFUs in the lungs, respectively. One-way ANOVA and Bonferroni's multiple-comparison test showed that strains of group IV (BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG -Tice) and group III (BCG-China, BCG-Danish, BCG-Prague, and BCG-Glaxo) afforded significantly better protection than strains of group II (BCG-Sweden and BCG-Birkhaug) in vaccinated mice.
Figure 3.
Variable protective efficacy of BCG groups. (a) Comparison of effectiveness of BCG groups based on tandem duplication DU2. The data of Figure 2a were redrawn by combining BCG strains of the DU2 same group. One-way analysis of variance (ANOVA) and Bonferroni's multiple comparisons were performed for statistical analysis. **P < 0.01, ***P < 0.001. (b) Comparison of effectiveness of BCG strains based on virulence. The 13 BCG strains were divided into three major groups based on virulence: the most virulent group: BCG-Phipps, BCG-Pasteur, BCG-Tice, and BCG-Frappier; the intermediate virulent group: BCG-China, BCG-Russia, BCG-Moreau, and BCG-Danish; and the least virulent group: BCG-Japan, BCG-Birkhaug, BCG-Sweden, BCG-Glaxo, and BCG-Prague. The data of Figure 2a were redrawn by combining data of the same group. One-way ANOVA and Bonferroni's multiple comparisons were performed for statistical analysis. ***P < 0.001. BCG, Bacille Calmette–Guérin; CFU, colony-forming unit; PBS, phosphate-buffered saline.
The most protective BCG group, DU2 group IV, also had the highest level of virulence in SCID mice, and there appeared to be a correlation between virulence and efficacy. In Figure 3b, we replotted the data of Figure 2a by dividing the BCG strains into three groups based on the virulence level (Figure 1a), the most virulent group (BCG-Phipps, BCG-Frappier, BCG-Pasteur, and BCG-Tice), the intermediate virulent group (BCG-China, BCG-Russia, BCG-Moreau, and BCG-Danish), and the least virulent group (BCG-Japan, BCG-Glaxo, BCG-Prague, BCG-Sweden, and BCG-Birkhaug). The mean M. tb burden for these three groups were 6.47 log10, 6.64 log10, and 6.81 log10 CFUs in the lungs, respectively, and the difference between the most virulent group and the least virulent group was statistically significant (P < 0.0001, one-way ANOVA and Bonferroni's test).
Discussion
In this study, we performed a head-to-head comparison of the virulence and efficacy of 13 BCG strains representing different genetic lineages in murine models. Previously, there had been over a dozen studies comparing the effectiveness of different BCG strains using several animal models including mice, guinea pigs, hamsters, and bank vole (reviewed in ref. 19). In the majority of these studies, the number of BCG strains included for comparative analyses were limited, and only three studies compared ≥10 strains of BCG.19 Differences in the animal model and study designs make it difficult to compare results from different studies. As such, no consistent conclusion can be drawn from them.19 Early studies in the 1970s by Ladefoged and coworkers compared 11 BCG strains in bank voles and 12 BCG strains in guinea pigs.26,27 However, in the bank vole study, the 11 BCG strains were compared in six separate experiments, each involving 5 strains, and BCG-Danish was the only strain common in all experiments, which limits the comparison of the results.26 In the guinea pig study, the M. tb strain used in the challenge was inadvertently attenuated and the data on protective efficacy was unavailable.27 A more recent study by Castillo-Rodal et al. compared the effectiveness of 10 BCG strains in BALB/c mice.28 However, 6 of the 10 BCG strains (BCG-Phipps, BCG-Tice, BCG-Pasteur, BCG-Frappier, BCG-Connaught, and BCG-Mexico) included in this study are from the DU2 group IV, while only 1 strain each from group I (BCG-Moreau) and group III (BCG-Danish) and 2 strains from group II (BCG-Sweden and BCG-Birkhaug) were included. Therefore, the BCG strains chosen for this study are not equally represented. Recognizing the limitations of the previous studies, we performed