Abstract
We have previously demonstrated that a low dose of cyclophosphamide (Cy) combined with gene therapy of interleukin-12 (AdIL-12) has a synergistic, although limited, antitumoral effect in mice with colorectal carcinoma. The main mechanism involved in the efficacy of Cy+AdIL-12 was the induction of a specific immune response mediated by cytotoxic T lymphocytes. Our current aims were to evaluate the effects of 4-methylumbelliferone (4Mu), a selective inhibitor of hyaluronan (HA) synthesis, on tumor microenvironment (TME) and to investigate how 4Mu affects the therapeutic efficacy of Cy+AdIL-12. The results showed that 4Mu significantly reduced the amount of tumoral HA leading to a significant decrease in tumor interstitial pressure (TIP). As a consequence, tumor perfusion was improved allowing an increased adenoviral transgene expression. In addition, treatment with 4Mu boosted the number of cytotoxic T lymphocytes that reach the tumor after adoptive transfer resulting in a potent inhibition of tumor growth. Importantly, we observed complete tumor regression in 75% of mice when 4Mu was administrated in combination with Cy+AdIL-12. The triple combination 4Mu+Cy+AdIL-12 also induced a shift toward antiangiogenic factors production in tumor milieu. Our results showed that TME remodeling is an interesting strategy to increase the efficacy of anticancer immunotherapies based on gene and/or cell therapy.
Introduction
In the last decade, a variety of immunotherapy strategies have been evaluated in patients with different types of cancer including colorectal carcinomas; however, the clinical responses that have been obtained are far from being satisfactory.1,2 Experimental and clinical evidence showed that there are, at least, two major obstacles for the success of immunotherapy-based strategies against cancer: (i) the ability of cancer cells to evade the immune response, known as immunoescape and, (ii) the reduced homing of immune cells inside the tumors.3
Tumors escape from immune-mediated rejection by complex mechanisms originated mainly within the tumor microenvironment (TME) including selective recruitment and expansion of a variety of regulatory cells (tolerogenic dendritic cells, natural and inducible regulatory T cells, myeloid-derived suppressor cells, tumor-associated macrophages), induction of inappropriate antigen presentation mechanisms and inactivation of costimulatory signals.4 These potent mechanisms of immunosuppression are active during tumor progression, leading to the escape of cancer cells from the immune system and limiting the effectiveness of anticancer immunotherapy.5 However, cancer immunotherapy fails not only due to cancer-induced immunosuppressive mechanisms but also to the lack of specific T-cell responses. Thus, a successful immunotherapy against tumors requires the combination of two sequential/simultaneous manipulations of the immune system: elimination of the tolerance and stimulation of an efficient immune response. In this sense, our previous studies in different murine gastrointestinal carcinoma models showed that the sequential administration of low-dose cyclophosphamide (Cy) plus subtherapeutic doses of an adenovirus expressing IL-12 genes (AdIL-12) satisfies both requirements; Cy mediates removal of immunosuppressive factors and IL-12 generates antitumor cytotoxic T lymphocytes activity. This combined treatment induced a synergistic, although limited, potentiation of the effect of both agents with regard to their antitumoral activities as single agents.6,7
Cancer cells do not act alone in promoting tumor growth and dissemination3; the crosstalk among tumor cells and all components of TME is crucial for tumor progression. TME is a heterogeneous mixture of different cell types, soluble factors, signaling molecules, extracellular matrix (ECM) components, and is characterized by an increased number of fibroblasts that stimulates the expansion of ECM and the increment of matrix tension.8 This is due, in part, to the synthesis of an abnormally large amount of collagen fibers, HA, proteoglycans, and proteolytic enzymes.9 In addition, tumor interstitial compartment contains leaky and tortuous blood vessels, poorly functioning lymphatic system, and increased plasma protein extravasation, contributing to the generation of a high TIP. Elevated TIP hampers an effective cancer treatment through a reduced uptake and heterogeneous distribution of drugs, effector immune cells, and gene therapy-based tools into tumor interstitium.10
Increased HA production and deposit have been observed in different tumors including colorectal carcinoma, ovarian and breast cancer. These studies have correlated the presence of high HA levels in TME with poor clinical outcome.11,12,13 4-Methylumbelliferone (4Mu), an orally available agent, has been reported to inhibit HA synthesis in several cell types14 and it has showed antitumor effects through the inhibition of cancer cell proliferation, migration, and invasion in vitro and in vivo.15,16 4Mu is able to inhibit HA synthesis through the reduction of HA synthase mRNA levels and by depletion of the HA precursor UDP-glucuronic acid. These effects were demonstrated in human fibroblasts14 and in melanoma, breast, pancreatic, and ovarian cancer cells17,18
The TME is complex, highly dynamic, and has a key role in tumor development and progression. Therefore, it is been proposed that TME modulation is particularly important to improve tumor response to cancer therapies.19
The objectives of our study were to evaluate the effects of 4Mu, a selective inhibitor of HA synthesis, on TME and to investigate how 4Mu affects the therapeutic efficacy of Cy+AdIL-12 against advanced colorectal carcinoma.
Results
4Mu inhibits HA production by colorectal carcinoma cells in vitro and in vivo
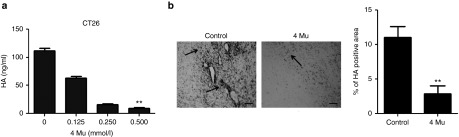
We first analyzed the effect of 4Mu on HA expression in vitro in CT26 cells using an enzyme-linked immunosorbent assay (ELISA)-like assay in culture supernatants. When CT26 cells were pretreated with 4Mu for 24 hours, we observed an inhibition on production of HA in a dose-dependent manner. HA production by CT26 cells was slightly reduced at a dose of 0.125 mmol/l of 4Mu whereas the levels of HA were significantly reduced at a dose of 0.5 mmol/l (8.9 ± 1.9 ng/ml; P < 0.01 (Figure 1a). CT26 cells viability was not affected by 4Mu at any doses assayed (data not shown). We also analyzed the effect of 4Mu on the HA production in tumor stroma. BALB/c mice were inoculated with CT26 cells (day 0). On day 6, CT26 tumor-bearing mice were orally treated with 200 mg/kg/day of 4Mu for 8 days or left untreated (control). At day 14, animals were sacrificed and tumor samples were taken for histochemistry using a HA-binding protein (HAbP). We observed that untreated tumors (control group) expressed significantly higher amounts of HA (11.1 ± 0.7% of positive area) in comparison with tumors from 4Mu-treated mice (Figure 1b). These results showed the capability of 4Mu to inhibit, both in vitro and in vivo, HA production by CT26 cells.
Figure 1.
4Mu inhibits hyaluronan (HA) production by CT26 cells in vitro and in vivo. (a) Quantification of HA by an enzyme-linked immunosorbent assay (ELISA)-like assay in CT26 cells-free supernatants obtained from 5 × 105 CT26 cells treated or not with 4Mu at different concentrations (0,125; 0.25 and 0.5 mmol/l) cultured at 37 °C for 24 hours. **P < 0.01: control versus 4Mu 0.5 mmol/l; Kruskal-Wallis test. (b) Histochemistry of HA in tumor samples of untreated or 4Mu treated-CT26 tumor-bearing mice. HA deposits are seen in black (arrows). Scale bar = 50 μm (left panel). Quantification of HA-positive area was done by densitometry. Bars represent the average of measures of each group (n = 8/group) ± SEM. **P < 0.01: control versus 4Mu; Mann–Whitney test (right panel).
4Mu treatment reduces intratumoral pressure and increases transgene expression
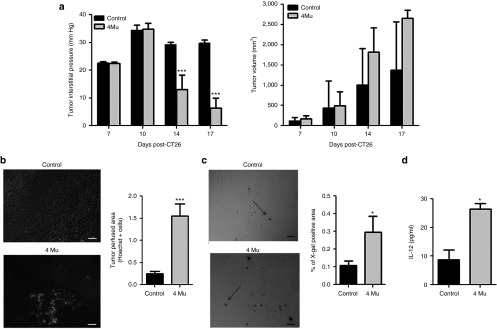
Accumulation of HA in TME increases TIP.20 Therefore, we investigated whether treatment with the HA synthesis inhibitor 4Mu could decrease TIP in CT26 tumor model. TIP was measured in CT26 tumor-bearing mice treated or not with 4Mu, on days 7, 10, 14, and 17 following tumor cells inoculation. Tumor growth was evaluated in parallel to exclude the effect of tumor volume on pressure values. We observed that until day 10, tumor volume and TIP were similar in both untreated and 4Mu-treated animals. Then, although tumor volume of both groups was increasing over time, treatment with 4Mu significantly reduced TIP compared with untreated tumors (Figure 2a). These results showed that treatment with 4Mu alone did not alter tumor growth, but significantly decreased TIP.
