Abstract
Osteoporosis, caused by a relative increase of bone resorption over bone formation, is characterized by decreased bone mass and bone strength, resulting in an increased incidence of bone fractures, which often leads to further disability and early mortality in the elderly due to impaired bone healing ability. The majority of therapeutics currently used in clinics for the treatment of osteoporosis are antiresorptive agents that exert their clinical effect by decreasing the rate of bone resorption. However, strategies solely aimed at antiresorption have limited therapeutic efficacy in restoring bone remodeling balance and enhancing osteoporotic fracture healing. Here, we report that miR-26a plays a critical role in modulating bone formation during osteoporosis. We found that miR-26a treatment could effectively improve the osteogenic differentiation capability of mesenchymal stem cells isolated from littermate-derived ovariectomized osteoporotic mice both in vitro and in vivo. MiR-26a exerts its effect by directly targeting Tob1, the negative regulator of BMP/Smad signaling pathway by binding to the 3′-untranslated region and thus repressing Tob1 protein expression. Our findings indicate that miR-26a may be a promising therapeutic candidate to enhance bone formation in treatment of osteoporosis and to promote bone regeneration in osteoporotic fracture healing.
Introduction
Normal physiological bone structure and function are maintained by the constant process of bone remodeling, which is regulated by the balance and coupling of osteoblast-mediated bone formation and osteoclast-mediated bone resorption.1 Osteoporosis, the most frequent bone remodeling disease, is caused by a relative increase of bone resorption over bone formation.2 Osteoporosis is characterized by decreased bone mass and strength, which results in a high incidence of bone fractures. The osteoporotic fracture often leads to further disability and premature mortality in the elderly due to impaired bone healing ability.3 Worldwide, approximately one in two women and one in four men over the age of 40 are expected to suffer from osteoporotic fractures.4
Although a variety of therapeutics have been used clinically for the treatment of osteoporosis, the vast majority are antiresorptive agents, which exert their effect by decreasing the rate of bone resorption.5 Less attention has been paid to a strategy aimed at reversal of reduced bone formation and rebuilding of damaged bone tissues. As such, only parathyroid hormone (PTH) has been approved by the FDA to be used in clinics to stimulate bone formation with intermittent injection periods encompassing up to 2 years.6,7,8 Because continuous treatment with PTH may cause bone resorption, as well as being costly over the 2-year administration,7,8,9,10 there is a need for the development of novel anabolic therapeutics targeting bone formation.
Alternatively, mesenchymal stem cells (MSCs) are the basic cellular unit of embryologic bone formation. Due to their self-renewal and osteogenic capability and as a promising means of tissue regeneration and repair, they have been widely used in bone regenerative applications.11,12 Moreover, it is conceivable that autologous MSCs could be used for such applications, thereby avoiding potential immune responses. However, our previous studies showed that the osteogenic capability of MSCs derived from osteoporotic animals was impaired.13,14 Therefore, it is important to discover novel anabolic molecules regulating MSC function under osteoporotic conditions.
MiRNAs, the noncoding ~22-nucleotide RNAs serving as repressors of gene expression at the post-transcriptional regulation level, are involved in a broad spectrum of biological processes.15 Several miRNAs have been identified to be positively or negatively involved in regulating osteogenic differentiation, using an in vitro cell differentiation model.16,17,18,19,20,21,22,23,24 However, most of these miRNAs have only been identified in vitro, and their functional roles in the pathophysiological mechanisms responsible for impaired bone formation in skeletal disorders remain to be established before they can be targeted clinically. Furthermore, the majority of miRNA studies among the previous reports focused on inhibiting miRNA functions. This study sought to identify means to mimic the activity of endogenous miRNA, which may provide a more applicable way for miRNA to be used in clinics.
In the present study, a miRNA, namely miR-26a, was identified to be negatively correlated with bone loss in estrogen deficiency-induced osteoporotic mice. Our findings further demonstrated that therapeutic overexpression of miR-26a in osteoporosis-impaired MSCs can lead to repression of Tob1 expression, which may coincide with the promotion of osteogenic capacity in osteoporosis-impaired MSCs. This observation provides the first clinical insight into the contribution of a miRNA to the restoration of osteogenic ability under osteoporotic conditions by exerting an anabolic effect.
Results
MiR-26a expression was negatively correlated with bone loss in osteoporotic mice
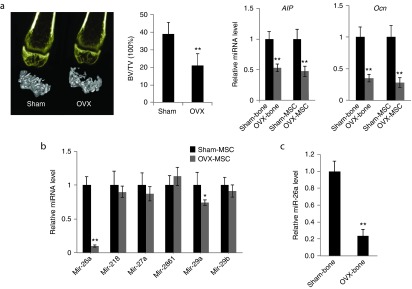
To investigate the miRNA expression profile during osteoporosis, an estrogen deficiency–induced osteoporotic mice model was generated and confirmed by quantification of the bone volume/tissue volume (BV/TV) ratio in the distal portion of femur using micro-CT and by analysis of gene expression of osteogenic markers including alkaline phosphatase (Alp) and osteocalcin (Ocn) using RT-qPCR. Alp is an early-stage osteogenic marker and Ocn is a marker of mineralization. The results revealed that BV/TV was significantly reduced in OVX group and that the osteogenic markers Alp and Ocn were correspondingly downregulated in both distal femur bone tissues and isolated MSC (P < 0.01) (Figure 1a). The miRNA microarray analysis was comprehensively performed on OVX-MSCs and MSC harvested from sham-treated mice (sham-MSCs). Among the differentially expressed miRNAs, several were selected because of obvious downregulation in the two groups. We further validated the altered expression profiles of selected miRNAs in both distal femur and MSCs harvested from the OVX or sham mice using RT-qPCR analysis, repeated three times. The results consistently showed that expression levels of miR-26a decreased significantly more than other miRNAs in the OVX group. In our previous study, we reported that miR-26a could significantly enhance bone repair and regeneration ability by positively regulating osteoblastic activity.25 We also screened miRNAs that had previously been shown to have a role in osteogenic differentiation including miR-29b, miR-2861, miR-27, miR-29a, and miR-21820,22,23,24,26 and identified that miR-26a exhibited profoundly diminished (P < 0.01) expression in the OVX group than in the others (Figure 1b). Therefore, miR-26a was identified as a target miRNA due to its obvious potential to enhance bone formation in osteoporosis.