comparative studies of 13 BCG strains in the same experiment, which has the advantage of removing variability between experiments. The BCG strains chosen for our experiments are also well characterized genetically (e.g., the availability of genome sequences), and these BCG strains represent all genetic lineages identified so far, including three, two, four, and four strains from DU2 groups I–IV, respectively. In addition, we also compared the virulence of these 13 BCG strains in SCID mice, which, to the best of our knowledge, has never been performed previously. One limitation of the present study is that laboratory strains instead of commercial BCG vaccines were used for experiments. However, analyses of commercial BCG vaccines are complicated by the observation that commercial preparations could have mixed BCG strains.29,30
Our results show that BCG strains exhibit variable virulence and efficacy. The segregation of the virulence of BCG strains generally coincides with their genetic clustering based on genome tandem duplications (Figure 1). The most virulent strains (BCG-Phipps, BCG-Pasteur, BCG-Frappier, and BCG-Tice) all belong to the DU2 group IV (Figure 1), and the two strains of the DU2 group II (BCG-Sweden and BCG-Birkhaug) are among the least virulent group. Tandem duplications are a major mechanism of BCG adaptation to in vitro growth conditions,9 thus it is not surprising that gene amplification via tandem duplication has a major influence on BCG properties. Comparison of the DU2 groups reveals that groups III and IV contain a 15765-bp (3,590,902–3,606,667) duplication that does not occur in groups I and II (Table 1). This duplication includes two regulatory genes: sigH and whiB1. SigH plays a critical role in the oxidative stress responses in M. tb31 and WhiB1 is a nitric oxide-responsive transcription factor.32 Elevated expression of sigH and whiB1 in BCG strains of groups III and IV9 may enhance their replication, thereby exhibiting higher virulence in SCID mice. BCG-Glaxo is less virulent than BCG-China and BCG-Danish of the same group (group III) likely because BCG-Glaxo is naturally deficient in the production of phthiocerol dimycocerosates and phenolic glycolipids.33 Phthiocerol dimycocerosates and phenolic glycolipids are multiple methyl-branched fatty acid-containing lipids in the mycobacterial cell wall and their critical roles in virulence have been demonstrated in multiple pathogenic mycobacteria including M. tb, M. bovis, and M. marinum.34,35,36,37,38,39,40 The low virulence of BCG-Prague may be related to the phoP mutation in this strain.10 PhoP is a response regulator of the PhoP-PhoR two-component system.41 PhoPR controls the synthesis and export of multiple virulence factors in M. tb including EsxA, an effector of the type VII secretion system ESX-1, and lipids of polyacyltrehalose and sulfolipid families, and therefore is critical for M. tb virulence.42,43,44 However, since BCG has lost the RD1 region encoding the ESX-16,7, in addition to the impaired phoPR regulation system in M. bovis and BCG due to single nucleotide polymorphisms in this locus,45 the extent to which the phoP mutation contributes to the attenuation of BCG-Prague remains unknown. BCG-Japan has a lower level of virulence than BCG-Russia and BCG-Moreau in group I strains presumably because it is deficient in the production of phthiocerol dimycocerosates/phenolic glycolipids and triacylglycerols.33 For BCG-Sweden and BCG-Birkhaug of group II, the deletion of whiB3, a reductive stress regulator, and trcR, a response regulator of the trcR-trcS two-component system, may account for their low virulence.10 Strain-specific single nucleotide polymorphisms revealed by whole genome sequencing11,12 may also account for the differential virulence of individual BCG strains. For example, BCG-Russia and BCG-Moreau of group I are more virulent than BCG-Danish but less virulent than BCG-China of the same group (group III). Future studies are required to test these hypotheses.
