Abstract
Wear particles induce periprosthetic inflammation and osteolysis through activation of nuclear factor kappa B (NF-κB), which up-regulates the downstream target gene expression for proinflammatory cytokines in macrophages. It was hypothesized that direct suppression of NF-κB activity in the early phases of this disorder could be a therapeutic strategy for preventing the inflammatory response to wear particles, potentially mitigating osteolysis. NF-κB activity can be suppressed via competitive binding with double stranded NF-κB decoy oligodeoxynucleotides (ODNs) that blocks this transcription factor from binding to the promoter regions of targeted genes. In this murine calvarial study, clinically relevant polyethylene particles (PEs) with/without ODN were subcutaneously injected over the calvarial bone. In the presence of PE particles, macrophages migrated to the inflammatory site and induced tumor necrosis factor alpha (TNF-α) and receptor activator of nuclear factor kappa B ligand (RANKL) expression, resulting in an increase in the number of osteoclasts. Local injections of ODN mitigated the expression of TNF-α, RANKL, and induced the expression of two anti-inflammatory, antiresorptive cytokines: interleukin-1 receptor antagonist and osteoprotegerin. Local intervention with NF-κB decoy ODN in early cases of particle-induced inflammation in which the prosthesis is still salvageable may potentially preserve periprosthetic bone stock.
Keywords: total joint replacement, wear particles, osteolysis, NF-κB, murine calvarial model
INTRODUCTION
Wear of the bearing surfaces of total joint replacements (TJRs) is expected with continued use. Despite the introduction of novel biomaterials for TJR, wear particle-induced inflammation and osteolysis are still unsolved problems.1 Nuclear factor kappa B (NF-κB) is the key transcription factor in particle-induced inflammation, and up-regulates downstream target gene expression for proinflammatory cytokines, chemokines and other proinflammatory molecules, including tumor necrosis factor α (TNF-α), interleukin-1 (IL-1) and monocyte chemotactic protein-1 (MCP1) and others.2,3 These proinflammatory cytokines further activate NF-κB signaling in a positive feedback loop. Osteoclastogenesis is also enhanced by NF-κB; proinflammatory cytokines such as TNF-α and IL-1 also enhance receptor activator NF-κB ligand (RANKL) expression.4–6 Because of its central role in inflammation and its regulation of macrophage-osteoclast lineage cells, direct modulation of NF-κB is a logical therapeutic strategy to mitigate wear particle-induced inflammation and osteolysis via suppression of proinflammatory cytokines.7
Oligodeoxynucleotides (ODNs) are molecules that can be used as “decoy” cis-elements to block the binding of nuclear factors to the promoter regions of targeted genes, resulting in the inhibition of gene activation.8 Synthetic NFκB decoy ODN can suppress NFκB activity through competitive binding to NF-κB, resulting in the prevention of NF-κB interaction and activation of NF-κB-promoting target gene expression.9 These effects are very specific, with a low occurrence of adverse events, and have been applied to several in vivo or in vitro immune-mediated disease models.10,11
The purpose of this study was to examine the effects of NF-κB decoy ODN in the early phase of wear particle-induced inflammation using a modified murine calvarial model. Our novel modification of an existing mouse calvarial model simulates the early stage of wear particle-associated inflammation, rather than the end stage of particle disease, that is associated with more extensive osteolysis. Thus the model and NF-κB decoy ODN treatment provide a unique opportunity for clinical translation; this intervention could be instituted to treat an existing joint arthroplasty that is still functional but exhibiting early clinical signs associated with wear of the bearing surfaces.
MATERIAL AND METHODS
Animals and injections
A total of 45 C57BL/6 male mice aged 8 to 10 weeks (Jackson Laboratories, ME) were housed in our Animal Facility 48 h prior to usage to allow for acclimatization. The experimental design was approved by our Institutional Administration Panel for Laboratory Animal Care on May 22, 2014. NIH and our university's guidelines for the care and use of laboratory animals were observed.
