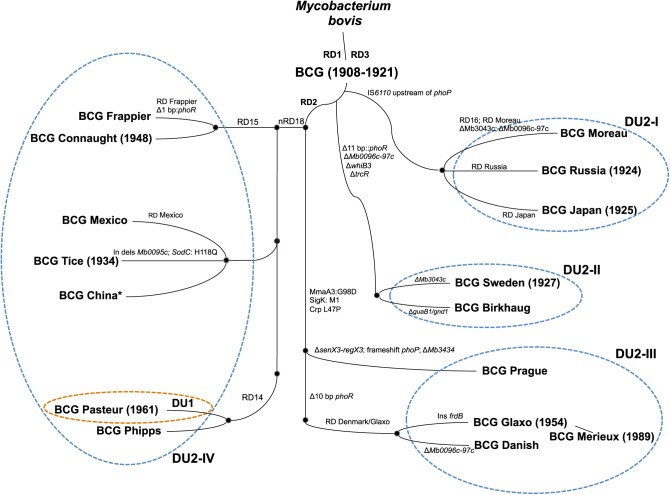
The bacillus Calmette-Guérin (BCG) is of central importance to the vaccination programs of many countries with a high incidence of tuberculosis (TB).1 However, despite its well-recognized efficacy against miliary and meningeal TB in young children, vaccination with BCG confers only limited and/or variable protection against pulmonary TB in adolescents and adults, with an efficacy ranging from 0 to 80% according to several clinical trials.1 The overall interpretation of BCG vaccine efficacy and the resulting recommendations are further complicated by the fact that BCG is not a single, pharmacologically well-defined vaccine but, rather, a pool of different BCG daughter strains (Figure 1) that have acquired phenotypic and genotypic variations during decades of in vitro culturing in different laboratories.2 As reported in this issue of Molecular Therapy, Zhang and colleagues have now compared phenotypic and genotypic information of 13 different BCG strains with data on their virulence and vaccine efficacy in severe combined immunodeficient (SCID) and BALB/c mice, respectively.3 The authors concluded that the distinct levels of virulence of the various strains might be linked to strain-specific duplications and deletions of genomic regions. Moreover, the authors observed a general trend whereby BCG strains showing higher virulence in SCID mice induced better protection against a Mycobacterium tuberculosis challenge in BALB/c mice3 relative to less virulent BCG strains. These observations have important implications for current BCG vaccination programs and are of particular relevance for both ongoing and future alternative TB vaccine development approaches.
Figure 1.
Representation of genealogy of BCG daughter strains. Comparative genomic analyses identified several gene-specific single-nucleotide polymorphisms (SNPs) as well as large-sequence polymorphisms (genomic deletions, tandem duplications, insertion sequences IS6110) both in BCG vaccines relative to virulent strains of M. bovis and M. tuberculosis, and among the different BCG daughter strains. The scheme shows regions of difference (RD), insertions (in), deletions (del), and SNPs, which differentiate the various BCG strains. The brown and blue dashed ellipses indicate tandem duplications DU1 (exclusively restricted to BCG Pasteur) and DU2 (present in the BCG substrains in four possible forms), which enable classification of BCG strains into four major lineages. *Note that in other phylogenies (e.g., ref. 10), BCG China/BCG Beijing belongs to a cluster closely related to BCG Danish. It seems likely that two different groups of BCG exist that are both named BCG China or BCG Beijing. Adapted from refs. 8 and 9.
BCG, an attenuated anti-TB vaccine, is one of the most well-known examples of globally used vaccines developed in the twentieth century. Originally obtained by Albert Calmette and Camille Guérin in the early 1920s at the Institut Pasteur of Lille by serially passaging a virulent Mycobacterium bovis isolate on potato slices soaked in glycerol and ox bile, it remains one of the most widely used vaccines today (more than 120 million doses each year). Although numerous efforts are being undertaken to develop novel and improved anti-TB vaccines,4 potential alternatives have only recently entered clinical development.5,6 BCG has a good safety profile in immunocompetent individuals and is successfully used in babies and young children to prevent disseminated forms of TB. However, as noted above, protection conferred against pulmonary TB is often insufficient. Although significant progress has been made recently in the genetic and genomic characterization of the different BCG strains,7,8,9,10,11 the phenotypic characterization of BCG strains, with emphasis on systematic virulence comparison and protective efficacy in selected model systems, mostly date from the pregenome era.12,13 Hence, studies such as those of Zhang and colleagues3 are useful to provide deeper insights into this important field.
