Abstract
Objective:
The purpose of this study is to describe a case series of 4 sisters with discordant clinical phenotypes associated with fragile X–associated tremor/ataxia syndrome (FXTAS) that may be explained by varying CGG repeat sizes and activation ratios (ARs) (the ratio of cells carrying the normal fragile X mental retardation 1 [FMR1] allele on the active X chromosome).
Methods:
Four sisters with premutation size FMR1 gene repeats underwent detailed clinical characterization. CGG repeat length was determined by PCR, and AR was determined using a newly developed commercial methylation PCR assay and was compared with the results from Southern blot with densitometric image analysis.
Results:
Sister 1 had the largest CGG expansion (82) and the lowest AR (12%), with the most severe clinical presentation. Sister 2 had a lower CGG expansion (70) and an AR of 10% but had a milder clinical presentation.Sister 3 had a similar CGG expansion (79) but a slightly higher AR of 15% and less neurologic involvement. Sister 4 had a similar CGG expansion size of 80 but had the largest AR (40%) and was the only sister not to be affected by FXTAS or have any neurologic signs on examination.
Conclusions:
These results suggest that premutation carrier women who have higher ARs may be less likely to show manifestations of FXTAS. If larger studies show similar patterns, AR data could potentially be beneficial to supplement CGG repeat size when counseling premutation carrier women in the clinic.
Fragile X–associated tremor/ataxia syndrome (FXTAS) is caused by a “premutation” size 55 to 200 CGG repeat expansion in the fragile X mental retardation 1 (FMR1) gene.1 Individuals with a normal FMR1 gene have fewer than 41 CGG repeats at the 5′ untranslated region of the gene. Three discrete disorders that increase morbidity of individuals carrying an expansion have been characterized, each associated with different lengths of the CGG tract. Fragile X syndrome (FXS), the most common inherited cause of intellectual disability in boys, results from a CGG expansion of >200 repeats (full mutation) and is characterized by neurocognitive and developmental abnormalities, including a high incidence of autism, anxiety, and attention-deficit/hyperactivity disorder. FXTAS, first described in 2001, is caused by a “premutation” with a CGG length of 55 to 200 repeats and manifests as kinetic tremor, cerebellar gait ataxia, executive dysfunction, and psychiatric symptoms in premutation carriers, typically over age 55.1 Although premutation carrier men are more frequently and severely affected, recent studies report more neurologic involvement in women than was previously believed, despite the presence of a normal FMR1 allele on the other X chromosome.2 A third disorder, fragile X–associated primary ovarian insufficiency (FXPOI), occurs in approximately 20% of women who carry a premutation size allele. Women with FXPOI experience ovarian dysfunction and early estrogen deficiency resulting in premature menopause.3 In addition, premutation carrier women have been reported to have a host of milder neurologic signs and other medical illnesses that have not yet been well defined.2,4,5
Because of the observation that women with FXTAS show a milder clinical presentation of the disease than do men, attention has been given to the possible role of X-inactivation in mediating the phenotypic outcome of premutation carrier women. X chromosome inactivation is the transcriptional silencing of 1 X chromosome in the somatic cells of women. For premutation carrier women, this means that a percentage of cells will contain an active abnormal FMR1 allele and the remaining cells will contain an active normal FMR1 allele. On average, 50% of cells from a carrier woman should contain an active premutation allele and 50% an active normal allele, but some individuals have skewing of this ratio such that the percentages are unequal.6 It has been hypothesized that variation in the activation ratio (AR) (the ratio of cells carrying the normal FMR1 allele on the active X chromosome) may result in substantial phenotypic heterogeneity among premutation carrier women.
There are few published studies correlating disease severity and AR in FXTAS. A case report described 2 sisters with similar premutation expansion sizes (69 and 83) but differing ARs.7 The sister with 78% AR did not meet the clinical criteria for FXTAS, whereas the sister with 29% AR had definite FXTAS. A much larger study including 82 premutation carrier women demonstrated that a lower AR in association with increasing CGG repeats was correlated with the risk for FXTAS and symptom severity.8 Recently, we reported that higher AR was associated with better posturography balance scores in carrier women with and without FXTAS.9
The current case series describes a family of 4 sisters with similar premutation size FMR1 alleles. The AR was measured in each sister, and clinical phenotypes were compared using these ratios. The purpose of a detailed study of this family was to determine whether AR, in addition to CGG repeat size, could account for the varying neurologic phenotypes.