Figure 1.
miR-26a expression was profoundly down-regulated during the process of estrogen deficiency-induced osteoporosis. (a) Micro-CT analysis of trabecular bone volume in the distal femur at 8 weeks postovariectomy. The distal femur cancellous bone histomorphometry was assessed by measuring BV/TV. Alp and Ocn mRNA levels in bone tissue and isolated mesenchymal stem cells (MSCs) were quantified by real time quantitative polymerase assay (RT-qPCR). (b) Expression variations of miRNA candidates (miR-29b, miR-2861, miR-27, miR-29a, and miR-218) in OVX-MSC compared to sham-MSC using RT-qPCR analysis (c) RT-qPCR analysis of miR-26a expression levels in isolated MSC in femoral bone tissues of OVX-mice compared to sham-treated mice. The level of miR-26a was normalized to U6. Data represent means ± SD. *P < 0.05, **P < 0.01 (n = 6 femurs/3 mice each group).
The altered expression profile of miR-26a was further validated by RT-qPCR in distal femur bone tissue from the OVX or sham mice. The expression changes were confirmed in distal femur bone tissue with fourfold decreased expression (P < 0.01) in OVX mice (Figure 1c).
MiR-26a overexpression partially rescued osteogenic function of OVX-MSC in vitro
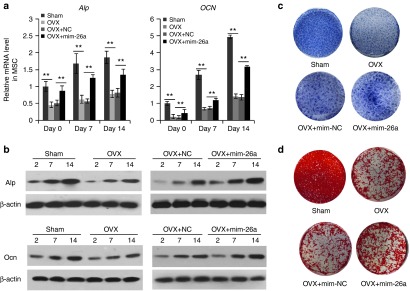
To investigate the role of miR-26a in regulating osteogenic function of OVX-MSC, expression levels of the two osteogenic marker genes Alp and Ocn were quantified in four groups including OVX-MSC with no treatment, sham-MSC with no treatment, OVX-MSC transfected with miR-26a mimics (mim-26a), and OVX transfected with negative control (mim-NC) through both RT-qPCR and western blotting. Mature miR-26a expression levels were evaluated by RT-qPCR analysis. Two days after transfection, miR-26a expression levels were increased 50-fold in OVX-MSCs under miR-26a treatment, compared to treatment with the negative control counterpart. The expression levels remained elevated (P < 0.01) through a 14-day observation period (Supplementary Figure S1). Consequently, the gene expression levels of the two osteogenic marker genes (Alp and Ocn) were decreased under osteoporotic conditions and enhanced in OVX-MSC transfected with mim-26a during the osteogenic induction period of 14 days (P < 0.05) (Figure 2a). Alp and Ocn protein expression was substantially enhanced in the mim-26a-treated group (Figure 2b). Consistent with the elevation of protein expression levels, activity of Alp was significantly enhanced in OVX-MSC after treatment with mim-26a (Figure 2c). In addition, matrix mineralization was enhanced by augmented miR-26a expression (Figure 2d). These data collectively indicate that the reduced osteogenic function of OVX-MSC can be partially rescued by miR-26a overexpression.
Figure 2.
miR-26a expression partially rescued osteogenic function of OVX-MSC in vitro. (a) RT-qPCR analysis of Alp and Ocn mRNA expression levels in OVX-MSC, sham-mesenchymal stem cells (MSC) and miR-26a or negative control-treated OVX-MSC at 0, 7 and 14 d post-osteogenic induction. (b) Western blot analysis of protein expression levels in OVX-MSC, sham-MSC and miR-26a or negative control treated OVX-MSC at 2, 7, and 14 days postosteogenic induction. (c) Alp staining and (d) Alizarin red S staining of OVX-MSC, sham-MSC, miR-26a or negative control treated OVX-MSC after 7 and 21 days of osteogenic induction, respectively. Data represent means ± SD. *P < 0.05, **P < 0.01 (n = 3 mice each group).
