Over the past three decades, a dogma has emerged regarding the uniform safety and nonpathogenicity of adeno-associated virus (AAV), for three excellent reasons. First, natural AAV infections in humans are common and until recently had not been associated with disease. Second, the recombinant AAV (rAAV) genome remains predominantly episomal, greatly reducing the risk of insertional mutagenesis and genotoxicity that was observed in clinical trials using retroviral vectors. Third, numerous human clinical trials and preclinical gene delivery studies in both small- and large-animal models have revealed no recognized vector toxicity other than the development of humoral and cellular immunity to the rAAV capsid. Although the lack of natural pathogenicity and the capacity for effective gene delivery coupled with stable, long-term gene expression have supported the advancement of rAAV as an optimal vector for human clinical trials, recent studies in mice have challenged the belief that rAAV is an innocuous gene therapy vector.
Additionally, a new report claiming that insertional mutagenesis by wild-type AAV serotype 2 can contribute to the development of hepatocellular carcinoma (HCC) in humans1 has increased concerns over the potential of rAAV-related genotoxicity in the setting of clinical gene therapy. In this commentary we discuss the preclinical mouse studies that documented rAAV-associated toxicity and consider the implications for human gene therapy.
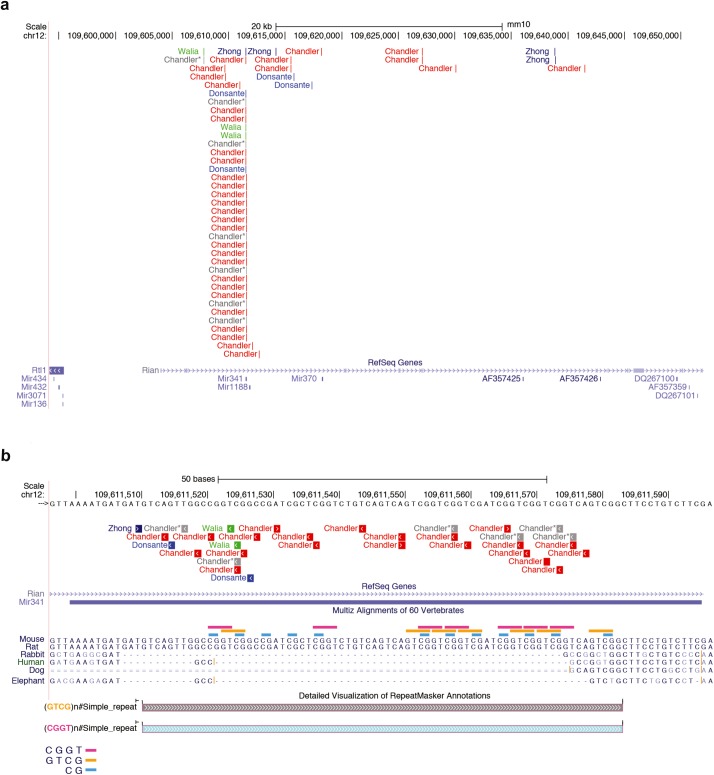
Three preclinical studies in mice have reported an increased incidence of HCC following gene delivery using single-strand rAAV (ssAAV) vectors associated with rAAV integrations into the Rian locus (Figure 1a).2,3,4 Although the vector serotypes have varied, all the studies delivered relatively high doses of rAAV during the murine neonatal period, a time during mouse development that is more akin to a premature human gestation. The first observation of a possible rAAV-HCC association5 had, at the time, an irreconcilable feature of very low copy number of vector genomes in the tumors. If rAAV integrations were a causative and an important event in the clonal evolution of the tumors, why was the vector genome not present at a high copy number? The most likely explanation, and one now supported by a number of studies, is that the rAAV genome underwent a recombination event upon or after integration, leaving behind only an inverted terminal repeat and some remnant of the vector. Indeed, a later study in mice treated with the same rAAV vector succeeded in capturing one side of the rAAV–tumor DNA junctions in four different HCCs; all the integrations mapped into the Rian locus and were associated with increased expression of multiple microRNAs (miRNAs) embedded within Rian.3 However, the small number of tumors analyzed and apparent lack of supportive findings from other preclinical murine gene therapy studies prevented the community from fully embracing the conclusion that significant genotoxicity mediated by insertional mutagenesis could be observed after therapeutic rAAV gene delivery in mice.