There appears to be a general trend that more virulent BCG strains are also more protective. BCG strains of the most virulent group (BCG-Phipps, BCG-Pasteur, BCG-Frappier, BCG-Tice) demonstrated better protection than BCG-Sweden and BCG-Birkhaug of group II. Among the group I strains, BCG-Japan is the least virulent and also less protective than BCG-Russia and BCG-Moreau. Consistent with this notion, a previous study found that recombinant BCG strains complemented with the RD1 region exhibited increased virulence in SCID mice but also better protection in C57BL/6 mice and guinea pigs.46,47 The correlation is less straightforward when comparing strains in group III (BCG-Danish, BCG-China, BCG-Prague, and BCG-Glaxo). For example, BCG-Prague is quite attenuated but well protecting, suggesting that multiple factors are involved. Current strategies to develop the next generation of TB vaccines include the construction of recombinant BCG.48,49 Candidates that have entered clinical trials include rBCG3050,51 and VPM1002 (rBCG::ΔureC hly+),52,53 which in the preclinical animal studies (mice or guinea pigs), consistently reduced the M. tb burden by 0.5–1.0 log10 CFUs compared to the corresponding parental BCG strains. Notably, we found that mice vaccinated with BCG-Phipps and BCG-Frappier had 0.5–0.8 log10 fewer M. tb than those vaccinated with BCG-Birkhaug, BCG-Sweden, BCG-Japan, or BCG-Glaxo (Figure 2a), highlighting the importance of selecting specific BCG strain(s) for the construction of recombinant BCG.
Currently, all BCG strains are considered “equal” in clinical use. The most widely used BCG vaccines include both early (BCG-Japan, BCG-Moreau, BCG-Russia) and late BCG (BCG-Pasteur, BCG-Danish, BCG-Connaught, distributed after 1927) strains.54 Reviews of clinical trial data found no evidence that efficacy was associated with the BCG strain.17,18 However, it should be noted that these analyses were limited by the paucity of randomized trials directly comparing different BCG strains. The conclusion drawn was based on comparisons between different clinical trials, which is compounded by multiple factors including differences in trial method and the population of the study. A randomized trial study comparing two BCG strains in 300,000 infants in Hong Kong found that a more virulent strain, BCG-Pasteur, administered at a lower dosage, provided a significantly greater (40%) protection against childhood forms of TB than a less virulent strain, BCG-Glaxo.55 However, in a retrospective analysis of cohorts in Kazakhstan, vaccination of neonates by one of the least virulent strains, BCG-Japan, reduced the risk of TB by 69%, by 43% after BCG-Serbia vaccination, and only by 22% after BCG-Russia.56 While it is difficult to compare results between clinical trial studies, and between animal and human studies, these results suggest there are significant differences in effectiveness against TB between BCG strains. Multiple studies have also demonstrated that BCG exhibits strain-dependent variations in immune responses in humans.57,58 Taken together, these pieces of evidence calls for a clinical trial study directly comparing the effectiveness of different BCG strains. Based on the findings of our study, we suggest that the trial should include BCG strains from a diverse genetic background—that is, representatives of each of the four major groups (DU2 group I–IV). The outcome of such a clinical trial may not only identify the most effective BCG strain(s) for current clinical use, but also uncover genetic factors that influence the vaccine effectiveness, which will be useful for the development of the next generation of TB vaccines.
Materials and Methods
Bacterial strains and culture conditions. All BCG strains included in this study have been previously described.10 BCG strains and M. tb H37Rv were grown at 37 °C in Middlebrook 7H9 broth (Difco) supplemented with 0.2% glycerol, 10% albumin–dextrose–catalase (ADC; BD BBL, Shanghai, China), and 0.05% Tween-80 or on Middlebrook 7H11 agar (Difco) supplemented with 0.5% glycerol and 10% oleic acid–albumin–dextrose–catalase (OADC; BD BBL).
Analysis of BCG virulence in SCID mice. All of the animal procedures were approved by the local animal care committees. Female SCID mice were purchased from Beijing HFK Bioscience and the mice were age matched (7–8 weeks) within each experiment. Mice (27 per group) were infected i.v. via the tail vein with 107 CFU of the different BCG strains in 0.1 ml PBS/0.01% Tween-80. At day 1 postinfection, two mice from each group were sacrificed and the lungs and spleens were harvested, homogenized in PBS, and plated on 7H11 agar to enumerate bacterial burden. This was performed to confirm the actual infection dosage. To analyze the replication of BCG strains in SCID mice, four mice from each group at week 1 and week 4 postinfection were sacrificed and the CFUs of BCG in lungs and spleen were determined. The survival of the remaining the mice was monitored over 18 weeks.