METHODS
Standard protocol approvals, registrations, and patient consents.
The sisters were recruited through the Fragile X–Associated Disorders Program at Rush University. This study was approved by the Rush University Institutional Review Board.
Clinical and molecular methods.
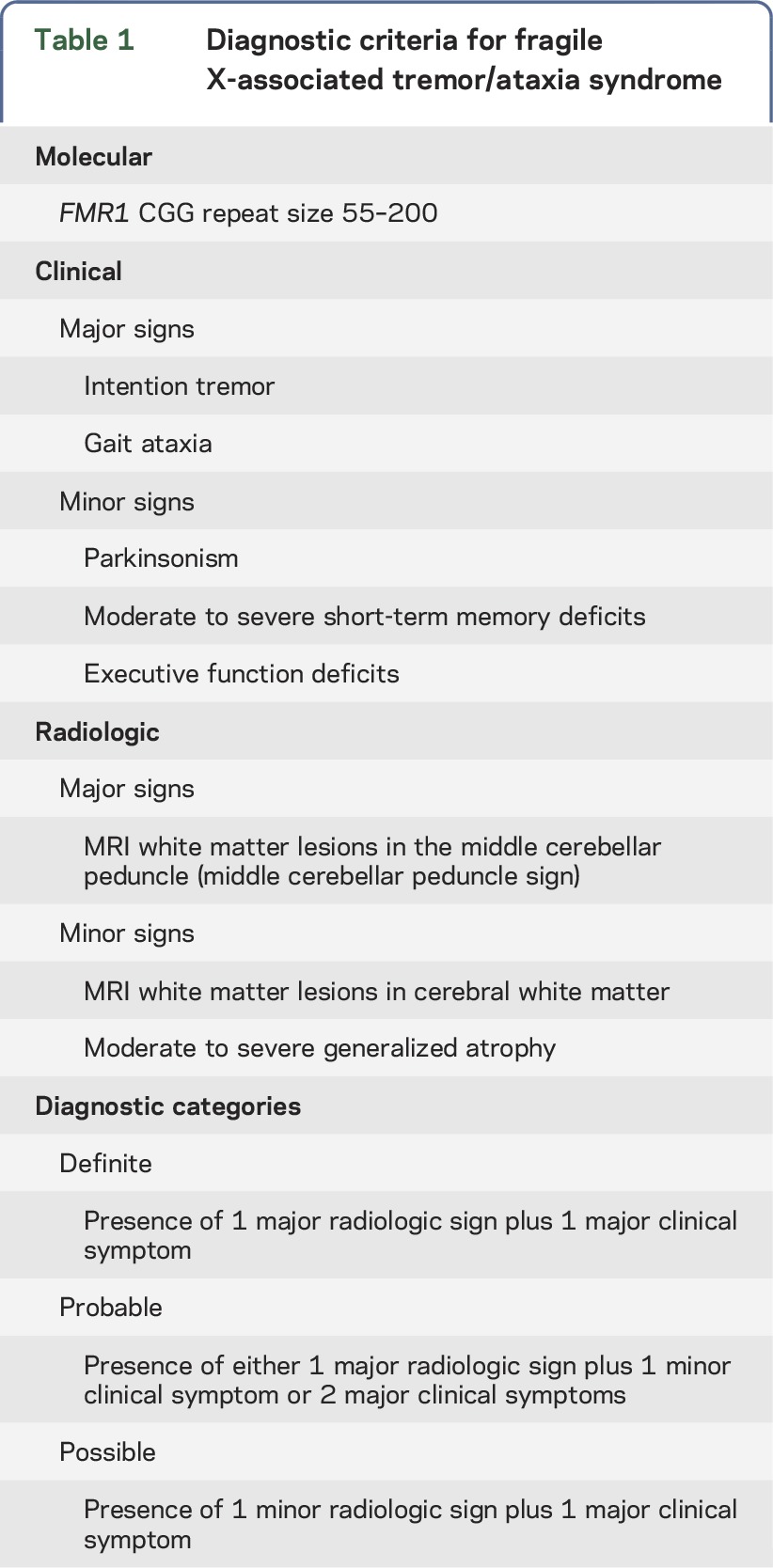
All sisters were scored using the FXTAS Rating Scale (FXTAS-RS), which rates tremor, postural sway, gait, parkinsonism, coordination, dystonia, speech, and oculomotor deficits to assess the presence and severity of FXTAS signs.8 The scale was created using items from the Unified Parkinson's Disease Rating Scale, the Clinical Rating Scale for Tremor, the International Cooperative Ataxia Rating Scale, and a tandem item from the Unified Huntington's Disease Rating Scale.10–13 Medical history, neurologic examination, and MRI, when available, were used to diagnose FXTAS according to clinical and radiologic criteria (table 1).14 Two sisters underwent additional posturography testing using the Neurocom Smart Balance Master system (Natus Medical, Pleasanton, CA)15 and gait analysis using inertial sensors.16
Table 1.
