Abstract
RNA interference (RNAi) technology using short hairpin RNAs (shRNAs) expressed via RNA polymerase (pol) III promoters has been widely exploited to modulate gene expression in a variety of mammalian cell types. For certain applications, such as lineage-specific knockdown, embedding targeting sequences into pol II-driven microRNA (miRNA) architecture is required. Here, using the potential therapeutic target BCL11A, we demonstrate that pol III-driven shRNAs lead to significantly increased knockdown but also increased cytotoxcity in comparison to pol II-driven miRNA adapted shRNAs (shRNAmiR) in multiple hematopoietic cell lines. We show that the two expression systems yield mature guide strand sequences that differ by a 4 bp shift. This results in alternate seed sequences and consequently influences the efficacy of target gene knockdown. Incorporating a corresponding 4 bp shift into the guide strand of shRNAmiRs resulted in improved knockdown efficiency of BCL11A. This was associated with a significant de-repression of the hemoglobin target of BCL11A, human γ-globin or the murine homolog Hbb-y. Our results suggest the requirement for optimization of shRNA sequences upon incorporation into a miRNA backbone. These findings have important implications in future design of shRNAmiRs for RNAi-based therapy in hemoglobinopathies and other diseases requiring lineage-specific expression of gene silencing sequences.
Introduction
RNA interference (RNAi) mediated by short interfering RNAs (siRNA) or microRNAs (miRNA) is a powerful method for posttranscriptional regulation of gene expression. RNAi has been extensively used for the study of biological processes in mammalian cells and could constitute a therapeutic approach to human diseases in which selective modulation of gene expression would be desirable. RNA polymerase (pol) III-driven short hairpin RNAs (shRNAs) are most commonly used in biological experimental settings. ShRNAs can be abundantly expressed to provide efficient knockdown, but at high multiplicities of infection (MOI), oversaturation of the endogenous RNAi machinery has been reported in some cases to be associated with cytotoxic effects due to the dysregulation of endogenous miRNAs.1,2,3,4,5 Additionally, activation of innate immune responses triggered by small RNAs in a sequence-specific as well as nonspecific manner may mediate cytotoxic side effects6,7 (reviewed in Jackson and Linsley8). These effects have been implicated in increased mortality in mice in some experimental transgenic model systems.9,10
ShRNAs mimic the structure of miRNA precursor intermediates but bypass the first cleavage step of endogenous miRNA processing. Endogenous miRNAs are transcribed as primary transcripts which are cleaved by the Microprocessor complex,11 exported from the nucleus, and processed by Dicer. The resulting siRNA duplex binds to the Ago-protein subunit of the RNA-induced silencing complex (RISC), where strand selection occurs.12 The guide strand is incorporated into the RISC, while the passenger strand is degraded (reviewed in Winter et al.13). The loading of guide versus passenger strands into RISC largely depends on the 5′ end stability of the siRNA, with the less stable strand preferentially incorporated into RISC.14,15 Additional factors influencing, such as 5′ nucleotide identity, also strongly influence strand loading.16,17 The 5′ end of the guide strand contains the “seed region,” which is critical for target identification.18,19 Precise processing is therefore important for the generation of guide RNAs with defined seed regions that mediate efficient binding to the appropriate target mRNAs. Inaccurate processing results in binding to off-target molecules, but a shift in cleavage sites also alters the nucleotide composition of duplex ends, which may have a profound effect on strand loading into RISC.20
For clinical translation of RNAi-based therapeutics, alternative expression systems utilizing pol II promoters will likely be required. This class of promoters allows for lineage or even cell type–specific expression. Mammalian pol II promoters also provide lower levels of expression compared to pol III promoters, which may obviate oversaturation of the processing machinery that has been reported in cells transduced at high MOIs.4,5 The use of pol II promoters for shRNA expression requires embedding of the shRNA sequences into flanking regions that are typically derived from endogenous miRNA precursors. ShRNAs flanked by a miRNA scaffold mimic the structure of endogenous miRNAs2,21 and are termed shRNAmiR. To date, flanking regions derived from human miRNA-30 and miRNA-223 have been widely used for the incorporation of recombinant shRNAs, and there have been numerous efforts to better understand and to improve this expression strategy.22,23 miRNA-223 has previously been shown to be effective when used as scaffold for shRNA expression in hematopoietic cells. It supports substantial knockdown of some target mRNAs and has low passenger strand activity in several hematopoietic cell types.22,24 However, despite progress, RNAi design based on bioinformatic prediction remains unreliable, and optimal sequences still need to be identified by screening of multiple candidates. Due to the high knockdown efficiency, easy design, and availability of commercial libraries, U6 or H1 RNA pol III promoter-driven shRNAs are frequently used for such screens. For possible therapeutic applications, potent shRNA candidates identified in these screens suggest good candidate shRNA target sites but optimally need to be transferred into a pol II expression vector. However, comparative data using pol II and pol III expression systems are limited, and the factors influencing the relative knockdown efficacy in both systems remain ill defined.
Here, we utilized BCL11A as a target to study the optimization of shRNAmiRs for potential therapeutic applications. BCL11A is a validated therapeutic target for reactivation of the γ-globin gene which in turn increases protective hemoglobin F (HbF) expression in the major hemoglobinopathies, sickle cell disease, and β-thalassemias. Down modulation or genetic deletion of BCL11A relieves γ-globin repression,25 and inactivation of BCL11A in the erythroid lineage of genetically engineered mice prevents red blood cell sickling and other sickle cell disease–associated phenotypes, such as hemolysis and organ toxicities.26 More recent studies have demonstrated that erythroid-specific expression is dependent in part on enhancer sequences located in an intronic region of the BCL11A gene,27 a finding of specific translational relevance since BCL11A appears critical for lymphoid and neuronal development28,29,30,31 and Sankaran et al. (submitted). Our goal in the studies reported here was to develop a clinically applicable viral vector that would lead to knockdown of BLC11A which would simultaneously induce γ-globin and reduce expression of the mutant beta-sickle hemoglobin (βs) as a therapy for sickle cell disease. Use of pol III directed shRNA targeting BCL11A led to efficient knockdown of the target protein but was accompanied by significant toxicity in several cell lines. Embedding the BCL11A shRNAs into a miRNA architecture to facilitate pol II-directed expression was associated with less toxicity but uniformly decreased knockdown efficiency due to miRNA processing that resulted in alternate guide strands. Thus, we designed shRNAmiRs that mimicked the mature guide strand sequences produced by effective pol III-driven shRNAs. Incorporation of these modifications into a pol II-driven mammalian expression vector led to significantly improved knockdown of BCL11A protein, reduction in the pol III-directed shRNA-induced toxicity in cell lines, and re-induction of fetal hemoglobin in primary erythroid cells used to model the fetal to adult globin switch. Thus, our data demonstrate that the toxicity seen in cells expressing shRNA sequences driven by pol III promoter can be reduced using shRNAmiRs expressed via pol II promoters. We describe critical features of RNA processing relevant to the use of shRNAs in different vector contexts. Our findings have important implications for design of miRNA-embedded shRNAs and their application in RNAi-based gene therapy approaches.
