Abstract
Extranodal NK/T-cell lymphoma (ENKTCL) is associated with latent Epstein-Barr virus (EBV) infection and frequent relapse even after complete response (CR) to intensive chemotherapy and radiotherapy. The expression of EBV proteins in the tumor provides targets for adoptive immunotherapy with antigen-specific cytotoxic T cells (CTL). To evaluate the efficacy and safety of EBV latent membrane protein (LMP)-1 and LMP-2a-specific CTLs (LMP1/2a CTLs) stimulated with LMP1/2a RNA-transferred dendritic cells, we treated 10 ENKTCL patients who showed complete response to induction therapy. Patients who completed and responded to chemotherapy, radiotherapy, and/or high-dose therapy followed by stem cell transplantation (HDT/SCT) were eligible to receive eight doses of 2 × 107 LMP1/2a CTLs/m2. Following infusion, there were no immediate or delayed toxicities. The 4-year overall survival (OS) and progression-free survival (PFS) were 100%, and 90% (95% CI: 71.4 to 100%) respectively with a median follow-up of 55·5 months. Circulating IFN-γ secreting LMP1 and LMP2a-specific T cells within the peripheral blood corresponded with decline in plasma EBV DNA levels in patients. Adoptive transfer of LMP1/2a CTLs in ENKTCL patients is a safe and effective postremission therapeutic approach. Further randomized studies will be needed to define the role of EBV-CTLs in preventing relapse of ENKTCL.
Introduction
Extranodal natural killer (NK)/T-cell lymphoma (ENKTCL), nasal type is a rare and highly aggressive disease with distinct clinicopathological features. While the majority of patients with ENKTCL have localized disease within the nasal cavity, the overall prognosis is poor and it is often difficult to accurately identify and predict high-risk patients for appropriate treatment. Many observational studies recommend early or up-front radiotherapy as primary treatment in early-stage ENKTCL patients without additional adverse factors as it shows greater response rate than chemotherapy in localized disease.1,2 However, radiotherapy alone is not sufficient to prevent recurrence of diseases outside of the radiation field.3,4 Recently, concurent chemoradiotherapy regimens have been suggested to improve both local and systemic disease control, especially in patients presenting with adverse risk factors.5,6
Concurrent chemoradiotherapy regimens involving nonanthracycline chemotherapy7,8 based on ifosfamide, methotrexate, and carboplatin, can successfully improve treatment outcome of patients by reducing local and systemic relapse rates. However, even with the recent progress in the treatment for ENKTCL, a substantial proportion of treated patients eventually relapse, in which no standard treatment is available. In addition to high expression of multidrug resistance gene,9,10 EBV's role in lymphomagenesis may explain the difficulty to cure this disease. Patients with systemic EBV infection in the peripheral blood (PB) or bone marrow are categorized as high-risk group and are correlated with an unfavorable prognosis.11,12 The re-emergence of EBV infection in blood sample often indicates relapse after treatment. These observations indicate that host immunity to EBV after tumor eradication can be important to maintain long-term disease control.
The targeted immunotherapy using EBV-CTLs is a safe and robust method that can specifically target and eradicate tumor cells without damaging normal tissues. Furthermore, the infusion of these cells can restore host antitumor immunity, which can therefore establish long-term maintenance of EBV-CTL responses in patients. The majority of the clinical experience with EBV-CTLs has been in EBV-associated post-transplant lymphoproliferative disease (PTLD)13,14 and these studies have showed that EBV-CTLs can be safely infused without complications while controlling EBV DNA concentrations to normal range in EBV-reactivated patients. The success of EBV-CTLs in PTLD has fueled the development of adoptive immunotherapy of EBV-CTLs for other EBV-associated malignancies including EBV-positive Hodgkin's disease lymphoma,15 nasopharyngeal carcinoma,16 and angiocentric lymphoma.17 These studies have proved adoptive immunotherapy of EBV-CTLs as an effective strategy to reconstitute EBV-specific immunity and to treat EBV-associated malignancies. In this study, we aimed to evaluate the efficacy of EBV LMP1/2a CTLs as postremission therapy in ENKTCL patients. We demonstrate the safety and efficacy of LMP1/2a CTLs in ENKTCL patients with long-term follow-up data and provide encouraging results that show the high efficacy of EBV-CTLs in preventing relapse in ENKTCL.