Diagnostic criteria for fragile X-associated tremor/ataxia syndrome
DNA was isolated from blood samples, and molecular testing on the samples was performed at the Rush University Molecular Diagnostic Laboratory. FMR1 PCR with quantification of allele-specific CGG repeat length and identification of AGG interspersions17,18 and AR measurement using a new methylation PCR (mPCR) assay19 were performed using commercially available kits (Asuragen, Inc., Austin, TX). The FMR1 mPCR19 was performed according to the manufacturer's instructions on 20 to 80 ng of DNA quantified with a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). The mPCR methodology involved gene-specific PCR of genomic DNA that was treated with Hpa II (methylation-sensitive) and amplified with hexachlorofluorescein (HEX)-specific primers (amplifies only the methylated allele) or mock-treated and amplified with fluorescein (FAM)-specific primers (amplifies both alleles), followed by separation and visualization by capillary electrophoresis. The FMR1 (DNA) locus was analyzed by Southern blot with probe StB12.3,20 following Eco RI/Eag I digestion. ARs for the normal allele were quantified by densitometric scanning of bands corresponding to unmethyated (active) DNA on the Southern blot. AR was calculated as signal from the normal-containing band divided by total signal in both the premutation-containing and normal bands with densitometric image analysis, as described in previous studies.7 Correspondingly, ARs using mPCR were determined relative to the unmethylated component of the normal allele, as described previously.19 The percentage methylation on the normal allele is proportional to the ratio of the total peak area in the HEX relative to the FAM channel. Thus, the AR is 1 − (HEX area/FAM area) for that allele. AR results were confirmed by Southern blot with densitometric image analysis as described in previous studies.7
RESULTS
Case reports.
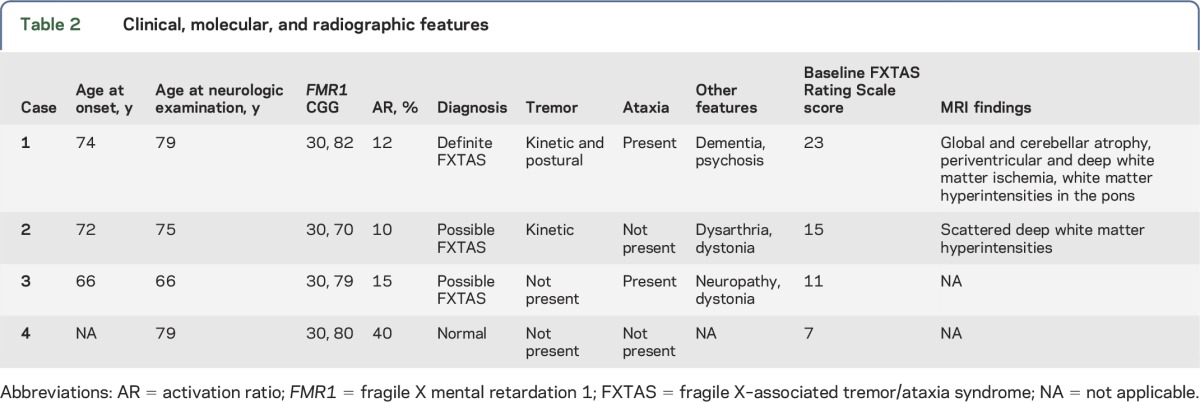
Four sisters are presented to illustrate phenotypic variability, CGG repeat size, and AR (table 2).