Figure 1.
rAAV integrations in Rian and Mir341 after gene therapy. rAAV integrations from published data sets,2,4,11 designated in the figure by first author, were extracted and filtered for those that mapped into (a) Rian and (b) Mir341 and then uploaded to the UCSC Genome Browser (Genome Reference Consortium Mouse Build 38) to display the exact location of each integration.18,19 A MULTIZ alignment of 60 vertebrate genomes centered on the murine Mir341 locus depicts only six vertebrate sequences, because no homology was detected for other vertebrates. The locations within Mir341 of two simple repeats—CTCG (pink) and CGGT (orange)—as well as CpGs (blue) are displayed. Chandler and colleagues' gray AAV integrations mapped into Mir341 but were not associated with HCC, as detailed in ref. 2.
In the past year, two independent studies, using different metabolic mutant mice, have also observed an rAAV-HCC association after therapeutic gene delivery in the neonatal period, and are consistent with the existence of an rAAV-HCC locus in rodents. One model was that of Sandhoff disease, and the other was of methylmalonic acidemia (MMA). When mice with Sandhoff disease were given large doses of an AAV 2/9 vector expressing the hexosaminidase b-subunit under the control of the cytomegalovirus promoter via superficial temporal vein injection, 7 of 10 treated mice developed HCC by 43 weeks with rAAV insertions that mapped into the Rian locus.4 A single treated mouse developed lung cancer and was found to have an rAAV integration in fibroblast growth factor receptor 2 (Fgfr2), a gene known to have altered expression in cancer.
The recent studies on the MMA mice, which relied upon large numbers of animals treated with varied rAAV vectors and serotypes in the neonatal period, have been the largest reported to date, and may be instructive for others who plan to contend with rAAV genotoxicity in preclinical models.2 Like the other models, the MMA mice also received large doses of rAAV vectors in the neonatal period.
However, these mice were maintained and aged with a relatively large group of 51 untreated control littermates that allowed the strain incidence of HCC to be measured as <10%, which was in agreement with estimates based on the dominant parental strain, C57BL6. Both unaffected control and MMA mice treated with therapeutic or reporter rAAVs developed HCC at a rate of 50–75% by 2 years, and the risk of HCC dropped when the dose was reduced from 1 × 1011 to 1 × 1010 genome copies (gc) per pup. In summary, the observations from the MMA mice were entirely consistent with rAAV exposure causing HCCs, because the cancers occurred at a high frequency in the rAAV-treated mice, in a genotype- and transgene-independent fashion, but not in the untreated and aged controls, and at a much lower rate in the mice that received lower doses of rAAV.
The adoption of a sensitive integration capture method initially developed for zebrafish genomics afforded an opportunity to directly characterize integration events in the HCC and unaffected controls tissues using high-throughput sequencing. Approximately 40 samples yielded thousands of rAAV integration events. All the rAAVs displayed a propensity to integrate into or near highly expressed genes with abundant transcripts in the neonatal liver, such as albumin and alpha-fetal protein, which were frequent targets of integration in both tumor and surrounding nontumor tissue. However, only the Rian locus—specifically the miRNA Mir341—emerged as a locus associated with increased frequency and abundance of integrations in the HCCs. This finding is consistent with several studies showing that insertional mutagenesis by lentivirus, transposons, or targeted-rAAV integration of the Rian locus causes HCCs in mice.6,7,8 The fact that 6- to 8-week-old mice treated with ssAAV in a hemophilia study9 were not noted to harbor integrations in Mir341 or Rian supports this concept, perhaps because Rian, like alpha-fetal protein, is highly expressed early in the neonatal period in the mouse liver.
The rAAV-associated HCCs described by Chandler et al.2 had a distinct gene and miRNA expression signature of widespread upregulation of many embedded miRNAs and genes within Rian and were consistent with the studies by Donsante et al.3 Although the roles that the dysregulated miRNAs play in AAV-associated HCC are not well understood, the upregulation of Rtl1, a gene located approximately 15 kb upstream from Rian in AAV-associated HCCs,2 has been noted in other types of murine HCCs. Rtl1 was independently identified as an HCC driver in studies that assessed hepatic genotoxicity after lentiviral and transposon exposure in the neonatal murine liver and therefore may be one of the key genes responsible for the genotoxicity of rAAV in mice.6,7,10
A particularly unexpected finding was that one of the vectors studied integrated into Mir341 but did not display an upregulation of miRNAs or genotoxicity as assessed by an increased tumor risk in the treated mice. Presumably this lack of genotoxicity is due to ineffectiveness of the regulatory sequences at strong local transactivation within the Rian locus after integration. This observation suggests that rAAV vector design can reduce or perhaps eliminate toxicity.