Three animal groups had percutaneous injections into the subcutaneous bursa overlying the calvaria with 3 different treatments, with 15 animals randomly assigned to each group. Negative control animals had 100 μL phosphate-buffered saline (PBS) injected on day 1 (group 1, PBS). Group 2 (+PE) received 3 mg (approximately 4 × 108) ultrahigh-molecular-weight polyethylene (PE) particles in 100 μL PBS on day 1, with subsequent injections of 100 μL PBS alone every other day for 14 days. Group 3 (PE + ODN) animals received PE particles and 5 μM of NF-κB decoy ODN on the first day, and then 5 μM NFκ ODN injections in 100 κL PBS every other day for 14 days. The amount of PE particles injected was lower than previous studies in order to mimic the early stages of particle induced inflammation.12–14
The subcutaneous injections were performed using an established protocol.15 Briefly, animals were anaesthetized with 2–3% isoflurane in 100% oxygen at a flow rate of 1 L/min. The cranium was carefully shaved and sterilized with Betadine Scrub® (Purdue Product, CT). With the use of sterile technique, the treatments were injected percutaneously at the intersection of the median sagittal line and the coronal line 1 mm anterior to a line connecting the ears. A 28.5-gauge needle was inserted subcutaneously over the calvarial bone, at a 45° angle from an anterior approach, until contact with the bone was obtained and the needle slightly withdrawn. The solution was then slowly injected, resulting in a temporary subcutaneous “bubble” overlying the calvarium. In the same manner, PBS with/without NF-κB decoy ODN was injected in each group every other day for 14 days. The animals were then euthanized and their calvaria underwent microcomputed tomography (micro-CT) analysis. Calvaria from ten animals then underwent sectioning and histological staining, whereas the remaining calvaria were placed in organ culture for enzyme-linked immunosorbent assay (ELISA) of supernatants.
Decoy ODN
The NF-κB decoy ODN sequences were as follows:
5′-CCTTGAAGGGATTTCCCTCC-3′
3′-GGAACTTCCCTAAAGGGAGG-5′
The synthetic ODNs were washed with 70% ethanol, dried and dissolved in sterile Tris–EDTA (10 mM Tris, 1 mM EDTA), and the supernatant was purified over a NAP-10 column (GE Healthcare, Piscataway, NJ). NF-κB decoy ODN was prepared at a concentration of 5 μM in PBS, according to previous preliminary experiments.
Ultrahigh-molecular-weight polyethylene particles
Conventional ultrahigh-molecular-weight PE particles (a gift from Dr. Timothy Wright Hospital for Special Surgery, New York) were obtained from joint simulator tests and isolated according to an established density centrifugation protocol. Frozen aliquots of the serum containing particles were lyophilized for 4–7 days. The dried material was digested in 5 M sodium hydroxide at 60°C for 1 h, followed by ultrasonication for 10 min. The digested particle suspension was centrifuged through a 5% sucrose gradient at 40k rpm (285k ×g at rmax) at 10°C for 3 h. The collected particles at the surface of the sucrose solution were incubated at 80°C for 1 h and ultrasonicated, and centrifuged again through an isopropanol gradient (0.96 and 0.90 g/cm3) at 40k rpm at 10°C for 1 h. The purified particles at the interface between the two layers of isopropanol were harvested, and the isopropanol was evaporated from the particle mixture then lyophilized until dry. Particles were then re-suspended in 95% ethanol, which was evaporated completely. The particles tested negative for endotoxin by means of a Limulus Amebocyte Lysate (LAL) Kit (Lonza, Allendale, NJ). The mean diameter of the particles was 1.0 ± 0.1 μm (mean ± standard error, averaged from 125 scanned particles) measured by electron microscopy.
Micro-CT imaging
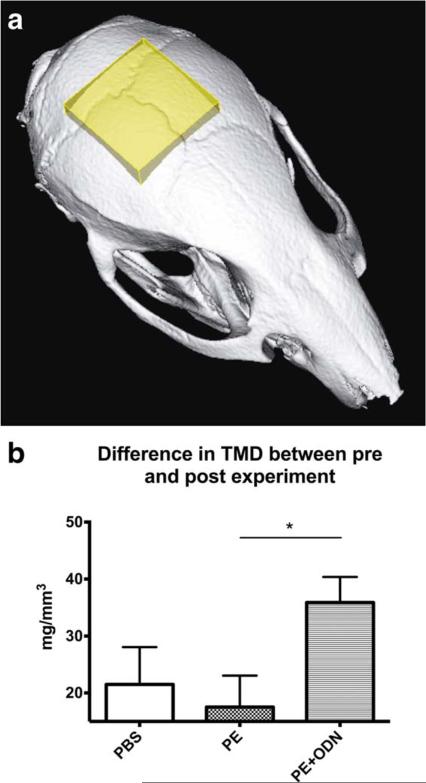
Micro-CT scans were performed in vivo with the eXplore Locus RS150 micro-CT scanner (GE Healthcare, Fairfield, CT) with the setting of 49 μm resolution during 8 min. A phantom which mimics hydroxyapatite and contains water and air inclusions was used for calibration. After scanning, a 3D region of interest was created at the level of the parietal bones in MicroView software (GE Medical Systems, Waukesha, WI) and a 5 × 5 × 2 mm cubic volume of interest was created after threshold normalization for all images [Fig. 1(a)]. The tissue mineral density (TMD) which was defined as the measurement restricted to within the volume of calcified bone tissue was detected for all animals and the time-dependent difference between pre and post experiment was evaluated in all groups.