Figure 2.
Effects of 4Mu on TIP and tumor perfusion result in increased transgene expression. (a) TIP measurement in mice treated with 4Mu or saline beginning at day 6 post-CT26 cells inoculation (n = 12/group). ***P < 0.001: control versus 4Mu at day 14 post-CT26 (8 days of 4Mu treatment), and ***P < 0.001: control versus 4Mu at day 17 post-CT26 (11 days of 4Mu treatment); ANOVA-Bonferroni post-test (left panel). Tumor volume of CT26-tumor-bearing mice treated with 4Mu or saline. No significant differences were observed between both groups (right panel). (b) Tumor perfusion was determined at 5 minutes after intravenous injection of Hoechst 33342 dye (15 mg/kg) (n = 4/group). Scale bar = 50 μm. Quantification of Hoechst 33342 dye-positive area was performed by densitometry. Bars represent the average of measures of each group ± SEM. ***P < 0.01: control versus 4Mu; Mann-Whitney test. (c) Effects of 4Mu on adenoviral transgene expression. X-gal staining for β-galactosidase in CT26 tumors (black arrows). Quantification of X-gal-positive area was performed by densitometry. Bars represent the average of measures of each group (n = 5/group) ± SEM. *P < 0.05: control versus 4Mu; Mann-Whitney test. (d) Intratumoral IL-12 expression was measured by ELISA after 48 hours of AdIL-12 transfection. Bars represent the average of measures of each group (n = 4/group) ± SEM. *P < 0.05: control versus 4Mu; Mann-Whitney test. Experiments are shown as the representative of two independent assays.
To assess the impact of 4Mu-induced TIP decrease on the permeability of tumor microvasculature, we analyzed tumor perfusion by injection of Hoechst 33342 dye. We observed individual cell clusters stained with Hoechst 33342 dye in 4Mu-treated tumors (Figure 2b), indicating the presence of perfused blood vessels. Contrarily, cells stained by Hoechst 33342 were practically undetectable in untreated mice.
Then, we hypothesized that the effects of 4Mu on TIP and tumor perfusion might have an impact on the adenovirus-mediated gene transfer into tumors. Therefore, tumor-bearing mice were treated with saline or 4Mu. On day 9, animals were intratumorally injected with Ad-βgal or AdIL-12. The results in the Figure 2c show a significant increase in βgal expression in 4Mu-treated mice compared with untreated mice. In addition, IL-12 protein expression was also higher in 4Mu-treated mice than in untreated mice (26.4 ± 1.9 versus 8.8 ± 3.3 pg/ml, Figure 2d). These results clearly show that treatment with 4Mu improve the efficacy of adenovirus-mediated gene transfer into CT26 tumors.
TME modulation achieved by 4Mu therapy increases the homing of specific antitumor T lymphocytes into CT26 s.c. tumors
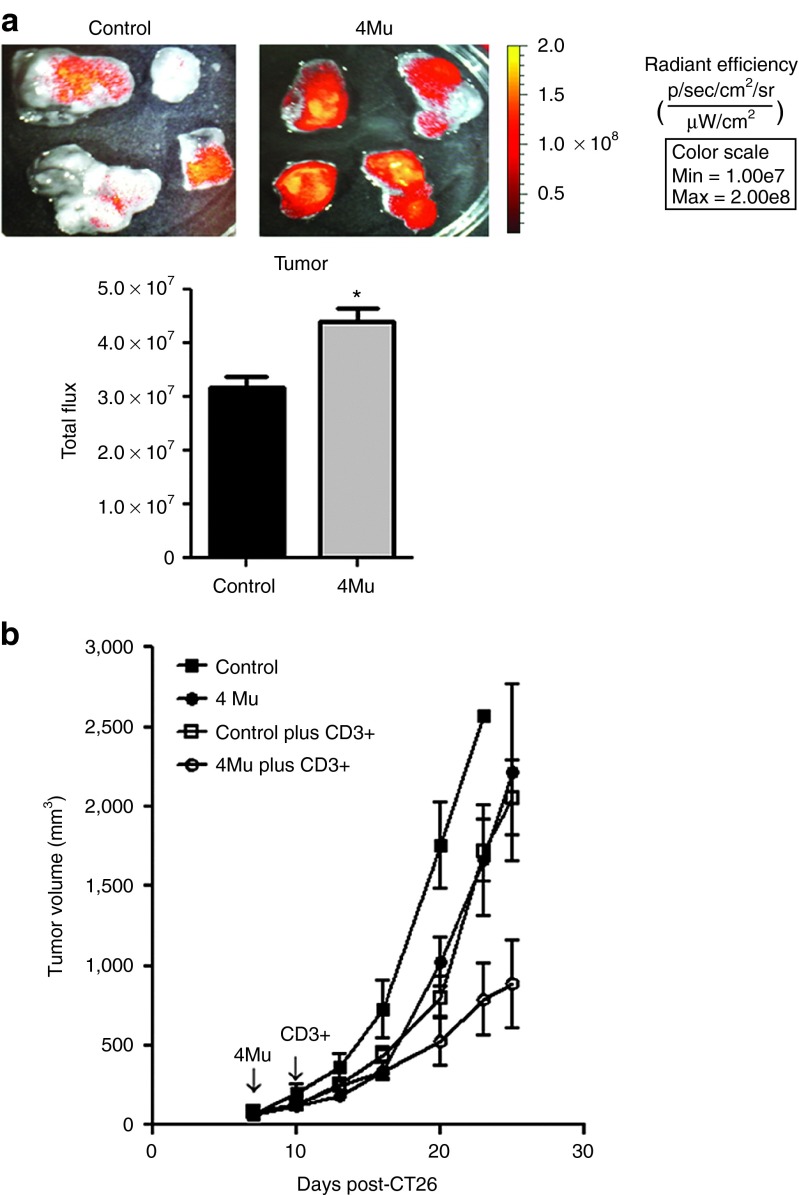
We decided to investigate whether the effects on TIP and functional perfusion induced by 4Mu in TME modify the homing of T lymphocyte into tumors. To this end, mice carrying CT26 s.c. tumors were treated or not with 4Mu and then received specific T lymphocytes obtained from cured mice treated with Cy + AdIL-12. Twenty-four hours after receiving CD3+T cells, mice were sacrificed and tumor-associated fluorescence was analyzed. The total fluorescence imaging in both groups of animals were similar, indicating no differences in the amount of injected CD3+DiR+ T cells (data not shown). Importantly, we found that tumors from animals treated with 4Mu showed a significantly higher signal in comparison with control mice (Figure 3a). Interestingly, when specific T lymphocytes were adoptively transferred in mice pretreated with 4Mu, a significant inhibition of tumor growth was achieved compared with mice nontreated with 4Mu (Figure 3b). These results suggest that the reversal of elevated TIP induced by 4Mu has the ability to restore, at least in part, the functional tumor perfusion resulting in an increased homing of therapeutic T cells into tumors.
Figure 3.
4Mu therapy increases the homing of specific antitumor T lymphocytes into CT26 s.c. tumors and inhibits tumor growth. (a) In vivo tracking of CD3+DiR+-specific T lymphocytes. Fourteen days after CT26 cells inoculation, tumor-bearing mice previously treated with 4Mu or saline during 7 days (n = 4/group) were intravenously injected with CD3+DiR+ cells (1 × 106). DiR fluorescence quantification was performed using bioluminescence imaging 24 hours after T-cells injection. Images represent the radiant efficiency and results were expressed as total flux of radiant efficiency. *P < 0.05: control versus 4Mu; Mann-Whitney test. Experiments are shown as the representative of three independent assays. (b) Antitumoral effect of adoptive T cells therapy on untreated or 4Mu-treated tumor-bearing mice. Administration of 4Mu plus adoptive transfer of specific T cells induced a significant inhibition of tumor growth in comparison with each therapy alone. Bars represent the average of measures of each group (n = 8/group) ± SEM; *P < 0.05. Kruskal-Wallis test. Experiments are shown as the representative of two independent assays.
