Abstract
Objective
International Classification of Diseases (ICD-9-CM) diagnosis codes are increasingly used to identify healthcare-associated infections, often with insufficient evidence demonstrating validity of the codes used. Absent medical record verification, we sought to confirm a claims algorithm to identify surgical site infections (SSIs) by examining the presence of clinically expected SSI treatment.
Methods
We performed a retrospective cohort study using private insurer claims data from persons < 65 years with ICD-9-CM procedure or CPT-4 codes for anterior cruciate ligament (ACL) reconstruction from 1/2004–12/2010. SSIs occurring within 90 days after ACL reconstruction were identified by ICD-9-CM diagnosis codes. Antibiotic utilization, surgical treatment, and microbiology culture claims within 14 days of SSI codes were used as evidence to support the SSI diagnosis.
Results
Of 40,702 procedures, 401 (1.0%) were complicated by SSI, 172 (0.4%) of which were specifically identified as septic arthritis. Most SSIs were associated with an inpatient admission (n=232, 58%), and/or surgical procedure(s) for treatment (n=250, 62%). Temporally-associated antibiotics, surgical treatment procedures, and cultures were present for 84% (338/401), 61% (246/401), and 59% (238/401) respectively. Only 5.7% (23/401) of procedures coded for SSI post-procedure had no antibiotics, surgical treatments, or cultures within 14 days of the SSI claims.
Conclusions
Over 94% percent of patients identified by our claims algorithm as having an SSI received clinically expected treatment for infection including antibiotics, surgical treatment, and culture, suggesting this algorithm has very good positive predictive value. This method may facilitate retrospective SSI surveillance and comparison of SSI rates across facilities and providers.
INTRODUCTION
Increasingly, billing or claims data are being used to identify healthcare-associated infections, including surgical site infections (SSIs). The accuracy of International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes to identify infections has been reported in a number of studies, with varying results depending on the surgical procedures studied and the diagnosis codes used to indicate infection.1-10 Anterior cruciate ligament (ACL) reconstruction is a surgical procedure for which there has been no validation of the ICD-9-CM diagnosis codes to identify subsequent SSI.
Infection following ACL reconstruction is rare, and most reports in the literature are single-center studies with a small number of infections. Among studies with five or more cases of septic arthritis or SSI, reported infection rates range from 0.14–1.96%;11-25 twelve of the 15 studies reported rates of less than 1.0%. Infection after ACL reconstruction is uncommon, but it can lead to poor outcomes including articular cartilage destruction, arthrofibrosis, loss of range of motion, and reduced activity level.11-15;17;23;26 While there are no defined treatment guidelines, typical treatment of ACL reconstruction-related infection includes antibiotics and arthroscopic or open drainage of the knee.27;28
We sought to determine whether additional information could be obtained from administrative claims data to support the diagnosis of SSI following ACL reconstruction. In the absence of a gold standard such as medical chart review, we used clinically expected treatments available in the administrative claims– specifically antibiotic utilization, use of microbiology cultures, and surgical treatment for infection– to support the coding of SSI.
METHODS
Data source
We conducted a retrospective cohort study using the HealthCore Integrated Research Database (HIRDSM). Individuals represented in the HIRDSM include lives from 14 WellPoint-affiliated plans. WellPoint is an independent licensee of the Blue Cross and Blue Shield Association and serves its members as the Blue Cross licensee for California; the Blue Cross and Blue Shield (BCBS) licensee for Colorado, Connecticut, Georgia, Indiana, Kentucky, Maine, Missouri (excluding 30 counties in the Kansas City area), Nevada, New Hampshire, New York (as the Blue Cross Blue Shield licensee in ten New York City metropolitan and surrounding counties and as the Blue Cross or Blue Cross Blue Shield licensee in selected upstate counties only), Ohio, Virginia (excluding the Northern Virginia suburbs of Washington, DC), and Wisconsin. Thirteen plans were used for this research. Data in the HIRDSM include all fully adjudicated claims submitted for reimbursement from providers, facilities, and outpatient pharmacies and are linked to health plan enrollment information.
Fully insured members 6 months to 64 years of age who were enrolled in a health plan that included medical coverage of hospital and physician services were eligible for selection into the study cohort. Prescription drug coverage was also required in order to assess antibiotic utilization. Exclusions included members with an ICD-9-CM diagnosis code or prescription claim indicating HIV-positive status at any time (for patient privacy), and members likely to have incomplete data (e.g., enrolled in a capitated plan or enrolled in multiple plans at the time of surgery). We also excluded members enrolled in a plan with hospital coverage only, since up to 60% of SSIs are identified and managed in the ambulatory setting.29 Medical and pharmacy claims were restricted to paid claims.