FIGURE 1.
Micro-CT analysis. The tissue mineral density (TMD) was analyzed in the three groups; injection of PBS, PE particles or PE particles + NF-κB decoy ODN (PBS, PE, and PE + ODN groups) in order to evaluate the effect of NF-κB decoy ODN on PE particle induced inflammation and osteolysis. (a) The volume of interest was defined as a 5 × 5 × 2 mm cuboid which has an anterior limit located at the beginning of the sagittal suture. (b) Bar diagram showing the TMD in the three groups during the 14-day experiment. ** p < 0.01. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Histomorphology
Animals were euthanized on the 14 day by CO2 inhalation and cervical dislocation. The calvaria were removed with dermal tissue by dissecting bone free from the underlying brain tissue, thus extracting an elliptical plate of bone bound by the foramen magnum, auditory canals, and orbits. After fixation in 4% paraformaldehyde (PFA) for 3 days, the calvaria were decalcified in ethylenediamine-tetra-acetic acid (EDTA) for 10 days, and then embedded on optimal cutting temperature compound (Sakura Finetek, Torrance, CA). The frozen specimens were stored at −80°C and subsequently were cut coronally into sections of 6 μm in width using a cryostat to include the beginning and end of the sagittal suture, the site of particle injection.
Hematoxylin and eosin (H&E) staining (Sigma Aldrich, St. Louis, MO) was performed for histomorphologic analysis by taking the most posterior section at the sagittal suture and two adjacent sections from each calvarium.
Immunohistochemistry and cell counting
Macrophages were detected by direct immunostaining with the use of anti CD11b antibody. After permeabilization with 0.4% Triton X in PBS for 15 min and blocking with 3% BSA + 2% Goat serum + 0.1% Triton X in PBS for 1 h, FITC Anti-Mouse CD11b Rat IgG2b, κ (3.5 μg/mL; BD, Franklin Lakes, NJ) was applied with incubation overnight. The slides were mounted with ProLong® Gold Antifade Mount with DAPI (Life Technologies, Grand Island, NY).
Avidin–biotin complex (ABC) immunohistochemistry with use of antialkaline phosphatase (anti-ALP) antibody was performed to identify osteoblasts. After blocking with 10% rabbit normal serum in 0.1% BSA-PBS for 1 h, a primary antibody (Anti-Mouse ALP Goat IgG, 2.5 μg/mL; R&D Systems, Minneapolis, MN) in 0.1% BSA-PBS was applied to the frozen sections on slides followed by incubation overnight. The sections then underwent endogenous peroxidase blocking in 0.3% H2O2 in PBS for 15 min. The sections were incubated with secondary antibody (Biotinylated Rabbit anti-Goat IgG, 7.5 μg/mL; Vector Laboratories, Burlingame, CA) in 0.1% BSA-PBS for 1 h, followed by labeling with Vectastain Elite ABC Kit (Vector Laboratories). Vector NovaRed Peroxide Substrate Kit and Mayer's hematoxylin (Vector Laboratories) were used for obtaining the color reaction and counter staining, respectively. After dehydration in graded ethanol, the slides were mounted with VectaMount Permanent Mounting Medium (Vector Laboratories).
Osteoclast-like cells were also identified using a leukocyte tartrate resistant acid phosphatase (TRAP) kit (Sigma Aldrich) as large multinucleated cells located on the bone perimeter within a resorption lacuna. Slides were imaged (Axio Observer 3.1; Zeiss, Oberkochen, Germany) and the stained cells in the sagittal suture were counted manually and normalized by the area of sagittal suture.