Protection against M. tb challenge. Groups of 12 female BALB/c mice were vaccinated s.c. on the scruff of the neck with 106 CFU of the BCG strains in 0.1 ml PBS/0.01% Tween-80 or PBS/0.01% Tween-80 alone as a control. At 8 weeks postvaccination, mice were aerogenically challenged with 100 CFU of M. tb H37Rv using a GlasCol nebulizer. Mice were euthanized at 4 and 9 weeks postchallenge (five mice per group per time point) to harvest the lungs and spleen, which were then homogenized and plated on 7H11 agar to enumerate the burden of M. tb. Plates were incubated at 37 °C and counted after 2.5–3 weeks. Actual infection dose was confirmed by homogenizing whole lungs of two mice at day 1 postinfection and plating on 7H11 to enumerate M. tb.
Statistical analysis. SCID mice survival was plotted using the Kaplan–Meier method and differences between curves were analyzed using the log-rank test. One-way ANOVA with Tukey's multiple comparisons were performed for M. tb burdens (log10-transformed CFU data) when there are more than six groups. One-way ANOVA and Bonferroni's multiple comparisons were performed for six or fewer groups.
Acknowledgments
This study was supported by grants from the China's 12th Five Year Programs for the prevention and cure of great infectious diseases (No. 2012ZX10003008-009, 2013ZX10003007-003) and Shanghai Science and Technology Commission (13DZ2252000), and a grant from Canadian Institutes of Health Research (MOP-106559).
References
- Colditz, GA, Berkey, CS, Mosteller, F, Brewer, TF, Wilson, ME, Burdick, E et al. (1995). The efficacy of bacillus Calmette-Guérin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96(1 Pt 1): 29–35. [PubMed] [Google Scholar]
- Trunz, BB, Fine, P and Dye, C (2006). Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet 367: 1173–1180. [DOI] [PubMed] [Google Scholar]
- Brewer, TF (2000). Preventing tuberculosis with bacillus Calmette-Guérin vaccine: a meta-analysis of the literature. Clin Infect Dis 31 (suppl. 3): S64–S67. [DOI] [PubMed] [Google Scholar]
- WHO (2007). Revised BCG vaccination guidelines for infants at risk for HIV infection. Wkly Epidemiol Rec 82: 181–196. [PubMed] [Google Scholar]
- Liu, J, Tran, V, Leung, AS, Alexander, DC and Zhu, B (2009). BCG vaccines: their mechanisms of attenuation and impact on safety and protective efficacy. Hum Vaccin 5: 70–78. [DOI] [PubMed] [Google Scholar]
- Mahairas, GG, Sabo, PJ, Hickey, MJ, Singh, DC and Stover, CK (1996). Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr, MA, Wilson, MA, Gill, WP, Salamon, H, Schoolnik, GK, Rane, S et al. (1999). Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284: 1520–1523. [DOI] [PubMed] [Google Scholar]
- Brosch, R, Gordon, SV, Buchrieser, C, Pym, AS, Garnier, T and Cole, ST (2000). Comparative genomics uncovers large tandem chromosomal duplications in Mycobacterium bovis BCG Pasteur. Yeast 17: 111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosch, R, Gordon, SV, Garnier, T, Eiglmeier, K, Frigui, W, Valenti, P et al. (2007). Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA 104: 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, AS, Tran, V, Wu, Z, Yu, X, Alexander, DC, Gao, GF et al. (2008). Novel genome polymorphisms in BCG vaccine strains and impact on efficacy. BMC Genomics 9: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, Y, Yang, X, Duan, J, Lu, N, Leung, AS, Tran, V et al. (2011). Whole-genome sequences of four Mycobacterium bovis BCG vaccine strains. J Bacteriol 193: 3152–3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, W, Zhang, Y, Zheng, H, Pan, Y, Liu, H, Du, P et al. (2013). Genome sequencing and analysis of BCG vaccine strains. PLoS One 8: e71243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki, M, Honda, I, Fujita, I, Yano, I, Yamamoto, S and Koyama, A (2009). Whole genome sequence analysis of Mycobacterium bovis bacillus Calmette-Guérin (BCG) Tokyo 172: a comparative study of BCG vaccine substrains. Vaccine 27: 1710–1716. [DOI] [PubMed] [Google Scholar]