Table 2.
Clinical, molecular, and radiographic features
Case 1.
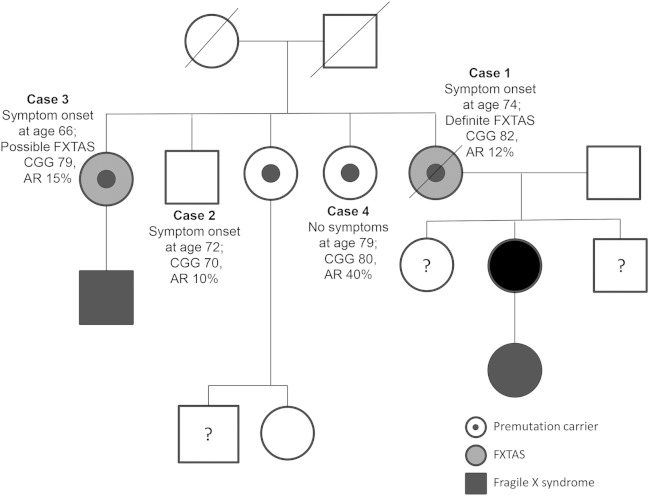
A 79-year-old woman developed balance problems at age 74. She had 3 sisters and 1 brother, as well as a nephew, a daughter, and a granddaughter with FXS (figure 1). Examination at age 75 revealed transient end-gaze nystagmus and absent reflexes in all 4 extremities. She had anterocollis, mild tremor, and dysdiadochokinesia in her left hand, increased tone in the right upper extremity, and bradykinesia in her left leg. She was unable to stand or walk in tandem without falling. Posturography revealed significant abnormalities in the vestibular control of balance, reduced limits of postural stability, and delayed automatic postural reflexes. Her FXTAS-RS score was 23 (figure 2). MRI of the brain showed severe global and cerebellar atrophy, periventricular and deep white matter ischemia, and white matter hyperintensities in the pons (figure 3). Her FMR1 CGG repeat sizes were 30 and 82 with an AR of 12%, and she was diagnosed with definite FXTAS.
Figure 1. Pedigree.
Abbreviations: AR = activation ratio; FXTAS = fragile X-associated tremor/ataxia syndrome.
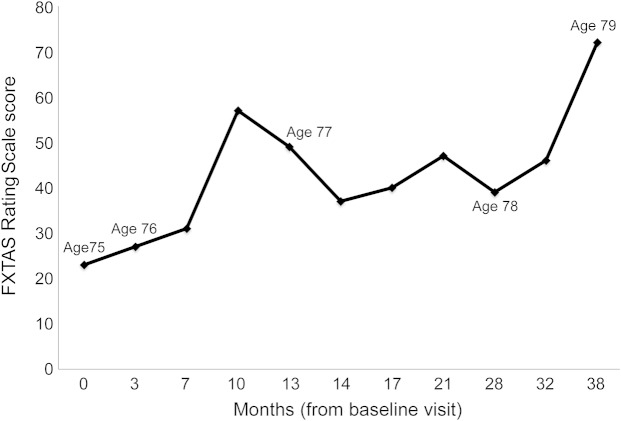
Figure 2. FXTAS Rating Scale scores for case 1.
Fragile X-associated tremor/ataxia syndrome (FXTAS) Rating Scale scores for case 1 show gradual progression over time. Worsening of the score was seen when the patient was admitted for psychosis at 10 months. Upon treatment of the psychosis and aggressive rehabilitation, the score improved. The score peaks again at 21 months when she had surgery. The last score was right before her death.
Figure 3. MRI for case 1.