The claims data available for this study contained up to five ICD-9-CM diagnosis codes per claim. Facility (hospital or ambulatory) claims included Uniform Billing (UB-92/UB-04) revenue and Healthcare Common Procedure Coding System (HCPCS) codes. Hospitals included up to five ICD-9-CM procedure codes per claim, while ambulatory facilities reported CPT-4 procedure codes. Provider claims included both CPT-4 and HCPCS codes.
We utilized the American Hospital Association Annual Survey of Hospitals (Health Forum, LLC, Chicago, IL) and the Outpatient Surgery Center Profiling Solution data (IMS Health, Plymouth Meeting, PA) in order to determine whether the ACL reconstruction was performed at a hospital or freestanding ambulatory surgery center. The facility information from these two data sources was matched to the operative facility using National Provider Identifier (NPI) codes, where available, otherwise matching was performed using facility name and address fields.
ACL reconstruction patient population
We identified ACL reconstruction procedures performed on an inpatient or outpatient basis at a hospital or freestanding ambulatory surgery center using ICD-9-CM and CPT-4 procedure codes from all facility (other than home health agencies) and provider claims among members eligible for cohort entry aged 6 months to 64 years between January 1, 2004 and December 31, 2010 (Table 1). The ACL reconstruction patient population was refined by excluding procedures likely to have erroneous claims for ACL reconstruction, procedures in members whose enrollment ended on the day of the surgical procedure, complicated procedures and procedures in patients considered medically complicated, and procedures in which the surgery date could not be determined from the available information in the claims (see below for description).
Table 1.
Codes Used to Identify Anterior Cruciate Ligament (ACL) Reconstruction, Procedure Exclusions, and Evidence for Surgery
| CPT-4 codes | ICD-9-CM procedure codes | UB-04 revenue codes | ICD-9-CM diagnosis codes | |
|---|---|---|---|---|
| Codes used to identify ACL reconstruction procedure | ||||
| ACL reconstruction | 27407, 27409, 27427–27429, 29888 | 81.43, 81.45 | ||
| Codes used for ACL reconstruction exclusion | ||||
| End-stage renal disease | 585.6, V45.1, V45.11, V45.12, V56.0, V56.1, V56.2, V56.8 | |||
| Septicemia | 038.0–038.9, 790.7 | |||
| Partial ostectomy, limb lengthening procedure, internal fixation of bone of leg, open reduction of fracture of leg, patellectomy | 27228, 27236, 27244, 27245, 27248, 27254, 27269, 27350, 27506, 27507, 27511, 27513, 27514, 27535, 27536, 27540, 27758, 27759, 27766, 27769, 27784, 27792, 27814, 27822, 27823, 27826-27828 | 77.85, 77.86, 77.87, 77.89, 78.35, 78.37, 78.39, 78.55, 78.56, 78.57, 78.59, 79.25, 79.26, 79.35, 79.36, 79.55, 79.56 | ||
| Codes used as additional evidence for ACL reconstruction surgery | ||||
| Anesthesia | 01320, 01380, 01400 | |||
| Tendon graft | 20924, 20926 | |||
| Surgery-related revenue codes | 0201, 0360, 0361, 0369, 0370, 0379, 0490, 0499, 0963, 0964, 0975 | |||
NOTE. CPT-4, Current Procedural Terminology, 4th edition; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; UB, Uniform Billing.
Identification and exclusion of erroneous claims for ACL reconstruction
We created an algorithm to identify problematic claims, which we defined as facility claims that contained apparent CPT-4, HCPCS, or UB-04 revenue codes truncated to four digits and populated in the fields reserved for ICD-9-CM procedure codes. This error appeared to occur during processing of certain types of non-inpatient facility claims (AEW, MAO, unpublished data). Claims in which an ACL procedure code was the only procedure code present, with no other claims submitted for the same date, were also classified as problematic claims and excluded.
Exclusion of complicated patients and procedures
The overall aim of this research study was to estimate the risk of SSI after ACL procedures by surgical facility type. For this reason, we excluded ACL reconstruction procedures performed in medically complicated patients who would be very unlikely to undergo surgery in an ambulatory setting and would have a very different risk profile from most ACL patients. We defined medically complicated patients as persons with end-stage renal disease or septicemia between 7 days before to 1 day after the ACL procedure date (Table 1).