Results
Pol III-driven shRNA sequences demonstrate cellular toxicity compared to pol II expressed shRNAmiRs
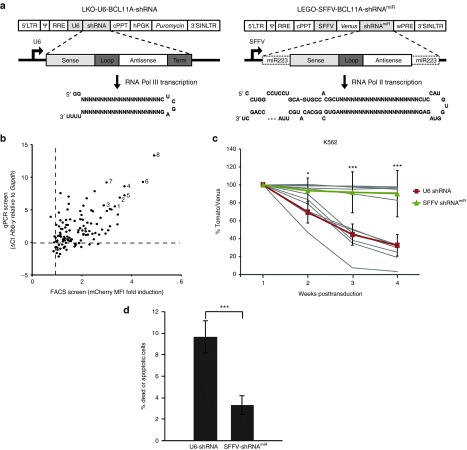
To identify candidate shRNAs mediating effective knockdown of BCL11A, a lentiviral library of 118 shRNAs targeting coding sequences of BCL11A mRNA conserved between humans and mice was screened in MEL cells. ShRNAs were expressed from a pol III-based U6 promoter (Figure 1a, left panel) in the LKO lentivirus backbone32 containing a puromycin resistance gene for selection named LKO-U6-BCL11A-shRNA (hereafter U6-shRNA). MEL cells, a commonly used cell line for the study of globin gene regulation, were transduced with the lentivirus vectors expressing shRNAs at a MOI of 2. The normalized expression of embryonic mouse Hbb-y mRNA, which serves as a functional homolog of the human γ-globin gene33 provides an indirect readout of BCL11A knockdown (Figure 1b, y-axis). As a second readout, the shRNA pool was also screened using a MEL-reporter cell line harboring a mCherry knock-in at the Hbb-y locus (D. Bauer, unpublished data). Fluorescent reporter induction was analyzed by flow cytometry (Figure 1b, x-axis). Eight shRNAs (labeled and named as shRNA1 through 8 in Figure 1b) that consistently induced Hbb-y and mCherry reporter expression in MEL cells were identified. We utilized these shRNAs to generate pol II-based vectors with the ultimate goal of developing lineage-specific expression vectors for knockdown of BCL11A. In a pilot experiment, one shRNA was embedded into human miRNA-223 (miR-223), miRNA-451, or miRNA-144 flanking and loop sequences to create synthetic miRNAs (shRNAmiR).4 Due to superior induction of Hbb-y in MEL cells, the miRNA-223 scaffold was chosen for subsequent experiments and cloning of all eight shRNA candidates (data not shown). For initial analysis, this cassette was incorporated in the pLeGO lentiviral vector34 (Figure 1a, right panel) into the 3′ untranslated region of the Venus fluorescent reporter under control of the very strong and ubiquitously expressed spleen focus forming virus (SFFV) promoter/enhancer named LEGO-SFFV-BCL11A-shRNAmiR (hereafter SFFV-shRNAmiR).
Figure 1.
Screening of shRNAs targeting BCL11A in pol III system and assessment of cytotoxicity among pol III and pol II expression systems. (a) Schematic representation of LKO-U6-BCL11A-shRNA (left side) and LEGO-SFFV-BCL11A-shRNAmiR (right side). The light gray boxes represent the sense strand; white boxes represent the antisense strand; dark gray boxes represent the loop structure, and the miRNA223 scaffold is indicated by a dotted line. The hairpin structures are shown below. (b) High-throughput screening of multiple shRNA sequences targeting BCL11A mRNA for knockdown efficiency using pol III-based lentivirus vectors. Both induction of Hbb-y mRNA by qRT-PCR and induction of mCherry reporter by FACS (as a surrogate for ɛ -y induction in a reporter cell line) were used as a functional readout for BCL11a knockdown. Normalized expression of Hbb-y mRNA relative to nontargeting control is plotted on y-axis and fold induction of mCherry expression (by mean fluorescence intensity, MFI) relative to nontransduced control is plotted on x-axis. (c) Comparative assessment of shRNA and shRNAmiR induced cytotoxicity. K562 cells were transduced with lentiviral vectors expressing each of the shRNAs numbered 1–8 in b either in U6-shRNA or SFFV-shRNAmiR and the expression levels were monitored by flow cytometry for 5 weeks posttransduction. The mean percentages of all U6-shRNA (Tomato) and SFFV-shRNAmiRs (Venus) were represented on top of the individual set of candidates respectively. (d) The percentage of apoptotic cells was detected by Annexin V and 7AAD staining on day 11 of posttransduction. Data represent mean ± SD from a representative experiment of three independent experiments conducted in triplicates. *P < 0.05; **P < 0.01; ***P < 0.001. n.s., not significant.
Since previous studies have demonstrated that high levels of shRNA expression may result in toxicity,9,10 we first compared the toxicities induced by U6 shRNA or SFFV shRNAmiR in MEL, K562, and Jurkat cell lines. The latter two cell lines do not express BCL11A, which excludes any confounding effect due to depletion of BCL11A message. For these experiments, the puromycin gene in the constructs used in Figure 1 was replaced with a fluorescent protein (dTomato) coding region to avoid any confounding toxicity associated with puromycin selection. K562, MEL, and Jurkat cells were transduced with U6 shRNA or SFFV shRNAmiR expressing lentiviral vectors at MOIs to attain similar transduction rates of 50–70% based on flow cytometric analysis. Cytotoxicity was determined by monitoring the fraction of U6 shRNA (Tomato) or SFFV shRNAmiR (Venus) transduced cells in vitro over 5 weeks. While the fraction of gene-modified cells remained relatively stable in populations transduced with SFFV shRNAmiR constructs, a continuous loss of transduced cells was observed for U6-shRNA–treated groups (Figure 1c). As no appreciable differences between different targeting sequences were observed, the mean of all eight shRNA which were used is shown. Similar data was seen in both MEL and Jurkat T cells (Supplementary Figure S1a,b) where there was significant difference between the U6 shRNA and SFFV shRNAmiR expressing cells in terms of loss of reporter expression. To determine whether the loss of U6 shRNA expressing cells in culture was due to reduced cell proliferation or increased apoptosis, the cells were stained with Annexin V and 7AAD on day11 posttransduction to measure cell death. As seen in Figure 1d, K562 cells expressing shRNAs via a pol III promoter demonstrated significantly higher apoptosis compared with shRNAmiR-expressing constructs.