Calvarial organ culture and ELISA
From each group, five calvaria, from which all soft tissues were removed, were obtained as a whole under sterile conditions and assigned for organ culture. Each calvarium was cultured with 2 mL of Dulbecco's modified Eagle's medium (DMEM) with glutamine (Gibco, Grand Island, NY), and 1% antimycotic/antibiotic solution in 12-well plates, and incubated for 24 h at 37°C with 5% CO2. The culture supernatants were then collected and stored at −80°C for subsequent ELISA analysis of TNF-α, interleukin-1 receptor antagonist (IL-1ra), RANKL and osteoprotegerin (OPG) release using specific kits (R&D Systems).
Statistics
All cell counts and ELISAs were evaluated in triplicate. Data were reported as mean ± standard deviation. Data used for statistical analysis were first assessed using a Kolmogorov–Smirnov test to ensure Gaussian distribution using Prism 6.0 (Graphpad Software, La Jolla, CA). For cell count and ELISA analyses, a One-way ANOVA with Tukey's post hoc test was performed. For micro-CT analysis and the ratio of IL-1ra/TNF-α and RANKL/OPG, the Kruskal–Wallis test followed by Dunn's multiple comparisons test was performed. Two-sided p values less than 0.05 was set as the threshold of significance.
RESULTS
Micro-CT imaging
Micro-CT analysis showed that NF-κB decoy ODN reversed the qualitative decrease in bone caused by PE. The difference in the TMD between pre and post experiments (post–pre experiment in the TMD) was 35.9 ± 15.6 mg/mm3 in the PE+ ODN group, demonstrating a significant increase compared with 17.5 ± 20.6 mg/mm3 in the PE group (p = 0.03). TMD in the PE + ODN group was increased compared to the PBS group (21.5 ± 22.7 mg/mm3), however, statistical significance was not reached [p = 0.22; Fig. 1(b)].
Histomorphology and immunohistochemistry
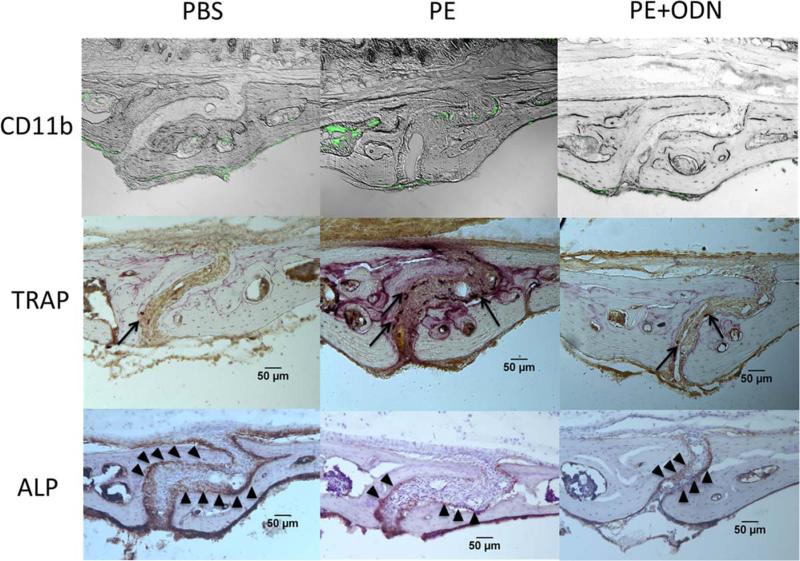
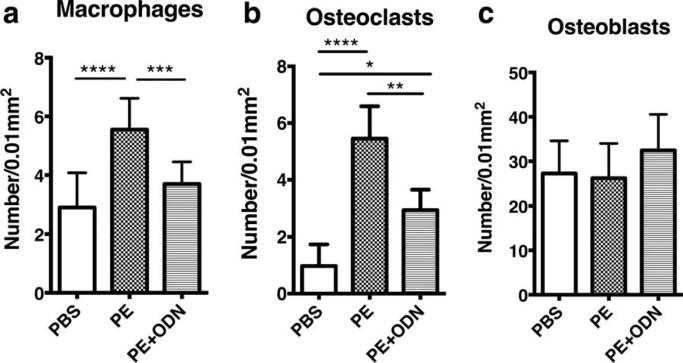
Macrophages and osteoclasts migrated into the area of the sagittal suture in the PE group, although the bone present was normal in appearance (Fig. 2). The number of CD11b positive macrophages and TRAP positive osteoclasts in the sagittal suture of the calvaria showed significant differences among the groups [Fig. 3(a,b)]. Greater numbers of CD11b positive cells were seen in the PE group compared with the PBS group (5.56 ± 1.07 vs. 2.91 ± 1.17/0.01mm2, respectively, p < 0.0001) and the PE + ODN group (vs. 3.70 ± 0.76/mm2, p = 0.001). The PE + ODN group showed similar CD11b positive cells as the PBS group (p = 0.2). Similarly, TRAP staining was increased in the PE particle group compared to the PBS group (5.5 ± 1.14 vs. 1.0 ± 0.76/0.01 mm2, p < 0.0001); the increase in the number of osteoclast-like cells was mitigated with NF-κB decoy ODN injection (2.95 ± 0.72/0.01 mm2, p = 0.002, vs. the PE group). The PE + ODN group demonstrated the greatest number of ALP positive cells among the three groups, however, the differences did not reach statistical significance [27.25 ± 7.34, 26.19 ± 7.81, and 32.46 ± 8.10/mm2 in the PBS, PE, and PE + ODN groups, respectively, p = 0.12; Fig. 3(c)].