MiR-26a directly targeted Tob1
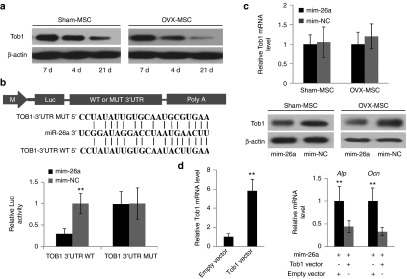
To further investigate the mechanism underlying the observation of the “rescue effect” of miR-26a overexpression in OVX-MSC, TargetScan release 6.2 ((http://genes.mit.edu/targetscan) was used to predict molecular targets of miR-26a. Only miRNAs conserved among the different species that target conserved gene transcripts were considered. A total of 747 putative targets were obtained. We focused on Tob1, a negative regulator of the BMP/Smad signaling pathway, and which has been reported to suppress bone formation in adult animals without altering bone resorption.27 Tob1 deficiency has been found to enhance osteoblastic activity in estrogen deficiency–induced osteoporotic mice by increasing osteoblast numbers.28 We found the Tob1 gene harbors a miR-26a binding site in its 3′-untranslated region (UTR). During osteogenic induction, miR-26a expression levels increased gradually, while Tob1 expression was decreased in both sham-MSC and OVX-MSC (Figure 3a). To test whether miR-26a directly targeted Tob1, luciferase reporter gene constructs including a wild-type (WT) Tob1 3′UTR or a Tob1 3′UTR containing mutant sequences of the miR-26a binding site (MUT), were generated. WT or MUT luciferase reporter was cotransfected with mim-26a or mim-NC. MiR-26a treatment significantly inhibited the luciferase reporter activity of WT Tob1 3′UTR, whereas the luciferase reporter activity of MUT Tob1 3′UTR was not repressed by miR-26a treatment (Figure 3b). We also observed Tob1 protein expression was downregulated in MSC by miR-26a treatment, whereas Tob1 mRNA expression remained unaltered (Figure 3c). To determine whether miR-26a functionally targeted Tob1 in rescuing the osteogenic function of OVX-MSC, miR-26a and Tob1 overexpression- or empty-vectors were cotransfected in OVX-MSC. It was found that, accompanying upregulated Tob1 expression levels, Alp and Ocn mRNA levels were decreased (P < 0.05) in OVX-MSC after Tob1 expression vector treatment compared to OVX-MSC treated with empty vector (Figure 3d). Taken together, these data indicate that Tob1 was the direct functional target of miR-26a under osteoporotic conditions.
Figure 3.
miR-26a directly targeted Tob1. (a) Western blot analysis of Tob1 protein expression in mesenchymal stem cells (MSC) during osteogenic induction. (b) Schematic illustration of putative miR-26a target site in mouse Tob1 3′UTR and alignment of miR-26a with wild-type (WT) and mutant (MUT) 3′UTR region showing complementary base-pairing to Tob1. MSCs were cotransfected with luciferase reporter constructs carrying WT 3′UTR or MUT 3′UTR, phRL-null (renilla plasmid) and miR-26a mimics or corresponding controls. Effects of miR-26a and corresponding controls on luciferase activity of reporter constructs were determined 48 hours post-transfection. Firefly luciferase activity values were normalized to renilla luciferase activity values. (c) RT-qPCR and Western blot analysis of Tob1 expression in MSC treated with miR-26a or corresponding control. (d) RT-qPCR analysis of Alp and Ocn mRNA levels in OVX-MSC after cotransfection of miR-26a mimics and Tob1 expression vector or empty vector. Data represent means ± SD. *P < 0.05, **P < 0.01 (n = 3 mice each group).
To further confirm that the effect of miR-26a on osteogenesis under osteoporotic conditions can, to some extent, be attributed to Tob1, we treated the MSCs with Tob1 siRNA to downregulate its expression and further used two models (subcutaneous implantation in NOD/SCID mice and bone defect regeneration model in OVX mice) to test the bone regeneration and repair ability of OVX-MSCs with the deficient expression of Tob1. We found that deficient Tob1 expression lead to both enhanced bone regeneration and repair ability in OVX-MSCs, as shown in Supplementary Figures S2 and S3.
Based on the above evidence, we concluded that Tob1 expression was central to osteoporosis. Furthermore, Tob1 played a key role in the regulatory process of miR-26a on osteogenesis under osteoporotic conditions.
MiR-26a overexpression partially rescued ectopic bone formation capability of OVX-MSC in vivo
To further investigate whether increased miR-26a expression could rescue the ectopic bone formation capability of OVX-MSC in vivo, nontransfected sham-MSCs, OVX-MSCs and OVX-MSCs treated with miR-26a, or negative control were loaded on HA-TCP scaffolds and implanted subcutaneously into NOD/SCID mice. At 8 weeks postimplantation, gene expression levels of Alp and Ocn were detected by RT-qPCR analysis. H&E staining showed that bone mass was significantly reduced in the OVX-MSC group compared with that of sham-MSC group (P < 0.01), whereas bone mass was enhanced in miR-26a treated OVX-MSC group when compared with negative control treated OVX-MSC group (P < 0.01) (Figure 4a). The data revealed that Alp and Ocn were both downregulated in the OVX-MSC group when compared with sham-MSC group (P < 0.01) (Figure 4b). Meanwhile, both markers were upregulated in mim-26a-treated OVX-MSC group in comparison to the negative control treated OVX-MSC group (P < 0.01) (Figure 4b). Taken together, these data reveal that increased miR-26a expression can rescue ectopic bone formation capability of OVX-MSC in vivo.
Figure 4.
miR-26a treatment promoted ectopic bone formation ability of OVX-MSC in vivo. Sham-mesenchymal stem cells (MSC), OVX-MSC, OVX-MSC treated with miR-26a or corresponding controls were seeded on HA-TCP scaffolds and the constructs implanted into NOD/SCID mice. (a) H&E staining and (b) RT-qPCR analysis of Alp and Ocn expression were performed after 8 weeks of implantation. Formation of bone (B) and connective tissue (CT) around the HA-TCP (HA) are indicated. Data represent means ± SD. Scale bar: 100 μm. **P < 0.01 (n = 3 mice each group).