Decreased knockdown efficiency of BCL11A by shRNAs embedded in a miRNA scaffold (shRNAmiR) compared to simple stem-loop shRNAs
We next tested the RNAi activity of both U6 shRNAs and SFFV shRNAmiR vectors. We directly compared the Bcl11a knockdown efficacy of shRNAs/shRNAmiRs that incorporated the same 21 nucleotide target-matching sequences in MEL cells using a nontargeting (NT) shRNA as negative control. Both the XL isoform of Bcl11a, which is considered critical for γ-globin repression,35 and the L isoform of BCL11A protein were detected by immunoblot in cell lysates from MEL cells transduced at an MOI of 2 (Figure 2a). In the majority of shRNAs examined, knockdown of BCL11A was less efficient in cells expressing SFFV-shRNAmiRs compared to U6-shRNAs (Figure 2a). To confirm the functional significance of this difference, we measured induction of Hbb-y mRNA levels by qRT-PCR (Figure 2b) in homogeneous populations of transduced cells obtained either by puromycin selection or by fluorescence-activated cell sorting. In every case, the reduced knockdown efficiency of SFFV-shRNAmiRs as compared to U6-shRNAs (Figure 2a) translated into significantly less induction of Hbb-y by SFFV-shRNAmiRs with the differences in Hbb-y induction appearing even more pronounced than the differences in BCL11A knockdown.
Figure 2.
Evaluation of shRNAs targeting BCL11A in pol III and pol II expression systems. (a) Comparison of knockdown efficiency of selected shRNAs (labeled as 1 through 8) in pol III (U6)- and pol II (SFFV)-based systems. BCL11A protein levels are shown by immunoblot with β-actin as control. (b) Fold induction of normalized expression of Hbb-y compared to nontargeting control measured by qPCR. Black bars represent the relative expression by U6 promoter-driven shRNAs, and white bars represent SFFV promoter-driven shRNAs. Data represent mean ± SD from a representative experiment of three independent experiments conducted in triplicates. *P < 0.05 is comparison between U6-shRNAs and SFFV-shRNAmiRs.
SFFV-shRNAmiR and U6-shRNAs give rise to different mature guide strand sequences
To understand the molecular basis for the differences in knockdown efficiency, we performed sequencing of small RNAs from cells transduced with various U6-shRNAs and their corresponding SFFV-shRNAmiR counterparts. We hypothesized that different mature guide strand sequences would be produced from shRNA versus shRNAmiR expression cassettes, thereby contributing to the significant differences in Hbb-y induction. We assessed the processed guide strand sequences produced from U6-shRNAs and SFFV-shRNAmiRs in MEL cells (Figure 3a,b). The most abundant mature guide RNAs produced from SFFV-shRNAmiRs closely corresponded to the in silico predicted mature sequence (Figure 3b). In contrast, most of the U6-shRNAs yielded mature guide strand sequences consisting of ~22 nt of the 3′ end of the pol III transcript, including a stretch of 3–5 nt derived from the pol III termination signal (Figure 3a). These sequences lacked the corresponding number of nucleotides of the target-matched sequence at the 5′ end. A similar distribution of processed products were observed in a large-scale screen of 247 different U6-shRNAs in Jurkat, A549, MCF7, and U937 cell lines, in which the predominant guide strand sequence has an average length of 22 nt with its 5′ end starting 4 bases from the constant loop sequence (Supplementary Figure S2). These findings indicate the importance of considering the processing events that generate mature sequences from pol II shRNAmiR and pol III shRNA transcripts when transferring shRNA sequences between vectors. The very similar distributions of mature sequences observed for the five cell types that we studied suggests that these processing patterns will generalize across different cellular contexts. We conclude that differences in the mature guide strand sequence generated in pol III versus pol II based vectors contribute substantially to differential BCL11A downregulation observed with U6-shRNAs compared to SFFV-shRNAmiRs. These data suggest that predicted conversions between pol III and pol II vectors may be possible by considering the Drosha and Dicer cleavage of pol II shRNAs compared to the Dicer cleavage only of pol III shRNAs.
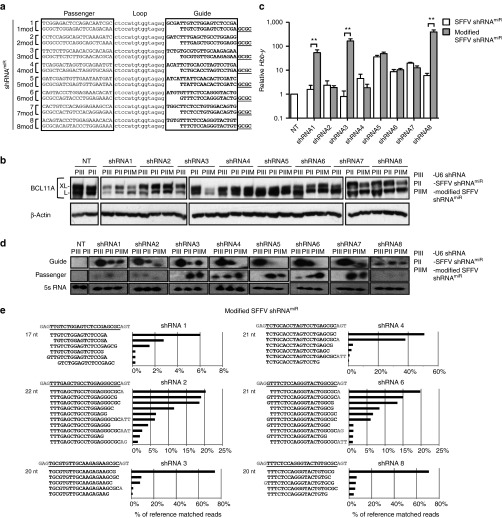
Figure 3.
Small RNA sequencing analysis reveals differential processing between pol III vs pol II transcripts. (a, b) Small RNA sequencing results of MEL cells transduced with U6-shRNAs and SFFV-shRNAmiRs 1, 2, 3, 4, 6, or 8. The RNA sequences were aligned to the corresponding reference guide strand sequence, shown at the top of each panel in bold underlined and the flanking sequences in grey. nt length indicates size of predominant species. The five most abundant variants of guide strands produced from (a) U6 shRNAs or (b) SFFV-shRNAmiRs are plotted on the y-axis. The relative % contribution of each variant is indicated on the x-axis calculated based on the total number of reads matching the reference shRNA sequence.
Modification of shRNA sequences in a pol II-based vector leads to improved knockdown efficiency
Based on these findings, we hypothesized that using the empirically determined mature sequences from pol III shRNA vectors for transfer into SFFV-shRNAmiR would lead to enhanced knockdown efficiency. Since the major determinant of knockdown efficiency lies within the seed region, we designed a set of SFFV-shRNAmiRs containing a 4-nucleotide shift in the 5′ end of the guide strand sequence (Figure 4a). At the 3′ end the nucleotides, GCGC were added to achieve higher 3′-end thermodynamic stability in the siRNA duplex which should promote preferential RISC loading of the intended guide strand. The effect of modifications on knockdown efficiency and Hbb-y induction was evaluated in MEL cells by immunoblot and qRT-PCR, respectively. Improved knockdown efficiency of BCL11A protein was observed in 5/8 of the modified shRNAs, including SFFV-shRNAmiR1, 3, 5, 7, and 8 (Figure 4b). The enhanced knockdown correlated with a 50- to 400-fold increased induction of Hbb-y transcripts for SFFV-shRNAmiR1, 3, and 8, with a nonsignificant change for SFFV-shRNAmiR 5 and 7 (Figure 4c). The other SFFV-shRNAmiRs did not show an appreciable increase in knockdown efficiency, possibly due to one of the less abundant mature guide strands produced from each construct being the biologically active one. To understand more fully the mechanism underlying the improved efficiency of the best three modified SFFV-shRNAmiRs (1, 3, and 8), we analyzed the abundance of guide and passenger strand small RNAs and their ratios by northern blot. First, a higher abundance of guide strand was seen for pol III versus pol II vectors in all cases. Furthermore, particularly for modified SFFV-shRNAmiRs 1 and 3, a higher abundance and higher guide to passenger strand ratios were found versus the unmodified shRNAmiRs (Figure 4d). Deep sequencing of small RNAs was performed to evaluate the impact of the modification on guide strand sequences and to correlate it with the changes observed in BCL11A knockdown. Generally, the resulting processed sequences reflected the introduced 4 nt shift, resulting in a guide strand with seed regions similar to the sequences obtained from pol III shRNAs expressed in the LKO backbone. For SFFV-shRNAmiRs 1, 3, and 8, a single dominant sequence was found, which contrasts the less effective SFFV-shRNAmiRs which showed a broader distribution of sequences.