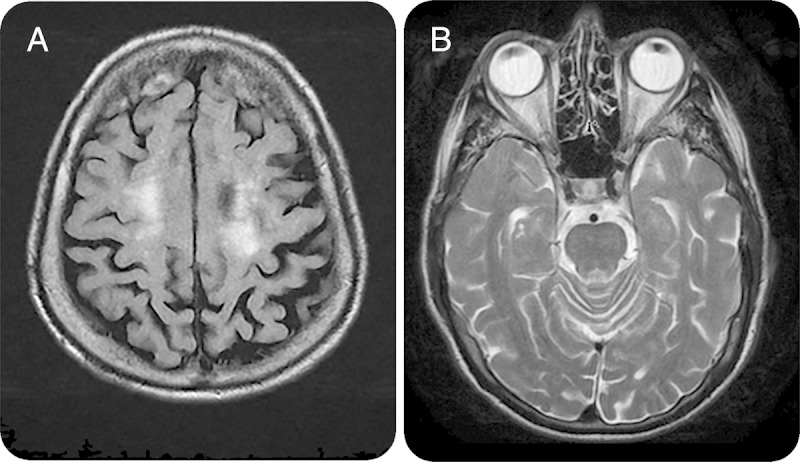
(A) T2-weighted axial fluid-attenuated inversion recovery brain MRI showing white matter hyperintensities in the cortex. (B) T2-weighted axial brain MRI showing white matter hyperintensities in the pons.
At age 76, she fell twice and on examination her ataxia and tremor had worsened, with an increased FXTAS-RS score of 27. She then developed mild cognitive impairment, hallucinations, and paranoid delusions. Her examination showed continued postural instability, wide-based gait and ataxia, festination, short steps, difficulty with turns, and ineffective use of walker. At age 77, her Mini-Mental State Examination score was 5/30,21 but her FXTAS motor signs were stable. Memantine was prescribed to slow her cognitive decline. Over the next 2 years, her FXTAS-RS score continued to worsen, reaching 72. However, she appeared unaware of her balance difficulties; when self-reporting her balance confidence for numerous activities of daily living, she scored in the range that represents a high level of perceived balance function, despite the fact that she had fallen several times in the previous year. She died at age 80 due to a presumed pulmonary embolus.
Case 2.
The first sister of case 1 was a 75-year-old woman who developed problems with balance and memory at age 72. She had fallen recently, and she described neck pain and numbness in the toes. Associated symptoms included problems with memory and depression starting at age 71. Her medical history was remarkable for FXPOI. She had a daughter who was healthy and a son with a learning disability and unknown gene status. Examination revealed persistent horizontal gaze-evoked nystagmus, mild dysarthria, mild left hand action tremor, and cervical dystonia. MRI of the brain showed scattered deep white matter hyperintensities. Her FXTAS-RS score was 15, but she met clinical criteria for possible FXTAS. Her FMR1 CGG repeat sizes were 30 and 70 with an AR of 10%.
Case 3.
The second sister of case 1 was a 66-year-old woman who presented with balance problems and dizziness. She described curling of her toes on the right foot, turning in of the right foot when walking, and numbness and tingling of the legs. She reported short-term memory problems, anxiety, and occasional depression. Neurologic examination showed increased stance, increased body sway with her eyes closed, and difficulty with tandem gait. Vibration was decreased by 50% in her feet. Her FXTAS-RS score was 11. Her FMR1 CGG repeat sizes were 30 and 79 with an AR of 15%. She met clinical criteria for possible FXTAS, although she did not undergo brain MRI.
Case 4.
The third sister of case 1 was a 79-year-old woman with no medical problems and no neurologic symptoms. She took no prescription medications and was a competitive dancer and body builder and taught exercise classes. Her neurologic examination, posturography, and gait testing were normal. Her FXTAS-RS score was 7, normal for her age. Her FMR1 CGG repeat sizes were 30 and 80 with an AR of 40%.
DISCUSSION
This case series describes a family of 4 sisters with premutation size FMR1 alleles who presented with a high degree of phenotypic variability (table 2). Case 1 had the largest CGG expansion, lowest AR, and the most severe clinical presentation. Case 2 had an AR similar to that of case 1 but a lower CGG expansion and milder neurologic symptoms. Case 3 had a CGG expansion in between cases 1 and 2 but a slightly higher AR and less neurologic involvement. Case 4 was the only sister not affected by FXTAS despite having a similar CGG repeat expansion; this may be attributed to her having the largest AR of the family. Although the AR varies only between 10% and 40% in these sisters, it is in the expected direction: the sister with the lowest AR has the most severe neurologic signs and the sister with the highest ratio has no signs of FXTAS. The difference in ratios alone does not seem to be sufficient to explain such a large difference in outcomes and may suggest that another unknown secondary gene effect plays a role as well.