We also excluded ACL reconstruction procedures performed at the time of or after another surgical procedure during the same admission since these procedures would be complex and attribution of an SSI to a particular procedure would not be possible. These additional surgical procedures were identified using CPT-4 and ICD-9-CM procedure codes from the National 7 Healthcare Safety Network (NHSN) list of procedures for SSI surveillance.30 We also excluded ACL reconstruction procedures in which any of the following procedures were coded within 7 days of ACL surgery, since these represent more complex ACL reconstructions: partial ostectomy, limb lengthening procedure, internal fixation of bone of leg, open reduction of fracture of leg, or patellectomy (Table 1).
Lastly, we excluded ACL reconstructions performed on or after calendar day 3 (where day 1 was the day of admission) of an inpatient admission.. The rationale for choosing the day 3 cutoff is that scheduled, elective surgical procedures are typically performed either on the day of admission or the following day. A surgical procedure performed on hospital day 3 or later would be unlikely to be the primary reason for admission. Therefore, these patients would not have had the opportunity to have the surgery performed at a freestanding ambulatory surgery center.
Establishing the surgery date and use of supporting evidence for surgery
ACL reconstruction dates within 7 days were collapsed into a single surgery due to potential inaccuracy in dates, particularly on provider claims.31 In these instances, we compared facility and provider surgery dates and incorporated supplemental evidence (e.g., claims for anesthesia and tendon graft procedures) from unique providers to determine the most likely surgery date. We excluded ACL reconstruction procedures coded by either a provider- or facility-only, unless there was additional evidence that a surgical procedure took place, i.e., claims for anesthesia services, tendon graft procedure, or a surgery-related UB-04 revenue code (Table 1).
Identification of surgical site infection
Claims for SSIs first recorded from 2 to 90 days after eligible procedures were identified using ICD-9-CM diagnosis codes (Table 2). We excluded individual SSI claims with locations that were not consistent with a provider diagnosis (e.g., laboratory, patient’s home) and those with CPT-4 codes for pathology services (88104–88399). This was done to avoid capturing an SSI that may have been a rule-out or working diagnosis.
Table 2.
Codes Used to Identify Surgical Site Infection (SSI) Following Anterior Cruciate Ligament Reconstruction
| ICD-9-CM diagnosis codes | |
|---|---|
| Knee-specific infection codes to identify SSI | |
| Septic arthritis | 711.06, 711.96 |
| Other infection to lower leg or joint prosthesis | 711.66, 730.06, 730.16, 730.26, 730.96, 996.66, 996.67 |
| General infection codes to identify SSI | |
| Postoperative infection | 998.5–998.59 |
| Infective myositis | 728.0 |
NOTE. ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification.
Timing of surgical site infection
The date of SSI onset was defined according to the timing and location of diagnosis. For SSI coded by an inpatient facility during the original operative admission, we assigned the date of SSI to the discharge date if the difference between the discharge and admission date was greater than or equal to 2 days. For SSI diagnosed during a subsequent inpatient admission, the date of SSI onset was assumed to be the date of hospital admission. For SSI diagnosed by a provider or in an ambulatory setting, the onset date was defined as the first service date with an ICD-9-CM diagnosis code for SSI.
The observation period for development of SSI was through 90 days after surgery, with earlier censoring for the end of insurance enrollment, subsequent ACL reconstruction, knee replacement, or other knee or leg surgery (i.e., partial ostectomy, limb lengthening procedure, internal fixation of bone of leg, open reduction of fracture of leg, patellectomy). In patients with subsequent surgeries, we censored 1 day after the subsequent surgery. Non-knee-specific ICD-9-CM diagnosis codes for infection (e.g., 998.59) were not classified as an SSI if they were first coded after a subsequent non-knee NHSN surgery within 90 days.
An ICD-9-CM diagnosis code for an SSI from 30 days before to 1 day after surgery was considered pre-existing infection. These ACL procedures were excluded from the study, since our goal was to identify incident cases of SSI.
Evidence supporting the diagnosis of surgical site infection
Prescription and medical claims for antibiotics, ICD-9-CM and CPT-4 procedure codes for surgical treatment, and CPT-4 codes for microbiology cultures were used to support the occurrence of an SSI (Table 3). Among persons with an incident SSI ICD-9-CM diagnosis code attributable to the ACL procedure, we considered antibiotic, surgical treatment, and culture claims 1 to 90 days after ACL reconstruction that were within 14 days from a date of an SSI diagnosis code and before applicable censoring to be supporting evidence for the coded SSI.
Table 3.