- Gomes, LH, Otto, TD, Vasconcellos, EA, Ferrão, PM, Maia, RM, Moreira, AS et al. (2011). Genome sequence of Mycobacterium bovis BCG Moreau, the Brazilian vaccine strain against tuberculosis. J Bacteriol 193: 5600–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behr, MA (2002). BCG–different strains, different vaccines? Lancet Infect Dis 2: 86–92. [DOI] [PubMed] [Google Scholar]
- Horwitz, MA, Harth, G, Dillon, BJ and Maslesa-Galić, S (2009). Commonly administered BCG strains including an evolutionarily early strain and evolutionarily late strains of disparate genealogy induce comparable protective immunity against tuberculosis. Vaccine 27: 441–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, TF and Colditz, GA (1995). Relationship between bacille Calmette-Guérin (BCG) strains and the efficacy of BCG vaccine in the prevention of tuberculosis. Clin Infect Dis 20: 126–135. [DOI] [PubMed] [Google Scholar]
- Mangtani, P, Abubakar, I, Ariti, C, Beynon, R, Pimpin, L, Fine, PE et al. (2014). Protection by BCG vaccine against tuberculosis: a systematic review of randomized controlled trials. Clin Infect Dis 58: 470–480. [DOI] [PubMed] [Google Scholar]
- Ritz, N, Hanekom, WA, Robins-Browne, R, Britton, WJ and Curtis, N (2008). Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev 32: 821–841. [DOI] [PubMed] [Google Scholar]
- Walker, KB, Brennan, MJ, Ho, MM, Eskola, J, Thiry, G, Sadoff, J et al. (2010). The second Geneva Consensus: recommendations for novel live TB vaccines. Vaccine 28: 2259–2270. [DOI] [PubMed] [Google Scholar]
- Barker, L, Hessel, L and Walker, B (2012). Rational approach to selection and clinical development of TB vaccine candidates. Tuberculosis (Edinb) 92 (suppl. 1): S25–S29. [DOI] [PubMed] [Google Scholar]
- North, RJ and Jung, YJ (2004). Immunity to tuberculosis. Annu Rev Immunol 22: 599–623. [DOI] [PubMed] [Google Scholar]
- Schell, RF, Ealey, WF, Harding, GE and Smith, DW (1974). The influence of vaccination on the course of experimental airborne tuberculosis in mice. J Reticuloendothel Soc 16: 131–138. [PubMed] [Google Scholar]
- Kelly, BP, Furney, SK, Jessen, MT and Orme, IM (1996). Low-dose aerosol infection model for testing drugs for efficacy against Mycobacterium tuberculosis. Antimicrob Agents Chemother 40: 2809–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme, IM (1988). A mouse model of the recrudescence of latent tuberculosis in the elderly. Am Rev Respir Dis 137: 716–718. [DOI] [PubMed] [Google Scholar]
- Ladefoged, A, Bunch-Christensen, K and Guld, J (1970). The protective effect in bank voles of some strains of BCG. Bull World Health Organ 43: 71–90. [PMC free article] [PubMed] [Google Scholar]
- Ladefoged, A, Bunch-Christensen, K and Guld, J (1976). Tuberculin sensitivity in guinea-pigs after vaccination with varying doses of BCG of 12 different strains. Bull World Health Organ 53: 435–443. [PMC free article] [PubMed] [Google Scholar]
- Castillo-Rodal, AI, Castañón-Arreola, M, Hernández-Pando, R, Calva, JJ, Sada-Díaz, E and López-Vidal, Y (2006). Mycobacterium bovis BCG substrains confer different levels of protection against Mycobacterium tuberculosis infection in a BALB/c model of progressive pulmonary tuberculosis. Infect Immun 74: 1718–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedwell, J, Kairo, SK, Behr, MA and Bygraves, JA (2001). Identification of substrains of BCG vaccine using multiplex PCR. Vaccine 19: 2146–2151. [DOI] [PubMed] [Google Scholar]