Antitumoral efficacy of Cy + AdIL-12 is improved by 4Mu
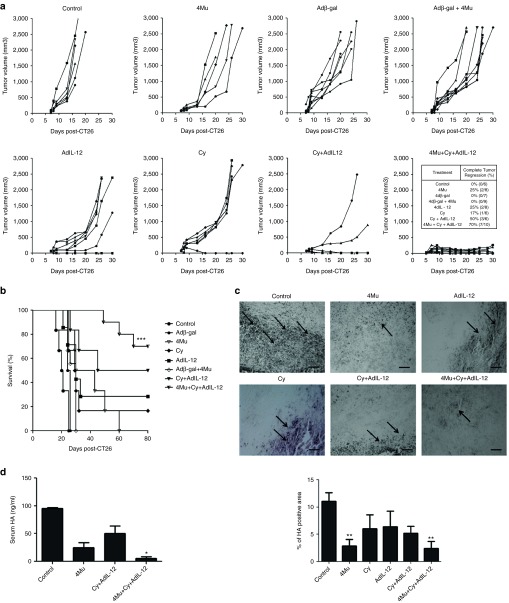
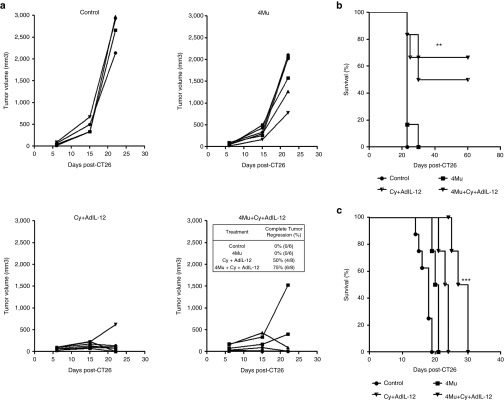
We previously demonstrated that AdIL-12 mediated a potent antitumor effect against colorectal carcinoma CT26 in mice pretreated with low doses of Cy. The antitumor effect achieved with this sequential combination was mediated by the induction of a potent specific immune response.6,7 Taking into account the effects mediated by 4Mu on TME and the significant reduction of tumor volume observed in 4Mu-treated animals that received adoptive cell therapy using specific T lymphocytes, we decided to investigate in vivo whether the antitumor effect of Cy + AdIL-12 may be enhanced by modulation of TME by 4Mu. Therefore, BALB/c mice were subcutaneously inoculated with CT26 cells, tumors were allowed to grow for 6 days (tumor volume of ~90 mm3) and then treatments were initiated. Over the course of 30 days, we observed that CT26-bearing mice treated with each agent administered alone (4Mu, Cy, or AdIL-12) showed a slight and nonsignificant inhibition of tumor growth; no complete tumor regressions were observed (Figure 4a). Contrarily, the combination Cy + AdIL-12 resulted in a significant tumor volume reduction and complete tumor regression in 50% of animals. Importantly, the addition of 4Mu to the combination Cy + AdIL-12 achieved a 70% of complete tumor regressions. Survival of mice receiving triple combined therapy (4Mu + Cy + AdIL-12) was significantly increased compared with mice receiving each single treatment or saline (Figure 4b; P < 0.001).
Figure 4.
In vivo effects of 4Mu + Cy + AdIL-12 on subcutaneous CT26 tumors. (a) Antitumoral efficacy: CT26 cells were injected s.c. at a dose of 5 × 105 cells into the right flank of BALB/c mice (day 0). Tumors were allowed to reach approximately 90 mm3 in size before treatment was initiated. Animals were distributed in different groups and treated with saline i.p. (control group, n = 6); 4-Mu (200 mg/kg/day, oral administration, o.a., during 3 weeks, from day 6, n = 6); Cy (50 mg/kg i.p., day 8, n = 6); Adβ-gal (109 TCID50 intratumoral (i.t.), day 9, n = 7); Adβ-gal + 4Mu (n = 9); AdIL-12 (109 TCID50 i.t., day 9, n = 8); Cy + AdIL-12 (n = 6) or 4Mu + Cy + AdIL-12 (n = 10). Then, mice were monitoring during 30 days and tumor volumes were measured. Tumor complete regressions (%) were recorded for each group. (b) Survival. ***P < 0.001 Kaplan Meier, log rank test. Data are representative of four independent experiments. (c) Quantification of hyaluronan (HA) in CT26 tumors by histochemistry with HAbP. Black arrows indicate HA deposits. Scale bar = 50 μm (left panel). Quantification of HA-positive area was done by densitometry. Bars represent the average of measures of each group (n = 8/group) ± SEM. **P < 0.01: control versus 4Mu and control versus 4Mu + Cy + AdIL-12; Kruskal-Wallis test (right panel). (d) Levels of serum HA. *P < 0.05: control versus 4Mu + Cy + AdIL-12; Kruskal-Wallis test.
Histochemistry with HAbP revealed that oral administration of 4Mu significantly reduced HA levels in CT26 tumors of 15 days of evolution (Figure 4c; P < 0.01). Moreover, when 4Mu was administered in combination with Cy + AdIL-12 a higher decrease in HA content was obtained (Figure 4c; P < 0.01). Cy and AdIL-12, alone or in combination, produced a slight decrease in intratumoral HA deposits. Regarding circulating levels of HA, we observed that control tumor-bearing mice expressed significantly higher amounts of serum HA (95.2 ± 2.0 ng/ml) in comparison with mice treated with the triple combination 4Mu + Cy + AdIL-12 (5.8 ± 2.0 ng/ml; P < 0.05) (Figure 4d).
In order to examine whether triple combined treatment exert antitumor effects in a more aggressive tumor model, we investigated its therapeutic efficacy in two liver metastatic colorectal carcinoma models using CT26 cells. For the first model, CT26 cells were injected directly into the liver of mice (day 0), treated with 4Mu (day 6), Cy (day 8) and 24 hours later with AdIL-12. Figure 5a shows that no significant antimetastatic effect was observed when 4Mu was administered alone. Although the combined therapy Cy + AdIL-12 showed a significant reduction in metastases growth and induced complete metastases regressions in 50% of animals, the addition of 4Mu produced a superior therapeutic effect, achieving complete metastases regressions in six out of eight animals (75 versus 0% in saline and 4Mu groups; Figure 5a). In addition, survival of mice receiving triple combined therapy was significantly higher than the controls (Figure 5b; P < 0.001). The second and more aggressive model consisted in the inoculation of CT26 cells through portal vein, with the aim to mimic the way of dissemination of colorectal carcinomas into de liver. As a result, animal survival in the triple combination group (4Mu + Cy + AdIL-12) was significantly higher than controls (Figure 5c; P < 0.05).
Figure 5.
Effects of 4Mu + Cy + AdIL-12 combination on liver metastases of colorectal carcinoma. (a) Antimetastatic efficacy. BALB/c mice received intrahepatic inoculation of 5 × 105 CT26 cells (day 0). Then, mice were distributed in experimental groups and treated with saline (control group, n = 6; 4Mu (200 mg/kg/day, oral administration, o.a., during 3 weeks, from day 6, n = 6); Cy (50 mg/kg i.p., day 8) + AdIL-12 (109 TCID50 intravenously, i.v., day 9, n = 8) or 4Mu + Cy +AdIL-12 (n = 8). At day 21, animals were sacrificed and the volume of metastatic nodules was measured with caliper. Metastases complete regressions (%) were recorded for each group. (b) Survival. **P < 0.01 Kaplan Meier (4Mu+Cy+AdIL-12 versus Cy+AdIL-12), log rank test. Data are representative of three independent experiments. (c) Animal survival analysis in the metastatic model induced by the administration of CT26 cells into portal vein. Mice were distributed in experimental groups and treated with saline (control group, n = 8); 4Mu (200 mg/kg/day, oral administration, from day 7, n = 8); Cy (50 mg/kg i.p., day 8) + AdIL-12 (109 TCID50 intravenously, i.v., day 9 n = 6) or 4Mu + Cy +AdIL-12 (n = 8). ***P < 0.001 Kaplan Meier (4Mu+Cy+AdIL-12 versus Cy+AdIL-12), log rank test. Data are representative of two independent experiments.
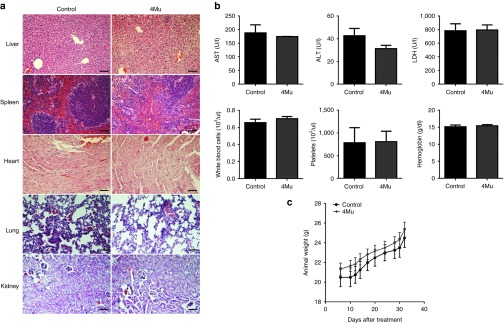
In addition, we assessed the toxicology profile of 4Mu therapy in mice and found that the employed oral scheme of 4Mu administration was well tolerated with no evident signs of clinical, biochemical and hematological toxicity within the studied period of time (Figure 6).
Figure 6.
Toxicity studies for 4Mu. (a) Representative H&E stained tissues from BALB/c mice treated or not with 4Mu. Magnification of tumor regions (20×) Scale bar = 50 μm. (b) Serum aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase measurement showed that 4Mu treatment does not induce liver damage. Quantification of white cells, platelets, and hemoglobin levels. (c) Animal weight curve were similar for both treated and nontreated mice. Experiments were done in duplicate. No significant, Mann-Whitney test.