Figure 4.
Modification of shRNA sequences leads to increased knockdown and improved guide versus passenger strand ratio in MEL cells. (a) shRNAmiRs were modified by deleting the first four bases from the guide sequence and the addition of GCGC to the 3′ end (shRNA modified). (b) Comparison of knockdown efficiency of modified and parental shRNAmiR sequences expressed from a SFFV-pol II promoter in MEL cells. (c) Fold induction of Hbb-y mediated by unmodified (white bars) and modified (gray bars) shRNAmiR sequences measured by qRT-PCR. Data represent mean ± SD. **P < 0.01. (d) Northern blot analysis of total RNA from cells transduced with multiple shRNAs and shRNAmiRs. Probes (20 nt) complementarity to the guide and passenger strands from positions 1 to 20 of shRNAs and shRNAmiRs were utilized to measure the abundance of processed small RNAs. 5S RNA was used as an internal control. (e) RNA-sequencing results of transduced MEL cells expressing shRNA1, 2, 3, 4, 6, or 8. The sequences of these RNAs were aligned to the corresponding reference guide strand sequence shown at the top of each panel. The sequences of different guide strand species are displayed on the y-axis and the frequency as percentage of aligned reads are shown on the x-axis. PIII: U6 shRNA; PII: SFFV shRNAmiR; PIIM: modified SFFV shRNAmiR.
Effect of shRNAmiR modification on BCL11A knockdown and γ-globin induction in primary human CD34+ derived erythroid cells
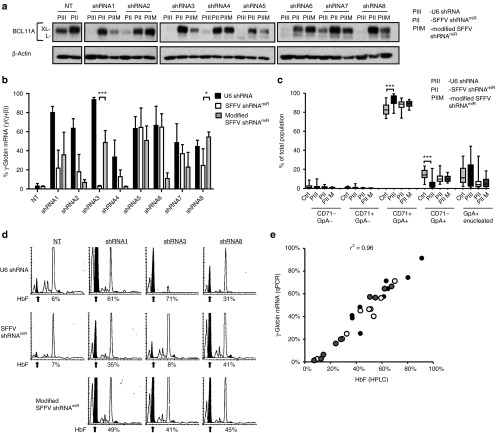
Reactivation of fetal globin with BCL11A knockdown has therapeutic potential for the treatment of sickle cell disease and β-thalassemia. To evaluate the effect of modified SFFV-shRNAmiR on BCL11A knockdown efficiency and induction of γ-globin and HbF expression in primary human cells, granulocyte-colony stimulating factor mobilized peripheral blood (mPB) CD34+ hematopoietic stem and progenitor cells from healthy volunteers were transduced with vectors expressing U6-shRNAs, SFFV-shRNAmiR, and modified SFFV-shRNAmiR and then subjected to erythroid differentiation. After 11 days in culture, BCL11A levels were determined via western blot (Figure 5a). Consistent with findings in MEL cells, enhanced knockdown was observed with modified SFFV-shRNAmiRs 1, 3, 5, 7, and 8, which also led to significantly increased induction of γ-globin transcripts with shRNAs 3 and 8 and a modest increase in shRNA 1 (Figure 5b). To determine if expression of the shRNA had any deleterious effect on erythroid differentiation, cells were assessed at day 18 of culture by flow cytometric analysis for surface expression of CD71 and GpA and enucleation. No significant differences were observed between SFFV-shRNAmiRs and control vector transduced samples (Figure 5c). In contrast, U6-shRNAs led to mild delay in the acquisition of differentiation markers during the later phases of maturation, which could indicate toxicity due to U6-promoter-mediated shRNA overexpression. The observations of high γ-globin mRNA induction were confirmed by increased HbF protein measured by high-performance liquid chromatography. All three tested modified shRNAmiRs yielded increased HbF output compared to unmodified SFFV-shRNAmiRs (Figure 5d), where between 40 and 50% of total hemoglobin in the erythroid cells was HbF. The correlation between γ-globin mRNA and HbF protein was high (r2 = 0.96), supporting the reliability of the analyses (Figure 5e).
Figure 5.
Modified shRNAmiRs lead to increased BCL11A knockdown efficiency and gamma globin induction in human CD34+ derived erythroid cells. (a) CD34+ cells transduced with U6-shRNA or SFFV-shRNAmiR vectors expressing different shRNAs with and without modification were selected either in the presence of puromycin (U6-shRNA) or sorted for Venus expression (SFFV-shRNAmiR and modified SFFV-shRNAmiR). BCL11A expression was measured by immunoblot with β-actin as a loading control on day 11 of differentiation. (b) Induction of γ-globin mRNA was determined on day 18 of differentiation by qRT-PCR. Data represents the percentage of γ-globin of total β-locus output (γ + β-globin) for U6-shRNAs (black bars), SFFV-shRNAmiR (white bars), and modified SFFV-shRNAmiR (grey bars). *P > 0.05; ***P > 0.001. (c) Quantification and statistical analysis of erythroid differentiation markers (CD71, GpA) and enucleation were assessed by flow cytometry. CTRL: control vectors SFFVshRNAmiRNT and SFFV-GFP; PIII: U6 shRNA; PII: SFFV shRNAmiR; PIIM: modified SFFV shRNAmiR. Data represents mean ± SD from three independent experiments. ***P > 0.001. (d) Hemoglobin F of cell lysates was measured by HPLC on day 18 of differentiation. The arrow indicates the HbF peaks and the percentage of HbF of total hemoglobin is shown below the chromatogram. (e) Correlation graph of γ-globin mRNA expression assessed by qRT-PCR versus HbF by HPLC. Black circles represent U6 shRNAs, open and gray circles represent SFFV shRNAmiRs or modified SFFV shRNAmiRs, respectively. Correlation coefficient (r2) is shown for all data.