The results of the study convincingly show that BCG strains of the duplication group DU2-IV (BCG Phipps, Frappier, Pasteur, and Tice) exhibit the highest levels of virulence in the SCID mouse infection model, whereas BCG strains of the DU2 group II (BCG Sweden and Birkhaug) were among the least virulent strains. Intriguingly, the differences observed between various BCG strains tested in the very sensitive SCID mouse model range from about 50 to 120 days of survival to the humane endpoint, depending on the lineage of the BCG strain.3 Given that the different BCG strains are a priori all considered as acceptable for human vaccination, the observed variations in virulence between strains in this model are striking. The findings thus confirm that selected BCG strains, such as BCG Pasteur, which overall has an excellent safety record in the vaccination of neonates and children, may show significantly increased virulence in this highly susceptible SCID mouse infection model relative to BCG strains of other lineages (e.g., BCG Danish). This point should be considered when novel vaccine candidates are evaluated for their suitability for further development. Such selection criteria are often focused primarily on maximal safety in SCID mice and therefore might result in the exclusion of otherwise promising candidates that show improved vaccine efficacy but partially increased virulence in SCID mice.
One such example is recombinant BCG::RD1, which shows heterologous expression of an important mycobacterial type VII secretion system, named ESX-1, that is present in M. tuberculosis and other members of the M. tuberculosis complex but absent from BCG strains because of the deletion of a 9.5-kb genomic region, termed region of difference 1 (RD1)14 (Figure 1). RD1 encodes part of this ESX-1 system, which is responsible for the secretion of the 6-kDa early secreted antigenic target ESAT-6, a key mycobacterial antigen and virulence factor.15,16 Pym et al.17 demonstrated that recombinant expression of full-length ESX-1 from M. tuberculosis in BCG increased the virulence of the recombinant strain in SCID mice but also led to increased immunogenicity and persistence, resulting in significant improvement of the protective efficacy of the recombinant BCG::RD1 strain against an M. tuberculosis challenge in mouse and guinea pig vaccination models. More recently, the finding that BCG::ESX-1 variants expressing virulence-neutral versions of the ESX-1 system were still able to induce improved protection in animal models18 provides further arguments for the development of ESX-1-based recombinant BCG vaccines. The expression of a functionally active ESX-1 system strongly affects the intracellular trafficking of mycobacteria, enabling selected ESX-1–proficient strains to gain access to the cytosol of infected macrophages and dendritic cells and induce specific signaling cascades that all seem to be important for generating appropriate immune responses and/or protection.19,20,21
As previously mentioned, Zhang et al. found a strong link between the BCG DU2 subgroups' virulence and vaccine efficacy in mouse models. These observations suggest that strains that cluster within the same groups also share common characteristics. However, researchers have yet to determine whether these characteristics are directly related to the DU2 regions or are due to other genomic polymorphisms shared between members of the different DU2 subgroups. The DU2 duplication might have originally arisen in BCG strains owing to culture conditions used by Calmette and Guérin in which the BCG strains needed to adapt to glycerol as the sole carbon source. Indeed, despite some variation in the size of the DU2 regions in the different BCG lineages, the core duplicated part of DU2 contains the gene glpD2, encoding glycerol 3-phosphate dehydrogenase.8 However, additional mutations in some individual BCG strains might also have contributed to the specific virulence phenotypes of certain strains. As an example, the low virulence shown by BCG Glaxo might have arisen from mutations leading