Angiogenesis is modulated by 4Mu therapy
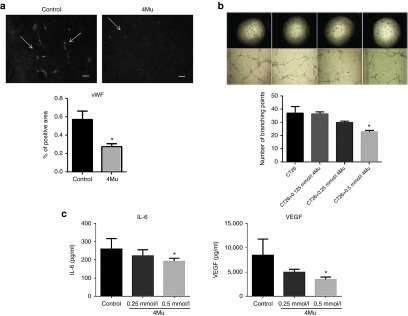
We first demonstrated that 4Mu reduced TIP and improved tumor perfusion. Considering that the von Willebrand Factor (vWF) is a glycoprotein produced by endothelial cells that regulates the blood vessel formation,21 we decided to study the microvessel density of CT26 tumors by immunostaining for vWF in mice treated or not with 4Mu. We observed a reduced expression of vWF on tumor tissue from 4Mu-treated CT26-bearing mice compared with untreated mice (Figure 7a). It is known that angiogenesis process involves a number of events such as vascular endothelial cells proliferation, migration and tube formation which is induced by growth factors as vascular endothelial growth factor (VEGF).22 We recently reported that vascular endothelial cells (HMEC-1) produced low levels of HA, which were not influenced by 4Mu.23 In addition, HMEC-1 proliferation was not affected by the different doses of 4Mu. We analyzed tube formation capability of HMEC-1 cells incubated with supernatant derived from CT26 cells and observed that the number of branching points were significantly reduced when HMEC-1 cells were incubated with supernatant obtained from 0.5 mmol/l 4Mu-pretreated CT26 cells (Figure 7b). We further quantified by ELISA whether 4Mu was able to modulate the expression levels of the proangiogenic VEGF and IL-6 factors produced by cancer cells. Figure 7c clearly shows that 4Mu induced a significant decrease of both factors in the supernatants of 4Mu-treated CT26 cells (P < 0.05).
Figure 7.
Antiangiogenic effects of 4Mu. (a) Expression of von Willebrand Factor (vWF) on tumor tissue from untreated or 4Mu-treated mice at day 14 post-CT26 cells inoculation (n = 4/group) (left panel). Quantification of vWF-positive area by densitometry. Bars represent the average of measures of each group (n = 4/group) ± SEM. *P < 0.05: control versus 4Mu; Mann-Whitney test (right panel). (b) Tube formation assay was performed stimulating HMEC cells sealed on a Matrigel coat with supernatants of CT26 cells pretreated with different doses of 4Mu. Images were taken at 20 and 40×. Quantification of branching points *P < 0.05 CT26 without pretreatment versus CT26+ 4Mu 0.5 mmol/l; Kruskal-Wallis test. (c) Quantification of VEGF (right panel) and IL-6 (left panel) by enzyme-linked immunosorbent assay in supernatants of CT26 cells untreated or pretreated with different doses of 4Mu. *P < 0.05 CT26 without pretreatment versus CT26+ 4Mu 0.5 mmol/l; Kruskal-Wallis test.
The combination of 4Mu + Cy + AdIL-12 promotes an unbalanced shift toward antiangiogenic factors
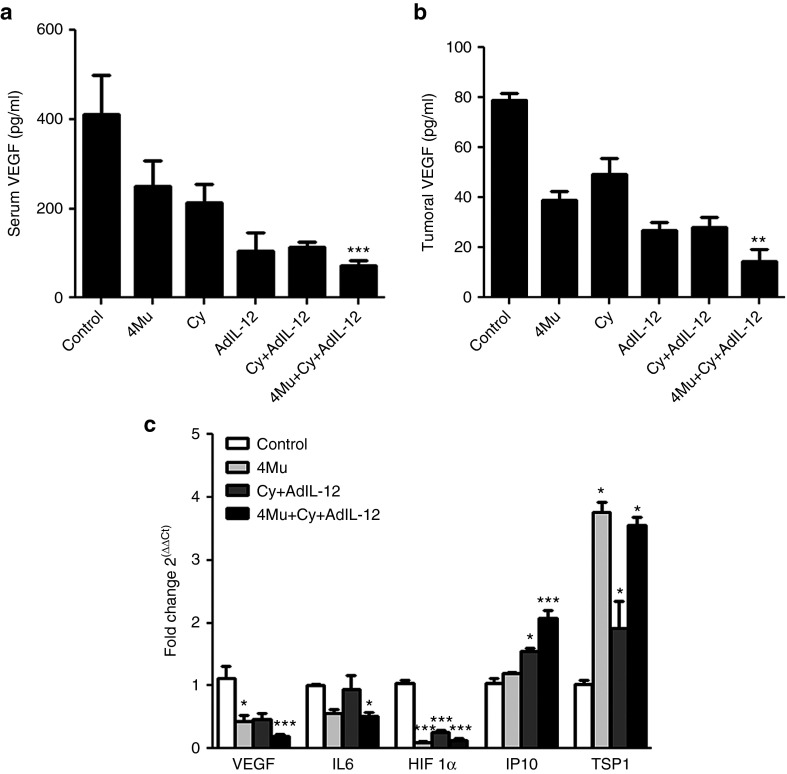
We decided to investigate if changes in the regulation of angiogenesis process mediated by soluble factors might explain, at least in part, the enhanced antitumoral efficacy observed with 4Mu + Cy + AdIL-12. Figure 8a,b shows levels of VEGF from untreated and treated CT26-bearing mice measured by ELISA. The triple combination 4Mu + Cy + AdIL-12 induced a significant decrease of both serum and tumoral VEGF (P < 0.001 and P < 0.01 respectively). Also, mRNA levels of VEGF, hypoxia-inducible factor 1-α (HIF1-α), interleukin 6 (IL-6) and the antiangiogenic molecules Thrombospondin-1 (TSP-1) and Interferon-inducible protein 10 (IP-10) were determinated by real-time PCR in tumor samples from untreated and treated tumor-bearing mice. Treatment with 4Mu + Cy + AdIL-12 diminished the expression of proangiogenic factors VEGF, HIF1-α, and IL-6 whereas induced an increase in the expression of TSP-1 and IP-10 (Figure 8c). These results suggest that triple combination could modify the balance of molecules in favor of suppression of tumor angiogenesis that lead to inhibition of tumor growth.
Figure 8.
The combination of 4Mu + Cy + AdIL-12 modulates the expression of proangiogenic and antiangiogenic factors. (a) Quantification of serum VEGF by enzyme-linked immunosorbent assay (ELISA) at day 14. ***P < 0.001: control versus 4Mu + Cy + AdIL-12, Kruskal-Wallis test. (b) Quantification of VEGF in tumor samples by ELISA at day 14. **P < 0.01: control versus 4Mu + Cy + AdIL-12, Kruskal-Wallis test. (c) Intratumoral mRNA levels of VEGF, HIF1-α, IL-6, TSP-1, and IP-10 by real-time polymerase chain reaction at day 14. VEGF: *P < 0.05: control versus 4Mu; ***P < 0.001: control versus 4Mu + Cy + AdIL-12; IL6: *P < 0.05: control versus 4Mu + Cy + AdIL-12; HIF1-α: ***P < 0.001: control versus 4Mu + Cy + AdIL-12; control versus Cy + AdIL-12 and control versus 4Mu; IP-10: *P < 0.05: control versus Cy + AdIL-12, ***P < 0.001: control versus 4Mu + Cy + AdIL-12; TSP-1: *P < 0.05: control versus 4Mu, control versus Cy + AdIL-12 and control versus 4Mu + Cy + AdIL-12. Kruskal-Wallis test. Bars represent the average of measures of each group (n = 6/group) ± SEM. Experiments are shown as the representative of two independent assays.
Discussion
A number of therapeutic approaches to generate effective antitumoral responses by either reverting immunosuppression or potentiating immune responses are under evaluation in the clinic.24 Therefore, a successful immunotherapy effort depends not only on the ability to induce a potent host immune response but also on the capacity to surpass several obstacles imposed by tumors.25 In this sense, it has been widely demonstrated that blood and lymphatic vasculature, fibroblasts, immune cells, and ECM components create a hostile TME. This abnormal TME is also characterized by hypoxia and high interstitial fluid pressure that resulted in tumor progression and treatment resistance.26 Therefore, cancer immunotherapy strategies need to be able to break the immune tolerance and also to overcome the intratumoral resistance to therapies.27
Several factors from TME are recognized as drivers for tumor growth and dissemination. Therefore, there is a growing interest in targeting the TME to improve therapeutic outcome. Elevated TIP has been identified as one of the most important barrier for an effective intratumoral distribution of cancer treatment. The genesis of increased TIP is multifactorial including the presence of tortuous tumor vasculatures, the lack of functional intratumoral lymphatic vessels, altered ECM components, and the stress exerted by proliferating cancer cells within a limited space.10,28 HA is a glycosaminoglycan present in most tissues and is also an important component of tumor ECM. Recently, it has been reported that high levels of HA in the TME constitutes a physical barrier that limits the access of monoclonal antibody therapy and the immune cells to tumors, suggesting a novel mechanism of resistance to immunotherapy in primary breast tumors and head and neck squamous cell carcinomas.29 In addition, the excessive accumulation of HA in TME during tumor progression causes an expansion of ECM, which contributes to the increased TIP.30,31
We decided to investigate the effects of the modulation of TME by the specific HA synthesis inhibitor 4Mu on the efficiency of combined immunotherapy based on AdIL-12 plus a single low dose of Cy in advanced colorectal carcinoma animal models. The abundance of HA produced by CT26 cells and the capacity of HA to increase TIP prompted us to specifically evaluate whether systemically delivered 4Mu could diminish HA accumulation within tumor stroma and, consequently, ameliorate TIP.