Results
Patient characteristics
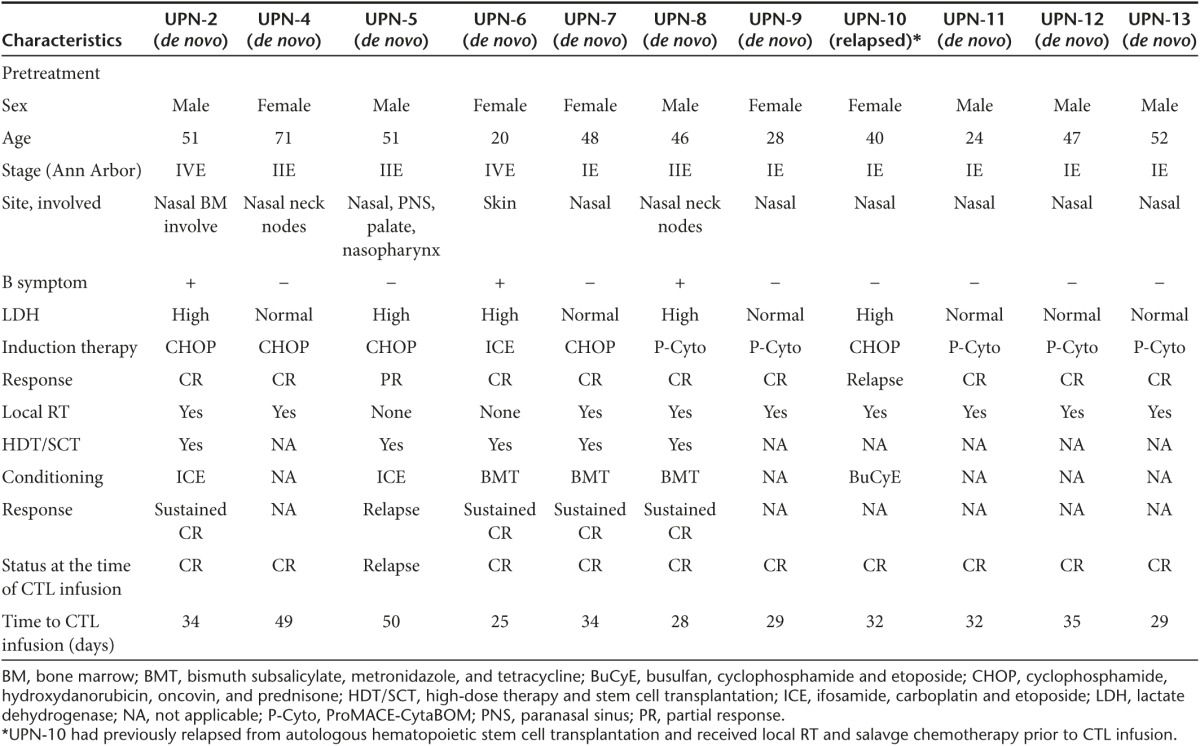
A total of 13 patients diagnosed with ENKTCL were enrolled. All tissue and/or blood samples from patients were confirmed to be EBV positive by PCR analysis (data not shown). Two patients died before LMP1/2a CTL generation. The remaining 11 patients included four high-risk patients (45%) presenting at least one risk factor and six low-risk patients (54%) presenting no risk factors. In addition, nine patients (81%) showed disease mainly involved in the nasal cavity or cervical nodes (Ann Arbor stage, IE-IIE), whereas two patients presented advanced-stage disease (Ann Arbor stage, IVE) with bone marrow and disseminated involvement in extralymphatic organs at diagnosis. Ten de novo patients were initially treated with standard chemotherapy ((CHOP), n = 4; (ICE), n = 1; (ProMACE-CytoBOM), n = 5) and eight of them were followed by local radiotherapy. Two patients were unable to receive local radiotherapy: UPN-5 presented extensive localized disease and UPN-6 presented multiple cutaneous manifestations with systemic involvement. Five patients further received high-dose therapy followed by autologous peripheral blood stem cell transplantation (HDT/SCT). UPN-10 was recruited into the study as a relapsed patient following autologous hematopoietic stem cell transplantation. This patient received local radiotherapy and salvage chemotherapy prior to the CTL infusion. Overall, 10 out of 11 patients showed complete response (CR) following induction therapy and 1 patient had relapsed at the time of CTL therapy. Therefore, only 10 patients who were in initial remission of disease at the time of CTL-therapy were eligible to receive CTL infusions and for further monitoring. Baseline patient characteristics are listed in Table 1.
Table 1. Patient characteristics.
Characterization of in vitro expanded EBV-CTLs specific for LMP-1 and LMP-2a
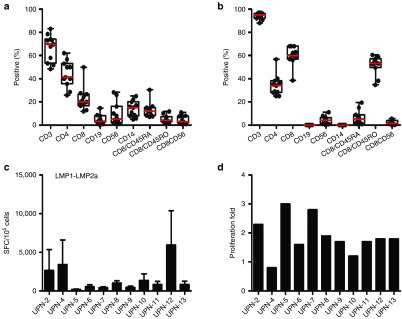
Phenotypic analyses of the initial leukaphresis samples collected from patients are presented in Figure 1. After stimulation with LMP-1 and LMP-2 electroporated DCs, the final products contained both CD8+ T cells (median, 60%; range, 38.5–68.1%) and CD4+ T cells (median, 31.5%; range, 25–56.7%) with the absence of CD19+ B-cell and CD14+ monocytes. CD8+ T cells were predominantly CD45RO+ memory cells (median, 53.4%; range, 34.7–60.5%). Furthermore, only low levels of NK cells (median, 4.2%; range, 0–11%) and NKT cells (median, 1.4%; range, 0–5.5%) were present. The specificity of EBV-CTLs against both LMP-1 and LMP-2a was determined by IFN-γ enzyme-linked immunospot (ELISPOT) assays.
Figure 1.
Characteristics of latent membrane protein (LMP)-1- and LMP-2a-specific cytotoxic T-lymphocytes (CTLs) derived from extranodal NK/T cell lymphoma (ENKTCL) patients. (a) Phenotype of leukaphresis products prior to stimulation showing mainly CD3+ and CD4+ T cells. Following stimulations with LMP-1 and LMP-2a transduced dendritic cells (DCs), (b) LMP-1- and LMP-2a-specific CTLs (LMP-1/2a CTLs) were generated composed of both CD4+ and CD8+ T cells. Red lines indicate median value. (c) Antigen-specific activity for each patient was evaluated by coculturing CTLs with LMP-1/2a transduced DCs. The number of spots corresponding to IFN-γ-secreting cells was measured using interferon-γ (IFN-γ) enzyme-linked immunospot (ELISPOT) assay. The measured ELISPOT are shown as mean values, with error bars indicating the standard errors of the means (SEM) from triplicate wells. (d) Proliferation fold of LMP-1/2a CTLs generated from each patient.