In summary, we have demonstrated that identical hairpins expressed either as shRNAs or embedded into a miRNA scaffold are processed to yield differing mature siRNAs in transduced cells. The target-matched sequence of the mature guide RNA derived from the pol III promoter expressed shRNAs is uniformly shifted 3′ by 3–5 nt, and this difference was associated with significant differences in knockdown efficiency of the target transcript. In the case of BCL11A, a potential therapeutic target, this led to appreciable differences in the reactivation of γ-globin expression. These data demonstrate the importance of validation of several shRNA sequences and design optimization when transferring these sequences into a miRNA scaffold to allow for pol II-mediated expression.
Discussion
The majority of previously validated effective shRNA sequences are derived from analyses performed using vectors incorporating pol III promoters, and the majority of commercially available knockdown systems are based on pol III promoters. Using these sequences in clinical gene therapy applications requires adaption of the hairpin into a pol II-promoter driven system. In spite of significant research in this area, guidelines for conversion of shRNA sequences derived from effective pol III-based vectors into pol II-based shRNAmiR vectors are lacking. Here, by comparing the results of RNA processing from cells transduced with both types of vectors in parallel, we confirmed that different small RNA products are generated with respect to the target-matched sequences resulting in a markedly reduced efficiency of target knockdown via pol II-based vectors. The mature guide strand sequences produced from pol II versus pol III systems containing identical target mRNA-matched sequences are generally shifted by 3–5 nt relative to each other. Addition of 3–5 U-residues from the pol III termination signal to the 3′ end of the shRNA transcript leads to a corresponding shift of the Dicer cleavage site, proving the dominant role of the 3′-counting rule for Dicer cleavage.36,37 The shift of the guide strand in pol III versus pol II has a major impact on knockdown efficiency, as the seed region is altered and the thermodynamical properties and terminal nucleotide identity of the small RNA duplex changes, thereby impacting guide strand incorporation into the RISC–effector complex.14,15,16,38 Re-engineering shRNAmiRs to mimic the mature guide strand sequences produced by pol III-driven shRNAs led to enhanced processing and improved knockdown of the target mRNA in ~50% of the modified sequences tested while reducing the toxicity associated with pol III-based vectors.
Using an optimized lentiviral vector containing a miRNA adapted shRNA (shRNAmiR) expressed from pol II promoter, we achieved HbF levels of >50% of total hemoglobin in primary erythroid cells derived from transduced CD34+ hematopoietic stem and progenitor cells. This level of HbF induction is likely to be clinically effective and compares favorably with previously published vectors25,33,39 utilizing pol III-driven expression cassettes that lack lineage specificity and the safety profile of SIN lentivirus vectors reported here. The effective use of pol II promoters will also allow for transcriptional targeting of shRNAmiRs in an erythroid-specific fashion which appears critical in the case of BCL11A. This approach should bypass the negative impact of knockdown of BCL11A on lymphoid cell development28,30 and engrafting hematopoietic stem cells (C. Brendel and D. A. Williams, unpublished data), avoids toxicity related to shRNA overexpression,1,3,10 and improves the safety profile of the vector system,40 while maintaining the therapeutic efficacy. Regulatory elements derived from the β-globin locus may be particularly suitable for this purpose, as they have been extensively studied and optimized in the context of lentiviral vectors.39,41,42,43,44 This approach should be applicable for the development of vectors targeting other genes using pol II promoters, including other lineage-specific expression cassettes.
In summary, our data demonstrate the requirement for validating multiple targeting sequences and critical features of RNA processing relevant to the use of shRNA in different vector contexts and also provide a strategy for lineage-specific gene knockdown that circumvents adverse consequences of widespread expression. Our findings have important implications for design of miRNA-embedded shRNAs and their application in RNAi-based gene therapy approaches.
Materials and Methods
Design and screening of shRNAs. U6 promoter-driven lentiviral vectors (pol III-puro) expressing different shRNAs targeting BCL11A/Bcl11a mRNA were obtained from the Broad Institute (Cambridge, MA). The pol III-puro has hPGK promoter-driven puromycin selection marker. More than 100 shRNAs targeting either both XL/L forms or only XL form and 3′UTR region were screened in MEL cells in a 96-well format using a Qiagen Turbocapture plate and with a multiplexed Taqman qRT-PCR reaction measuring Gapdh and Hbb-y.
Construction of shRNAmiR constructs. The shRNAmiR vectors were constructed by cloning the shRNA sequences with flanking mir223 sequences into the lentiviral LeGO-V2 vector containing a SFFV-driven Venus reporter.34 The shRNAmiR sequences with mir223 loop were synthesized by genscript USA. (NJ), and the resulting shRNAmiRs were cloned into the pol II backbone downstream of the Venus coding sequence using Xba1 and BamH1 sites. All the sequences of shRNAs are listed in Figure 3a. A nontargeting control shRNA sequence was designed and named as SFFV-shRNAmiRNT. The SFFV-GFP vector, not containing any shRNA cassette and expressing GFP via an SFFV promoter, was used as a mock control.45
Virus production and titration. Lentiviral vector supernatants were generated by cotransfecting 10 μg of lentiviral transfer vectors, 10 μg of gag-pol, 5 μg of rev, and 2 μg of VSVG packaging plasmids into HEK293T cells in a 10 cm culture dish using calcium phosphate reagent (Invitrogen, Carlsbad, CA). Supernatants were collected at 24 and 48 hours after transfection, filtered through a 0.4 µm membrane (Corning, NY), and subsequently concentrated by ultracentrifugation at 23,000 rpm for 2 hours in a Beckmann XL-90 centrifuge using SW-28 swinging buckets. To determine the titer, NIH3T3 cells were infected with the virus in the presence of polybrene (8 µg/ml) and analyzed 48 hours posttransduction by fluorescence-activated cell sorting for Venus expression (pol II constructs) or by puromycin selection (1 mg/ml, pol III constructs).
Cell culture. 3T3, 293T, MEL, Jurkat, and K562 cells were maintained in Dulbecco's modified Eagle's or RPMI medium supplemented with 10% fetal calf serum, 2% penicillin–streptomycin, and 2 mmol/l glutamine, respectively.
In vitro erythroid differentiation culture. Frozen stocks of primary human CD34+ cells were obtained from mobilized peripheral blood of healthy donors (Center of Excellence in Molecular Hematology at Fred Hutchinson Cancer Research Center, Seattle or the Flow Core at Boston Children's Hospital) according a protocol approved by the BCH Institutional Review Board. Erythroid differentiation protocol used is based on a three-phase protocol adapted from Giarratana et al.46 The cells were cultured in erythroid differentiation medium (EDM) based on Iscove modified Dulbecco's medium (Cellgro, Manassas, VA) supplemented with stabilized glutamine, 330 μg/ml holo-human transferrin (Sigma-Aldrich, St Louis, MO), 10 μg/ml recombinant human insulin (Sigma-Aldrich), 2 IU/ml heparin Choay (Sigma-Aldrich), and 5% solvent/detergent virus inactivated (S/D) plasma. During the first phase of expansion (days 0 to 7), CD34+ cells were cultured in EDM in the presence of 10–6 mol/l hydrocortisone (Sigma-Aldrich), 100 ng/ml stem cell factor (SCF) (R&D Systems, Minneapolis, MN), 5 ng/ml IL-3 (R&D Systems), and 3 IU/ml Epo (Amgen, Thousand Oaks, CA). On day 4, cells were resuspended in EDM containing SCF, IL-3, Epo, and hydrocortisone. In the second phase (days 7 to 11), the cells were resuspended in EDM supplemented with SCF and Epo. In the third phase (days 11 to 18), the cells were cultured in EDM supplemented with Epo alone. The cultures were maintained at 37 °C in 5% CO2 in air.