FIGURE 2.
Calvarial histopathology. Immunohistochemical and histochemical stains were performed to evaluate inflammation, osteoclast and osteoblast activity. (a) Immunostaining macrophages with mouse FITC-labeled anti CD11b antibodies. In PE group, CD11b positive cells migrate into sagittal suture area. (b) Osteoclasts were stained by a leukocyte tartrate resistant acid phosphatase (TRAP). Osteoclasts that migrated into the sagittal suture were stained as purple (arrows). (c) Avidin–Biotin Complex (ABC) immunohistochemistry with use of antialkaline phosphatase (ALP) antibody showed osteoblasts as brown-stained cells at the perimeter bone in sagittal suture (arrowhead). Ratio at 200× magnification. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
FIGURE 3.
Cell numbers in the calvarial immunostaining sections. (a and b) macrophages and osteoclasts were detected using murine anti CD11b and TRAP staining, respectively. The number of these cells was reduced with the administration of NFκB decoy ODN in the presence of PE particles. (c) The number of osteoblasts immunohistochemically stained using anti-mouse ALP showed no significant differences among the groups. *, p < 0.05; **, p < 0.01; ***, p < 0.001; and ****, p < 0.0001.
ELISA analysis
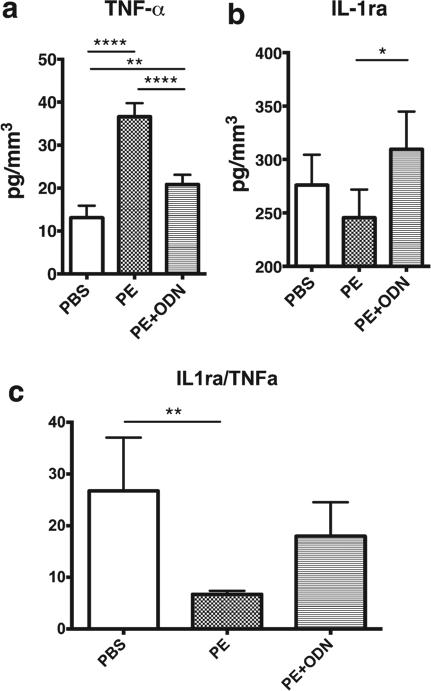
A significantly higher concentration of TNF-α was released from the calvaria treated with PE only (36.6 ± 3.14 pg/mm3) compared with PBS [13.1 ± 2.80 pg/mm3, p < 0.0001; Fig. 4(a)]. TNF-α secretion with PE + ODN (20.84 ± 2.26 pg/mm3) was significantly decreased compared to the PE treatment alone (p < 0.0001), however, the PE + ODN value was greater than for the PBS treatment (p = 0.002). IL-1ra secretion was significantly higher in the PE + ODN group compared with the PE group [309.6 ± 35.27 vs. 245.5 ± 26.38 pg/mm3. respectively, p = 0.02; Fig. 4(b)]. IL-1ra secretion in the PBS group was 276.0 ± 28.40 pg/mm3, and showed no differences between other groups (p = 0.32 and 0.29, vs. the PE and the PE + ODN group, respectively). Analysis of the IL-1ra/TNF-α ratio showed that a significant decrease in the PE group compared with PBS group [6.7 ± 0.7 vs. 26.7 ± 10.3, p = 0.003; Fig 4(c)]. NF-κB decoy ODN injection increased the ratio in PE + ODN group (18.0 ± 6.5), however, no significant difference was seen between the PE and PE + ODN groups (p = 0.12).
FIGURE 4.