Critical-sized calvarial bone defect regeneration with miR-26a-treated OVX-MSCs
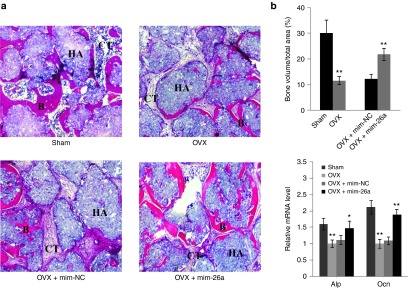
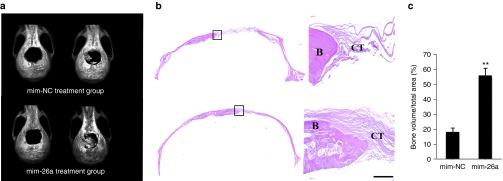
A 5-mm critical-sized calvarial bone defect was generated in osteoporotic mice. Littermate-derived OVX-MSCs were mixed with antagomir-26a or corresponding control delivery hydrogel and then placed into the defect area. The mature miR-26a expression level within the defect area was evaluated using RT-qPCR analysis. Three days after transfection, miR-26a expression levels were elevated eightfold under miR-26a treatment when compared to treatment with negative control counterpart. At 3 months postimplantation, miR-26a expression within the defect area had returned to original levels (Supplementary Figure S4). Live micro-CT was used to track the neo-bone formation from day 0 to 12 weeks after surgery. The results showed that, after 12 weeks of implantation, the miR-26a-treated group showed significantly more bone repair than the negative control group (Figure 5a). Histological examination confirmed the results of the micro-CT observations (Figure 5b). Furthermore, quantification analysis revealed that more than 50% new bone was regenerated under mim-26a treatment. In contrast, less than 20% new bone was formed in the negative control group (Figure 5c).
Figure 5.
MiR-26a treatment improved littermate-derived OVX-MSCs mediated critical-sized calvarial bone defect repair. Live Micro-CT at day 0 and week 12 and H&E staining images of neo-bone after 12 weeks of implantation. Formation of bone (B) and connective tissue (CT) are indicated. Scale bar: 100 µm. **P < 0.01(n = 3 mice each group).
To further investigate whether local miR-26a delivery in vivo would also have an effect on other tissue of the body, we measured miR-26a expression in various tissues, including muscle, brain, heart, liver, and lung and found that these were unaffected by miR-26a treatment, as displayed in Supplementary Figure S5.
To further investigate the role of miR-26a in osteoporosis, we applied antagomir-26a or its negative control via intravenous injection so as to silence endogenous miR-26a expression in vivo. At 3 months postapplication, proximal tibia from both groups were collected for micro-CT analysis and BV/TV further quantified as shown in Supplementary Figure S6. We found that decreased miR-26a expression prompted a 34% lower BV/TV value than did negative controls under osteoporotic conditions. Therefore, we conclude that miR-26a has a substantial effect on osteoporosis.
Discussion
This study identified that miR-26a is an important regulator of osteoporosis. Decreased expression of miR-26a in osteoporotic mice leads to impaired bone formation. Our results suggest that therapeutic enhancement of miR-26a expression in OVX-MSC can promote bone formation ability by exerting an anabolic effect.
Multiple miRNAs are reported to be involved in osteogenesis-related signaling pathways by regulating bone protein expression, post-transcriptionally.16,20,26,29,30,31 Less work has been performed on the role of miRNAs in regulating bone remodeling and in disorders such as osteoporosis. In this study, we identified a novel anabolic agent, miR-26a, able to restore the osteogenic capacity of osteoporosis-impaired MSCs. We found that elevated miR-26a expression levels accompanied the increased expression of osteoblast marker genes including Alp and Ocn in osteoporosis-impaired MSCs. Furthermore, the impaired bone-forming ability of OVX-MSC was partially rescued by enhanced miR-26a expression in vivo. This observation provides the first clinical insight into the contribution of a miRNA to the restoration of bone mass under osteoporotic circumstances by exerting an anabolic effect.
In previous reports, miR-26a expression has been found to be consistently up- or down-regulated in a wide range of tumors, such as hepatocellular32 and nasopharyngeal33 carcinomas, lung34 and breast35 cancers, glioma,36 and cholangiocarcinoma,37 indicating that miR-26a is a critical regulator in carcinogenesis and tumor progression, resembling oncogene or tumor suppressor gene activity in various cancers. In our study, we define a new mechanism whereby miR-26a is involved in the regulation of the abundance of Tob1 protein in osteoporotic MSCs. Tob1, a protein highly expressed in bone tissue, is a negative regulator of BMP/Smad signaling in osteoblasts.27 Tob1 deficiency greatly enhanced osteoblast activity in osteoporosis. The amount of Tob1 protein is strictly regulated through transcriptional, translational and post-translational mechanisms in different cellular contexts. Our data demonstrated that elevated miR-26a expression was accompanied by significantly decreased Tob1 protein expression. Furthermore, our experimental evidence from in vitro studies suggests that Tob1 is a functional target of miR-26a and mediates its regulation role in bone formation.
Bone formation is a remodeling process and depends on a delicate balance between bone formation and bone resorption. However, bone resorption levels can exceed those of bone formation, resulting in osteoporosis and accompanying increases in bone fragility and impaired bone healing ability. Approximately 200 million people are estimated to suffer from this disease worldwide.38 A major need in the treatment of this disease is to identify safe anabolic agents that can increase bone formation on a long-term basis to such an extent that they compensate for the increase in bone resorption. Thus far, the major focus has been on preventing bone resorption through inhibition of osteoclast activity. Only parathyroid hormone (PTH) has been used in clinics to stimulate bone formation with intermittent injection spanning up to 2 years. However, the way may increase bone resorption but has no effect on promoting osteoporotic fracture at all. Therefore, further research may yield means to overcome these difficulties. Our study provides a new finding: that miRNA in OVX-MSCs enhanced bone formation capacity in vitro and in vivo and that the specific miRNA can be targeted in these cells to rescue impaired bone formation as a form of cell therapy.