Clinical response to EBV-CTL therapy
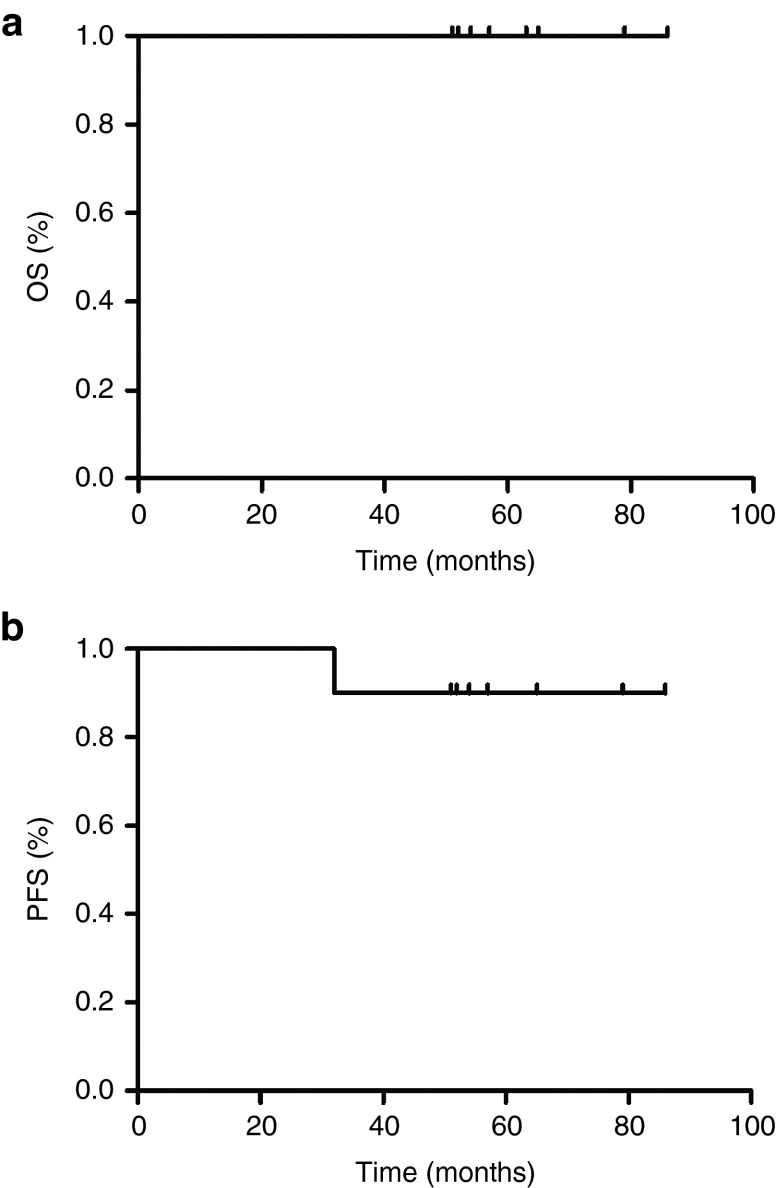
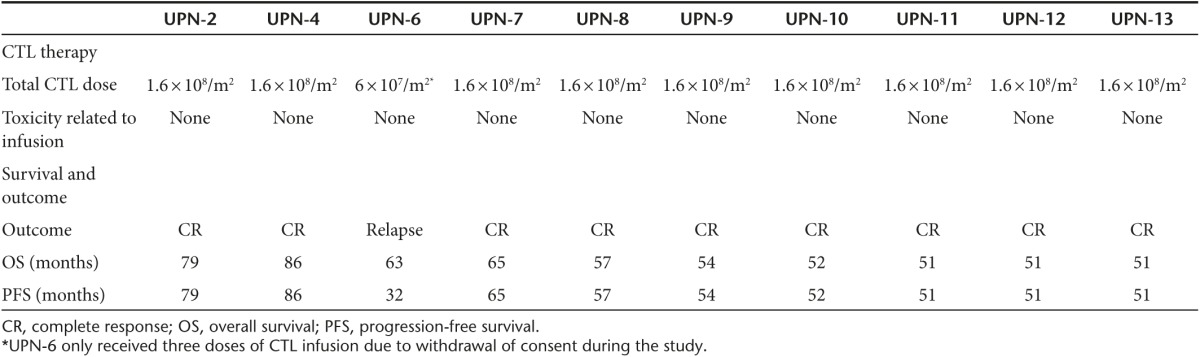
Patients were infused with four weekly doses of CTLs 2 × 107 cells/m2 autologous LMP1/2a CTLs and were reinfused with another four weekly doses after an interval of one month. Nine patients received all eight doses and one patient received three doses due to withdrawal of consent. The median time to CTL infusion after the last treatment was 32 days (range, 8–50). There were no immediate or delayed toxicities related to the CTL infusion. The 4-year OS and progression-free survival (PFS) since first CTL infusion were 100%, and 90% (95% CI: 71.4 to 100%), respectively with a median follow-up of 55.5 months. UPN-6 who had not completed the CTL therapy dose, relapsed after CTL therapy (Figure 2). The outcomes of patients are summarized in Table 2.
Figure 2.
Outcomes in patients receiving latent membrane protein (LMP)-1- and LMP-2a-specific cytotoxic T lymphocyte (CTLs; LMP-1/2a CTLs) as postremission therapy. (a) Overall survival and (b) progression-free survival for 10 patients treated with LMP-1/2a CTLs.
Table 2. Outcome of CTL therapy.
Immunologic effects and follow-up after adoptive transfer
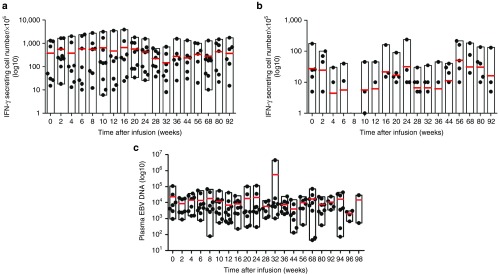
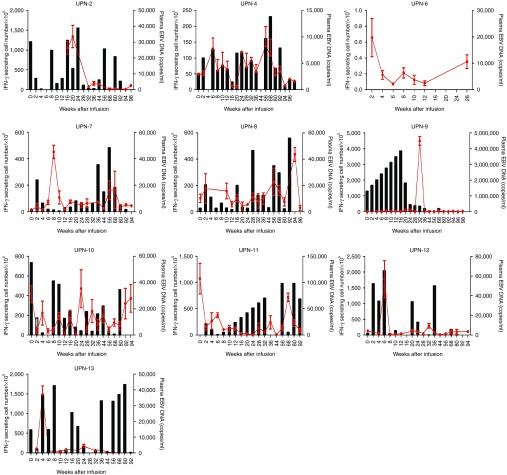
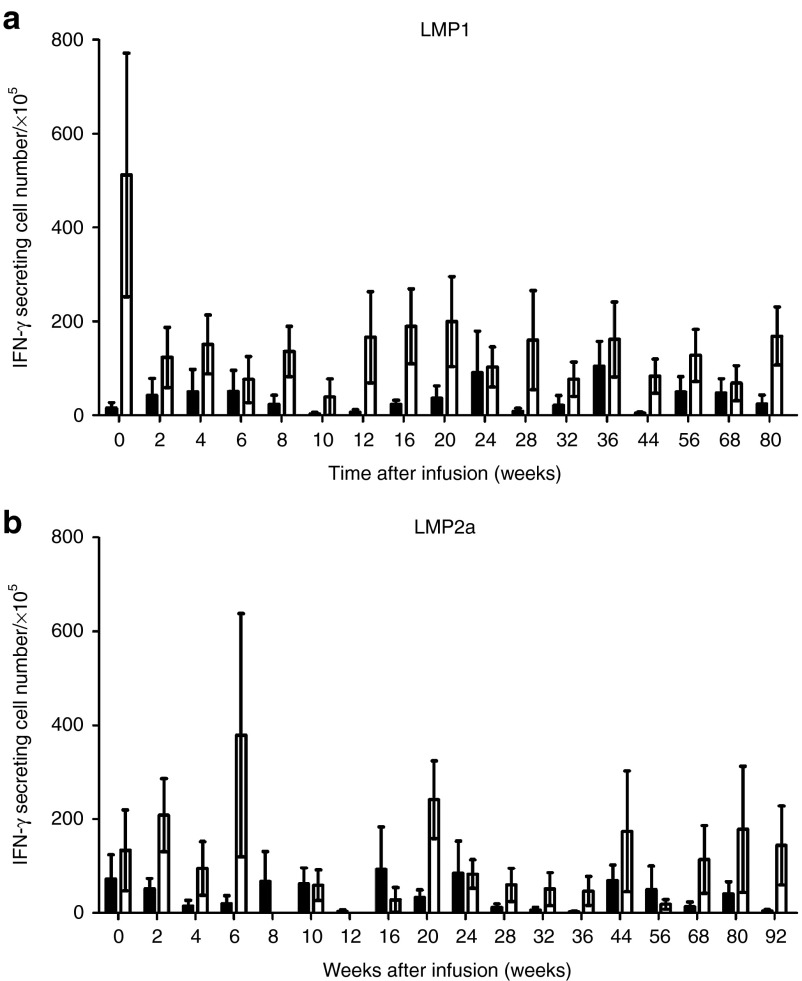
We measured the frequency of LMP1/2a CTLs every 2 weeks after the first CTL infusion using IFN-γ ELISPOT assays. Figure 3a,b shows that all patients had circulating LMP1/2a CTLs. Overall, the LMP-1-specific responses outweighed the LMP-2a-specific responses. In association with the LMP-specific CTL responses, we also measured the levels of EBV in the patient's plasma using real-time-quantitative polymerase chain reaction (Figure 3c). Overall, the plasma EBV levels varied between patients and the correlation between CTL infusion and EBV immunity was not univocal as demonstrated in Figure 4. In patients 2 and 11, high EBV DNA levels were followed by an increase in IFN-γ secreting T cells. Subsequently, the increases in CTL responses corresponded with a drop in EBV DNA levels indicating some correlation between CTL therapy and EBV control. In contrast, some patients, such as patients 9, 12, and 13, already had high immune responses before CTL infusions, whereas others had numerous fluctuations over time. Furthermore, we observed increasing EBV-DNA levels in patient 10, a relapsed patient from a previous transplantation, indicating that CTL therapy may not have induced control. Similarly, patient 6, who did not complete the treatment, showed an increase in EBV-DNA levels. Although, responses against LMP-2a antigen were measured for each individual, these responses were minimal compared to the responses against LMP-1. To further investigate the T-cell responses against each antigen, we isolated CD4+ and CD8+ T-cell populations and performed IFN-γ ELISPOT assays against LMP-1 and LMP-2a separately (Figure 5). As expected, the responses against both antigens consisted mainly of CD8+ T cells. However, the frequency of LMP-specific CD4+ T cells was also present throughout the monitoring period. Also, low levels of LMP-2a-specific responses were present in both CD4+ and CD8+ T cells.
Figure 3.