Codes Used as Supplemental Evidence for Surgical Site Infection (SSI) Following Anterior Cruciate Ligament Reconstruction
| ICD-9-CM or CPT-4 procedure codes | Antibiotic | |
|---|---|---|
| Antibiotic | aminoglycosides, aztreonam, cephalosporins, cilastatin and imipenem, colistin, daptomycin, doripenem, ertapenem, erythromycin-sulfisoxazole, fluoroquinolones, imipenem-cilastatin, lincosamides, linezolid, loracarbef, meropenem, penicillins, quinupristin-dalfopristin, rifampin, sulfamethoxazole-trimethoprim, sulfonamides, tetracyclines, tigecycline, trimethoprim, and vancomycin | |
| Knee-specific surgical treatment for SSI | 27301, 27303, 27310, 27330, 27331, 27334, 27335, 27360, 29870, 29871, 29873, 29875, 29876, 29884, 80.06, 80.16, 80.26 | |
| General surgical treatment for SSI | 10060, 10061, 10180, 20000, 20005, 20680 | |
| Microbiology culture | 87040, 87070, 87071, 87073, 87075, 87076, 87077 |
NOTE. ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; CPT-4, Current Procedural Terminology, 4th edition.
Statistical analysis
All statistical analyses were performed using the chi-square test. All data management and analyses were performed using SAS v9.3 (SAS Institute Inc., Cary, NC). This study was approved by the Washington University Human Research Protection Office.
RESULTS
A total of 41,837 ACL reconstruction procedures met all eligibility criteria and were identified during the 7-year study period. The number of distinct procedures was reduced to 40,702 procedures among 38,883 patients after removing procedures with no supporting evidence for the procedure (n=686), complicated ACL procedures and procedures in medically complicated patients (n=393), and ACL reconstructions performed at the time of a pre-existing SSI (n=56).
More than one ACL reconstruction was performed during the study period in 4.4% of patients. The procedures were evenly distributed over the study years. Most procedures were performed as day surgery at a hospital or at a freestanding ambulatory surgery center. Most ACL reconstruction procedures involved males, and the median age was 29 years (range 2–64 years) (Table 4).
Table 4.
Characteristics of Anterior Cruciate Ligament Reconstruction Procedures in 38,883 Patients
| Characteristic | n (%) |
|---|---|
| Total procedures | 40,702 |
| Age, median (range) | 29 (2–64) |
| Age < 18 years | 7,436 (18.3) |
| Male | 24,490 (60.2) |
| Location of procedurea | |
| Inpatient | 1,953 (4.8) |
| Day surgery at hospital | 15,769 (38.7) |
| Ambulatory surgery center | 12,526 (30.8) |
| Missing facility typeb | 10,454 (25.7) |
| Procedures per year | |
| 2004 | 5,664 (13.9) |
| 2005 | 5,874 (14.4) |
| 2006 | 6,041 (14.8) |
| 2007 | 5,989 (14.7) |
| 2008 | 6,134 (15.1) |
| 2009 | 5,751 (14.1) |
| 2010 | 5,249 (12.9) |
Inpatient and day surgery matched to a facility in the American Hospital Association (AHA) Annual Survey of Hospitals (Chicago, IL); inpatient was based on an inpatient designation in the HealthCore claims data. Ambulatory surgery center matched to a facility in the IMS Health Outpatient Surgery Center Profiling Solution data (Plymouth Meeting, PA).
Missing facility type due to no facility claim for procedure (n=4,065), no match to a facility in the AHA Annual Survey of Hospitals or the IMS Health Outpatient Surgery Center Profiling Solution data (n=6,366), or a match to multiple facilities (n=23).
SSIs were identified by ICD-9-CM diagnosis codes after 401 (1.0%, 95% confidence interval [CI] 0.9–1.1%) procedures. The median time to onset was 20 days (interquartile range 10–33 days), with 293 (73%) SSIs identified ≤30 days after the ACL reconstruction procedure. Fifty-four percent (n=218) had at least one knee-specific SSI code, as defined in Table 2. Fifty-eight percent (n=232) of patients had a hospital admission associated with their SSI. A total of 250 patients (62%) had one or more surgical procedures for treatment (median = 1, range 0–5). Among those with an SSI, 43% (n=172) had at least one code for septic arthritis for an overall incidence of septic arthritis of 0.4% (95% CI 0.4–0.5). Persons with septic arthritis were more likely to have had an inpatient admission at the time of infection and more likely to have had surgical treatment than persons coded for SSI but not septic arthritis (Table 5).
Table 5.
