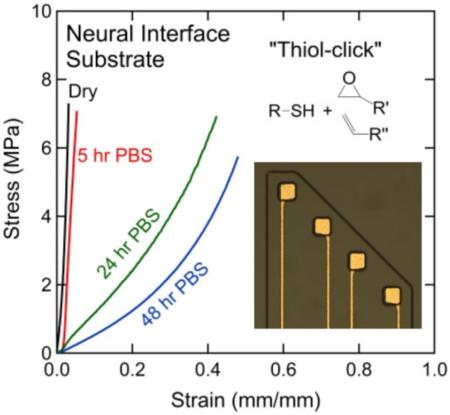
Abstract
Neural interfaces provide an electrical connection between computers and the nervous system: current penetrating devices are orders-of-magnitude stiffer than surrounding tissue. In this work, recent efforts in softening electronics and utilize thiol-ene and thiol-epoxy “click” reactions are built upon to incorporate fluid-sensitive hydrogen bonding into smart substrates for high electrode density neural interfaces. The modulus of these substrates drops more than two orders of magnitude in response to physiological conditions, despite fluid uptake of less than 6%, and can be tuned by the covalent crosslink density and degree of hydrogen bonding in the polymer network. Intracortical and intrafascicular electrode arrays are fabricated and characterized with impedance spectroscopy and cyclic voltammetry.
Keywords: mechanical properties, stimuli-sensitive polymers, structure–property relations, thermosets, thin films
1. Introduction
The field of flexible electronics has rapidly progressed to provide flexibility and stretchability to devices intended to interact with the body.[1] For the design of these devices, attention has traditionally focused on using commercially available polymers as substrates.[2] Substrates such as polydimethylsiloxane, Parylene-C, and polyimides, with recognized hydrophobicity, biostability, and biocompatibility, have enabled a breadth of implantable devices. Multi-functional polymers with tunable physiological responses promise to advance the capabilities of flexible electronics intended to function in vivo.[3]
Neural interfaces are devices capable of recording or stimulating activity in the nervous system and a significant embodiment of the potential of implantable flexible electronics.[4] To record high-fidelity neural activity, the electrode portion of a neural interface is often directly implanted into neural tissue, necessitating an implant with sufficient stiffness to penetrate the tissue.[5] After insertion, micromotion in the neural tissue leads to strain in the tissue surrounding the stiff implant.[6] Several strategies have been proposed to mitigate the mechanical insult to the tissue surrounding implantable neural interfaces. These strategies include coating stiff implants with compliant hydrogels[7] or more flexible implants with degradable stiff polymers.[8] Mechanically responsive substrates, that are stiff for implantation and subsequently soften after exposure to physiological conditions, also offer a promising solution. The result is a reduction both of induced strain in tissue and of modulus mismatch and at the biotic–abiotic interface, which may lessen the immune response to the implant.[9,10] In addition to mitigating the extent of the scarring response, a neural interface device must also maintain a stable electrical connection between computer and tissue throughout the duration of recording.[11] Fluid uptake of insulating and supporting layers must be closely controlled for polymer-encapsulated electrodes to maintain the dielectric properties of the substrate and to prevent volume changes that may cause failure of brittle thin film materials used for the conductors and electrodes. At the electrode–tissue interface, electrode materials that are stable, have low electrochemical impedance and/or encourage ingrowth of neurites are especially promising.[12,13] In the opinion of the authors, it is highly likely that future neural interfaces must include advances in how the implant interacts with tissue mechanically, biochemically and electrochemically.
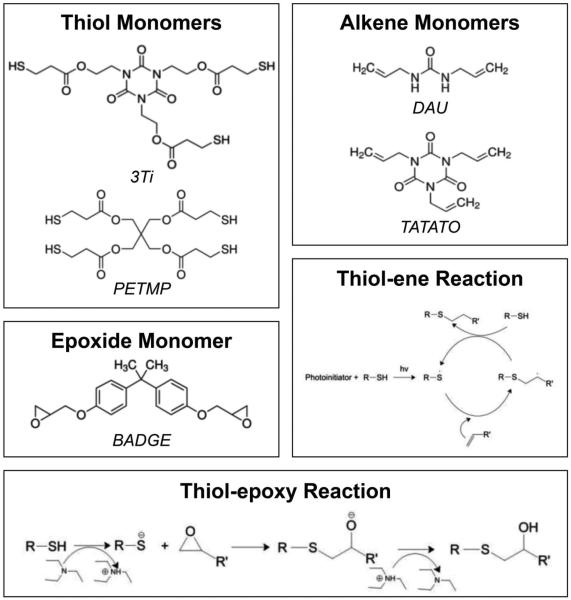
The stringent requirements for both biocompatibility and electrical stability of neural interfaces have motivated researchers to design substrates that are suitable for microelectronics processing techniques and have tunable mechanical and surface properties. Previously, it has been shown that thiol-ene/acrylate substrates can be designed to function as mechanically responsive substrates for intracortical electrodes.[14] Network polymers derived from other subsets of the family reactions known as “thiol-click” may have the versatility needed to enable further capabilities in flexible electronics. The thiol-ene reaction can proceed through a radical type mechanism that is depicted in Scheme 1.[15,16] This mechanism nominally proceeds through the formation of a thiyl radical, which readily attacks many types of alkenes forming a thioether linkage. This resulting carbon-centered radical then abstracts the hydrogen from another thiol and the reaction proceeds elsewhere. It should be noted that a wide range of polymer networks, including polyureas and polyisocyanurates, can be formed by using thiol or alkene functionalized monomers such as those shown in Scheme 1. In addition to the thiol-ene reaction, the thiol-epoxy reaction can also be utilized to achieve useful network structures. The thiol-epoxy reaction mechanism is also depicted in Scheme 1.[17] Nominally this reaction proceeds by a nucleophilic ring-opening mechanism between the epoxide and a base-generated thiolate anion.