Overall immune responses in patients following latent membrane protein (LMP)-1- and LMP-2a-specific cytotoxic T lymphocyte (CTLs; LMP-1/2a CTLs) therapy. Peripheral blood mononuclear cells were cocultured with (a) LMP-1 or (b) LMP-2 transduced dendritic cells. Number of interferon-γ (IFN-γ) secreting cells per 1 × 105 mononuclear cells was measured in enzyme-linked immunospot assays. (c) Epstein Barr virus DNA levels were measured from the patient's plasma. Red lines indicate mean value. The y-axis is presented in Log10 scale.
Figure 4.
Individual immune responses after latent membrane protein (LMP)-1- and LMP-2a-specific cytotoxic T lymphocyte (CTLs; LMP-1/2a CTLs) therapy. Black bars indicate the IFN-γ secreting cell numbers per 1 × 105 mononuclear cells over time. Red lines indicate the plasma EBV DNA copies per mL over time. Enzyme-linked immunospot assays were not performed for UPN 6.
Figure 5.
CD4+ and CD8+ T-cell responses against latent membrane protein (LMP)-1 and LMP-2a antigens. CD4+ and CD8+ T cells were first isolated from peripheral blood mononuclear cells and were then cocultured with (a) LMP-1 or (b) LMP-2 transduced dendritic cells. Black bar indicates CD4+ T-cell responses and white bar indicates CD8+ T-cell responses for each time point. Number of interferon-γ (IFN-γ) secreting cells per 1 × 105 mononuclear cells was measured in enzyme-linked immunospot (ELISPOT) assays. The measured ELISPOT are shown as mean values, with error bars indicating the standard errors of the means from triplicate wells.
Discussion
In this study, we demonstrate the long-term effects of LMP1/2a CTLs as postremission therapy in ENKTCL patients. Bollard et al.18 previously reported the use of LMP-2 specific single antigen CTLs and also LMP-1- and LMP-2a-specific double-antigen CTLs19 for the treatment of lymphoma patients, which included a population of NK/T cell lymphoma patients. Similarly, the current study focuses on demonstrating the feasibility of EBV-CTLs in a similar yet unique entity of NK/T cell lymphoma patients, which is rare in Western countries. We administered LMP1/2a CTLs to 10 patients with ENKTCL who had reached initial remission through chemotherapy, radiotherapy, and/or HDT/SCT. Among 10 patients who had received CTL therapy, 9 patients showed sustained remission and 1 patient had relapsed at the last follow-up. Responses were associated with increased frequencies of LMP-1- and LMP-2a-specific T cells in the peripheral blood after CTL infusion and this corresponded with the control of plasma EBV DNA levels.
Developments in treatment regimens have improved initial CR rates in ENKTCL patients; however, effective postremission therapy is still necessary to prevent further relapse. Currently, combined chemotherapy and radiotherapy is considered standard treatment for patients with localized early-stage disease.20,21 While optimized timing of radiotherapy remains to be elucidated, combined chemoradiotherapy regimens are still insufficient for majority of patients who present poor risk factors, including the presence of B-symptoms, extranasal involvement, high lactate dehydrogenase levels, and EBV viral load.22 Our study included six patients with poor risk factors, and five of these patients were able to achieve a CR following induction therapy based on investigator's choice (Table 1). We additionally treated patients presenting risk factors or advanced stage disease with autologous hematopoietic stem cell transplantation (or HDT/SCT) for consolidation. Limited studies on HDT/SCT in ENKTCL patients suggest that HDT/SCT may improve OS and PFS of ENKTCL patients.23,24 However, whether HDT/SCT truly offer a survival advantage to high-risk or advanced patients remains to be determined.