to a defect in the synthesis of phthiocerol dimycocerosate and phenolic glycolipids,3,22 which are important virulence-associated lipids in tubercle bacilli. Similarly, mutations affecting the PhoP/PhoR two-component system, which positively regulates the expression of numerous virulence factors of tubercle bacilli, might also have an important impact on selected strains. BCG Prague, for example, shows a frameshift mutation in PhoP, and has been found to be one of the least virulent BCG strains tested. Previous work had also shown that mutations in the PhoR sensor and/or the promoter of phoP are probably responsible for the overall absence of human-to-human aerosol transmission of M. bovis strains.23 Moreover, the deletion of phoP constitutes the molecular basis for the attenuation of the vaccine candidate MTBVAC, presently in clinical development.6
In conclusion, we emphasize that BCG vaccination is far from being an optimal strategy for preventing TB infection and/or TB disease in susceptible individuals, although the exact contribution of disease prevention by BCG is difficult to estimate. However, as alternative and more efficient anti-TB vaccines are still not licensed, investigations into the phenotypic and genotypic characteristics of BCG strains remain very relevant. The use of BCG in vaccination and research has pitfalls. As noted before, although BCG shows good efficacy in preventing disseminated forms of TB in young children, its widespread use has not prevented the pandemic spread of the disease. At the same time, BCG generally performs very well in protecting laboratory animals against a challenge with M. tuberculosis. It is therefore often very difficult or impossible to identify novel vaccine candidates during the development phase that are able to confer better protection than BCG in animal models. This effect could theoretically hamper the advancement of promising new vaccines to enter advanced preclinical and/or clinical development. The study by Zhang and colleagues highlights some of these features and may help overcome the shortcomings linked to the outstanding, yet only available, anti-TB vaccine represented by the pool of different BCG strains.
Acknowledgments
The authors appreciate the support of BCG-related work by the Fondation pour la Recherche Medicale (DEQ20130326471), the European Community (H2020-PHC-64338), and the Institut Pasteur (PTR 441).
References
- Ritz, N, Hanekom, WA, Robins-Browne, R, Britton, WJ and Curtis, N (2008). Influence of BCG vaccine strain on the immune response and protection against tuberculosis. FEMS Microbiol Rev 32: 821–841. [DOI] [PubMed] [Google Scholar]
- Oettinger, T, Jorgensen, M, Ladefoged, A, Haslov, K and Andersen, P (1999). Development of the Mycobacterium bovis BCG vaccine: review of the historical and biochemical evidence for a genealogical tree. Tuber Lung Dis 79: 243–250. [DOI] [PubMed] [Google Scholar]
- Zhang, L, Ru, H-w, Chen, F-z, Jin, C-y, Sun, R-f, Fan, X-y et al. (2016). Variable virulence and efficacy of BCG vaccine strains in mice and correlation with genome polymorphisms. Mol Ther 24: 398–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whole Mycobacteria Cell Vaccines for Tuberculosis Summary Group (2015). Developing whole mycobacteria cell vaccines for tuberculosis: workshop proceedings, Max Planck Institute for Infection Biology, Berlin, Germany, 9 July 2014. Vaccine 33: 3047–3055. [DOI] [PubMed] [Google Scholar]
- Grode, L, Ganoza, CA, Brohm, C, Weiner, J 3rd, Eisele, B and Kaufmann, SH (2013). Safety and immunogenicity of the recombinant BCG vaccine VPM1002 in a phase 1 open-label randomized clinical trial. Vaccine 31: 1340–1348. [DOI] [PubMed] [Google Scholar]
- Spertini, F, Audran, R, Chakour, R, Karoui, O, Steiner-Monard, V, Thierry, ACet al. (2015). Safety of human immunisation with a live-attenuated Mycobacterium tuberculosis vaccine: a randomised, double-blind, controlled phase I trial. Lancet Respir Med 3: 953–962. [DOI] [PubMed] [Google Scholar]