- Markey, K, Ho, MM, Choudhury, B, Seki, M, Ju, L, Castello-Branco, LR et al. (2010). Report of an international collaborative study to evaluate the suitability of multiplex PCR as an identity assay for different sub-strains of BCG vaccine. Vaccine 28: 6964–6969. [DOI] [PubMed] [Google Scholar]
- Raman, S, Song, T, Puyang, X, Bardarov, S, Jacobs, WR Jr. and Husson, RN (2001). The alternative sigma factor SigH regulates major components of oxidative and heat stress responses in Mycobacterium tuberculosis. J Bacteriol 183: 6119–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, LJ, Stapleton, MR, Fullstone, GJ, Crack, JC, Thomson, AJ, Le Brun, NE et al. (2010). Mycobacterium tuberculosis WhiB1 is an essential DNA-binding protein with a nitric oxide-sensitive iron-sulfur cluster. Biochem J 432: 417–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, JM, Islam, ST, Ren, H and Liu, J (2007). Differential productions of lipid virulence factors among BCG vaccine strains and implications on BCG safety. Vaccine 25: 8114–8122. [DOI] [PubMed] [Google Scholar]
- Reed, MB, Domenech, P, Manca, C, Su, H, Barczak, AK, Kreiswirth, BN et al. (2004). A glycolipid of hypervirulent tuberculosis strains that inhibits the innate immune response. Nature 431: 84–87. [DOI] [PubMed] [Google Scholar]
- Camacho, LR, Ensergueix, D, Perez, E, Gicquel, B and Guilhot, C (1999). Identification of a virulence gene cluster of Mycobacterium tuberculosis by signature-tagged transposon mutagenesis. Mol Microbiol 34: 257–267. [DOI] [PubMed] [Google Scholar]
- Cox, JS, Chen, B, McNeil, M and Jacobs, WR Jr. (1999). Complex lipid determines tissue-specific replication of Mycobacterium tuberculosis in mice. Nature 402: 79–83. [DOI] [PubMed] [Google Scholar]
- Rousseau, C, Winter, N, Pivert, E, Bordat, Y, Neyrolles, O, Avé, P et al. (2004). Production of phthiocerol dimycocerosates protects Mycobacterium tuberculosis from the cidal activity of reactive nitrogen intermediates produced by macrophages and modulates the early immune response to infection. Cell Microbiol 6: 277–287. [DOI] [PubMed] [Google Scholar]
- Tsenova, L, Ellison, E, Harbacheuski, R, Moreira, AL, Kurepina, N, Reed, MB et al. (2005). Virulence of selected Mycobacterium tuberculosis clinical isolates in the rabbit model of meningitis is dependent on phenolic glycolipid produced by the bacilli. J Infect Dis 192: 98–106. [DOI] [PubMed] [Google Scholar]
- Hotter, GS, Wards, BJ, Mouat, P, Besra, GS, Gomes, J, Singh, M et al. (2005). Transposon mutagenesis of Mb0100 at the ppe1-nrp locus in Mycobacterium bovis disrupts phthiocerol dimycocerosate (PDIM) and glycosylphenol-PDIM biosynthesis, producing an avirulent strain with vaccine properties at least equal to those of M. bovis BCG. J Bacteriol 187: 2267–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, J, Tran, V, Li, M, Huang, X, Niu, C, Wang, D et al. (2012). Both phthiocerol dimycocerosates and phenolic glycolipids are required for virulence of Mycobacterium marinum. Infect Immun 80: 1381–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters, SB, Dubnau, E, Kolesnikova, I, Laval, F, Daffe, M and Smith, I (2006). The Mycobacterium tuberculosis PhoPR two-component system regulates genes essential for virulence and complex lipid biosynthesis. Mol Microbiol 60: 312–330. [DOI] [PubMed] [Google Scholar]
- Martin, C, Williams, A, Hernandez-Pando, R, Cardona, PJ, Gormley, E, Bordat, Y et al. (2006). The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine 24: 3408–3419. [DOI] [PubMed] [Google Scholar]