Although we cannot make a definitive conclusion due to heterogenous treatment protocols, we show that EBV-CTLs may be beneficial for patients with risk factors in preventing systemic relapse. All patients who were in initial remission at the time of CTL infusion and received all doses of CTLs (excluding UPN-6), remain in complete remission. Furthermore, our study included a patient (UPN-10) with a poor prognosis who had previously relapsed from autologous hematopoietic stem cell transplantation and this patient was able to achieve durable remission following EBV-CTL therapy.
In order to assess the response to treatment, we monitored the EBV DNA levels in the peripheral blood throughout the study. EBV infection is consistently associated with ENKTCL and studies have showed evidence that the circulating EBV DNA levels correlate with tumor load, disease activity, and relapse.25,26 We observed that even patients with initial remission prior to CTL infusion had residual levels of EBV DNA (Figure 4) and these residual levels may suggest possible EBV reactivation. Currently, existing antiviral chemotherapeutics have low efficacy targeting EBV. We previously demonstrated the potential benefit of antiviral immunotherapy using pegylated interferon α-2a for the treatment of ENKTCL27; however, it was also associated with hematologic toxicities at higher doses. While we did observe increased CTL responses corresponding with the control of the plasma EBV DNA level in some patients, majority of patients had numerous fluctuations in EBV DNA levels and immune responses. Therefore, the correlation between CTL infusions and EBV DNA control remains to be elucidated and needs to be addressed in larger studies.
In addition, the characteristics of the infused CTLs may have affected the outcome of CTL response. In our study the generated CTL products had a homeogenous characteristic with majority of CTLs expressing CD3+CD8+45RO+ phenotype. Despite the homogenous phenotype of the CTLs infused, the outcome showed high variation between patients. It has previously been reported by Haque et al. that patients who received higher numbers of CD4+ cells in infused CTL lines showed better responses, indicating that CD4 cells within the CTL product may provide help to CD8+ T cells to enhance survival in vivo.28 Furthermore, while CD4+ T cells have been traditionally viewed as helper T cells, there is increasing evidence that suggest CD4+ T cells may also exhibit cytotoxic potential with granzyme B and perforin containng granules.29 Therefore, CD4+ T cells included in the final CTL product may also contribute to the cytotoxic potential of CTLs. While most CTL lines consisted mainly of CD8+ T cells, one CTL line generated from UPN-8 consisted of 60% CD4+ T cells versus 40% CD8+ T cells. However, this did not correspond with the patient's CTL response or EBV DNA control in vivo following infusion (Figure 4). Furthermore, additional analysis of the memory T-cell subsets may provide insight into the outcome of CTL infusions including durability of treatment. It has been previously noted that central memory T cells persist longer in vivo than effector memory T cells following administration.30 Although we did not analyze the memory T-cell subsets at the time of generation, we evaluated CTL lines that were available from four patients. Similar to a previous report by Weber et al.,31 the CTL lines generated during the study consisted of subsets with predominance of CD8+/CD45RO+/CD62L-/CCR7- effector memory T cells (data not shown). However, the presence of CD8+/CD45RO+/CD62L+/CCR7+ central memory cells was also noted. The higher levels of effector memory T cells may explain the weak correlation between the CTL infusion and disease control (EBV DNA) in our study. Therefore, it will be important to produce CTLs consisting of high levels of central memory T cells in the future for durable treatment.
For future clinical trials, it may be recommended to begin leukapheresis for EBV-CTL generation after confirming CR in patients. This approach may prevent expenses of generating EBV-CTLs for patients who are not appropriate to receive CTL infusions, such as those who do not achieve CR at the time of infusion or those who die during treatment. However, repeated chemotherapy cycles during induction therapy may limit the efficient generation of dendritic cells and EBV-CTLs. Therefore, novel approaches in generating adequate therapeutic doses of EBV-CTLs even after chemotherapy is needed.
In conclusion, we have shown the feasibility and efficacy of LMP-1/2a-specific CTLs in preventing relapse in high-risk ENKTCL patients with 4 years of follow-up. Our results suggest that controlling the EBV viral load after initial remission can help achieve durable remissions without long-term toxicities.
Materials and Methods
Patients. Patients with histologically verified extranodal NK/T-cell lymphoma, nasal type, according to the WHO classification who had achieved CR following induction or salvage therapy were eligible for the current study. Patients who did not achieve complete remission were excluded. Thirteen consecutive patients: 11 newly diagnosed and 2 relapsed patients with the median age of 47 years (range, 20–71 years) with histologically verified extranodal NK/T-cell lymphoma, nasal type, were enrolled. All patients received conventional therapy prior to infusion of LMP1/2a CTLs including five patients who underwent high-dose chemotherapy with autologous stem cell transplantation (HDT/SCT). Ten patients achieved CR, one patient relapsed after induction therapy, and two patients died of disease progression before EBV-CTL production. Overall, 10 patients were eligible for the study.
Response to treatment was assessed according to WHO criteria. Complete response was defined as the disappearance of all known disease for at least 4 weeks. Partial response was defined as a decrease of 50% in the sum of the products of the tumor's longest dimension and its widest perpendicular dimension for at least 4 weeks, without the appearance of new lesions or progression of any other lesion. Progressive disease was defined as a >25%increase in one or more of the measurable lesions or the appearance of a new lesion. Further patient characteristics are provided in Table 1.
The current study was registered and approved by the Korea Food and Drug Administration on 9 May 2007 (Approval Number: Korea Food and Drug Administration Biosimilar Products No. 362, 2007.5.9) All patients signed an informed consent form approved by the Institutional Review Board of the Seoul St. Mary's Hospital, the Catholic University of Korea and followed the Declaration of Helsinki protocols. Furthermore, the study was registered and approved by Clinical Research Information Service, Republic of Korea in the WHO Registry Network (KCT0001271).
Generation and characterization of EBV LMP1/2a-specific CTLs. Peripheral blood was obtained from each patient and mononuclear cells were isolated by using Ficoll-Hypaque (Amersham Pharmacia Biotech, Piscataway, NJ) density gradient centrifugation and cryopreserved. Human leukocyte antigen (HLA)-A subtypes of the donors were determined with sequence based typing in HLA laboratory. Consent forms and approval for this study were obtained from the donors and the Institutional Review Board of The Catholic University of Korea, College of Medicine
Following density separation, CD14+ monocytes, CD4+ T and CD8+ T cells were isolated by MACS System (MiltenyiBiotec, Bergisch Gladbach, Germany) according to the manufacturer's instructions. DCs were generated as described previously from CD14+ cells.32 Briefly, selected CD14+ cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum, 100 ng/ml GM-CSF (GM-CSF; Gentaur, Brussels, Belgium), and 100 ng/ml IL-4 (IL-4; Gentaur) every 3 days for 6–7 days in a humidified 37 °C, 5% CO2 incubator. On the sixth or seventh day, immature DCs were harvested and then electroporated for mRNA transduction.