Characteristics of Surgical Site Infection (SSI) and Septic Arthritis Following 40,702 Anterior Cruciate Ligament Reconstruction Procedures
| Characteristic | Total SSI | Septic arthritis | SSI, no septic arthritis | Pa |
|---|---|---|---|---|
| Total | 401 | 172 | 229 | |
| Incidence per 100 procedures, % | 1.0 | 0.4 | 0.6 | |
| Infection coded during an inpatient admission, n (%) | 232 (57.9) | 137 (79.7) | 95 (41.5) | < 0.01 |
| Surgical treatment for SSIb in postoperative period, n (%) | 250 (62.3) | 149 (86.6) | 101 (44.1) | < 0.01 |
As determined by the chi-square test.
See methods for specific ICD-9-CM and CPT-4 procedure codes used for surgical treatment.
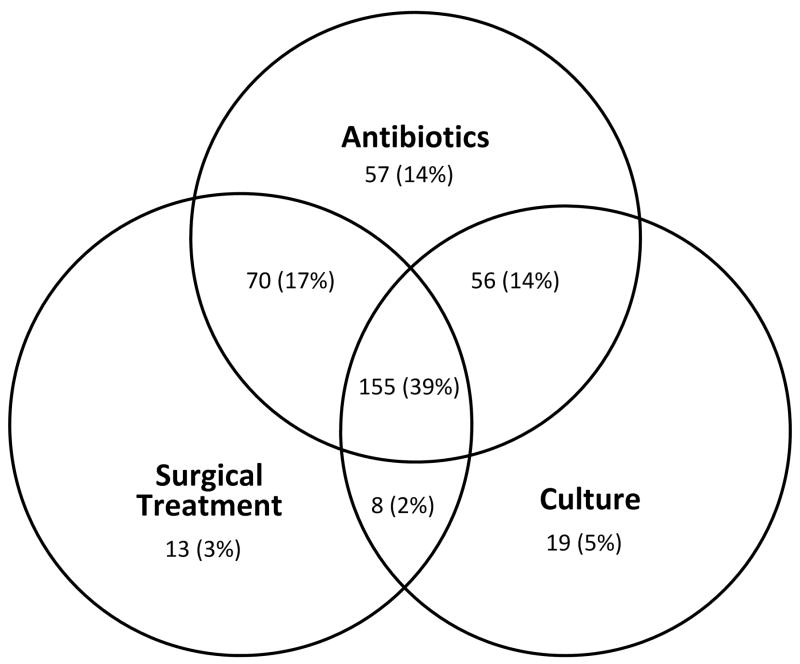
Overall, 84.3% (338/401) of SSIs had a temporally-associated antibiotic claim, 61.4% (246/401) had a temporally-associated claim coded for surgical treatment, and 59.4% had a temporally-associated claim for microbiology culture (238/401). In total, 155/401 (38.7%) SSIs had three types of supporting evidence (i.e., antibiotics, surgical treatment, and culture) as seen in the centermost overlapping circles in the Figure, 134 (33.4%) had two types of evidence (i.e., 70 SSIs had surgical treatment and antibiotics, 56 SSIs had antibiotics and culture, and 8 SSIs had surgical treatment and culture), 89 (22.2%) had one type of supporting evidence. Only 23 (5.7%) SSIs had no additional claim for an antibiotic, surgical treatment, or culture to support the diagnosis of SSI, for a positive predictive value (PPV) of 94.3% (378/401) (Figure). The PPV of our SSI algorithm was 89.5% (359/401) when only antibiotics and surgical treatment were considered as supporting evidence. The proportion of antibiotic, surgical treatment, and culture claims data associated with SSI did not vary significantly based on whether the onset of SSI was ≤30 days or 31–90 days following the ACL reconstruction procedure (Table 6).
Figure. Description of supporting evidence for 401 cases of surgical site infection (SSI) after anterior cruciate ligament reconstruction proceduresa.
a Note: 23 (5.7%) of 401 SSI identified by International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) diagnosis codes had no subsequent claims for antibiotics, surgical treatment for SSI, or microbiology cultures.
Table 6.
Surgical Treatment and Use of Antibiotics and Culture Within 14 days of Anterior Cruciate Ligament Reconstruction-Associated Surgical Site Infection (SSI) by SSI Onset
| Characteristic | Total SSI n (%) | SSI onset ≤30 days from procedure n (%) | SSI onset > 30 days from procedure n (%) | Pa |
|---|---|---|---|---|
| Total | 401 | 293 | 108 | |
| ≥1 claims for antibiotics | 338 (84.3) | 250 (85.3) | 88 (81.5) | 0.35 |
| ≥1 claims for surgical treatment | 246 (61.4) | 178 (60.8) | 68 (63.0) | 0.69 |
| ≥1 claims for culture | 238 (59.4) | 173 (59.0) | 65 (60.2) | 0.84 |
| ≥1 claims for antibiotics, surgical treatment, and/or culture | 378 (94.3) | 278 (94.9) | 100 (92.6) | 0.38 |
As determined by the chi-square test.
