Abstract
Dysregulation of Trp-Kyn pathway is the most recent hypothesis of mechanisms of schizophrenia. In particular, over-production of kynurenic acid (KYNA), one of the three immediate downstream metabolites of kynurenine (Kyn) along tryptophan (Trp): Kyn pathway, has been considered as a new target for therapeutic intervention in schizophrenia. Up-regulation of KYNA formation was suggested to occur at the expense of down-regulated production of 3-hydroxyKyn (3-HK), the second immediate downstream metabolite of Kyn. We were interested to assess the third immediate downstream Kyn metabolite, anthranilic acid (AA). Serum AA concentrations were evaluated in schizophrenia patients and control subjects by HPLC-mass spectrometry method. We found 2-fold increase of AA and 3-fold decrease of 3-HK concentrations in serum of schizophrenia patients. Up regulated formation of AA might contribute to mechanisms of schizophrenia considering experimental evidences of AA augmentation of autoimmune processes in rat and mice; clinical findings of AA elevation in rheumatoid arthritis and type 1 diabetes, autoimmune diseases diametrical to schizophrenia; and involvement of autoimmunity in development of schizophrenia. Present data warrant further studies of AA as biological marker in, at least, a subgroup (associated with autoimmune mechanisms) of schizophrenia patients and as a new target for therapeutic intervention.
Keywords: Anthranilic acid, Schizophrenia, Kynurenines, Rheumatoid arthritis, Type 1 diabetes, Microbiome
INTRODUCTION
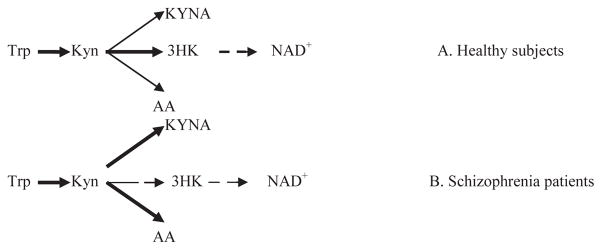
Schizophrenia is a severe and debilitating psychiatric disorder with 1% lifetime prevalence relatively independent of geographic, cultural, and socioeconomic variables. A etiopathogenetic mechanisms of schizophrenia are not completely defined and require further investigation. The original dopaminergic hyperactivity hypothesis [1] was recently supplemented by glutamatergic hypoactivity hypothesis of aetiopathogenetic mechanisms of schizophrenia [2]. Experimental and clinical studies revealed psychotomimetic effects of glutamate receptor antagonists [2]. One of them, kynurenic acid (KYNA), is one of the three immediate downstream metabolites of kynurenine (Kyn) along tryptophan (Trp) : Kyn pathway, a major source of NAD+ biosynthesis in humans [3] (Figure 1). Trp conversion into Kyn (via N-formyl-Kyn) is catalyzed by indoleamine (or Trp) 2,3-dioxygenases (IDO and TDO, respectively). KYN conversion into 3-hydroxykynurenine (3-HK), catalyzed by riboflavindependent kynurenine 3-monoxygenase (KMO), is key step of formation of NAD+ [4]. Inhibition of KMO activity increases utilization of Kyn as a substrate for formation of KYNA, catalyzed by kynurenine aminotransferase II (KAT), and, thus, contributes to elevation of KYNA content in brains and cerebrospinal fluid of schizophrenia patients [2,5]. Besides 3-HK and KYNA, KYN is a substrate for formation of anthranilic acid (AA), catalyzed by kynureninase (KYNU) (Figure 1A). AA was detected in concentrations ranged from 0.5 to 1.8 pmol/mg protein in rat central (frontal cortex, hypothalamus, hippocampus, cerebellum, medulla, thalamus, striatum, olfactory bulb, spinal cord and retina) and peripheral organs (kidney, liver, lung, heart, pancreas, spleen, testis and adrenal gland), and serum (around 130 nM) and urine (10 nmol/mg creatinine) [6]. The same study revealed AA localization in glial cells based on a significant increase of AA content in brain tissue lesioned by intrastriatally injected quinolinate. However, we could not find in available literature reports of AA evaluation in schizophrenia patients.