The coding sequence for LMP1, LMP2a was obtained by extraction of total RNA from B95-8 transformed LCL using an RNeasy kit (Qiagen, Valencia, CA) and, after reverse transcription, LMP1, LMP2a cDNA was amplified by PCR with specific primers as follows: LMP1 forward primer, 5′-CAGTGTGCTGGAATTCATGGAACACGACCTTGAGAG; reverse primer, 5′-GATTCTCCTCCACGTCACCGCATGTTAGAAGACTTCCTCTGCCCTCCTCATAGCTTAGCTGAACTG, LMP2a forward primer, 5′- GAGGAAGTCTTCTAACATGCGGTGACGTGGAGGAGAATCCCGGCCCTATGGGGTCCCTAGAAATGGT; reverse primer, 5′- TAGATGCATG CTCGAG TTATACAGTGTTGCGATATG. The full-length of LMP1, LMP2a fragments were then ligated into pcDNA3 (+) (Invitrogen Carlsbad, CA) using its EcoRI and XhoI sites. Clones were sequenced to verify their identity. Resulting plasmid DNA was linearized and transcribed in vitro by using a mMESSAGE and mMACHINE kit (Ambion Austin, TX) according to the manufacturer's instructions. A 3′-poly (A) tail was added by using poly(A) polymerase (Ambion) followed by purification with an RNeasy kit. Electroporation of IVT mRNA into DCs was performed as described previously.2 In brief, DCs were washed twice with serum-free Opti-MEM, after mixing with 20 μg mRNA, they were then electroporated in a 2 mm cuvette by using an Electro Square Porator ECM 830 (Harvard Apparatus), under conditions of 450 V and 500 μSec for DCs. DCs were subsequently cultured in DC medium supplemented with GM-CSF and IL-4 for 24 hours, followed with tumor necrosis factor α (PeproTech Rocky Hill, NJ), IL-6 (PeproTech), IL-1β (PeproTech) and prostaglandin E2 (Sigma) for maturation.
EBV-specific CTLs were generated using autologous LMP1 and LMP2a electroporated DC as previously published.28 Briefly, purified CD8+ and Th1 polarized CD4+ T cells were used as responder cells and stimulated with LMP1 and LMP2a mRNA electroporated DCs at a stimulator: responder ratio of 1: 20. On the seventh day, CTLs were harvested, counted to assess viability using trypan blue exclusion, and restimulated with RNA-electroporated DCs at a S:R ratio of 1:10. Cells were cultured in CTLs media for 7 more days with 10 U/ml IL-2 (Proleukin; Chiron, Emeryville, CA) twice weekly from the 14th day. On the 20th day of initial stimulation, cells were harvested, counted, and analyzed for their phenotype, specificity, and functional capacity.
Immunophenotyping. LMP1/2a CTLs were stained with pairs of monoclonal antibodies that recognized the cell surface molecules CD3, CD4, CD8, CD19, CD56, CD45RA, CD45RO (BD Pharmingen, San Diego, CA) and combined them in pairs for dual flow cytometer analysis (FACSCalibur; BD Medical Systems, Franklin Lakes, NJ).
Treatment schedule. Patients completed all cycles of induction treatment including chemotherapy, radiotherapy, and/or HDT/SCT based on investigator's choice before the infusion of LMP1/2a CTLs. Cryopreserved CTLs were thawed and administered intravenously. Patients were monitored for vital signs before and immediately after each infusion. LMP1/2a CTLs were infused at weekly intervals for the first 4 weeks and again after 4 weeks to deliver four more doses. In total, patients received eight doses with each dose of 2 × 107 CTLs/m2.
Enzyme-linked immunospot assay. For detection of cells secreting interferon-γ (IFN-γ), ELISPOT assays were performed with BD ELISPOT assay kit, and the procedure was conducted according to the manufacturer's instructions. The CTL populations were serially diluted from 5 × 105 to 5 × 104, cells/well, and EBV antigen-specific activity was measured using unelectroporated or electroporated DC with LMP1/LMP2a mRNA. Each culture condition was run in triplicate. The number of spots corresponding to IFN-γ-secreting cells was counted using AID-ELISPOT-Reader (AID). ELISPOT assays were also performed using the patient's peripheral blood after EBV-CTL therapy to assess the contribution of the infused CTLs to the patient's EBV immune response.
EBV monitoring
EBV polymerase chain reaction analyses. To determine the EBV status of the patients, total RNA was purified from lymphoma biopsies and/or peripheral blood using RNAzol BTM (Tel-Test, Friendswood, TX). cDNA was synthesized by incubating 2 μg RNA at 42 °C for 60 minutes in a mixture containing 2.5 μmol/l of oligo (dT) primer, 1 mmol/l of dNTPs, and 0.5μl AMV reverse transcriptase (Behringer Mannheim, Mannheim, Germany).33 LMP1-specific primer pair (forward: 5′-AGCCCTCCTTGTCCTCTATTCCTT-3′, reverse: 5′-ACCAAGTCGCCAGAGCATCTCCAA-3′), LMP2A-specific primer pair (forward: 5′-ATGACTCATCTCAACACATA-3′, reverse: 5′-CATGTTAGGCAAATTGCAAA-3′), BARF1-specific primer pair (forward: 5′-CCAGAGCAATGGCCAGGTTC-3′, reverse: 5′-CAAGGTGAAATAGGCAAGTGCG-3′), GAPDH-specific primer pair (forward: 5′-ACCACAGTCCATGCCATCAC-3′, reverse: 5′-TCCACCACCCTGTTGCTGTA-3′) were used to amplify each mRNA cDNA. The amplified products were separated on a 3% agarose gel and visualized by ethidium bromide staining.