Transduction and flow cytometry for in vitro culture. MEL and CD34+cells were transduced with lentiviral vectors expressing U6-shRNA or SFFV-shRNAmiR in the presence of polybrene (8 µg/ml) (Sigma-Aldrich, St Louis, MO) for MEL cells and 10 µmol/l prostaglandin E2 (Cayman Chemical, Ann Arbor, MI) and 2 µg/ml polybrene for CD34+ cells rand centrifuged for 2 hours at (2000 rpm) at room temperature. Live cells were either sorted for Venus expression (pol II vectors) 48 hours posttransduction by using BD FACS Aria II (BD Biosciences, San Jose, CA) or cells were selected in the presence of puromycin (1 mg/ml, pol III constructs). For fluorescence-activated cell sorting analysis, 7AAD (Invitrogen) was included as dead cell marker. K562 cells were stained with 5 µl of Annexin-V-FITC and 5 µl of 7AAD (BD Pharmingen, San Diego, CA) and incubated for 15 minutes in dark before flow cytometry. CD34+ cells were labeled with allophycocyanin (APC), phycoerythrin (PE), and PE-Cyanine7 conjugated antibodies. Anti-CD235 (glycophorin A)-PE, anti-CD71-APC, or anti-CD71-PE-Cyanine7 antibodies and DRAQ-5 (all eBioscience, San Diego, CA) were used for phenotyping. Analyses were performed on LSR-II flow cytometer (BD Biosciences) using Diva or FlowJoX (Treestar, Ashland, OR) software.
RNA extraction and qRT-PCR. Total RNA was extracted from MEL cells 7 days after sorting/postselection with puromycin, or freshly sorted cells on day 18 of erythroid differentiation of CD34+ cells, using the Qiagen RNA Plus micro kit (Valencia, CA). CDNA was generated using random hexamer primers and superscript III (Invitrogen, Carlsbad, CA). Quantitative PCR was performed using SYBR Green PCR master mix (Applied Biosystems, Warrington, UK) with intron spanning mouse Hbb-y and Gapdh primers (Hbb-y forward 5′-TGGCCTGTGGAGTAAGGTCAA-3′, reverse 5′-GAAGCAGAGGACAAGTTCCCA-3′), (Gapdh forward 5′-TCACCACCATGGAGAAGGC-3′, reverse 5′-GCTAAGCAGTTGGTGGTGCA-3′), and human HBG, HBB, and GAPDH primers (HBG forward 5′-TGGATGATCTCAAGGGCAC-3′, reverse 5′- TCAGTGGTATCTGGAGGACA-3′) (HBB forward 5′-CTGAGGAGAAGTCTGCCGTTA-3′, reverse 5′-AGCATCAGGAGTGGACAGAT-3′) and GAPDH forward 5′- ACCCAGAAGACTGTGGATGG-3′, reverse 5′- TTCAGCTCAGGGATGACCTT-3′). The PCR amplification conditions were: 95 °C for 10 minutes, followed by 40 cycles of 15 seconds at 95 °C and 1 minute at 60 °C. The qPCRs were performed on an ABI 7500 machine (Applied BioSystems, Foster City, CA). A standard curve using serial dilutions of cDNAs was used to determine the precise amplification efficacy for each reaction. Hbb-y and γ-globin expression levels were normalized to GAPDH as an internal control, and relative gene expression (ΔΔCt method) was used for analysis of PCR data, including correction for differential amplification efficiencies.
Northern blot analysis. MEL cells transduced with U6-shRNAs and SFFV-shRNAmiRs were sorted and collected after puromycin selection culturing for 7 days. Total RNA was isolated using 1 ml TRIzol reagent (Ambion, Austin, TX), and 15 µg were resolved on a 15% acrylamide gel. Small transcript sizes were determined using the Decade Ladder (Ambion, Austin, TX). RNA was transferred to Hybond-XL membrane (Amersham, Piscataway, NJ) and UV-crosslinked. Blots were prehybridized using UltraHyb-Oligo (Ambion) at 35 °C, probed with γ-32P-labeled oligonucleotides (4 polynucleotide kinase; Amersham, Piscataway, NJ) at 37 °C for 1 hour, washed in 2× sodium citrate, 0.1% sodium dodecyl sulphate at 30–35 °C, and exposed to film. Probe sequences for detecting mature miRNA were as follows: shRNA1, 5′ CGGAGACTCCAGACAATCGC 3′; shRNA2, 5′ CTCCAGGCAGCTCAAAGATC 3′; shRNA3, 5′ TCTCTTGCAACACGCACAGA 3′; shRNA4, 5′ CAGGACTAGGTGCAGAATGT 3′; shRNA5, 5′ ATCGAGTGTTGAATAATGAT 3′; shRNA6, 5′ GTACCCTGGAGAAACACAT 3′; shRNA7, 5′ ACTGTCCACAGGAGAAGCCA 3′; shRNA8, 5′ CAGTACCCTGGAGAAACACA 3′.
Western blot analysis. Transduced MELs and CD34+ cells were lysed in lysis buffer (RIPA) with protease (Roche, Mannheim, Germany) and phosphatase inhibitors (Santa Cruz Biotechnology, Dallas, TX), pepstatin, and leupeptin (Sigma, St Louis, MO). Protein lysates were estimated by BCA protein assay (Thermo Scientific, Carlsbad, CA). Twenty-five micrograms of protein was suspended in 2× laemmli sample buffer, boiled, and loaded on to an 8% SDS–polyacrylamide gel and subsequently transferred to a polyvinylidene fluoride membrane (Millipore, Billerica, MA). Following blocking in PBS with 0.1% Triton–X100 and 5% nonfat dry milk, the polyvinylidene fluoride membrane was incubated with a monoclonal mouse anti-BCL11A antibody (Abcam, Cambridge, MA) or mouse anti-β-actin (Sigma). Anti-mouse IgG HRP-linked secondary antibody (Cell Signaling, Danvers, MA) was used for detection by chemiluminescence 20× LumiGLO Reagent and 20× Peroxide (Cell Signaling).