Among the 338 persons with an SSI and a temporally-associated antibiotic, the most common classes of antibiotics prescribed were cephalosporins (59%), vancomycin (28%), and fluoroquinolones (21%). Among the 246 persons with an SSI and an associated surgical procedure for treatment, 164 (67%) had an arthroscopy procedure, 101 (41%) had an arthrotomy procedure or removal of implant, and 92 (37%) had another incision and drainage procedure.
DISCUSSION
The use of administrative data to identify healthcare-associated infection identification is challenging, but these data can be an important resource for relatively rare events, such as SSIs. Some authors have concluded that billing and claims data cannot be reliably used for SSI surveillance.4;9;10 We found that 94.3% of patients identified as having an SSI by our rigorous claims algorithm also received clinically expected treatment for infection; a more conservative PPV estimate excluding culture was still very high at 89.5%. While we could not confirm the SSIs with medical chart review, our results suggest that the claims algorithm we used to identify SSIs has very good PPV.
We only used ICD-9-CM diagnosis codes that were specific to SSIs and/or were consistent with the NHSN clinical SSI definition. Studies that used specific SSI ICD-9-CM diagnosis codes (e.g., 998.5, 998.51, and 998.59)1-3 were more likely to report a higher PPV than studies using a larger range of diagnosis codes including ones less specific for SSI.4;9;10 We also used available information to distinguish pre-existing and incident infections, and censored at the time of any subsequent procedures. This censoring reduces the likelihood of attributing an SSI after a subsequent surgery to the index ACL reconstruction, which has been reported previously as a source of misclassification bias when using administrative data.3;10
In our large, geographically diverse study population, we found the incidence of SSI following ACL reconstruction to be 1.0%. This rate is higher than 12 of the 15 studies we identified in the published, English language literature.11;13-18;20;21;23-25 There are several potential reasons for our findings. We used claims data from across the spectrum of care, rather than from re-admission20 or single center medical record review to identify infections.11-19;22-25 The inclusion of outpatient claims has been shown by others to capture at least twice as many SSIs as inpatient surveillance alone.7;29 Only two studies that reported low SSI rates were from multiple institutions. Maletis et al. reported an overall SSI rate of 0.46% (0.3% deep SSI, 0.1% superficial SSI) using a Kaiser Permanente registry with all outcomes verified by chart review.21 Jameson et al. utilized data from the English National Health Service and reported a rate of 0.25% for deep infection within 30 days and 0.75% for wound complication (infection and hematoma), but identification of complications relied solely on hospital re-admissions.20 Another explanation for the lower SSI rates in the literature is that most studies only included more severe SSIs (e.g., septic arthritis).11-20;22;24;25 For example, in 12 published studies, all patients with reported SSIs received intravenous antibiotics;11-19;22;24;25 while in another 12 studies all cases with SSIs required surgical treatment.11-20;22;25 While the incidence of total SSIs in our current study is about twice that of reported rates, our reported incidence of septic arthritis (0.4%) and more severe infections requiring hospital admission (0.6%), and/or surgical treatment (0.6%) is consistent with the SSI rates reported in the literature.
Limitations of claims data for SSI surveillance includes issues common to secondary analysis of data collected for other purposes (i.e., billing and reimbursement). Therefore, some data elements that are important for SSI risk prediction surveillance, such as procedure dates, may be less accurate since they do not impact reimbursement. There is also likely undercoding of SSIs, particularly minor infections during the 90-day global surgical provider reimbursement period.32 Thus our calculation of SSI incidence after ACL reconstruction likely underestimates the true infection rate, since minor infections that occurred within the global reimbursement period may not be coded. Additionally, our findings may not be generalizable to all ACL reconstruction procedures since we limited our surgical population to less complex procedures. While medical chart review is considered the gold standard for validation, medical records were not available for private insurer claims data study. However, medical chart review is often limited to single-center studies, while our data represent hundreds of facilities and providers which increase the generalizability of our findings. Future studies could use medical chart review as gold standard to confirm our findings, but would require procedures from various practice settings (e.g., urban/rural, ambulatory/inpatient). Our use of temporally-associated clinical treatment for SSI to support coding of SSI is reproducible and allows patients to be tracked across the spectrum of care.
Over 94% percent of patients identified by our claims algorithm as having an SSI received clinically expected treatment for infection, suggesting the algorithm has very good positive predictive value. This method may facilitate retrospective surveillance and comparison of SSI rates across facilities and providers.