In order to monitor the levels of EBV in the patient's plasma, semiquantitative polymerase chain reaction was performed. DNA was extracted from plasma using QIAamp DNA Blood Mini kit (Qiagen) according to the manufacturer's instructions.34 To determine EBV copy number, we used EBV nuclear antigen-1 (EBNA-1)-specific primer sets (forward; 5′-GGATGCGATTAAGGACCTTGTT-3′, and reverse 5′-AAAGCTGCACACAGTCACCCT-3′) and TaqMan probe for EBNA-1 (5′-TGACAAAGCCCGCTCCTACCTGCAAT-3′). The PCR mixture was prepared using recommended volume of Premix EX TaqTM Kit (Takara Biotechnology, Japan) and 2.5μl of template DNA was extracted from plasma samples according to the manufacturer's instructions. The PCR reaction was performed by incubating the mixtures at 95 °C for 5 minutes, and then by 45 cycles of 95 °C for 5 seconds and 55 °C for 30 seconds.
Statistical analysis. Descriptive statistics were calculated to summarize CTL characteristics and immune monitoring data. Normally distributed continuous data were presented mean ± SD, and data that were not approximately normal in distribution were calculated median (range). Categorical data were presented by frequencies and percentages. Overall survival (OS) was defined as the time from the date of first CTL infusion until the date of death from any cause. Progression-free survival (PFS) was defined as the time from the date of infusion until the date of disease relapse/progression or death from any cause. The survival curves of OS and PFS were plotted using the Kaplan–Meier method. We calculated 95% confidence intervals for OS and PFS rates at 4 years were estimated using the Greenwood method.
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea (H12C0718) and the Catholic Institute of Cell Therapy from August 2007 to November 2010. We thank S.Y. Kim (Department of Hematology, Kunkuk University), S.E. Lee (Department of Hematology, Catholic University of Korea), and S.H. Kim (Department of Nuclear Medicine, Catholic University of Korea) for their assistance and support throughout the study. S.-G.C., and T.-G.K. designed the study. S.-G.C., H.-J.S., S.K.L., S.T.O., H.-J.L., H.-I.C., S.-E.J., G.P., J.H.O., B.-O.C., S.W.K., S.W.K., N.G.C., J.W.L., and Y.S.H. collected data. S.-G.C., N.K., H.-J.S., S.K.L., H.W.Y., and T.-G.K. analyzed and interpreted data. S.-G.C., N.K., H.-J.S., S.K.L., S.T.O., H.-J.L., H.-I.C., H.W.Y., S.-E.J., G.P., J.H.O., B.-O.C., S.W.K., S.W.K., N.G.C., J.W.L., Y.S.H., and T.-G.K. wrote and revised the manuscript. S.-G.C., N.K., H.-J.S., S.K.L., S.T.O., H.-J.L., H.-I.C., H.W.Y., S.-E.J., G.P., J.H.O., B.-O.C., S.W.K., S.W.K., N.G.C., J.W.L., Y.S.H., and T.-G.K. approved the final version. The authors declare that there are no conflicts of interest.
References
- Chim, CS, Ma, SY, Au, WY, Choy, C, Lie, AK, Liang, R et al. (2004). Primary nasal natural killer cell lymphoma: long-term treatment outcome and relationship with the International Prognostic Index. Blood 103: 216–221. [DOI] [PubMed] [Google Scholar]
- Li, YX, Yao, B, Jin, J, Wang, WH, Liu, YP, Song, YW et al. (2006). Radiotherapy as primary treatment for stage IE and IIE nasal natural killer/T-cell lymphoma. J Clin Oncol 24: 181–189. [DOI] [PubMed] [Google Scholar]
- Cheung, MM, Chan, JK, Lau, WH, Foo, W, Chan, PT, Ng, CS et al. (1998). Primary non-Hodgkin's lymphoma of the nose and nasopharynx: clinical features, tumor immunophenotype, and treatment outcome in 113 patients. J Clin Oncol 16: 70–77. [DOI] [PubMed] [Google Scholar]
- Kim, GE, Lee, SW, Chang, SK, Park, HC, Pyo, HR, Kim, JH et al. (2001). Combined chemotherapy and radiation versus radiation alone in the management of localized angiocentric lymphoma of the head and neck. Radiother Oncol 61: 261–269. [DOI] [PubMed] [Google Scholar]
- Kim, SJ, Kim, K, Kim, BS, Kim, CY, Suh, C, Huh, J et al. (2009). Phase II trial of concurrent radiation and weekly cisplatin followed by VIPD chemotherapy in newly diagnosed, stage IE to IIE, nasal, extranodal NK/T-Cell Lymphoma: Consortium for Improving Survival of Lymphoma study. J Clin Oncol 27: 6027–6032. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M, Suzuki, R, Kwong, YL, Kim, WS, Hasegawa, Y, Izutsu, K et al. (2008). Phase I study of dexamethasone, methotrexate, ifosfamide, L-asparaginase, and etoposide (SMILE) chemotherapy for advanced-stage, relapsed or refractory extranodal natural killer (NK)/T-cell lymphoma and leukemia. Cancer Sci 99: 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito, Y, Kimura, H, Maeda, Y, Hashimoto, C, Ishida, F, Izutsu, K et al. (2012). Pretreatment EBV-DNA copy number is predictive of response and toxicities to SMILE chemotherapy for extranodal NK/T-cell lymphoma, nasal type. Clin Cancer Res 18: 4183–4190. [DOI] [PubMed] [Google Scholar]
- Yamaguchi, M, Kwong, YL, Kim, WS, Maeda, Y, Hashimoto, C, Suh, C et al. (2011). Phase II study of SMILE chemotherapy for newly diagnosed stage IV, relapsed, or refractory extranodal natural killer (NK)/T-cell lymphoma, nasal type: the NK-Cell Tumor Study Group study. J Clin Oncol 29: 4410–4416. [DOI] [PubMed] [Google Scholar]
- Drénou, B, Lamy, T, Amiot, L, Fardel, O, Caulet-Maugendre, S, Sasportes, M et al. (1997). CD3- CD56+ non-Hodgkin's lymphomas with an aggressive behavior related to multidrug resistance. Blood 89: 2966–2974. [PubMed] [Google Scholar]
- Yamaguchi, M, Kita, K, Miwa, H, Nishii, K, Oka, K, Ohno, T et al. (1995). Frequent expression of P-glycoprotein/MDR1 by nasal T-cell lymphoma cells. Cancer 76: 2351–2356. [DOI] [PubMed] [Google Scholar]