In conclusion, this study demonstrated that therapeutic overexpression of miR-26a in osteoporosis-impaired MSCs may lead to the repression of Tob1 expression, which may coincide with the promotion of osteogenic ability in osteoporosis-impaired MSCs, both in vitro and in vivo. Our findings have identified that miR-26a may be a promising therapeutic target for potent osteoporosis and osteoporotic fracture treatments.
Materials and Methods
Animals. C57BL/6J and immunocompromised mice were obtained from the Laboratory Animal Research Centre of the Fourth Military Medical University, Xi'an, China. All animal experiments were conducted in accordance with the committee guidelines of Animal Ethics and Experimental Safety of the Fourth Military Medical University and met the NIH guidelines for the care and use of laboratory animals.
Ovariectomy (OVX)-induced osteoporotic mouse model. C57BL/6J female mice at age of 6–8 weeks were randomly divided into two groups to receive either sham or bilateral OVX surgery. At 8 weeks postsurgery, bilateral femurs from sham-operated or OVX mice were collected and further analyzed using microCT. The osteoporotic model was confirmed by quantification of the bone BV/TV of the distal femurs.
Cell culture. MSC was isolated from an aspirate of bone marrow harvested from the tibia and femur in mice undergoing sham or OVX surgery and cultured as previously described.39 Briefly, after euthanasia, the hind limbs were aseptically removed and tibia and femur were further dissected free of soft tissues. Bone marrow was flushed from the dissected bones with growth medium containing α-MEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS, 1% penicillin/streptomycin. The cell suspension was seeded in 10-cm dishes and maintained in the growth medium in a humidified atmosphere of 5% CO2 at 37 °C. The medium was changed every 2–3 days and nonadherent cells were removed. Confluent cells were digested using 0.25% trypsin containing 10 mmol/l EDTA and cells at passage 3–5 were used for the experiments.
Osteogenic differentiation. A total of 1 × 105 MSC were seeded into each well of a six-well plate. At 80% confluence, the cell culture medium was replaced with osteogenic medium containing 100 nmol/l dexamethasone (Sigma-Aldrich, St. Louis, MO), 5 mmol/l β-glycerophosphate, and 50 μg/ml ascorbic acid. After 21 days of induction, the cells were fixed and stained with 40 mmol/l alizarin red S (ARS, Sigma-Aldrich).
MiRNA transfection. MSC were transfected with miR-26a mimics, or their respective negative control (NC, Guangdong Ruibo, China) using siPORT NeoFX transfection reagent (Life Technologies, Carlsbad, CA) following the instruction of the manufacturer. Briefly, 5 μl transfection reagent was diluted in 50 μl Opti-MEM I (Life Technologies) and mixed with 50 nmol/l miR-26a mimics, or equal amounts of its respective negative control. After being incubated for 10 minutes at room temperature, the mixture was dispensed together with 2.5 ml MSC suspension (1 × 106 cells/ml) into six-well plates. At 6 hours after transfection, the medium was replaced with fresh cell culture medium. The transfected cells were further induced in osteogenic medium at 40–50% confluence and harvested at day 0, 2, 7, and 14. Total RNA was extracted and the expression level of miR-26a and osteogenic markers including Alp and Ocn were analyzed by real-time-quantitative polymerase chain reaction (RT-qPCR). All transfection experiments were repeated in triple.
RT-qPCR. Osteogenic gene expression level was examined by RT-qPCR. The primer information of Alp and Ocn were shown in Supplementary Table S1. RT-qPCR was performed according to the published protocol.14 Total RNA was extracted using Trizol Reagent (Invitrogen) according to the manufacturer's protocol. Complementary DNAs (cDNAs) were synthesized using a PrimeScript RT reagent kit (TaKaRa, Dalian, China) following conditions: 37 °C for 15 minutes, 85 °C for 5 seconds, hold at 4 °C. For miRNAs expression analysis, miRNA was reverse-transcribed using specific RT primers (RiboBio, Guangzhou, China) following conditions: 37 °C for 30 minutes, 85 °C for 5 seconds, hold at 4 °C.
RT-qPCR reactions were performed using the SYBR Premix Ex Taq II kit (TaKaRa) and detected on the ABI Prism 7500 HT sequence detection system (Applied Biosystems, Foster City, CA). RT-qPCR was performed using the following cycles: 3 minutes at 95 °C, 15 seconds at 95 °C and 30 seconds at 60 °C for 40 cycles. β-actin and U6 were used as loading controls for quantitation of mRNA and miRNAs respectively. The Bulge-Loop miRNA RT-qPCR Primer Set (Ribo Bio, Guangzhou, China) were used for RT-qPCR of miR-26a (Product ID: miRQ0003495-1–2), miR-218 (Product ID: miRQ0004665-1-1), miR-27a (Product ID: miRQ0004633-1-1), miR-2861(Product ID:miRQ0013803-1-1), miR-29a(Product ID:miRQ0004631-1-1), miR-29b(Product ID:miRQ0017063-1-1), and U6 (Product ID: MQP-0202). Raw data can then be analyzed with SDS Relative Quantification Software version 2.2.3 (Applied BioSystems), generally using the automatic cycle threshold (Ct) setting for assigning baseline and threshold for Ct determination. The standard curve, melt profile and melt profile with no template control and RT control of the genes (Alp, Tob1, Ocn, β-actin, miR-26a and U6) detected by RT-qPCR could be found in Supplementary Figure S10.