Acknowledgments
We thank Cherie Hill for database and computer management support.
Financial support
Funding for this project was provided by the Agency for Healthcare Research and Quality (AHRQ) 5R01HS019713 (MAO). The findings and conclusions in this document are those of the authors, who are responsible for its content, and do not necessarily represent the views of AHRQ. No statement in this report should be construed as an official position of AHRQ or of the U.S. Department of Health and Human Services.
MAO reports consultant work with Pfizer and Sanofi Pasteur and grant funding through Cubist Pharmaceuticals and Sanofi Pasteur for work outside the submitted manuscript.
Footnotes
Potential conflicts of interest
All other authors report no conflicts of interest relevant to this article.
References
- 1.Yokoe DS, Noskin GA, Cunningham SM, et al. Enhanced identification of postoperative infections among inpatients. Emerg Inf Dis. 2004;10:1924–1930. doi: 10.3201/eid1011.040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolon MK, Hooper D, Stevenson KB, et al. Improved surveillance for surgical site infections after orthopedic implantation procedures: extending applications for automated data. Clin Infect Dis. 2009;48:1223–1229. doi: 10.1086/597584. [DOI] [PubMed] [Google Scholar]
- 3.Olsen MA, Fraser VJ. Use of diagnosis codes and/or wound culture results for surveillance of surgical site infection after mastectomy and breast reconstruction. Infect Control Hosp Epidemiol. 2010;31:544–547. doi: 10.1086/652155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West J, Khan Y, Murray DM, Stevenson KB. Assessing specific secondary ICD-9-CM codes as potential predictors of surgical site infections. Am J Infect Control. 2010;38:701–705. doi: 10.1016/j.ajic.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Calderwood MS, Ma A, Khan YM, et al. Use of Medicare diagnosis and procedure codes to improve detection of surgical site infections following hip arthroplasty, knee arthroplasty, and vascular surgery. Infect Control Hosp Epidemiol. 2012;33:40–49. doi: 10.1086/663207. [DOI] [PubMed] [Google Scholar]
- 6.Hollenbeak CS, Boltz MM, Nikkel LE, Schaefer E, Ortenzi G, Dillon PW. Electronic measures of surgical site infection: implications for estimating risks and costs. Infect Control Hosp Epidemiol. 2011;32:784–790. doi: 10.1086/660870. [DOI] [PubMed] [Google Scholar]
- 7.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256:973–981. doi: 10.1097/SLA.0b013e31826b4c4f. [DOI] [PubMed] [Google Scholar]
- 8.Yokoe DS, Khan Y, Olsen MA, et al. Enhanced surgical site infection surveillance following hysterectomy, vascular, and colorectal surgery. Infect Control Hosp Epidemiol. 2012;33:768–773. doi: 10.1086/666626. [DOI] [PubMed] [Google Scholar]
- 9.Julian KG, Brumbach AM, Chicora MK, et al. First year of mandatory reporting of healthcare-associated infections, Pennsylvania: an infection control-chart abstractor collaboration. Infect Control Hosp Epidemiol. 2006;27:926–930. doi: 10.1086/507281. [DOI] [PubMed] [Google Scholar]
- 10.Stevenson KB, Khan Y, Dickman J, et al. Administrative coding data, compared with CDC/NHSN criteria, are poor indicators of health care-associated infections. Am J Infect Control. 2008;36:155–164. doi: 10.1016/j.ajic.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Van Tongel A, Stuyck J, Bellemans J, Vandenneucker H. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, management and outcome. Am J Sports Med. 2007;35:1059–1063. doi: 10.1177/0363546507299443. [DOI] [PubMed] [Google Scholar]
- 12.Schollin-Borg M, Michaelsson K, Rahme H. Presentation, outcome, and cause of septic arthritis after anterior cruciate ligament reconstruction: a case control study. Arthroscopy. 2003;19:941–947. doi: 10.1016/j.arthro.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Judd D, Bottoni C, Kim D, Burke M, Hooker S. Infections following arthroscopic anterior cruciate ligament reconstruction. Arthroscopy. 2006;22:375–384. doi: 10.1016/j.arthro.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Indelli PF, Dillingham M, Fanton G, Schurman DJ. Septic arthritis in postoperative anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 2002:182–188. doi: 10.1097/00003086-200205000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Abdel-Aziz A, Radwan YA, Rizk A. Multiple arthroscopic debridement and graft retention in septic knee arthritis after ACL reconstruction: a prospective case-control study. Int Orthop. 2013 doi: 10.1007/s00264-013-2123-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benner RW, Shelbourne KD, Freeman H. Infections and patellar tendon ruptures after anterior cruciate ligament reconstruction: a comparison of ipsilateral and contralateral patellar tendon autografts. Am J Sports Med. 2011;39:519–525. doi: 10.1177/0363546510388163. [DOI] [PubMed] [Google Scholar]