- Huang, WT, Chang, KC, Huang, GC, Hsiao, JR, Chen, HH, Chuang, SS et al. (2005). Bone marrow that is positive for Epstein-Barr virus encoded RNA-1 by in situ hybridization is related with a poor prognosis in patients with extranodal natural killer/T-cell lymphoma, nasal type. Haematologica 90: 1063–1069. [PubMed] [Google Scholar]
- Ishii, H, Ogino, T, Berger, C, Köchli-Schmitz, N, Nagato, T, Takahara, M et al. (2007). Clinical usefulness of serum EBV DNA levels of BamHI W and LMP1 for Nasal NK/T-cell lymphoma. J Med Virol 79: 562–572. [DOI] [PubMed] [Google Scholar]
- Heslop, HE, Slobod, KS, Pule, MA, Hale, GA, Rousseau, A, Smith, CA et al. (2010). Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood 115: 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney, CM, Smith, CA, Ng, CY, Loftin, S, Li, C, Krance, RA et al. (1995). Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet 345: 9–13. [DOI] [PubMed] [Google Scholar]
- Bollard, CM, Aguilar, L, Straathof, KC, Gahn, B, Huls, MH, Rousseau, A et al. (2004). Cytotoxic T lymphocyte therapy for Epstein-Barr virus+ Hodgkin's disease. J Exp Med 200: 1623–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straathof, KC, Bollard, CM, Popat, U, Huls, MH, Lopez, T, Morriss, MC et al. (2005). Treatment of nasopharyngeal carcinoma with Epstein-Barr virus–specific T lymphocytes. Blood 105: 1898–1904. [DOI] [PubMed] [Google Scholar]
- Cho, HI, Hong, YS, Lee, MA, Kim, EK, Yoon, SH, Kim, CC et al. (2006). Adoptive transfer of Epstein-Barr virus-specific cytotoxic T-lymphocytes for the treatment of angiocentric lymphomas. Int J Hematol 83: 66–73. [DOI] [PubMed] [Google Scholar]
- Bollard, CM, Gottschalk, S, Leen, AM, Weiss, H, Straathof, KC, Carrum, G et al. (2007). Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood 110: 2838–2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollard, CM, Gottschalk, S, Torrano, V, Diouf, O, Ku, S, Hazrat, Y et al. (2014). Sustained complete responses in patients with lymphoma receiving autologous cytotoxic T lymphocytes targeting Epstein-Barr virus latent membrane proteins. J Clin Oncol 32: 798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong, YL, Anderson, BO, Advani, R, Kim, WS, Levine, AM and Lim, ST; Asian Oncology Summit (2009). Management of T-cell and natural-killer-cell neoplasms in Asia: consensus statement from the Asian Oncology Summit 2009. Lancet Oncol 10: 1093–1101. [DOI] [PubMed] [Google Scholar]
- Tse, E and Kwong, YL (2013). How I treat NK/T-cell lymphomas. Blood 121: 4997–5005. [DOI] [PubMed] [Google Scholar]
- Kohrt, H, Lee, M and Advani, R (2010). Risk stratification in extranodal natural killer/T-cell lymphoma. Expert Rev Anticancer Ther 10: 1395–1405. [DOI] [PubMed] [Google Scholar]
- Kim, HJ, Bang, SM, Lee, J, Kwon, HC, Suh, C, Kim, HJ et al. (2006). High-dose chemotherapy with autologous stem cell transplantation in extranodal NK/T-cell lymphoma: a retrospective comparison with non-transplantation cases. Bone Marrow Transplant 37: 819–824. [DOI] [PubMed] [Google Scholar]
- Suzuki, R, Suzumiya, J, Nakamura, S, Kagami, Y, Kameoka, JI, Sakai, C et al.; NK-cell Tumor Study Group. (2006). Hematopoietic stem cell transplantation for natural killer-cell lineage neoplasms. Bone Marrow Transplant 37: 425–431. [DOI] [PubMed] [Google Scholar]
- Au, WY, Pang, A, Choy, C, Chim, CS and Kwong, YL (2004). Quantification of circulating Epstein-Barr virus (EBV) DNA in the diagnosis and monitoring of natural killer cell and EBV-positive lymphomas in immunocompetent patients. Blood 104: 243–249. [DOI] [PubMed] [Google Scholar]
- Suzuki, R, Yamaguchi, M, Izutsu, K, Yamamoto, G, Takada, K, Harabuchi, Y et al.; NK-cell Tumor Study Group. (2011). Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 118: 6018–6022. [DOI] [PubMed] [Google Scholar]
- Kim, SY, Cho, SG, Kim, SW, Choi, BO, Park, KS, Lim, J et al. (2011). Pilot study of pegylated interferon alpha-2a treatment during chemo- and radiotherapy and post-remission maintenance in patients with EBV-positive extranodal NK/T cell lymphoma. Ann Hematol 90: 693–699. [DOI] [PubMed] [Google Scholar]
- Merlo, A, Turrini, R, Bobisse, S, Zamarchi, R, Alaggio, R, Dolcetti, R et al. (2010). Virus-specific cytotoxic CD4+ T cells for the treatment of EBV-related tumors. J Immunol 184: 5895–5902. [DOI] [PubMed] [Google Scholar]
- Appay, V, Zaunders, JJ, Papagno, L, Sutton, J, Jaramillo, A, Waters, A et al. (2002). Characterization of CD4(+) CTLs ex vivo. J Immunol 168: 5954–5958. [DOI] [PubMed] [Google Scholar]
- Berger, C, Jensen, MC, Lansdorp, PM, Gough, M, Elliott, C and Riddell, SR (2008). Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest 118: 294–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, G, Gerdemann, U, Caruana, I, Savoldo, B, Hensel, NF, Rabin, KR et al. (2013). Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 27: 1538–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, JS, Sohn, HJ, Park, GS, Chung, YJ and Kim, TG (2011). Induction of antitumor immunity using dendritic cells electroporated with Polo-like kinase 1 (Plk1) mRNA in murine tumor models. Cancer Sci 102: 1448–1454. [DOI] [PubMed] [Google Scholar]
- Chen, H, Smith, P, Ambinder, RF and Hayward, SD (1999). Expression of Epstein-Barr virus BamHI-A rightward transcripts in latently infected B cells from peripheral blood. Blood 93: 3026–3032. [PubMed] [Google Scholar]
- Teramura, T, Tabata, Y, Yagi, T, Morimoto, A, Hibi, S and Imashuku, S (2002). Quantitative analysis of cell-free Epstein-Barr virus genome copy number in patients with EBV-associated hemophagocytic lymphohistiocytosis. Leuk Lymphoma 43: 173–179. [DOI] [PubMed] [Google Scholar]