- Behr, MA, Wilson, MA, Gill, WP, Salamon, H, Schoolnik, GK, Rane, S et al. (1999). Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284: 1520–1523. [DOI] [PubMed] [Google Scholar]
- Brosch, R, Gordon, SV, Garnier, T, Eiglmeier, K, Frigui, W, Valenti, Pet al. (2007). Genome plasticity of BCG and impact on vaccine efficacy. Proc Natl Acad Sci USA 104: 5596–5601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah, AM, Hill-Cawthorne, GA, Otto, TD, Coll, F, Guerra-Assunção, JA, Gao, Get al. (2015). Genomic expression catalogue of a global collection of BCG vaccine strains show evidence for highly diverged metabolic and cell-wall adaptations. Sci Rep 5: 15443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia Pelayo, MC, Uplekar S, Keniry, A, Mendoza Lopez, P, Garnier, T, Nunez Garcia, J et al. (2009). A comprehensive survey of single nucleotide polymorphisms (SNPs) across Mycobacterium bovis strains and M. bovis BCG vaccine strains refines the genealogy and defines a minimal set of SNPs that separate virulent M. bovis strains and M. bovis BCG strains. Infect Immun 77: 2230–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin, R, Coscolla, M, Efstathiadis, E, Gagneux, S and Ernst, JD (2014). Impact of in vitro evolution on antigenic diversity of Mycobacterium bovis bacillus Calmette-Guerin (BCG). Vaccine 32: 5998–6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagranderie, MR, Balazuc, AM, Deriaud, E, Leclerc, CD and Gheorghiu, M. (1996). Comparison of immune responses of mice immunized with five different Mycobacterium bovis BCG vaccine strains. Infect Immun 64: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubos, RJ and Pierce, CH (1956). Differential characteristics in vitro and in vivo of several substrains of BCG. Am Rev Tuberc 74: 655–717. [DOI] [PubMed] [Google Scholar]
- Mahairas, GG, Sabo, PJ, Hickey, MJ, Singh, DC and Stover, CK (1996). Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol 178: 1274–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe, M, Oettinger, T, Wiker, HG, Rosenkrands, I and Andersen, P (1996). Evidence for occurrence of the ESAT-6 protein in Mycobacterium tuberculosis and virulent Mycobacterium bovis and for its absence in Mycobacterium bovis BCG. Infect Immun 64: 16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlessi, L, Prados-Rosales, R, Casadevall, A and Brosch, R (2015). Release of mycobacterial antigens. Immunol Rev 264: 25–45. [DOI] [PubMed] [Google Scholar]
- Pym, AS, Brodin, P, Majlessi, L, Brosch, R, Demangel, C, Williams, A et al. (2003). Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat Med 9: 533–539. [DOI] [PubMed] [Google Scholar]
- Bottai, D, Frigui, W, Clark, S, Rayner, E, Zelmer, A, Andreu, Net al. (2015). Increased protective efficacy of recombinant BCG strains expressing virulence-neutral proteins of the ESX-1 secretion system. Vaccine 33: 2710–2718. [DOI] [PubMed] [Google Scholar]
- Etna, MP, Giacomini, E, Pardini, M, Severa, M, Bottai, D, Cruciani, Met al. (2015). Impact of Mycobacterium tuberculosis RD1-locus on human primary dendritic cell immune functions. Sci Rep. 5: 17078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majlessi, L and Brosch, R. Mycobacterium tuberculosis meets the cytosol: the role of cGAS in anti-mycobacterial immunity. Cell Host Microbe 17: 733–735. [DOI] [PubMed] [Google Scholar]
- Simeone, R, Sayes, F, Song, O, Gröschel, MI, Brodin, P, Brosch, Ret al. (2015). Cytosolic access of Mycobacterium tuberculosis: critical impact of phagosomal acidification control and demonstration of occurrence in vivo. PLoS Pathog 11: e1004650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, JM, Islam, ST, Ren, H and Liu, J (2007). Differential productions of lipid virulence factors among BCG vaccine strains and implications on BCG safety. Vaccine 25: 8114–8122. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Asensio, J, Malaga, W, Pawlik, A, Astarie-Dequeker, C, Passemar, C, Moreau, Fet al. (2014). Evolutionary history of tuberculosis shaped by conserved mutations in the PhoPR virulence regulator. Proc Natl Acad Sci USA 111: 11491–11496. [DOI] [PMC free article] [PubMed] [Google Scholar]