First, we confirmed that 4Mu is an effective agent to reduce the levels of HA in our tumor model, both in vitro and in vivo. Next, we demonstrated that oral administration of 4Mu to CT26 tumor-bearing mice significantly reduced TIP and improve tumor permeability. As a consequence of changes in tumor perfusion, we observed an increased delivery of adenoviruses and notably, a higher homing of tumor-specific T lymphocytes, indicating that the delivery of both genes and cells were effectively improved through the modulation of TME.
Different approaches targeting factors that contribute to the elevated TIP resulted in an improved intratumoral distribution of anticancer agents. These agents include vascular disrupting mediators (combretastatin A4, patupilone, paclitaxel); vascular targeting agents (bevacizumab; imatinib); proteolytic enzymes (hyaluronidase or collagenase); TGF-β inhibitors, among others.32,33,34,35,36 Many of them demonstrated potential as anticancer agents as well as limitations. For instance, normalization of tumor vessels is usually transient, and treatments with antiangiogenic agents might increase tumor hypoxia.37 On the other hand, the administration of proteolytic enzymes requires the knowledge of the optimal biologic dose to avoid toxicity.20,26,38,39
4Mu is a vegetal 7 hydroxy-4-methyl coumarin, which has been used safely in humans as a cholagogue as well as in clinical trials in patients with chronic viral hepatitis (ClinicalTrials.gov identifier: NCT00225537;40). In addition, the capability of 4Mu to control tumor growth by inhibition of HA synthesis has been demonstrated in many types of tumors including prostate, breast, and liver cancer,15,16,18 but never in combination with immunotherapy. We have previously reported a synergistic antitumor combination of Cy and AdIL-12 in mouse colorectal carcinoma (CRC). This combined immunotherapy showed capacity to revert immunosuppression induced by CD4+CD25+FoxP3+ T regulatory cells.7 The antitumoral activity achieved with this strategy also includes the induction of interferon (IFN)-γ secreting CD4 T-lymphocytes with cytotoxic activity.6,7 In order to evaluate whether the modulation of TME in CT26 tumor model is able to improve the efficacy of cancer immunotherapy, we decided to investigate the therapeutic activity of 4Mu plus the combined immunotherapy with Cy + AdIL-12 on advanced CRC. Our results indicated that the administration of this triple combination has an additive antitumor activity on established CT26 tumors, and further prolonged the survival of tumor-bearing mice. We observed that triple combined therapy induced a higher increase in antitumor efficacy, leading to 70% of complete tumor regressions in comparison with 50% of complete regression observed with Cy + AdIL-12 therapy. Moreover, when we tested the triple combination in a more aggressive model, liver metastatic CRC model, we found a superior efficacy (75% of complete tumor regressions). This enhanced therapeutic efficacy obtained in the metastatic model is probably due to 4Mu is metabolized in the liver, allowing an enhanced availability of the agent in the TME.40
One of the most important challenges that cancer immunotherapy has to face is the immunosuppressive TME.
Immunostimulatory monoclonal antibodies have the ability to unleash disease-destroying cellular immunity that negatively impacts the outcome of cancer patients. The anti-CTLA-4 mAb (ipilimumab) has been approved for metastatic melanoma41 and anti-PD-1 and anti-PD-L1 mAbs have shown extremely promising clinical activity.42 However, more aggressive cancers, such as pancreatic cancer, could be refractory to single therapy with these antibodies.43 In addition, TME is immunosuppressive, fibrotic, and poorly vascularized, and a major obstacle for a successful therapy. The administration of CD40 agonist antibodies resulted in the of activation antigen presenting cells (APCs) and in the induction of tumor-specific T cells.44 It has been reported that macrophages responding to agonist CD40 therapy infiltrate neoplastic lesions and facilitate depletion of type I collagen, a major component of the TME. However, these effects were limited to focal areas within the tumor mass, and some regions resulted resistant to therapy.45 Therefore, combination of immunotherapy with an agent that modulates the TME is an attractive therapeutic strategy. In this sense, the tumor vasculature could be remodeled using some kind of antiangiogenic agents resulting in a more suitable TME for immunotherapy.46 In this study we showed that treatment of mice with 4Mu together with the combined immunotherapy Cy + AdIL-12 reduced the expression levels of vWF in tumoral stroma and generates an unbalanced shift toward antiangiogenic factors expression. Indeed, VEGF, HIF1-alpha and IL-6 were downregulated whereas TSP-1 and IP-10 were upregulated. Mice treated with 4Mu + Cy + AdIL-12 exhibited increased expression of TSP-1, a molecule that can inhibit vascular endothelial cell proliferation and IP-10, an inflammatory chemokine induced by INF-γ, with angiostatic effects.47 Our results suggest that this antiangiogenic effect could be mediated mostly by 4Mu. The potential of 4Mu as antiangiogenic compound was recently described both in vitro and in vivo assays. It has been demonstrated that 4Mu affects several key steps of angiogenesis, including endothelial cell proliferation, adhesion, tube formation, and ECM remodeling.48 However, the molecular mechanisms underlying the antiangiogenic activity of 4Mu in cancer are not yet clarified. Our current results suggest for first time that 4Mu exerts antiangiogenic effects in CRC models.
Altogether, our results demonstrate that the reduction of TIP during tumor progression using the HA synthesis inhibitor 4Mu resulted critical for a more efficient delivery of gene and cell therapy. We demonstrated that the administration of 4Mu can potentiate the efficacy of the combined treatment with a single low dose of Cy plus gene therapy with AdIL-12 through breaking down the mechanism induced by tumors to avoid antitumor response and significantly enhancing the therapeutic efficiency. Although further studies will be necessary before it can be applied in clinical settings, this therapeutic strategy appears to be very attractive for potentiating the efficacy of immunotherapy against advanced CRC.
Materials and Methods
Cell lines. Mouse CT26 tumor cell line, an undifferentiated murine CRC cell line established from a N-nitroso-N-methylurethan-induced transplantable tumor in BALB/c (H-2d) mice was kindly provided by Prof. Prieto, University of Navarra, Spain. CT26 cells were cultured in complete Dulbecco's Modified Eagle Medium (2 mmol/l glutamine, 100 U/ml penicillin, 100 mg/ml streptomycin) and 10% heat-inactivated fetal bovine serum and incubated at 37 °C in a 5% CO2 humidified atmosphere.
Animals. Six-to-8-week-old male BALB/c mice were purchased from Fundación Balseiro, Buenos Aires, Argentina. Animals were maintained at our Animal Resources Facilities (School of Biomedical Sciences, Austral University) in accordance with the experimental ethical committee and the NIH guidelines on the ethical use of animals. The “Animal Care Committee” from School of Biomedical Sciences, Austral University, approved the experimental protocol. Mice entered the quarantine and were allowed to acclimatize to local conditions for 1 week before receiving injections of cancer cells.
Drugs. Cyclophosphamide (Filaxis, Argentina) was dissolved in sterile water at a concentration of 20 mg/ml and injected i.p. at the doses indicated. For in vitro assays, 4-methyl-umbilliferone (Sigma-Aldrich, St. Louis, MO) was dissolved in sterile Hank's balanced salt solution at different concentrations (0.125; 0.25, and 0.5 mmol/l). For in vivo experiments, 4Mu was dissolved in water at a concentration of 5 mg/ml and orally administered at the doses indicated.
Adenoviral vectors. Construction of a recombinant adenovirus encoding for IL-12 (AdIL-12) was previously described.6 Adenovirus carrying the lacZ reporter gene under the control of the CMV promoter (Adβ-Gal) was produced similarly. The concentration of vectors was expressed as 50% tissue culture infectious doses (TCID50) per milliliter.