High-performance liquid chromatography. Hemolysates were prepared from cells on day 18 of differentiation using osmotic lysis in water and three rapid freeze-thaw cycles. Hemoglobin electrophoresis with cellulose acetate and high-performance liquid chromatography were carried out with the lysates, in the clinical laboratories of the Brigham and Women's Hospital using clinically calibrated standards for the human hemoglobins.
RNA sequencing and analysis. Small RNAs were extracted from 6 × 106 MEL cells using mirVana miRNA isolation kit (Invitrogen) according to the manufacturer's instructions and sent out for deep RNA sequencing using Illumina Hiseq2000. A self-developed PERL script was used to remove the adaptor sequence, and 19–25 nt small RNAs were used for further analysis. Bowtie software (http://bowtie-bio.sourceforge.net/index.shtml) was used for alignment, and one mismatch was permitted. Expression level of small RNAs was normalized to one million of total reads of each library for comparison between different samples. For the experiment with 250 TRC shRNAs in four cell lines, lentivirus was prepared by the Broad Institute using a high-throughput virus preparation protocol, and cells were infected at high MOI with a single shRNA per well in 96-well plates (http://www.broadinstitute.org/rnai/public/resources/protocols). Puromycin was added at 1 day postinfection, and cells were lysed in Trizol at 4 days postinfection. All lysates were pooled for each cell line, followed by total RNA extraction and small RNA library preparation.47 Illumina reads were trimmed, collapsed to unique reads (>17 nt) with counts, and mapped to TRC shRNA expression vector sequences allowing no mismatches. Mature shRNA sequence distributions were calculated for each shRNA before averaging across shRNAs.
Statistical analysis. The GraphPad Prism 5.0 software package was used for statistical analysis. Results are expressed as mean ± SD. Statistical significance was assessed by t-test.
SUPPLEMENTARY MATERIAL Figure S1. Assessment of cytotoxicity among U6-shRNAs and SFFVshRNAmiRs in different cell lines. Figure S2. Deep sequencing of 247 processed TRC shRNA products in four cell lines.
Acknowledgments
We thank Maria Suarez and Natasha Rossi for administrative assistance and Dr. David Bartel for help with small RNA sequencing. We also thank Glenn Cowley from Broad Institute who helped in designing the shRNA library, Alicia Soriana for assistance in HPLC, Chad Harris and Jonathan Fogel for technical assistance, and Ronald Mathieu and Mahnaz Paktinat of the Pediatric Hematology/Oncology FACS facility for help with cell sorting. We thank Guliana Ferrari and Luigi Naldini for the GLOBE-lentiviral vector containing the β-globin LCR. We thank Axel Schambach for discussion and the lentiviral packaging plasmids. This work was supported by NIH U01HL117720-01 (R.R., S.H.O., D.A.W., P.D., and R.I.G.), F30DK103359-01A1 (M.C.C.), K08 DK093705 (D.E.B.), and The RNAi Consortium (TRC).
Supplementary Material
References
- Grimm, D, Streetz, KL, Jopling, CL, Storm, TA, Pandey, K, Davis, CR et al. (2006). Fatality in mice due to oversaturation of cellular microRNA/short hairpin RNA pathways. Nature 441: 537–541. [DOI] [PubMed] [Google Scholar]
- McBride, JL, Boudreau, RL, Harper, SQ, Staber, PD, Monteys, AM, Martins, I et al. (2008). Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: implications for the therapeutic development of RNAi. Proc Natl Acad Sci USA 105: 5868–5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan, AA, Betel, D, Miller, ML, Sander, C, Leslie, CS and Marks, DS (2009). Transfection of small RNAs globally perturbs gene regulation by endogenous microRNAs. Nat Biotechnol 27: 549–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giering, JC, Grimm, D, Storm, TA and Kay, MA (2008). Expression of shRNA from a tissue-specific pol II promoter is an effective and safe RNAi therapeutic. Mol Ther 16: 1630–1636. [DOI] [PubMed] [Google Scholar]
- Boudreau, RL, Martins, I and Davidson, BL (2009). Artificial microRNAs as siRNA shuttles: improved safety as compared to shRNAs in vitro and in vivo. Mol Ther 17: 169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persengiev, SP, Zhu, X and Green, MR (2004). Nonspecific, concentration-dependent stimulation and repression of mammalian gene expression by small interfering RNAs (siRNAs). RNA 10: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish, RJ and Kruithof, EK (2004). Short-term cytotoxic effects and long-term instability of RNAi delivered using lentiviral vectors. BMC Mol Biol 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, AL and Linsley, PS (2010). Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9: 57–67. [DOI] [PubMed] [Google Scholar]
- Grimm, D, Wang, L, Lee, JS, Schürmann, N, Gu, S, Börner, K et al. (2010). Argonaute proteins are key determinants of RNAi efficacy, toxicity, and persistence in the adult mouse liver. J Clin Invest 120: 3106–3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, JN, Wolken, N, Brown, T, Dauer, WT, Ehrlich, ME and Gonzalez-Alegre, P (2011). Lethal toxicity caused by expression of shRNA in the mouse striatum: implications for therapeutic design. Gene Ther 18: 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory, RI, Yan, KP, Amuthan, G, Chendrimada, T, Doratotaj, B, Cooch, N et al. (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature 432: 235–240. [DOI] [PubMed] [Google Scholar]
- Ha, M and Kim, VN (2014). Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15: 509–524. [DOI] [PubMed] [Google Scholar]
- Winter, J, Jung, S, Keller, S, Gregory, RI and Diederichs, S (2009). Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol 11: 228–234. [DOI] [PubMed] [Google Scholar]
- Khvorova, A, Reynolds, A and Jayasena, SD (2003). Functional siRNAs and miRNAs exhibit strand bias. Cell 115: 209–216. [DOI] [PubMed] [Google Scholar]
- Schwarz, DS, Hutvágner, G, Du, T, Xu, Z, Aronin, N and Zamore, PD (2003). Asymmetry in the assembly of the RNAi enzyme complex. Cell 115: 199–208. [DOI] [PubMed] [Google Scholar]
- Frank, F, Sonenberg, N and Nagar, B (2010). Structural basis for 5'-nucleotide base-specific recognition of guide RNA by human AGO2. Nature 465: 818–822. [DOI] [PubMed] [Google Scholar]
- Fellmann, C, Zuber, J, McJunkin, K, Chang, K, Malone, CD, Dickins, RA et al. (2011). Functional identification of optimized RNAi triggers using a massively parallel sensor assay. Mol Cell 41: 733–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, EC (2002). Micro RNAs are complementary to 3' UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet 30: 363–364. [DOI] [PubMed] [Google Scholar]