To further understand the genomic distribution of the integrations captured within the genomic context of the Mir341 gene, we have depicted all the reported rAAV integrations in this locus2,3,4,11 (Figure 1b). As can be seen in Figure 1, the 96–base pair sequence that makes up Mir341 is absent from all vertebrate genomes except for those of mice and rats. Several independent studies have recovered a striking number of rAAV integrations from this tiny sequence, raising the question of why this site seems to be a preferred target for rAAV integration. Although the Mir341 lacks homology to both human and mouse AAVS1 sequences,12 we note that it contains many elements that may make it recombinogenic. Mir341 contains multiple copies of two simple quadranucleotide repeats (CCGT and GGCT), some of which are internally repeated, as well as 11 CpG dinucleotides. Perhaps these elements cause the Mir341 locus to adopt an unusual secondary structure during or after unwinding that serves as a substrate for recombination with the rAAV inverted terminal repeat, but this hypothesis will require testing through further studies. Although Mir341 does not exist in humans, defining the mechanism(s) that drive rAAV integration at this locus in mice may help identify areas in the human genome that are susceptible to insertional mutagenesis by rAAV.
The implications from the results of the selected studies reviewed here, as well as others that have examined genotoxicity of rAAV, allow us to offer some general comments regarding preclinical study design, rAAV-associated HCC in mice, and the extrapolation of rodent safety data to humans. If a mouse is treated with a large doses of an ssAAV vector in the neonatal period (~1 × 1011 gc per pup, which approximates 1 × 1014 gc/kg in humans) and allowed to age for 2 years, it will have a high chance to develop an HCC if the vector promoter and enhancer are strong enough to transactivate local genes. In such HCCs, the rAAV will be present predominantly in the Rian locus—specifically in Mir341. The integration profile for self-complementary (sc) AAVs, which have been studied in a limited fashion in neonatal rats13 and in the context of an unusual cancer-prone mouse model,14 should be determined and might add information regarding mechanism(s) of integration. scAAV vectors have already been successfully administered to hemophiliacs in human clinical trials, and the treatment of infants with spinal muscular atrophy with very large doses of an scAAV (3.3 × 1014 vector genomes/kg) configured to express SMN under the control of a powerful hybrid cytomegalovirus enhancer/chicken-b-actin promoter, has been approved. Therefore, studies to determine whether scAAVs could have distinct integration and genotoxic profiles will be important. Because Mir341, which appears to be the nucleus of the rAAV HCC site in mice, is missing from the genomes of other large animals commonly used in gene therapy studies, such as rabbits, cats, dogs, and nonhuman primates, the general extension of murine data from rAAV-associated HCCs warrants critical assessment.
A more rational approach may be to conduct comprehensive studies with large animals aimed at defining the patterns of rAAV integrations when animals are treated at varying ages. Concurrent studies utilizing biomarker analyses and histopathology could permit closer examination into whether rAAV administration confers an increased risk to develop cancer in species other than mice, and, if so, whether there is a developmental component to genotoxicity. In addition, the possibility that species will differ with respect to rAAV integrations calls for the development of relevant cellular modeling to examine the question of genotoxicity in humans. Whether rAAV integrations, accrued over life in otherwise healthy humans who then developed HCC after exposure to wild-type AAV, are drivers or passengers in the HCC genome appears to be a distinct concern, with a different underlying mechanism.15,16,17 The many years of encouraging safety data from numerous animals and humans treated with rAAV suggests that the malignancy risk for humans might not be best predicted by experience with rodents. In other words, mice are not men when one considers genotoxicity after rAAV gene therapy. Given the lack of therapies for the many diseases that rAAV gene therapy promises to treat, such as lethal inborn errors of metabolism, the risk of toxicity imposed by rAAV exposure will need to be balanced against the significant benefits offered by effective gene therapy, which for some patients could be lifesaving.