In vivo experiments
Subcutaneous CRC model. CT26 cells were injected at a dose of 5 × 105 cells subcutaneously (s.c.) into the right flank of BALB/c mice (day 0). Tumors were allowed to reach approximately 90 mm3 in size before treatment was started. Animals were distributed in different groups and then treated with saline intraperitoneal (i.p.) (control group, n = 6); Adβ-gal (109 TCID50 intratumoral (i.t.), day 9, n = 7); 4-Mu (200 mg/kg/day, oral administration, o.a, during 3 weeks, from day 6, n = 6); Adβ-gal + 4Mu (n = 9); Cy (50 mg/kg i.p., day 8, n = 6); AdIL-12 (109 TCID50 i.t., day 9, n = 8); Cy + AdIL-12 (n = 6) or 4Mu + Cy +AdIL-12 (n = 10). Adenovirus were diluted in saline (final volume of 50 μl) and i.t. injected in a single site; no leakage of material was observed after inoculations. Tumor growth was assessed by caliper measurement.
Liver metastatic CRC model via intrahepatic inoculation of CT26 cells. BALB/c mice received an intrahepatic inoculation of 5 × 105 CT26 cells (day 0). The mice were distributed in experimental groups and treated with saline (control group, n = 6); 4Mu (200 mg/kg/day, o.a., during 3 weeks, from day 6 (n = 6); Cy (50 mg/kg i.p., day 8) + AdIL-12 (109 TCID50 intravenously, i.v., day 9, n = 8) or 4Mu + Cy + AdIL-12 (n = 8). At day 21, animals were sacrificed and the volume of metastatic nodules was measured with caliper.
Liver metastatic CRC model via portal vein administration of CT26 cells. BALB/c mice were administrated with 5 × 105 CT26 cells via portal vein (day 0). The mice were distributed in experimental groups and treated with saline (control group, n = 5); 4Mu (200 mg/kg/day, o.a., during 3 weeks, from day 10 (n = 5); or 4Mu + Cy + AdIL-12 (n = 8). Survival plots were performed when deaths of animals were documented.
TIP. CT26 tumors were subcutaneously induced as described above. The mice were distributed in experimental groups and treated with saline (control group, n = 12) or 4Mu (200 mg/kg/day, o.a during 3 weeks, from day 6, n = 12). At days 7, 10, 14, and 17, post-CT26 administration animals were anesthetized using isofluorane chamber and interstitial fluid pressure measurements were performed for each animal using the wick-in-needle technique. A standard 23-gauge needle filled with nylon floss and saline, embedded in heparin, was inserted into the centre of the tumor and connected to a pressure transducer (Multiparametric Monitor LEEX M8A, Emmed Medical, Tucumán, Argentina). Pressure values in subcutaneous tissue served as controls.
Administration of CD3+DiR+ cells. CT26 tumor cells were injected at a dose of 5 × 105 cells s.c. into the right flank of BALB/c mice (day 0). Tumors were allowed to reach approximately 90 mm3 in size before treatment was started. Animals were distributed in groups: control (saline, i.p.) and 4Mu (200 mg/kg/day, o.a., during 3 weeks, from day 6 until mice were sacrificed). On day 14, control and 4Mu-treated mice were injected i.v with 1 × 106 CD3+ T lymphocytes isolated from ex vivo expanded splenocytes of tumor-free mice after treatment with Cy (50 mg/kg; i.p) + AdIL-12 (109 TCID50). Briefly, CD3+ cells were obtained using anti-mouse CD3+ MicroBeads and magnetic cell sorting following the manufacturer's recommendations (Miltenyi Biotec, Cologne, Germany). Before administration, isolated CD3+ T lymphocytes were stained with the cell tracker DiR (Molecular Probes, Invitrogen, Carlsbad, CA) for fluorescence imaging. Briefly, isolated CD3+ cells were incubated with 5 mmol/l DiR solution for 5 minutes at 37 °C in 5% CO2-humidified atmosphere and for 15 minutes at 4 °C and then washed with PBS. Twenty-four hours after CD3+DiR+ T cells administration, mice were sacrificed and spleen, liver, tumor, lungs, and regional lymph nodes were excised from each animal. Fluorescence imaging was performed using the Xenogen In Vivo Imaging System (IVIS; Caliper Life Sciences, Hopkinton, MA)
Adoptive transfer of CD3+ T lymphocytes. BALB/c mice were s.c. injected with 5 × 105 CT26 cells (day 0) and tumors were allowed to reach 90 mm3 before adoptive therapy was started. Animals were distributed in groups: control (saline, i.p); 4Mu (200 mg/kg/day, o.a, during 3 weeks from day 6). On day 10, control and 4Mu-treated mice were inoculated or not with 2 × 106 CD3+ T cells isolated by magnetic cell sorting as it was described above. Control and 4Mu-treated mice that not received CD3+ T cells were used as control groups. Tumor growth was assessed by caliper measurement.
Ex vivo experiments
Efficiency of adenovirus transfection. Subcutaneous CT26 tumors were induced in BALB/c mice as described above. The mice were distributed in experimental groups and treated with saline i.p. or 4Mu (200 mg/kg/day, o.a from day 6 until mice were sacrificed). On day 9, animals were treated with Adβ-gal (109 TCID50 i.t) or AdIL-12 (109 TCID50 i.t). Forty-eight hours later, mice were sacrificed and tumor samples were taken to determine IL-12 levels by ELISA or β-galactosidase expression by X-gal staining reaction. Briefly, frozen tissue section were fixed for 5 minutes in paraformaldehyde 2% and incubated with 10 mg/ml X-gal (Promega, WI) into staining solution in a humidified chamber at 37̊C for several hours until blue color has developed to a maximum. Positive areas were calculated with ImageJ software.
Detection of perfused blood vessels. Subcutaneous CT26 tumors were induced in BALB/c mice as described above. The mice were distributed in experimental groups and treated with saline (control group) or 4Mu (200 mg/kg/day, o.a, for a week, from day 6). At day 14, animals received 10 mg/kg of Hoechst 33342 dye (Sigma- Aldrich) in PBS via tail vein (total volume of 100 μl). Five minutes later, the animals were sacrificed and tumor samples were taken, embedded in mounting medium (Cryoplast, Biopack, Buenos Aires, Argentina) and 10 μm sections were cut. Images were recorded and Hoechst 33342 profiles were counted using a particle analysis routine in ImageJ software.
Ex vivo expansion of splenocytes for CD3+ T cell isolation. BALB/c mice were injected with CT26 cells and treated with Cy + AdIL-12 as described above. Splenocytes from cured mice were isolated and pooled and 4 × 106 cells/ml were cocultured with mitomycin C–treated CT26 cells (4 × 105/ml) and mitomycin C–treated splenocytes (4 × 105/ml) in a 24-well plate (1 ml/well) with 10 UI/ml rmIL-2. Seven days later, viable cells were harvested and washed, adjusted to 4 × 106/ml, and cocultured again with mitomycin C–treated CT26 cells and splenocytes. The cytotoxic activity of harvested cells was confirmed. On day 14, viable cells were used for isolation of CD3+ T Lymphocytes.
Toxicity studies. BALB/c mice untreated or orally treated with 4Mu (200 mg/kg/day, during 3 weeks) were used to assess its toxicology profile. Mice were supervised for more than 30 days. White cells, platelets, and hemoglobin quantification were analyzed by a cell counter (Countess II FL Automated Cell Counter, Life Technologies, Invitrogen, Carlsbad, CA). Aspartate aminotransferase, alanine aminotransferase, and lactate dehydrogenase levels were measured by standard colorimetric methods. Finally, healthy mice or mice receiving 4Mu were sacrificed to collect liver, spleen, heart, lung, and kidney samples. Paraffin-embedded tissues were stained with hematoxylin and eosin for pathological analysis.
In vitro experiments
HA quantification by an ELISA-like assay. HA from CT26 cells-free supernatants was measured using a competitive ELISA-like assay as described elsewhere.49 Briefly, 96-well microplates (Nalgene International Corporation, NY) were coated with 100 μg/ml of HA (CPN spol.s.r.o. Czech Republic). Then, wells were incubated with 25 μl of sample or standard HA (0–1 μg/ml), in the presence of 0.75 μg/ml of biotinylated HA-Binding Protein (bHA-BP) (Calbiochem, Merck KGaA, Darmstadt, Germany) at 37 °C for 4 hours and then washed with PBS–0.05% Tween-20. The bHA-BP bound to the wells was determined using an avidin–biotin detection system (Sigma-Aldrich). Sample concentrations were calculated from a standard curve generated by plotting the absorbance at 490 nm against the concentration of HA.49
HA staining. HA staining was performed as described elsewhere.50 Briefly, paraffin tumor sections were incubated with 3% H2O2–methanol for 30 minutes at room temperature to block endogenous peroxidase, followed by avidin, biotin and protein-blocking solution (Vector Laboratories, Burlingame, CA). Then, 5 μg/ml of bHA-BP (Calbiochem, Merck KGaA, Darmstadt, Germany) diluted in 1% bovine serum albumin (BSA)–phosphate-buffered saline (PBS) was applied for 1 hour. Negative controls were stained with bHA-BP and pretreated with 100 U/ml of Streptomyces hyaluronidase (Calbiochem, Merck KGaA, Darmstadt, Germany) at 37 °C for 30 minutes. Peroxidase complex (Sigma-Aldrich) 1:10 in PBS was used as a revealing system. The signal was detected by 0.1% diaminobenzidine (Sigma-Aldrich), 4% glucose, 0.08% ClNH4, 5% nickel ammonium sulfate in 0.2 M AcNa and 0.05% H2O2. Positive areas were calculated with ImageJ software (National Institutes of Health, Bethesda, MD, USA; NIH).