Figure 1.
Proposed changes of Kyn down-stream metabolism in schizophrenia
Abbreviations: Trp: Tryptophan; Kyn : Kynurenine; KYNA : Kynurenic acid; AA : Anthranilic Acid; 3-HK : 3-HydroxyKynurenine; NAD+ : Nicotinamide Adenine Dinucleotide
Here we present preliminary results of our study of serum concentrations of downstream Kyn metabolites in schizophrenia patients
MATERIALS AND METHODS
Patients
Overnight fasting blood samples were collected from patients (three men and three women, age range from 38 to 56 years) with schizophrenia, diagnosed according to DSM-V criteria [7]. All patients were taking anti-psychotic medication: Abilify (three patients), Haloperidol (one patient), Haloperidol decanoate injections (one patient) and Saphris (one patient).
Healthy Subjects (Controls)
There were 12 subjects (6 females and 6 males, age range from 32 to 64 years). Study was approved by Tufts Medical Center IRB.
Assessment of kynurenine metabolites
Serum samples were stored at −50°C until analysis. AA, Trp, Kyn, KYNA and 3-HK concentrations were analyzed by HPLC:mass spectrometry method [8].
Statistical Analysis
Results are presented as mean ± standard error (Trp and Kyn in μM and AA, KYNA and 3-HK in nM). Statistical significance was assessed by Mann-Whitney test, two-tailed.
RESULTS AND DISCUSSION
Serum concentrations of Kyn and its metabolites
Assessment of peripheral AA is an appropriate approach in clinical studies considering that AA, in difference with KYNA, easily penetrates blood-brain barrier (via passive diffusion), and, therefore, peripherally produced AA might contribute significantly to it brain pools and act on targets in brain [9].
AA concentrations were increased (approximately two-fold) in schizophrenia patients in comparison with control subjects. AA increase was robust: all controls had AA serum concentrations lower than schizophrenia patients] (Table 1).
Table 1.
Down-stream kynurenine metabolites in serum of schizophrenia patients.
| Schizophrenia* (n=6) | Control* (n=12) | P (Mann-Whitney test, two-tailed) | |
|---|---|---|---|
| Tryptophan (μM) | 77.88±7.53 | 68.90± 2.49 | ns |
| Kynurenine (μM) | 2.06±0.27 | 1.76± 0.09 | ns |
| Kyn × 100 : Trp | 2.64±0.21 | 2.56±0.35 | ns |
| Anthranilic acid (nM) | 111.43±7.56 | 21.65±5.99 | 0.0001 |
| AA : Kyn | 60.89±11.98 | 12.22±3.22 | 0.0001 |
| 3-HK (nM) | 6.25±0.05 | 19.55± 3.14 | 0.03 |
| 3-HK : Kyn | 3.14±1.34 | 11.58±1.3 | 0.0003 |
| KYNA (nM) | 36.43±3.99 | 35.78± 3.59 | ns |
| KYNA : Kyn | 18.33±1.47 | 19.76±1.69 | Ns |
mean±standard error
Abbreviations: Kyn : Kynurenine; Trp : Tryptophan; AA : Anthranilic Acid; 3-HK : 3-HydroxyKynurenine; KYNA : Kynurenic acid.
3-HK concentrations were approximately three-fold lower in schizophrenia patients than in controls.
There was no statistically significant difference between Trp, Kyn and KYNA concentrations in serum of schizophrenia patients and controls.
End product: substrate ratios of Trp: Kyn metabolic pathway
In clinical studies, evaluation of end product: substrate ratios are used for assessment of activities of enzymes, catalyzing corresponding metabolic reactions [10].
AA: Kyn ratio was higher (1.6 fold) while 3-HK: Kyn ratio was lower (3.7 fold) in patients than in controls.
KYNA: KYN ratio did not differ between patients and controls (Table 1).