TNF-α and IL-1ra secretion in calvarial organ cultures. The calvaria, which were harvested from the skulls, were cultured ex vivo and the secretion levels of (a) TNF-α and (b) IL-1ra were quantified by ELISA. NFκ ODN induced IL-1ra and suppressed the secretion of TNF-α which was induced by PE particles. The decreased (c) the ratio of IL-1ra/TNF-α caused by PE particles was reversed in part via NFκ ODN injection. *, p < 0.05; **, p < 0.01; and ****, p < 0.0001.
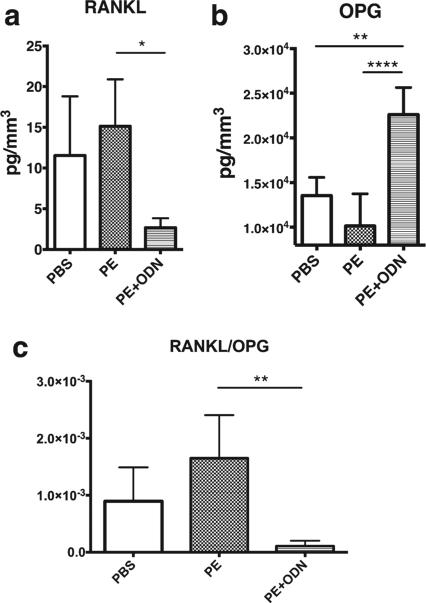
RANKL secretion was suppressed in the PE + ODN group compared with the PE group [2.67 ± 2.57 vs. 15.13 ± 5.77 pg/mm3, p = 0.01; Fig. 5(a)]. RANKL secretion in the PBS group was 11.53 ± 7.27 pg/mm3 in the PBS group, showing no differences between other groups. NF-κB decoy ODN stimulated OPG secretion in the PE + ODN group [22,620 ± 3018 pg/mm3; Fig. 5(b)]. The concentration of OPG in the PE + ODN group was significantly greater than other two groups (13,548 ± 2036 pg/mm3 in the PBS group and 10,135 ± 3600 pg/mm3 in the PE group, p = 0.001 and p < 0.0001, respectively). In the RANKL/OPG ratio analysis, NF-κB decoy ODN decreased the ratio in PE + –ODN group significantly compared with PE group [1.1 × 10−4 ± 9.5 × 10−5 vs. 1.7 × 10−3 ± 7.5 × 10−4, p = 0.006; Fig. 5(c)].
FIGURE 5.
RANKL and OPG secretion in calvarial organ cultures. Secretion level of (a) RANKL and (b) OPG by ELISA. NFκ ODN suppressed the expression of RANKL and strongly induced OPG secretion, resulting in the decrease in (c) the ratio of RANKL/OPG in the PE + ODN group. *, p < 0.05; **, p < 0.01; and ****, p < 0.0001.
DISCUSSION
In order to understand the pathogenesis of wear particle induced inflammation, appropriate animals models must be developed to simulate the early phases of this disorder, in which osteolysis is not or minimally present. The current study focused on this early phase of particle-induced inflammation in which a prosthesis might still be potentially salvageable via intervention with a biological agent, in this case, local treatment with an NF-κB decoy ODN. The model used in the present study is simple, minimally invasive, reproducible and cost-effective.
Micro-CT has been established as a reliable means to assess the fine structure and mineral density of rodent bone in various physiological and pathological conditions.16 In the current study, micro-CT was used to determine the TMD in the parietal bones surrounding the sagittal suture; although the resolution of the scanner used was relatively low, it still is high enough to analyze TMD in rodent calvarial bone.15 Micro-CT analysis showed a qualitative degradation in the TMD in the PE group, while NF-κB decoy ODN treatment partially prevented this particle-induced mineral loss. Corresponding changes in the infiltrating number of macrophages and the number of osteoclasts were seen in the histopathological analysis and in the production of TNF-α, IL-1Ra, RANKL, and OPG in the calvarial explant culture. However, no quantitative changes in the amount of bone between the treatment groups were seen in the bone histomorphometry analysis. It can be speculated that in this model of early low-grade particle-induced inflammation, histologically evident osteolytic lesions would subsequently follow progressive inflammation, increased osteoclastogenesis, and the loss of bone mineral as evident in the micro-CT.