Immunofluorescence. Immunofluorescence for von Willebrand factor was performed on frozen tumor sections. After 1 hour of incubation in blockage buffer (5% normal donkey serum, Jackson ImmunoResearch, PA, 1% BSA, 0.3% Triton-X in PBS; room temperature), tissue was incubated overnight at 4 °C with a rabbit anti-von Willebrand factor polyclonal antibody (vWF; 1:215; Sigma-Aldrich). After extensive washing, tissue was incubated with FITC-conjugated donkey anti-rabbit IgG secondary antibody (1:450; 2 hours, room temperature; Vector Laboratories). Slides were mounted and analyzed under a fluorescence microscope. Images were captured and quantified using the ImageJ software.
ELISA. Serum samples or tumor homogenates were tested in competitive ELISA using kits from R&D Systems (R&D Systems, MN) to quantify VEGF and IL-12 (DuoSet mouse VEGF, Cat DY493; DuoSet mouse IL-12, Cat DY2398) following the manufacturer's recommendations. CT26 cells (5 × 105 cells/well) were incubated with 4Mu (0, 0.125, 0.25, and 0.5 mmol/l) for 20 hours. Supernatant was subjected to VEGF and IL-6 quantification. IL-6 levels were determined using BD OptEIA Set Mouse IL-6 (BD Bioscience, CA) following the manufacturer's recommendations.
Tube formation assay. Tube formation assay was performed using Matrigel (BD Bioscience, CA) thawed at 4 °C to prevent polymerization. Forty microliters of Matrigel/well were seeded in a 96-well culture plate (GBO, Frickenhausen, Germany), and allowed to polymerize at 37 °C for at least 30 minutes. HMEC-1 cells (2 × 104), fetal bovine serum starved for 18 hours, were seeded on 90 µl of fetal bovine serum-free Dulbecco's Modified Eagle Medium, and stimulated with 10 µl of 4Mu-pretreated CT26 supernatants. After 6 hours of incubation at 37 °C, cells were fixed with 2% paraformaldehyde solution. Quantification was performed by analyzing the number of branching points/well at 20× (Nikon, Buenos Aires, Argentina) with Image J software (National Institutes of Health, Bethesda, MD).
Reverse transcription-polymerase chain reaction (RT-PCR). Total RNA from tumor was extracted using Trizol Reagent (Sigma-Aldrich). Total RNA (2 mg) was reverse transcribed with 200 U of SuperScript II Reverse Transcriptase (Invitrogen) using 500 ng of Oligo (dT) primers. cDNAs were subjected to real-time polymerase chain reaction (qPCR) (Stratagene Mx3005p, Stratagene, La Jolla, CA). For qRT-PCR, the mRNA levels of VEGF, hypoxia-inducible factor 1α (HIF1-α); interleukin-6 (IL-6); thrombospondin-1 (TSP-1), and interferon-inducible protein-10 (IP-10) were quantified by SYBRGreen (Invitrogen), using the following primers: VEGF forward 5′- GTGCACTGGACCCTGGCTTTA-3′ and reverse 5′- GGTCTCAATCGGACGGCAGTA-3′; HIF-1α forward 5′- GCAGCAGGAATTGGAACATT-3′ and reverse 5′- GCATGCTAAATCGGAGGGTA -3′; IL-6 forward 5′- CCCACCTCATGCACTGTTGA -3′ and reverse 5′-TTATGTGGCGGATTGGGCTT-3′; TSP-1 forward 5′- ATACAGATGGCGTCTCAGCC -3′ and reverse 5′- GAACAGGCCTAGTCTACCGC -3′; IP-10 forward 5′-CTGAGTGGGACTCAAGGGAT-3′ and reverse 5′- AGGCTCGCAGGGATGATTTC -3′. PCR amplifications were carried out using a cycle of 95 °C for 10 minutes and 40 cycles under the following parameters: 95 °C for 30 seconds, 56 °C for 30 seconds, 72 °C for 1 minute. At the end of PCR reaction, the temperature was increased from 60 to 95 °C at a rate of 2 °C/minute, and the fluorescence was measured every 15 seconds to construct the melting curve. Values were normalized to levels of glyceraldehyde- 3-phosphate dehydrogenase transcript (GAPDH; used as housekeeping) (forward 5′-CATCTCTGCCCCCTCTGCTG-3′ and reverse 5′-GCCTGCTTCACCACCTTCTTG-3′). Data were processed by the ΔΔCt method. The relative amount of the PCR product amplified from untreated cells was set as 1. A nontemplate control (NTC) was run in every assay, and all determinations were performed as triplicates for each animal (n = 5/group) in two separated experiments.
Statistic analysis. All experiments were performed in triplicate and repeated at least three times on different occasions. Values were expressed as the mean ± SEM. The Mann–Whitney or Kruskal–Wallis (ANOVA) tests were used to evaluate the statistical differences between two groups or more than two groups, respectively. Mice survival was analyzed by a Kaplan-Meier curve. A P value of <0.05 was considered as significant. Prism software (Graph Pad, San Diego, CA) was employed for the statistical analysis.
Acknowledgments
We would like to thank Guillermo Gastón, Santiago Cabrera, Gustavo Ortiz, and Vanina Ferreira for their expert technical assistance.
References
- Miamen, AG, Gustafson, MP and Roberts, LR (2014). Rethinking cancer immunotherapy: Using advanced cancer genetics in immune-mediated eradication of gastrointestinal cancers. Hepatology 60: 2121–2124. [DOI] [PubMed] [Google Scholar]
- Shapira, S, Lisiansky, V, Arber, N and Kraus, S (2010). Targeted immunotherapy for colorectal cancer: monoclonal antibodies and immunotoxins. Expert Opin Investig Drugs 19 Suppl 1: S67–S77. [DOI] [PubMed] [Google Scholar]
- Hanahan, D and Weinberg, RA (2011). Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Drake, CG, Jaffee, E and Pardoll, DM (2006). Mechanisms of immune evasion by tumors. Adv Immunol 90: 51–81. [DOI] [PubMed] [Google Scholar]
- Becker, JC, Andersen, MH, Schrama, D and Thor Straten, P (2013). Immune-suppressive properties of the tumor microenvironment. Cancer Immunol Immunother 62: 1137–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvicini, M, Rizzo, M, Alaniz, L, Piñero, F, García, M, Atorrasagasti, C et al. (2009). A novel synergistic combination of cyclophosphamide and gene transfer of interleukin-12 eradicates colorectal carcinoma in mice. Clin Cancer Res 15: 7256–7265. [DOI] [PubMed] [Google Scholar]
- Malvicini, M, Ingolotti, M, Piccioni, F, Garcia, M, Bayo, J, Atorrasagasti, C et al. (2011). Reversal of gastrointestinal carcinoma-induced immunosuppression and induction of antitumoural immunity by a combination of cyclophosphamide and gene transfer of IL-12. Mol Oncol 5: 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan, D and Coussens, LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21: 309–322. [DOI] [PubMed] [Google Scholar]
- Balkwill, FR, Capasso, M and Hagemann, T (2012). The tumor microenvironment at a glance. J Cell Sci 125(Pt 23): 5591–5596. [DOI] [PubMed] [Google Scholar]
- Heldin, CH, Rubin, K, Pietras, K and Ostman, A (2004). High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer 4: 806–813. [DOI] [PubMed] [Google Scholar]
- Ropponen, K, Tammi, M, Parkkinen, J, Eskelinen, M, Tammi, R, Lipponen, P et al. (1998). Tumor cell-associated hyaluronan as an unfavorable prognostic factor in colorectal cancer. Cancer Res 58: 342–347. [PubMed] [Google Scholar]
- Anttila, MA, Tammi, RH, Tammi, MI, Syrjänen, KJ, Saarikoski, SV and Kosma, VM (2000). High levels of stromal hyaluronan predict poor disease outcome in epithelial ovarian cancer. Cancer Res 60: 150–155. [PubMed] [Google Scholar]
- Auvinen, PK, Parkkinen, JJ, Johansson, RT, Agren, UM, Tammi, RH, Eskelinen, MJ et al. (1997). Expression of hyaluronan in benign and malignant breast lesions. Int J Cancer 74: 477–481. [DOI] [PubMed] [Google Scholar]
- Kakizaki, I, Kojima, K, Takagaki, K, Endo, M, Kannagi, R, Ito, M et al. (2004). A novel mechanism for the inhibition of hyaluronan biosynthesis by 4-methylumbelliferone. J Biol Chem 279: 33281–33289. [DOI] [PubMed] [Google Scholar]