Kyn: Trp ratio, a clinical index of IDO and TDO activity, did not differ between patients and controls. It is noteworthy that elevated serum AA concentrations might restrict utilization of Kyn: Trp ratio for assessment of IDO/TDO activity considering that AA is not only metabolite of Trp but a substrate of Trp formation by bacteria, e.g., by intestinal microbiome [11].
The main finding of the present communication is a robust and dramatic increase of AA concentrations in serum of schizophrenia patients. To the best of our knowledge, this is the first observation of increased AA concentrations in schizophrenia patients. Our results are in agreement with previously reported AA elevation in rat plasma in social isolation rearing model of schizophrenia [12]. It is unlikely that AA elevation depends on the use of anti-psychotic medication, considering that chronic administration of anti-psychotic drug risperidone did not affect Trp: Kyn metabolism in rat brain [5]. Elevation of AA concentrations could be caused by up-regulation of enzyme (KYNU), catalyzing conversion of Kyn into AA. Our finding of increased AA: Kyn ratio suggests up-regulation of KYNU activity. However, direct assessment of brain KYNU activity did not reveal its up-regulation in schizophrenia patients [5]. In the same vein, diet deficient of vitamin B6, a cofactor of KYNU, did not decrease urine AA excretion in baboons [13]. The other cause of AA elevation might be an increased formation of Kyn, a substrate for AA synthesis, from Trp, catalyzed by IDO/TDO, and/or on decreased conversion of Kyn into KYNA or 3-HK. We did not find increased Kyn: Trp (index of IDO/TDO activity) or decreased KYNA : Kyn ratio (index of KAT activity) in schizophrenia patients in agreement with observation of no changes of KAT activity in Broadmann areas of schizophrenia patients [5]. We did find decreased 3-HK : Kyn ratio (index of KMO activity) and drastic decrease of 3-HK serum concentrations in schizophrenia patients in agreement with previously reported reduction in KMO gene expression and KMO enzyme activity and 3-HK decrease in Broadmann areas of schizophrenia patients [5,14]. Administration of diet deficient of riboflavin, a KMO cofactor, increased AA (12-fold) and decreased 3-HK (10-fold) urine excretion in baboons suggesting that increased availability of substrate, Kyn, due to inhibition of KMO, up-regulates AA formation [13]. Effect of KMO inhibition on AA formation might be further supported by observation of increased AA excretion in riboflavin deficient rats [15] and AA elevation in brain, liver and plasma KMO(−/−) mice [16].
Therefore, the most likely cause of AA increase in schizophrenia is a shift of downstream Kyn metabolism from formation of 3-HK towards production of AA due to inhibition of KMO, an enzyme catalyzing Kyn conversion into 3-HK (Figure 1B).
Common features of schizophrenia with autoimmune diseases suggest that, at least, some group of schizophrenia could be an autoimmune disease itself [17]. Experimental study revealed that AA significantly enhanced the development of adjuvant-induced model of rheumatoid arthritis in rats and increased antibody formation to sheep erythrocytes in mice [18]. Protection against autoimmune-induced apoptosis is mediated by Trp degradation mediated by up-regulation of IDO activity [19]. AA might prevent Trp depletion by serving as a substrate for Trp formation by bacteria, e.g., intestinal microbiome, [11], and by inhibition of sodium-coupled transporter of uncharged solutes (e.g., Trp) across mammalian cell membranes [20].
Clinical studies reported drastic elevation of AA in organ-specific autoimmune disorders, rheumatoid arthritis [21] and Type 1 diabetes (T1D) [22]. AA elevation in schizophrenia, rheumatoid arthritis, and T1D suggests that AA contributes to common autoimmune etiology of these disorders. Inverse correlations between schizophrenia with rheumatoid arthritis [23] and T1D [24] suggests that these conditions are diametrically related, i.e., increased risk for one set of diseases commonly engenders decreased risk for another [25]. Diametrical diseases reflect evolutionary-genetic tradeoffs centrally mediating the expression of human adaptations and influencing the prevalence and forms of human maladaptation manifest in disease [25]. Considering that Trp: Kyn pathway is evolutionary conserved (and was described in flies, worms, bacteria, mammals and humans), it might be interesting to explore possible role of AA (and other Kyn metabolites), in mechanisms of diametrical disorders.