Interestingly, even with PE particles, the TMD had a tendency to be increased. This agrees with the work of Ferreira et al.17 in which low levels of IL-1β and TNF-α induced mesenchymal stem cells to differentiate into osteoblasts independent from the NF-κB signaling pathway. Higher levels of IL-1β and TNF-α can enhance osteoclastogenesis by direct stimulation of macrophages and osteoclasts.18 The magnitude of inflammatory factors in the local biological microenvironment appears to affect bone formation and resorption in somewhat contradictory ways.4,19
Previously, we showed that NF-κB decoy ODN inhibited the production of multiple cytokines and chemokines using primary mouse macrophages and human THP1 cells in vitro.9 The present data suggests that this strategy is a viable one using our mouse calvarial model. In agreement with this finding Alhawagri et al.20 showed that blocking the binding of NEMO with IKKα/β inhibited osteolysis via suppression of NF-κB activity. Thus, interference of this upstream biological pathway appears to be a more efficacious strategy, in preference to suppressing individual cytokines.
Interestingly, NF-κB decoy ODN was found to stimulate OPG secretion in the calvaria exposed to PE particles. It is widely recognized that OPG can suppress osteoclast activity as a decoy receptor and block the interaction between RANKL and RANK.21,22 However, little is known concerning the mechanisms by which suppression of the NF-κB signaling pathway can up-regulate the production of OPG from osteoblasts and stromal cells. Recently, NF-κB decoy ODN was shown to increase TGF-β1 and OPG in MSCs exposed to PE particles, and up-regulate OPG expression through a TGF-β1 dependent pathway.23
CONCLUSION
Local injections of NF-κB decoy ODN mitigated the expression of proinflammatory cytokines in the presence of clinically relevant PE wear particles. This scenario is comparable to the early phases of particle-induced inflammation in which extensive bone resorption and peri-implant osteolysis with loosening of implant components is not yet present. In cases of early particle-induced inflammation associated with unexpectedly high acetabular liner wear rates or diagnosed by emerging biomarkers of increased peri-implant bone turnover, local, intra-articular delivery of NF-κB decoy ODN may potentially preserve periprosthetic bone stock, and prolong the time to revision surgery.
Acknowledgments
Contract grant sponsor: NIH; contract grant number: 2R01 AR055650 and 1R01AR063717
Contract grant sponsor: Ellenburg Chair in Surgery in Stanford University
Footnotes
How to cite this article: Sato T, Pajarinen J, Lin T, Tamaki Y, Loi F, Yao Z, Egashira K, Goodman SB. 2015. NF-κB decoy oligodeoxynucleotide inhibits wear particle-induced inflammation in a murine calvarial model. J Biomed Mater Res Part A 2015:103A:3872–3878.
REFERENCES
- 1.Goodman SB. Wear particles, periprosthetic osteolysis and the immune system. Biomaterials. 2007;28:5044–5048. doi: 10.1016/j.biomaterials.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin TH, Tamaki Y, Pajarinen J, Waters HA, Woo DK, Yao Z, Goodman SB. Chronic inflammation in biomaterial-induced peri-prosthetic osteolysis: NF-kappaB as a therapeutic target. Acta Bio-mater. 2014;10:1–10. doi: 10.1016/j.actbio.2013.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston RB., Jr Current concepts: Immunology. Monocytes and macrophages. N Engl J Med. 1988;318:747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 4.Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL. IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest. 2005;115:282–290. doi: 10.1172/JCI23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dinh TD, Higuchi Y, Kawakami S, Yamashita F, Hashida M. Evaluation of osteoclastogenesis via NFkappaB decoy/mannosylated cationic liposome-mediated inhibition of pro-inflammatory cytokine production from primary cultured macrophages. Pharm Res. 2011;28:742–751. doi: 10.1007/s11095-011-0366-0. [DOI] [PubMed] [Google Scholar]
- 6.Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos Int. 2013;24:2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clohisy JC, Hirayama T, Frazier E, Han SK, Abu-Amer Y. NF-kB signaling blockade abolishes implant particle-induced osteoclasto-genesis. J Orthop Res. 2004;22:13–20. doi: 10.1016/S0736-0266(03)00156-6. [DOI] [PubMed] [Google Scholar]