Western blot. The protein expression levels of Alp and Ocn were examined by Western blot. Briefly, cells were washed with phosphate-buffered saline twice followed by scraped and lysed in lysis buffer (50 mmol/l Tris, pH 7.5, 250 mmol/l NaCl, 0.1% SDS, 2 mmol/l dithiothreitol, 0.5% NP-40, 1 mmol/l phenylmethylsulfonyl fluoride (PMSF), and protease inhibitor cocktail) on ice for 30 minutes. The lysates were clear and protein fractions were collected by centrifugation at 15,000 g at 4 °C for 10 minutes. The protein concentrations of cell lysis were determined with a Bradford protein assay. Hundred micrograms of protein per sample were loaded onto a 7.5% polyacrylamide gel. After electrophoresis, proteins were transferred to a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA). The membrane was blocked with 5% nonfat milk in phosphate-buffered saline and then incubated with antibodies against Alp, Ocn (sc-28904; sc-18319, Santa Cruz, Dallas, TX) or β-actin (ab8227, Abcam, Cambridge, MA) at 1: 1,000 dilutions for 3 hours. The membrane was then incubated with secondary antibodies conjugated with horseradish peroxidase for 1 hour. The Blot was processed using an ECL kit (Santa Cruz). Full scans of blots labeled with the intensity quantification were shown in Supplementary Figure S7.
Luciferase reporter assay. MiR-26a binding sites of Tob1 3′UTRs were amplified by PCR from mouse total cDNA. The length of miR-26a binding sites is 360 bp. It is not full-length Tob1 3'UTR. The PCR product was then subcloned into HindIII and SpeI sites of pMIR-Report vector. Wild-type and mutagenic binding sequence of the target gene Tob1 are listed in Figure 3b. Constructed wild-type luciferase reporter (WT), mutant luciferase reporter (MUT) or empty vectors were cotransfected with miR-26a mimics or its corresponding negative control into MSC. Firefly and Renilla luciferase activity were measured in cell lysates using a Dual-Luciferase Reporter Assay System (Promega, Fitchburg, WI) according to the manufacturer's instruction. Renilla luciferase (Rluc) was expressed from PMIR-report luciferase vector and Firefly Luciferase (Fluc) was expressed from pRL-SV40 Vector. The pRL-SV40 Vector was used for reported gene data normalization. The dual luciferase reporter assay was carried out according to the manufacturer's instructions (Promega). First, 20 μl of Luciferase Assay Reagent II (Promega) was added to the lysates for firefly luciferase activity measurement using a luminescence counter (Packard, Meriden, CT), and then 20 ul of Stop &GloReagent (Promega) was added to the lysates to quench firefly luciferase activity and activate Renilla luciferase activity for measurement using a luminescence counter (Packard). The relative luciferase activity of each sample was calculated as the ratio of firefly (reporter) luciferase activity to Renilla (normalization control) luciferase activity.
The Tob1 expression vector was designed to contain only the ORF, not the 3'UTR to avoid the interaction between the miR-26a and the vector. The exported document of the sequencing result of the Tob1 3′UTR construct was shown in Supplementary Figure S8.
Subcutaneous implantation. 4.0 × 106 untreated MSC, MSC transfected with miR-26a mimics or its negative control were mixed with 40 mg hydroxyapatite tricalcium phosphate (HA-TCP) ceramic particles (National Engineering Research Center for Biomaterials, Sichuan University, China). HA-TCP is a kind of osteo-conductive biological materials, which was often used to form into scaffold for bone tissue engineering. After centrifuge, the pellets were subcutaneously implanted into the dorsal pockets of 8-week-old immunocompromised mice. At 8 weeks after implantation, the mice were euthanized and the implants were harvested for analysis.
MSC-mediated mouse calvarial bone defect regeneration. For critical-sized calvarial bone defect repair, a 5-mm calvarial defect was generated in osteoporotic mice. Approximately 4.0 × 106 MSCs harvested from littermate-derived estrogen deficiency–induced osteoporotic mice (OVX-MSC) and 20 µl 50 nmol/l agomir-26a or equal amounts of its respective negative control were dispensed into a 250 µl HyStem-HP hydrogel (Glycosan Biosystems, Salt lake City, UT) and then were implanted within the defects. The gelation procedure followed the instruction of the manufacturer. To track neo-bone formation, mice were anesthetized every 3 weeks postsurgery and subjected to live microCT observations.
Micro-computed tomography (Micro CT) analysis. Micro-CT uses x-rays to create cross-sections of a physical object that can be used to recreate a virtual model (3D model) without destroying the original object. For the distal femur analysis, specimens were embedded in 1% agarose and placed in a 19-mm tube and scanned over the entire length of the tibia using a microCT system (Siemens AG, Germany). Scan settings were: voxel size 20 μm, 80 kVp, 500A. Analysis was performed using the manufacturer's evaluation software and a fixed global threshold of 28% (280 on a grayscale of 0–1,000) for cortical bone and 18% for trabecular bone was used to segment bone from nonbone.
A region of interest in secondary spongiosa was beginning at 0.1 mm from the most proximal aspect of the growth plate in which both condyles were no longer visible. All the trabecular bone from each selected slice was segmented for three-dimension reconstruction to calculate the following parameter BV/TV.
Therapeutic inhibition of miR-26a in OVX mice. OVX mice were treated with antagomir-26a (10 mg/kg body weight) or the negative control antagomir (Guangdong Ruibo, China) by tail vein injection every 2 weeks.