- Frigui, W, Bottai, D, Majlessi, L, Monot, M, Josselin, E, Brodin, P et al. (2008). Control of M. tuberculosis ESAT-6 secretion and specific T cell recognition by PhoP. PLoS Pathog 4: e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passemar, C, Arbués, A, Malaga, W, Mercier, I, Moreau, F, Lepourry, L et al. (2014). Multiple deletions in the polyketide synthase gene repertoire of Mycobacterium tuberculosis reveal functional overlap of cell envelope lipids in host-pathogen interactions. Cell Microbiol 16: 195–213. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Asensio, J, Malaga, W, Pawlik, A, Astarie-Dequeker, C, Passemar, C, Moreau, F et al. (2014). Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci USA 111: 11491–11496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pym, AS, Brodin, P, Brosch, R, Huerre, M and Cole, ST (2002). Loss of RD1 contributed to the attenuation of the live tuberculosis vaccines Mycobacterium bovis BCG and Mycobacterium microti. Mol Microbiol 46: 709–717. [DOI] [PubMed] [Google Scholar]
- Pym, AS, Brodin, P, Majlessi, L, Brosch, R, Demangel, C, Williams, A et al. (2003). Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9: 533–539. [DOI] [PubMed] [Google Scholar]
- Skeiky, YA and Sadoff, JC (2006). Advances in tuberculosis vaccine strategies. Nat Rev Microbiol 4: 469–476. [DOI] [PubMed] [Google Scholar]
- Andersen, P and Kaufmann, SH (2014). Novel vaccination strategies against tuberculosis. Cold Spring Harb Perspect Med 4: a018523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, MA, Harth, G, Dillon, BJ and Maslesa-Galic', S (2000). Recombinant bacillus calmette-guerin (BCG) vaccines expressing the Mycobacterium tuberculosis 30-kDa major secretory protein induce greater protective immunity against tuberculosis than conventional BCG vaccines in a highly susceptible animal model. Proc Natl Acad Sci USA 97: 13853–13858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz, MA and Harth, G (2003). A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun 71: 1672–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grode, L, Seiler, P, Baumann, S, Hess, J, Brinkmann, V, Nasser Eddine, A et al. (2005). Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guérin mutants that secrete listeriolysin. J Clin Invest 115: 2472–2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desel, C, Dorhoi, A, Bandermann, S, Grode, L, Eisele, B and Kaufmann, SH (2011). Recombinant BCG ΔureC hly+ induces superior protection over parental BCG by stimulating a balanced combination of type 1 and type 17 cytokine responses. J Infect Dis 204: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, N and Curtis, N (2009). Mapping the global use of different BCG vaccine strains. Tuberculosis (Edinb) 89: 248–251. [DOI] [PubMed] [Google Scholar]
- Milstien, JB and Gibson, JJ (1990). Quality control of BCG vaccine by WHO: a review of factors that may influence vaccine effectiveness and safety. Bull World Health Organ 68: 93–108. [PMC free article] [PubMed] [Google Scholar]
- Favorov, M, Ali, M, Tursunbayeva, A, Aitmagambetova, I, Kilgore, P, Ismailov, S et al. (2012). Comparative tuberculosis (TB) prevention effectiveness in children of Bacillus Calmette-Guérin (BCG) vaccines from different sources, Kazakhstan. PLoS One 7: e32567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, EJ, Webb, EL, Mawa, PA, Kizza, M, Lyadda, N, Nampijja, M et al. (2012). The influence of BCG vaccine strain on mycobacteria-specific and non-specific immune responses in a prospective cohort of infants in Uganda. Vaccine 30: 2083–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz, N, Dutta, B, Donath, S, Casalaz, D, Connell, TG, Tebruegge, M et al. (2012). The influence of bacille Calmette-Guerin vaccine strain on the immune response against tuberculosis: a randomized trial. Am J Respir Crit Care Med 185: 213–222. [DOI] [PubMed] [Google Scholar]