- 8.Osako MK, Nakagami H, Morishita R. Modification of decoy oligodeoxynucleotides to achieve the stability and therapeutic efficacy. Curr Top Med Chem. 2012;12:1603–1607. doi: 10.2174/156802612803531397. [DOI] [PubMed] [Google Scholar]
- 9.Lin TH, Yao Z, Sato T, Keeney M, Li C, Pajarinen J, Yang F, Egashira K, Goodman SB. Suppression of wear-particle-induced pro-inflammatory cytokine and chemokine production in macrophages via NF-kappaB decoy oligodeoxynucleotide: A preliminary report. Acta Biomater. 2014;10:3747–3755. doi: 10.1016/j.actbio.2014.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shimizu H, Nakagami H, Morita S, Tsukamoto I, Osako MK, Nakagami F, Shimosato T, Minobe N, Morishita R. New treatment of periodontal diseases by using NF-kappaB decoy oligodeoxynucleotides via prevention of bone resorption and promotion of wound healing. Antioxid Redox Signal. 2009;11:2065–2075. doi: 10.1089/ars.2008.2355. [DOI] [PubMed] [Google Scholar]
- 11.Desmet C, Gosset P, Pajak B, Cataldo D, Bentires-Alj M, Lekeux P, Bureau F. Selective blockade of NF-kappa B activity in airway immune cells inhibits the effector phase of experimental asthma. J Immunol. 2004;173:5766–5775. doi: 10.4049/jimmunol.173.9.5766. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhang C, Zhou X, Wang H, Mao Y, Wang X. Parthenolide inhibits polyethylene particle-induced mouse calvarial osteolysis in vivo. J Surg Res. 2014;187:176–181. doi: 10.1016/j.jss.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 13.Xiao F, Zhai Z, Jiang C, Liu X, Li H, Qu X, Ouyang Z, Fan Q, Tang T, Qin A, Gu D. Geraniin suppresses RANKL-induced osteoclasto-genesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp Cell Res. 2015;330:91–101. doi: 10.1016/j.yexcr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Zhou X, Zhang C, Wang X, An B, Zhang P, Zhu Z. Berberine inhibits lipopolysaccharide- and polyethylene particle-induced mouse calvarial osteolysis in vivo. J Surg Res. 2012;173:e47–e52. doi: 10.1016/j.jss.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 15.Rao AJ, Zwingenberger S, Valladares R, Li C, Lane Smith R, Goodman SB, Nich C. Direct subcutaneous injection of polyethyl ene particles over the murine calvaria results in dramatic osteolysis. Int Orthop. 2013;37:1393–1398. doi: 10.1007/s00264-013-1887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouxsein ML, Boyd SK, Christiansen BA, Guldberg RE, Jepsen KJ, Muller R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res. 2010;25:1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira E, Porter RM, Wehling N, O'Sullivan RP, Liu F, Boskey A, Estok DM, Harris MB, Vrahas MS, Evans CH, Wells JW. Inflammatory cytokines induce a unique mineralizing phenotype in mesenchymal stem cells derived from human bone marrow. J Biol Chem. 2013;288:29494–29505. doi: 10.1074/jbc.M113.471268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwarz EM, Lu AP, Goater JJ, Benz EB, Kollias G, Rosier RN, Puzas JE, O'Keefe RJ. Tumor necrosis factor-alpha/nuclear transcription factor-kappaB signaling in periprosthetic osteolysis. J Orthop Res. 2000;18:472–480. doi: 10.1002/jor.1100180321. [DOI] [PubMed] [Google Scholar]
- 19.Jules J, Feng X. In vitro investigation of the roles of the proinflammatory cytokines tumor necrosis factor-alpha and interleukin-1 in murine osteoclastogenesis. Methods Mol Biol. 2014;1155:109–123. doi: 10.1007/978-1-4939-0669-7_10. [DOI] [PubMed] [Google Scholar]
- 20.Alhawagri M, Yamanaka Y, Ballard D, Oltz E, Abu-Amer Y. Lysine392, a K63-linked ubiquitination site in NEMO, mediates inflammatory osteoclastogenesis and osteolysis. J Orthop Res. 2012;30:554–560. doi: 10.1002/jor.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 22.Goater JJ, O'Keefe RJ, Rosier RN, Puzas JE, Schwarz EM. Efficacy of ex vivo OPG gene therapy in preventing wear debris induced osteolysis. J Orthop Res. 2002;20:169–173. doi: 10.1016/S0736-0266(01)00083-3. [DOI] [PubMed] [Google Scholar]
- 23.Lin TH, Sato T, Barcay KR, Waters H, Loi F, Zhang R, Pajarinen J, Egashira K, Yao Z, Goodman SB. NF-kappaB decoy oligodeoxynucleotide enhanced osteogenesis in mesenchymal stem cells exposed to polyethylene particle. Tissue Eng Part A. 2015;21:875–883. doi: 10.1089/ten.tea.2014.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]