Histological observation. Samples were fixed in 4% paraformaldehyde and decalcified with 5% EDTA, followed by embedding with paraffin. Sections were stained with hematoxylin and eosin (H&E) following the procedures as previously described.40 Sample gross views of 1/4th the maximum cross-sectional area for each group analyzed by HE staining were shown in Supplementary Figure S9.
Statistical analysis. We used parametric test. Data were assessed for normal distribution and similar variance between groups before further analysis. Numerical data were presented as mean ± SD. All statistical analyses were performed using SPSS 11.0 software. Statistical differences among groups were analyzed by one-way ANOVA. Statistical differences between two groups were determined by the Student's t-test. To control type I error when performing multiple comparisons, post hoc test (Bonferroni correction) was applied. P < 0.05 was considered statistically significant.
SUPPLEMENTARY MATERIAL Figure S1. MiR-26a expression level in MSC was significantly increased after pre-miR-26a transfection, as determined by RT-qPCR at 0, 2, 7, and 14 days post transfection. Figure S2. The deficient expression of Tob1 promoted ectopic bone formation ability of OVX-MSC in vivo. Figure S3. The deficient expression of Tob1 improved littermate–derived OVX-MSCs mediated critical-sized calvarial bone defect repair. Figure S4. MiR-26a expression level within the defect area has returned to its original level at 3-month post implantation as determined by RT-qPCR. Figure S5. RT-qPCR analysis of miR-26a expression in various tissues of the mice treated with local miR-26a delivery. Figure S6. The effect of antagomir-26a in ovx mice determined by microCT examination. Figure S7. Full scans of blots labeled with the intensity quantification. Figure S8. The exported document of the sequencing result of the Tob1 3′UTR construct. Figure S9. Sample gross views of 1/4th the maximum cross-sectional area for each group analyzed by HE staining. Figure S10. The standard curve, melt profile and melt profile with no template control and RT control of the genes (Alp, Tob1, Ocn, β-actin, miR-26a and U6) detected by RT-qPCR. Table S1. Oligonucleotide primer sequences utilized in the RT-qPCR.
Acknowledgments
This work was supported by grants from the National Natural Science Research Program of China (2010CB944800 and 2010CB964700), the Preliminary 973 Program (2012CB526704), and the National Natural Science Research Program of China (81100732). Grant supporters: Yan Jin and Jihua Chen. All authors declare that they have no conflicts of interest.
Supplementary Material
References
- Bouxsein, ML, Myers, KS, Shultz, KL, Donahue, LR, Rosen, CJ and Beamer, WG (2005). Ovariectomy-induced bone loss varies among inbred strains of mice. J Bone Miner Res 20: 1085–1092. [DOI] [PubMed] [Google Scholar]
- Cho, SW, Sun, HJ, Yang, JY, Jung, JY, An, JH, Cho, HY et al. (2009). Transplantation of mesenchymal stem cells overexpressing RANK-Fc or CXCR4 prevents bone loss in ovariectomized mice. Mol Ther 17: 1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliuc, D, Nguyen, ND, Nguyen, TV, Eisman, JA and Center, JR (2013). Compound risk of high mortality following osteoporotic fracture and refracture in elderly women and men. J Bone Miner Res 28: 2317–2324. [DOI] [PubMed] [Google Scholar]
- Bodenner, D, Redman, C and Riggs, A (2007). Teriparatide in the management of osteoporosis. Clin Interv Aging 2: 499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoback, D (2007). Update in osteoporosis and metabolic bone disorders. J Clin Endocrinol Metab 92: 747–753. [DOI] [PubMed] [Google Scholar]
- Black, DM, Greenspan, SL, Ensrud, KE, Palermo, L, McGowan, JA, Lang, TF et al.; PaTH Study Investigators. (2003). The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349: 1207–1215. [DOI] [PubMed] [Google Scholar]
- Neer, RM, Arnaud, CD, Zanchetta, JR, Prince, R, Gaich, GA, Reginster, JY et al. (2001). Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- Cosman, F, Nieves, J, Zion, M, Woelfert, L, Luckey, M and Lindsay, R (2005). Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353: 566–575. [DOI] [PubMed] [Google Scholar]
- Lindsay, R, Nieves, J, Formica, C, Henneman, E, Woelfert, L, Shen, V et al. (1997). Randomised controlled study of effect of parathyroid hormone on vertebral-bone mass and fracture incidence among postmenopausal women on oestrogen with osteoporosis. Lancet 350: 550–555. [DOI] [PubMed] [Google Scholar]
- Leroux, L, Descamps, B, Tojais, NF, Séguy, B, Oses, P, Moreau, C et al. (2010). Hypoxia preconditioned mesenchymal stem cells improve vascular and skeletal muscle fiber regeneration after ischemia through a Wnt4-dependent pathway. Mol Ther 18: 1545–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, YL, Zhao, Q, Zhang, YC, Cheng, L, Liu, M, Shi, J et al. (2004). Autologous mesenchymal stem cell transplantation induce VEGF and neovascularization in ischemic myocardium. Regul Pept 117: 3–10. [DOI] [PubMed] [Google Scholar]
- Bang, OY, Lee, JS, Lee, PH and Lee, G (2005). Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol 57: 874–882. [DOI] [PubMed] [Google Scholar]
- Yang, N, Wang, G, Hu, C, Shi, Y, Liao, L, Shi, S et al. (2013). Tumor necrosis factor α suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 28: 559–573. [DOI] [PubMed] [Google Scholar]