Individuals harboring premutation alleles produce increased levels of expanded CGG-containing FMR1 mRNA, which is believed to result in neurologic disease due to RNA toxicity.22 Severity of motor impairment has been related to CGG repeat length in women, but only when controlling for AR.8 As such, it has been observed that sisters with similar CGG expansions may have highly variable phenotypic presentations, likely due to differences in AR.7,23
There are several limitations to this study. First, it describes only 1 family of sisters rather than a series. Second, the FXTAS diagnostic criteria (table 1) require a brain MRI to make a probable or definite FXTAS diagnosis. Cases 3 and 4 were not imaged because of mild or absent neurologic signs, so only a diagnosis of possible FXTAS could be reached in case 3. The presence of major radiologic criteria, such as the middle cerebellar peduncle sign, on imaging would increase the diagnostic certainty to definite despite the low FXTAS-RS score.
This case series suggests a possible role for AR in conferring risk for FXTAS and its severity in premutation carrier women. In current practice, counseling regarding premutation-related disorders is based on CGG repeat size alone. The addition of AR results may provide information that could inform the clinician regarding current or future phenotypes. Much larger studies are warranted to determine the true utility of this molecular measure in informing the clinician.
ACKNOWLEDGMENT
The authors thank Gary J. Latham for review of the manuscript and Stela Filipovic-Sadic for technical support.
GLOSSARY
- AR
activation ratio
- FAM
fluorescein
- FMR1
fragile X mental retardation 1
- FXPOI
fragile X–associated primary ovarian insufficiency
- FXS
fragile X syndrome
- FXTAS
fragile X–associated tremor/ataxia syndrome
- FXTAS-RS
FXTAS Rating Scale
- HEX
hexachlorofluorescein
- mPCR
methylation PCR
AUTHOR CONTRIBUTIONS
Deborah Hall: clinical data collection; drafting/revising the manuscript. Erin Robertson-Dick: drafting/revising the manuscript. Joan O'Keefe: clinical data collection; revising the manuscript. Andrew Hadd: revising the manuscript. Lili Zhou: clinical data collection; revising the manuscript. Elizabeth Berry-Kravis: clinical data collection; revising the manuscript.
STUDY FUNDING
This work was supported in part by awards from the Rush Translational Science Consortium (D.A.H.), the Rush Cohn Fellowship award (J.A.O.), and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (R44 HD060450-02, A.G.H.).
DISCLOSURE
Deborah A. Hall has received research funds from NIH, NINDS, Shapiro Foundation, National Parkinson Disease Foundation, Pfizer, and Neurocrine. Erin Robertson-Dick reports no disclosures. Joan A. O'Keefe has served on the scientific advisory board of NIH/Vtesse, Inc. and has received research support from Rush University Cohen's Fellowship Award and Rush University Rush Translational Consortium Award. Andrew Hadd is a full-time employee and options holder in Asuragen, Inc.; has been a consultant for Integrated DNA Technologies; has received research support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development; and holds stock/stock options/board of directors compensation in Asuragen, Inc. Lili Zhou has received funding from Roche for diagnostic FMR1 testing during clinical trials in FXS and has current grant funding from NIH. Elizabeth Berry-Kravis has served as the Chair of DSMB for Novartis Phase II trial of AFQ056 in fragile X syndrome; has served on the scientific advisory boards of Novartis, Roche, Neurotrope, and Vteese; holds a patent for Method for assay of CCHS-causing polyalanine repeat expansion for diagnosis; receives publishing royalties for The Fragile X Tremor Ataxia Syndrome (FXTAS), Springer, 2010; has been a consultant for Novartis, Roche, Seaside Therapeutics, and Neurotrope; was part of a Novartis Preceptorship on Fragile X Syndrome in February 2012; and has received research support from Neuropharm LTD, Seaside Therapeutics, Roche, Novartis, Neuren, Alcobra, Vtesse, Asuragen Inc., CDC, NIH, John Merck Fund, the Hope for Hayley Foundation, and the FRAXA Research Foundation. Go to Neurology.org/ng for full disclosure forms.