- 17.Binnet MS, Basarir K. Risk and outcome of infection after different arthroscopic anterior cruciate ligament reconstruction techniques. Arthroscopy. 2007;23:862–868. doi: 10.1016/j.arthro.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 18.Burks RT, Friederichs MG, Fink B, Luker MG, West HS, Greis PE. Treatment of postoperative anterior cruciate ligament infections with graft removal and early reimplantation. Am J Sports Med. 2003;31:414–418. doi: 10.1177/03635465030310031501. [DOI] [PubMed] [Google Scholar]
- 19.Fong SY, Tan JL. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Ann Acad Med Singapore. 2004;33:228–234. [PubMed] [Google Scholar]
- 20.Jameson SS, Dowen D, James P, Serrano-Pedraza I, Reed MR, Deehan D. Complications following anterior cruciate ligament reconstruction in the English NHS. Knee. 2012;19:14–19. doi: 10.1016/j.knee.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Maletis GB, Inacio MC, Funahashi TT. Analysis of 16,192 anterior cruciate ligament reconstructions from a community-based registry. Am J Sports Med. 2013;41:2090–2098. doi: 10.1177/0363546513493589. [DOI] [PubMed] [Google Scholar]
- 22.Sechriest VF, Carney JR, Kuskowski MA, Haffner JL, Mullen MJ, Covey DC. Incidence of knee sepsis after ACL reconstruction at one institution: the impact of a clinical pathway. J Bone Joint Surg Am. 2013;95:843–846. doi: 10.2106/JBJS.L.00408. [DOI] [PubMed] [Google Scholar]
- 23.Viola R, Marzano N, Vianello R. An unusual epidemic of Staphylococcus-negative infections involving anterior cruciate ligament reconstruction with salvage of the graft and function. Arthroscopy. 2000;16:173–177. doi: 10.1016/s0749-8063(00)90032-x. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Ao Y, Wang J, Hu Y, Cui G, Yu J. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction: a retrospective analysis of incidence, presentation, treatment, and cause. Arthroscopy. 2009;25:243–249. doi: 10.1016/j.arthro.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Williams RJ, III, Laurencin CT, Warren RF, Speciale AC, Brause BD, O’Brien S. Septic arthritis after arthroscopic anterior cruciate ligament reconstruction. Diagnosis and management. Am J Sports Med. 1997;25:261–267. doi: 10.1177/036354659702500222. [DOI] [PubMed] [Google Scholar]
- 26.Schub DL, Schmitz LM, Sakamoto FA, Winalski CS, Parker RD. Long-term outcomes of postoperative septic arthritis after anterior cruciate ligament reconstruction. Am J Sports Med. 2012;40:2764–2770. doi: 10.1177/0363546512461903. [DOI] [PubMed] [Google Scholar]
- 27.Mouzopoulos G, Fotopoulos VC, Tzurbakis M. Septic knee arthritis following ACL reconstruction: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2009;17:1033–1042. doi: 10.1007/s00167-009-0793-1. [DOI] [PubMed] [Google Scholar]
- 28.Scully WF, Fisher SG, Parada SA, Arrington EA. Septic arthritis following anterior cruciate ligament reconstruction: a comprehensive review of the literature. J Surg Orthop Adv. 2013;22:127–133. doi: 10.3113/jsoa.2013.0127. [DOI] [PubMed] [Google Scholar]
- 29.Sands K, Vineyard G, Platt R. Surgical site infections occurring after hospital discharge. Clin Infect Dis. 1996;173:963–970. doi: 10.1093/infdis/173.4.963. [DOI] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. National Healthcare Safety Network (NHSN) Procedure-Associated (PA) Module: Surgical Site Infection (SSI) event. Centers for Disease Control and Prevention; 2013. [November 14, 2013]. http://www.cdc.gov/nhsn/PDFs/pscManual/9pscSSIcurrent.pdf. [Google Scholar]
- 31.Li X, King C, deGara C, White J, Winget M. Validation of colorectal cancer surgery data from administrative data sources. BMC Med Res Methodol. 2012;12:97. doi: 10.1186/1471-2288-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Centers for Medicare & Medicaid Services. Global Surgery Fact Sheet. Centers for Medicare & Medicaid Services; 2013. [November 14, 2013]. http://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNProducts/downloads/GloballSurgery-ICN907166.pdf. [Google Scholar]