References
- Nault, JC, Datta, S, Imbeaud, S, Franconi, A, Mallet, M, Couchy, G et al. (2015). Recurrent AAV2-related insertional mutagenesis in human hepatocellular carcinomas. Nat Genet 47: 1187–1193. [DOI] [PubMed] [Google Scholar]
- Chandler, RJ, LeFave, MC, Varshney, GK, Trivedi, NS, Carrillo-Carrasco, N, Senac, JS et al. (2015). Vector design influences hepatic genotoxicity after adeno-associated virus gene therapy. J Clin Invest 125: 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante, A, Miller, DG, Li, Y, Vogler, C, Brunt, EM, Russell, DW et al. (2007). AAV vector integration sites in mouse hepatocellular carcinoma. Science 317: 477. [DOI] [PubMed] [Google Scholar]
- Walia, JS, Altaleb, N, Bello, A, Kruck, C, LaFave, MC, Varshney, GK et al. (2015). Long-term correction of Sandhoff disease following intravenous delivery of rAAV9 to mouse neonates. Mol Ther 23: 414–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donsante, A, Vogler, C, Muzyczka, N, Crawford, JM, Barker, J, Flotte, T et al. (2001). Observed incidence of tumorigenesis in long-term rodent studies of rAAV vectors. Gene Ther 8: 1343–1346. [DOI] [PubMed] [Google Scholar]
- Dupuy, AJ, Rogers, LM, Kim, J, Nannapaneni, K, Starr, TK, Liu, P et al. (2009). A modified sleeping beauty transposon system that can be used to model a wide variety of human cancers in mice. Cancer Res 69: 8150–8156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranzani, M, Cesana, D, Bartholomae, CC, Sanvito, F, Pala, M, Benedicenti, F et al. (2013). Lentiviral vector-based insertional mutagenesis identifies genes associated with liver cancer. Nat Methods 10: 155–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, PR, Xu, M, Toffanin, S, Li, Y, Llovet, JM and Russell, DW (2012). Induction of hepatocellular carcinoma by in vivo gene targeting. Proc Natl Acad Sci USA 109: 11264–11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, H, Malani, N, Hamilton, SR, Schlachterman, A, Bussadori, G, Edmonson, SE et al. (2011). Assessing the potential for AAV vector genotoxicity in a murine model. Blood 117: 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan, JD, Keng, VW, Tschida, BR, Scheetz, TE, Bell, JB, Podetz-Pedersen, KM et al. (2013). Identification of rtl1, a retrotransposon-derived imprinted gene, as a novel driver of hepatocarcinogenesis. PLoS Genet 9: e1003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong, L, Malani, N, Li, M, Brady, T, Xie, J, Bell, P et al. (2013). Recombinant adeno-associated virus integration sites in murine liver after ornithine transcarbamylase gene correction. Hum Gene Ther 24: 520–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutheil, N, Yoon-Robarts, M, Ward, P, Henckaerts, E, Skrabanek, L, Berns, KI et al. (2004). Characterization of the mouse adeno-associated virus AAVS1 ortholog. J Virol 78: 8917–8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauttier, V, Pichard, V, Aubert, D, Kaeppel, C, Schmidt, M, Ferry, N et al. (2013). No tumour-initiating risk associated with scAAV transduction in newborn rat liver. Gene Ther 20: 779–784. [DOI] [PubMed] [Google Scholar]
- Rosas, LE, Grieves, JL, Zaraspe, K, La Perle, KM, Fu, H and McCarty, DM (2012). Patterns of scAAV vector insertion associated with oncogenic events in a mouse model for genotoxicity. Mol Ther 20: 2098–2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell, DW and Grompe, M (2015). Adeno-associated virus finds its disease. Nat Genet 47: 1104–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büning H, Schmidt M. Adeno-associated vector toxicity-to be or not to be? Mol Ther. 2015. 23:1673-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns KI, Byrne BJ, Flotte TR, Gao G, Hauswirth WW, Herzog RW, Muzyczka N, et al . Adeno-associated virus type 2 and hepatocellular carcinoma? Hum Gene Ther. 2015. 26:779-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent, WJ, Sugnet, CW, Furey, TS, Roskin, KM, Pringle, TH, Zahler, AMet al. (2002). The human genome browser at UCSC. Genome Res 12: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raney, BJ, Dreszer, TR, Barber, GP, Clawson, H, Fujita, PA, Wang, T et al. (2014). Track data hubs enable visualization of user-defined genome-wide annotations on the UCSC Genome Browser. Bioinformatics 30: 1003–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]