CONCLUSION
Preliminary report from our studies reveals a drastic and robust elevation of AA serum concentrations in schizophrenia patients. Dysregulation of Trp - Kyn pathway is the most recent hypothesis of mechanisms of schizophrenia. However, possible involvement of AA in mechanisms of schizophrenia has not been considered so far. Present data warrant further studies of AA as biological marker of, at least, a subgroup of schizophrenia patients (e.g., associated with autoimmune mechanisms) and as a new target for therapeutic intervention in schizophrenia.
Acknowledgments
GF Oxenkrug is a recipient of NIMH104810 grant.
ABBREVIATIONS
- Trp
Tryptophan
- Kyn
Kynurenine
- KYNA
Kynurenic acid
- AA
Anthranilic Acid
- 3-HK
3-HydroxyKynurenine
- IDO
Indoleamine 2,3-dioxygenase
- TDO
Tryptophan 2,3-dioxygenase
- KMO
Kynurenine 3-Monoxygenase
- KAT
Kynurenine Amino Transferase II
- KYNU
Kynureninase
- NAD+
Nicotinamide Adenine Dinucleotide
Footnotes
Conflict of Interest
P. Summergrad is a non-promotional speaker for CME outfitters, Inc., and consultant and non-promotional speaker for Pri-med, Inc. Other authors have nothing to declare.
References
- 1.CARLSSON A, LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 2.Erhardt S, Schwieler L, Nilsson L, Linderholm K, Engberg G. The kynurenic acid hypothesis of schizophrenia. Physiol Behav. 2007;92:203–209. doi: 10.1016/j.physbeh.2007.05.025. [DOI] [PubMed] [Google Scholar]
- 3.Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ. Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci. 2012;13:465–477. doi: 10.1038/nrn3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okamoto H, Hayaishi O. Flavin adenine dinucleotide requirement for kynurenine hydroxylase of rat liver mitochondria. Biochem Biophys Res Commun. 1967;29:394–399. doi: 10.1016/0006-291x(67)90469-x. [DOI] [PubMed] [Google Scholar]
- 5.Sathyasaikumar KV, Stachowski EK, Wonodi I, Roberts RC, Rassoulpour A, McMahon RP, et al. Impaired kynurenine pathway metabolism in the prefrontal cortex of individuals with schizophrenia. Schizophr Bull. 2011;37:1147–1156. doi: 10.1093/schbul/sbq112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guidetti P, Walsh JL, Schwarcz R. A fluorimetric assay for the determination of anthranilic acid in biological materials. Anal Biochem. 1994;220:181–184. doi: 10.1006/abio.1994.1316. [DOI] [PubMed] [Google Scholar]
- 7.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5. Arlington, Va: American Psychiatric Publishing; 2013. [Google Scholar]
- 8.Toledo-Sherman LM, Prime ME, Mrzljak L, Beconi MG, Beresford A, Brookfield FA, et al. Development of a series of aryl pyrimidine kynurenine monooxygenase inhibitors as potential therapeutic agents for the treatment of Huntington’s disease. J Med Chem. 2015;58:1159–83. doi: 10.1021/jm501350y. [DOI] [PubMed] [Google Scholar]
- 9.Fukui S, Schwarcz R, Rapoport SI, Takada Y, Smith QR. Blood-brain barrier transport of kynurenines: implications for brain synthesis and metabolism. J Neurochem. 1991;56:2007–2017. doi: 10.1111/j.1471-4159.1991.tb03460.x. [DOI] [PubMed] [Google Scholar]
- 10.Ulvik A, Theofylaktopoulou D, Midttun Ø, Nygård O, Eussen SJ, Ueland PM. Substrate product ratios of enzymes in the kynurenine pathway measured in plasma as indicators of functional vitamin B-6 status. Am J Clin Nutr. 2013;98:934–940. doi: 10.3945/ajcn.113.064998. [DOI] [PubMed] [Google Scholar]
- 11.RYDON HN. Anthranilic acid as an intermediate in the biosynthesis of tryptophan by Bact. typhosum. Br J Exp Pathol. 1948;29:48–57. [PMC free article] [PubMed] [Google Scholar]