- Lokeshwar, VB, Lopez, LE, Munoz, D, Chi, A, Shirodkar, SP, Lokeshwar, SD et al. (2010). Antitumor activity of hyaluronic acid synthesis inhibitor 4-methylumbelliferone in prostate cancer cells. Cancer Res 70: 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioni, F, Malvicini, M, Garcia, MG, Rodriguez, A, Atorrasagasti, C, Kippes, N et al. (2012). Antitumor effects of hyaluronic acid inhibitor 4-methylumbelliferone in an orthotopic hepatocellular carcinoma model in mice. Glycobiology 22: 400–410. [DOI] [PubMed] [Google Scholar]
- Morohashi, H, Kon, A, Nakai, M, Yamaguchi, M, Kakizaki, I, Yoshihara, S et al. (2006). Study of hyaluronan synthase inhibitor, 4-methylumbelliferone derivatives on human pancreatic cancer cell (KP1-NL). Biochem Biophys Res Commun 345: 1454–1459. [DOI] [PubMed] [Google Scholar]
- Kultti, A, Pasonen-Seppänen, S, Jauhiainen, M, Rilla, KJ, Kärnä, R, Pyöriä, E et al. (2009). 4-Methylumbelliferone inhibits hyaluronan synthesis by depletion of cellular UDP-glucuronic acid and downregulation of hyaluronan synthase 2 and 3. Exp Cell Res 315: 1914–1923. [DOI] [PubMed] [Google Scholar]
- Jain, RK (2013). Normalizing tumor microenvironment to treat cancer: bench to bedside to biomarkers. J Clin Oncol 31: 2205–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariffin, AB, Forde, PF, Jahangeer, S, Soden, DM and Hinchion, J (2014). Releasing pressure in tumors: what do we know so far and where do we go from here? A review. Cancer Res 74: 2655–2662. [DOI] [PubMed] [Google Scholar]
- Starke, RD, Ferraro, F, Paschalaki, KE, Dryden, NH, McKinnon, TA, Sutton, RE et al. (2011). Endothelial von Willebrand factor regulates angiogenesis. Blood 117: 1071–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri, R (2003). Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer 3: 422–433. [DOI] [PubMed] [Google Scholar]
- Piccioni, F, Fiore, E, Bayo, J, Atorrasagasti, C, Peixoto, E, Rizzo, M et al. (2015). 4-Methylumbelliferone inhibits hepatocellular carcinoma growths by decreasing IL-6 production and angiogenesis. Glycobiology 8: 825–835. [DOI] [PubMed] [Google Scholar]
- Mellman, I, Coukos, G and Dranoff, G (2011). Cancer immunotherapy comes of age. Nature 480: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poschke, I, Mougiakakos, D and Kiessling, R (2011). Camouflage and sabotage: tumor escape from the immune system. Cancer Immunol Immunother 60: 1161–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang, H and Declerck, YA (2013). Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res 73: 4965–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura, D and Jain, RK (2007). Tumor microenvironment abnormalities: causes, consequences, and strategies to normalize. J Cell Biochem 101: 937–949. [DOI] [PubMed] [Google Scholar]
- Padera, TP, Stoll, BR, Tooredman, JB, Capen, D, di Tomaso, E and Jain, RK (2004). Pathology: cancer cells compress intratumour vessels. Nature 427: 695. [DOI] [PubMed] [Google Scholar]
- Singha, NC, Nekoroski, T, Zhao, C, Symons, R, Jiang, P, Frost, GI et al. (2015). Tumor-associated hyaluronan limits efficacy of monoclonal antibody therapy. Mol Cancer Ther 14: 523–532. [DOI] [PubMed] [Google Scholar]
- Toole, BP (2004). Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer 4: 528–539. [DOI] [PubMed] [Google Scholar]
- Friman, T, Gustafsson, R, Stuhr, LB, Chidiac, J, Heldin, NE, Reed, RK et al. (2012). Increased fibrosis and interstitial fluid pressure in two different types of syngeneic murine carcinoma grown in integrin ß3-subunit deficient mice. PLoS ONE 7: e34082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong, RT, Boucher, Y, Kozin, SV, Winkler, F, Hicklin, DJ and Jain, RK (2004). Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res 64: 3731–3736. [DOI] [PubMed] [Google Scholar]
- Liao, S, Liu, J, Lin, P, Shi, T, Jain, RK and Xu, L (2011). TGF-beta blockade controls ascites by preventing abnormalization of lymphatic vessels in orthotopic human ovarian carcinoma models. Clin Cancer Res 17: 1415–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brekken, C, Hjelstuen, MH, Bruland, ØS and de Lange Davies, C (2000). Hyaluronidase-induced periodic modulation of the interstitial fluid pressure increases selective antibody uptake in human osteosarcoma xenografts. Anticancer Res 20(5B): 3513–3519. [PubMed] [Google Scholar]
- Ferretti, S, Allegrini, PR, O'Reilly, T, Schnell, C, Stumm, M, Wartmann, M et al. (2005). Patupilone induced vascular disruption in orthotopic rodent tumor models detected by magnetic resonance imaging and interstitial fluid pressure. Clin Cancer Res 11: 7773–7784. [DOI] [PubMed] [Google Scholar]
- Taghian, AG, Abi-Raad, R, Assaad, SI, Casty, A, Ancukiewicz, M, Yeh, E et al. (2005). Paclitaxel decreases the interstitial fluid pressure and improves oxygenation in breast cancers in patients treated with neoadjuvant chemotherapy: clinical implications. J Clin Oncol 23: 1951–1961. [DOI] [PubMed] [Google Scholar]
- Jain, RK (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307: 58–62. [DOI] [PubMed] [Google Scholar]
- Thompson, CB, Shepard, HM, O'Connor, PM, Kadhim, S, Jiang, P, Osgood, RJ et al. (2010). Enzymatic depletion of tumor hyaluronan induces antitumor responses in preclinical animal models. Mol Cancer Ther 9: 3052–3064. [DOI] [PubMed] [Google Scholar]
- Brekken, C and de Lange Davies, C (1998). Hyaluronidase reduces the interstitial fluid pressure in solid tumours in a non-linear concentration-dependent manner. Cancer Lett 131: 65–70. [DOI] [PubMed] [Google Scholar]
- Takeda, S and Aburada, M (1981). The choleretic mechanism of coumarin compounds and phenolic compounds. J Pharmacobio-dyn 4: 724–734. [DOI] [PubMed] [Google Scholar]
- Postow, MA, Chesney, J, Pavlick, AC, Robert, C, Grossmann, K, McDermott, D et al. (2015). Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 372: 2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamid, O, Robert, C, Daud, A, Hodi, FS, Hwu, WJ, Kefford, R et al. (2013). Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369: 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares, KC, Rucki, AA, Wu, AA, Olino, K, Xiao, Q, Chai, Y et al. (2015). PD-1/PD-L1 blockade together with vaccine therapy facilitates effector T-cell infiltration into pancreatic tumors. J Immunother 38: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgueta, R, Benson, MJ, de Vries, VC, Wasiuk, A, Guo, Y and Noelle, RJ (2009). Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 229: 152–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty, GL (2013). Macrophage-based immunotherapy for the treatment of pancreatic ductal adenocarcinoma. Oncoimmunology 2: e26837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Y, Yuan, J, Righi, E, Kamoun, WS, Ancukiewicz, M, Nezivar, J et al. (2012). Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc Natl Acad Sci USA 109: 17561–17566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addison, CL, Nör, JE, Zhao, H, Linn, SA, Polverini, PJ and Delaney, CE (2005). The response of VEGF-stimulated endothelial cells to angiostatic molecules is substrate-dependent. BMC Cell Biol 6: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Vilas, JA, Quesada, AR and Medina, MÁ (2013). 4-methylumbelliferone inhibits angiogenesis in vitro and in vivo. J Agric Food Chem 61: 4063–4071. [DOI] [PubMed] [Google Scholar]
- Cordo-Russo, R, Garcia, MG, Barrientos, G, Orsal, AS, Viola, M, Moschansky, P et al. (2009). Murine abortion is associated with enhanced hyaluronan expression and abnormal localization at the fetomaternal interface. Placenta 30: 88–95. [DOI] [PubMed] [Google Scholar]
- Jameson, JM, Cauvi, G, Sharp, LL, Witherden, DA and Havran, WL (2005). Gammadelta T cell-induced hyaluronan production by epithelial cells regulates inflammation. J Exp Med 201: 1269–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]