REFERENCES
- 1.Hagerman RJ, Leehey M, Heinrichs W, et al. Intention tremor, parkinsonism, and generalized brain atrophy in male carriers of fragile X. Neurology 2001;57:127–130. [DOI] [PubMed] [Google Scholar]
- 2.Coffey SM, Cook K, Tartaglia N, et al. Expanded clinical phenotype of women with the FMR1 premutation. Am J Med Genet A 2008;146A:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman SL, Curnow EC, Easley CA, et al. Use of model systems to understand the etiology of fragile X-associated primary ovarian insufficiency (FXPOI). J Neurodev Disord 2014;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hunter JE, Rohr JK, Sherman SL. Co-occurring diagnoses among FMR1 premutation allele carriers. Clin Genet 2010;77:374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hall D, Todorova-Koteva K, Pandya S, et al. Neurological and endocrine phenotypes of fragile X carrier women. Clin Genet 2016;89:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang J, Yu R, Shete S. X-chromosome genetic association test accounting for X-inactivation, skewed X-inactivation, and escape from X-inactivation. Genet Epidemiol 2014;38:483–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol 2005;57:144–147. [DOI] [PubMed] [Google Scholar]
- 8.Leehey MA, Berry-Kravis E, Goetz CG, et al. FMR1 CGG repeat length predicts motor dysfunction in premutation carriers. Neurology 2008;70:1397–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahn S, Elton RL; UPDRS Program Members. Unified Parkinson's Disease Rating Scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB, editors. Recent Developments in Parkinson's Disease, vol 2 Florham Park, NJ: Macmillan Healthcare Information; 1987:153–163, 293–304. [Google Scholar]
- 10.Bain PG, Findley LG, Atchison P, et al. Assessing tremor severity. J Neurol Neurosurg Psychiatry 1993;56:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trouillas P, Takayanagi T, Hallett M, et al. International Cooperative Ataxia Rating Scale for the pharmacological assessment of the cerebellar syndrome. J Neurol Sci 1997;145:205–211. [DOI] [PubMed] [Google Scholar]
- 12.Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Mov Disord 1996;11:136–142. [DOI] [PubMed] [Google Scholar]
- 13.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet 2003;72:869–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natus Medical Incorporated. Balance Manager Systems, Clinical Interpretations Guide, Computerized Dynamic Posturography. Pleasanton, CA: Natus Medical Incorporated; 2009:D102559–00D:139–145. [Google Scholar]
- 15.O'Keefe JA, Robertson-Dick E, Dunn EJ, et al. Characterization and early detection of balance deficits in fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Cerebellum 2015;14:650–662. [DOI] [PubMed] [Google Scholar]
- 16.Salarian A, Horak FB, Zampieri C, Carlson-Kuhta P, Nutt JG, Aminian K. iTUG, a sensitive and reliable measure of mobility. IEEE Trans Neural Syst Rehabil Eng 2010;18:303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filipovic-Sadic S, Sah S, Chen L, et al. A novel FMR1 PCR method for the routine detection of low abundance expanded alleles and full mutations in fragile X syndrome. Clin Chem 2010;56:399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Tassone F, Berman RF, et al. Murine hippocampal neurons expressing Fmr1 gene premutations show early developmental deficits and late degeneration. Hum Mol Genet 2010;19:196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L, Hadd AG, Sah S, et al. High-resolution methylation polymerase chain reaction for fragile X analysis: evidence for novel FMR1 methylation patterns undetected in Southern blot analyses. Genet Med 2011;3:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rousseau F, Heitz D, Biancalana V, et al. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med 1991;325:1673–1681. [DOI] [PubMed] [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 22.Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol 2013;12:786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Revenga L, Pagonabarraga J, Gómez-Anson B, et al. Motor and mental dysfunction in mother-daughter transmitted FXTAS. Neurology 2010;75:1370–1376. [DOI] [PubMed] [Google Scholar]