- 12.Möller M, Jan L, Preez Du, Emsley Robin, Harvey Brian H. Social isolation rearing in rats alters plasma tryptophan metabolism and is reversed by sub-chronic clozapine treatment. Neuropharmacology. 2012;62:2499–2506. doi: 10.1016/j.neuropharm.2012.02.021. [DOI] [PubMed] [Google Scholar]
- 13.Verjee ZH. Tryptophan metabolism in baboons: effect of riboflavin and pyridoxine deficiency. Acta Vitaminol Enzymol. 1975;29:198–201. [PubMed] [Google Scholar]
- 14.Wonodi I, Stine CO, Sathyasaikumar KV, Robert RC, Mitchell BDL, Elliot Hong L, et al. Downregulated Kynurenine 3-Monooxygenase Gene Expression and Enzyme Activity in Schizophrenia and Genetic Association With Schizophrenia Endophenotypes. Arch Gen Psychiatry. 2011;68:665–674. doi: 10.1001/archgenpsychiatry.2011.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.CHARCONNET-HARDING F, DALGLIESH CE, NEUBERGER A. The relation between riboflavin and tryptophan metabolism, studied in the rat. Biochem J. 1953;53:513–521. doi: 10.1042/bj0530513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giorgini F, Huang SY, Sathyasaikumar KV, Notarangelo FM, Thomas MA, Tararina M, et al. Targeted deletion of kynurenine 3-monooxygenase in mice: a new tool for studying kynurenine pathway metabolism in periphery and brain. J Biol Chem. 2013;288:36554–36566. doi: 10.1074/jbc.M113.503813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Knight JG. Is schizophrenia an autoimmune disease? A review. Methods Find Exp Clin Pharmacol. 1984;6:395–403. [PubMed] [Google Scholar]
- 18.Kunitomo M, Tanaka Y, Yamada K, Yamaguchi Y, Bandô Y. Enhancing activity of anthranilic acid on adjuvant arthritis in rats and antibody formation in mice. Jpn J Pharmacol. 1989;50:507–510. doi: 10.1254/jjp.50.507. [DOI] [PubMed] [Google Scholar]
- 19.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20:469–473. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 20.Pajor AM. Molecular properties of the SLC13 family of dicarboxylate and sulfate transporters. Pflugers Arch. 2006;451:597–605. doi: 10.1007/s00424-005-1487-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Igari T, Tsuchizawa M, Shimamura T. Alteration of tryptophan metabolism in the synovial fluid of patients with rheumatoid arthritis and osteoarthritis. Tohoku J Exp Med. 1987;153:79–86. doi: 10.1620/tjem.153.79. [DOI] [PubMed] [Google Scholar]
- 22.Oxenkrug G, Van der Hart M, Summergrad P. Elevated anthranilic acid plasma concentrations in type 1 but not type 2 diabetes mellitus. Integr Mol Med. 2015;2:365–368. doi: 10.15761/IMM.1000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee SH, Byrne EM, Hultman CM, Kähler A, Vinkhuyzen AA, Ripke S, et al. New data and an old puzzle: the negative association between schizophrenia and rheumatoid arthritis. Int J Epidemiol. 2015 doi: 10.1093/ije/dyv136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juvonen H, Reunanen A, Haukka J, Muhonen M, Suvisaari J, Arajärvi R, et al. Incidence of schizophrenia in a nationwide cohort of patients with type 1 diabetes mellitus. Arch Gen Psychiatry. 2007;64:894–899. doi: 10.1001/archpsyc.64.8.894. [DOI] [PubMed] [Google Scholar]
- 25.Crespi BJ, Go MC. Diametrical diseases reflect evolutionary-genetic tradeoffs: Evidence from psychiatry, neurology, rheumatology, oncology and immunology. Evol Med Public Health. 2015;2015:216–253. doi: 10.1093/emph/eov021. [DOI] [PMC free article] [PubMed] [Google Scholar]