- Lewis, BP, Shih, IH, Jones-Rhoades, MW, Bartel, DP and Burge, CB (2003). Prediction of mammalian microRNA targets. Cell 115: 787–798. [DOI] [PubMed] [Google Scholar]
- Park, JE, Heo, I, Tian, Y, Simanshu, DK, Chang, H, Jee, D et al. (2011). Dicer recognizes the 5' end of RNA for efficient and accurate processing. Nature 475: 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng, Y, Wagner, EJ and Cullen, BR (2002). Both natural and designed micro RNAs can inhibit the expression of cognate mRNAs when expressed in human cells. Mol Cell 9: 1327–1333. [DOI] [PubMed] [Google Scholar]
- Fellmann, C, Hoffmann, T, Sridhar, V, Hopfgartner, B, Muhar, M, Roth, M et al. (2013). An optimized microRNA backbone for effective single-copy RNAi. Cell Rep 5: 1704–1713. [DOI] [PubMed] [Google Scholar]
- Knott, SR, Maceli, AR, Erard, N, Chang, K, Marran, K, Zhou, X et al. (2014). A computational algorithm to predict shRNA potency. Mol Cell 56: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola, M, Passerini, L, Pucci, F, Gentner, B, Bacchetta, R and Naldini, L (2009). Regulated and multiple miRNA and siRNA delivery into primary cells by a lentiviral platform. Mol Ther 17: 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaran, VG, Menne, TF, Xu, J, Akie, TE, Lettre, G, Van Handel, B et al. (2008). Human fetal hemoglobin expression is regulated by the developmental stage-specific repressor BCL11A. Science 322: 1839–1842. [DOI] [PubMed] [Google Scholar]
- Xu, J, Peng, C, Sankaran, VG, Shao, Z, Esrick, EB, Chong, BG et al. (2011). Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science 334: 993–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer, DE, Kamran, SC, Lessard, S, Xu, J, Fujiwara, Y, Lin, C et al. (2013). An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science 342: 253–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, P, Keller, JR, Ortiz, M, Tessarollo, L, Rachel, RA, Nakamura, T et al. (2003). Bcl11a is essential for normal lymphoid development. Nat Immunol 4: 525–532. [DOI] [PubMed] [Google Scholar]
- John, A, Brylka, H, Wiegreffe, C, Simon, R, Liu, P, Jüttner, R et al. (2012). Bcl11a is required for neuronal morphogenesis and sensory circuit formation in dorsal spinal cord development. Development 139: 1831–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y, Wang, J, Khaled, W, Burke, S, Li, P, Chen, X et al. (2012). Bcl11a is essential for lymphoid development and negatively regulates p53. J Exp Med 209: 2467–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ippolito, GC, Dekker, JD, Wang, YH, Lee, BK, Shaffer, AL 3rd, Lin, J et al. (2014). Dendritic cell fate is determined by BCL11A. Proc Natl Acad Sci USA 111: E998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffat, J, Grueneberg, DA, Yang, X, Kim, SY, Kloepfer, AM, Hinkle, G et al. (2006). A lentiviral RNAi library for human and mouse genes applied to an arrayed viral high-content screen. Cell 124: 1283–1298. [DOI] [PubMed] [Google Scholar]
- Xu, J, Sankaran, VG, Ni, M, Menne, TF, Puram, RV, Kim, W et al. (2010). Transcriptional silencing of {gamma}-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev 24: 783–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber, K, Mock, U, Petrowitz, B, Bartsch, U and Fehse, B (2010). Lentiviral gene ontology (LeGO) vectors equipped with novel drug-selectable fluorescent proteins: new building blocks for cell marking and multi-gene analysis. Gene Ther 17: 511–520. [DOI] [PubMed] [Google Scholar]
- Xu, J, Bauer, DE, Peng, C, Smith, EC and Orkin, SH (2013). Identification of BCL11A structure-function domains for fetal hemoglobin silencing. Blood 122: 435. [Google Scholar]
- Zhang, H, Kolb, FA, Jaskiewicz, L, Westhof, E and Filipowicz, W (2004). Single processing center models for human Dicer and bacterial RNase III. Cell 118: 57–68. [DOI] [PubMed] [Google Scholar]
- Vermeulen, A, Behlen, L, Reynolds, A, Wolfson, A, Marshall, WS, Karpilow, J et al. (2005). The contributions of dsRNA structure to Dicer specificity and efficiency. RNA 11: 674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, HR, Schoenfeld, LW, Ruby, JG, Auyeung, VC, Spies, N, Baek, D et al. (2010). Mammalian microRNAs: experimental evaluation of novel and previously annotated genes. Genes Dev 24: 992–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilber, A, Hargrove, PW, Kim, YS, Riberdy, JM, Sankaran, VG, Papanikolaou, E et al. (2011). Therapeutic levels of fetal hemoglobin in erythroid progeny of β-thalassemic CD34+ cells after lentiviral vector-mediated gene transfer. Blood 117: 2817–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zychlinski, D, Schambach, A, Modlich, U, Maetzig, T, Meyer, J, Grassman, E et al. (2008). Physiological promoters reduce the genotoxic risk of integrating gene vectors. Mol Ther 16: 718–725. [DOI] [PubMed] [Google Scholar]
- Roselli, EA, Mezzadra, R, Frittoli, MC, Maruggi, G, Biral, E, Mavilio, F et al. (2010). Correction of beta-thalassemia major by gene transfer in haematopoietic progenitors of pediatric patients. EMBO Mol Med 2: 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisowski, L and Sadelain, M (2007). Locus control region elements HS1 and HS4 enhance the therapeutic efficacy of globin gene transfer in beta-thalassemic mice. Blood 110: 4175–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumbeti, A, Higashimoto, T, Urbinati, F, Franco, R, Meiselman, HJ, Witte, D et al. (2009). A novel human gamma-globin gene vector for genetic correction of sickle cell anemia in a humanized sickle mouse model: critical determinants for successful correction. Blood 114: 1174–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero, Z, Urbinati, F, Geiger, S, Cooper, AR, Wherley, J, Kaufman, ML et al. (2013). β-Globin gene transfer to human bone marrow for sickle cell disease. J Clin Invest (epub ahead of print). [DOI] [PMC free article] [PubMed]
- Demaison, C, Parsley, K, Brouns, G, Scherr, M, Battmer, K, Kinnon, C et al. (2002). High-level transduction and gene expression in hematopoietic repopulating cells using a human immunodeficiency [correction of imunodeficiency] virus type 1-based lentiviral vector containing an internal spleen focus forming virus promoter. Hum Gene Ther 13: 803–813. [DOI] [PubMed] [Google Scholar]
- Giarratana, MC, Rouard, H, Dumont, A, Kiger, L, Safeukui, I, Le Pennec, PY et al. (2011). Proof of principle for transfusion of in vitro-generated red blood cells. Blood 118: 5071–5079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimson, A, Srivastava, M, Fahey, B, Woodcroft, BJ, Chiang, HR, King, N et al. (2008). Early origins and evolution of microRNAs and Piwi-interacting RNAs in animals. Nature 455: 1193–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.