- Liao, L, Yang, X, Su, X, Hu, C, Zhu, X, Yang, N et al. (2013). Redundant miR-3077-5p and miR-705 mediate the shift of mesenchymal stem cell lineage commitment to adipocyte in osteoporosis bone marrow. Cell Death Dis 4: e600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel, DP (2004). MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116: 281–297. [DOI] [PubMed] [Google Scholar]
- Li, Z, Hassan, MQ, Volinia, S, van Wijnen, AJ, Stein, JL, Croce, CM et al. (2008). A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA 105: 13906–13911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, J, Zhao, L, Xing, L and Chen, D (2010). MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 28: 357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, MQ, Gordon, JA, Beloti, MM, Croce, CM, van Wijnen, AJ, Stein, JL et al. (2010). A network connecting Runx2, SATB2, and the miR-23a~27a~24-2 cluster regulates the osteoblast differentiation program. Proc Natl Acad Sci USA 107: 19879–19884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, YJ, Bae, SW, Yu, SS, Bae, YC and Jung, JS (2009). miR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res 24: 816–825. [DOI] [PubMed] [Google Scholar]
- Li, Z, Hassan, MQ, Jafferji, M, Aqeilan, RI, Garzon, R, Croce, CM et al. (2009). Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284: 15676–15684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh, T, Nozawa, Y and Akao, Y (2009). MicroRNA-141 and -200a are involved in bone morphogenetic protein-2-induced mouse pre-osteoblast differentiation by targeting distal-less homeobox 5. J Biol Chem 284: 19272–19279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan, MQ, Maeda, Y, Taipaleenmaki, H, Zhang, W, Jafferji, M, Gordon, JA et al. (2012). miR-218 directs a Wnt signaling circuit to promote differentiation of osteoblasts and osteomimicry of metastatic cancer cells. J Biol Chem 287: 42084–42092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, T and Xu, Z (2010). miR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun 402: 186–189. [DOI] [PubMed] [Google Scholar]
- Kapinas, K, Kessler, C, Ricks, T, Gronowicz, G and Delany, AM (2010). miR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 285: 25221–25231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y, Fan, L, Liu, S, Liu, W, Zhang, H, Zhou, T et al. (2013). The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials 34: 5048–5058. [DOI] [PubMed] [Google Scholar]
- Li, H, Xie, H, Liu, W, Hu, R, Huang, B, Tan, YF et al. (2009). A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 119: 3666–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida, Y, Tanaka, S, Umemori, H, Minowa, O, Usui, M, Ikematsu, N et al. (2000). Negative regulation of BMP/Smad signaling by Tob in osteoblasts. Cell 103: 1085–1097. [DOI] [PubMed] [Google Scholar]
- Usui, M, Yoshida, Y, Tsuji, K, Oikawa, K, Miyazono, K, Ishikawa, I et al. (2004). Tob deficiency superenhances osteoblastic activity after ovariectomy to block estrogen deficiency-induced osteoporosis. Proc Natl Acad Sci USA 101: 6653–6658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskildsen, T, Taipaleenmäki, H, Stenvang, J, Abdallah, BM, Ditzel, N, Nossent, AY et al. (2011). MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc Natl Acad Sci USA 108: 6139–6144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugatani, T and Hruska, KA (2007). MicroRNA-223 is a key factor in osteoclast differentiation. J Cell Biochem 101: 996–999. [DOI] [PubMed] [Google Scholar]
- Cheng, P, Chen, C, He, HB, Hu, R, Zhou, HD, Xie, H et al. (2013). miR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J Bone Miner Res 28: 1180–1190. [DOI] [PubMed] [Google Scholar]
- Kota, J, Chivukula, RR, O'Donnell, KA, Wentzel, EA, Montgomery, CL, Hwang, HW et al. (2009). Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137: 1005–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J, He, ML, Wang, L, Chen, Y, Liu, X, Dong, Q et al. (2011). MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res 71: 225–233. [DOI] [PubMed] [Google Scholar]
- Liu, B, Wu, X, Liu, B, Wang, C, Liu, Y, Zhou, Q et al. (2012). MiR-26a enhances metastasis potential of lung cancer cells via AKT pathway by targeting PTEN. Biochim Biophys Acta 1822: 1692–1704. [DOI] [PubMed] [Google Scholar]
- Zhang, B, Liu, XX, He, JR, Zhou, CX, Guo, M, He, M et al. (2011). Pathologically decreased miR-26a antagonizes apoptosis and facilitates carcinogenesis by targeting MTDH and EZH2 in breast cancer. Carcinogenesis 32: 2–9. [DOI] [PubMed] [Google Scholar]
- Huse, JT, Brennan, C, Hambardzumyan, D, Wee, B, Pena, J, Rouhanifard, SH et al. (2009). The PTEN-regulating microRNA miR-26a is amplified in high-grade glioma and facilitates gliomagenesis in vivo. Genes Dev 23: 1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, J, Han, C and Wu, T (2012). MicroRNA-26a promotes cholangiocarcinoma growth by activating β-catenin. Gastroenterology 143: 246–56.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell, O and Kanis, JA (2004). An estimate of the worldwide prevalence, mortality and disability associated with hip fracture. Osteoporos Int 15: 897–902. [DOI] [PubMed] [Google Scholar]
- Kumar, S, Wan, C, Ramaswamy, G, Clemens, TL and Ponnazhagan, S (2010). Mesenchymal stem cells expressing osteogenic and angiogenic factors synergistically enhance bone formation in a mouse model of segmental bone defect. Mol Ther 18: 1026–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachos, T, Diggs, A, Weisbrode, S, Bartlett, J and Bertone, A (2007). Mesenchymal stem cell-mediated gene delivery of bone morphogenetic protein-2 in an articular fracture model. Mol Ther 15: 1543–1550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.