Scheme 1.
Pictorial representation of the alkene, epoxide, and thiol monomers and schematics of the reaction between thiols and epoxides or alkenes.
Previous research into thiol-ene substrate-based responsive neural interfaces has focused on networks with the onset of glass transition just above 37 °C. A glassy substrate enables facile implantation of electrodes into neural tissue, and subsequent substrate softening induced by network plasticization after a small amount of water uptake (<3%) provides modulus decrease for attempted tissue compliance matching.[14] Additionally, the thioether linkages in thiol-ene based substrates provide enhanced adhesion to gold, which is commonly used in flexible electronics as a conductor, and therein eliminate the need for brittle adhesion layers. The monomers utilized, primarily TATATO and 3Ti (shown in Scheme 1), provide the requisite glass transition temperature through a densely crosslinked network and not through strong physical interactions. However, the degree of plasticization in previous published results was not sufficient to drive the polymer from its glassy state to its rubbery state. Following a strategy that is often employed in physiologically responsive polymers, it is hypothesized that the degree of plasticization can be improved by incorporating hydrogen bonding in the final network and reduce the necessary crosslink density to achieve the desired glass transition temperature (Tg) allowing for a reduction in final modulus of the substrate.[18]
The goal of this work is to demonstrate tunability of network structure and physiological response (change in modulus and fluid uptake) in thiol-click networks and the suitability of these polymers as substrates for responsive penetrating neural interfaces. Specifically, hydrophobic monomers containing hydrogen bonding capable moieties, namely diallylurea and bisphenol A diglycidyl ether, are introduced into thiol-click networks. The thermomechanical properties of the resulting networks are characterized before and after exposure to simulated physiological conditions. Furthermore, arrays of microelectrodes are fabricated on several substrates, and electrochemical impedance and cyclic voltammetry is used to characterize these devices.
2. Experimental Section
2.1. Materials
1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione(TATATO),1,3diallylurea (DAU), bisphenol A diglycidyl ether (BADGE),triethylamine, pentaerythritol tetrakis(3-mercaptopropionate) (PETMP), isophorone diisocyanate (IPDI) and 2,2-dimethoxy-2-phenylacetophenone (DMPA) were purchased from Sigma–Aldrich. Tris[2-(3-mercaptopropionyloxy)ethyl] isocyanurate (3Ti) was purchased from Wako Chemicals. All chemicals were used as received.
2.2. Device Fabrication and Characterization
2.2.1. Polymer Synthesis, Characterization, and Device Fabrication
All samples were synthesized using a 1:1 molar ratio of functional groups (alkene or epoxide: thiol). Devices were fabricated using photolithography to pattern both gold and Parylene-C. Differential scanning calorimetry (DSC) on both dry and swollen samples was performed to measure Tg. Dry samples were heated from room temperature to 125 °C, cooled to −30 °C and subsequently heated to 200 °C. Swollen samples were loaded at 25 °C, cooled to −30 °C, and subsequently heated to 100 °C. Data shown for both are of only the final heating ramp. Further detailed polymer synthesis, thermomechanical characterization, and device processing can be found in Supplemental Information.
2.2.2. Impedance Spectroscopy
Impedance spectroscopy and cyclic voltammetry were utilized to characterize the fabricated electrodes. The electrochemical cell consisted of the fabricated gold electrode as the working electrode, a large platinum wire as the counter electrode and a Ag/AgCl reference electrode in PBS. A CH Instruments potentiostat was used to measure impedance and the phase angle between 10 kHz and 100 Hz using a 5 mV sinusoidal potential and to perform cyclic voltammetry between −0.5 and 0.5 V with a scan rate of 0.1 V s−1.
3. Results and Discussion
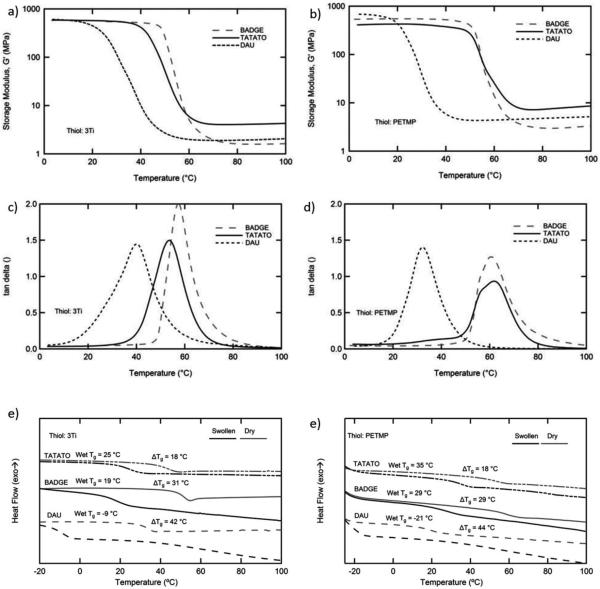
We introduce strong secondary interactions into thiol-click networks to tune both network structure and network response to physiological conditions to enable a new generation of softening neural interfaces. Monomers with hydrogen bonding moieties enable design of polymer networks that show a large glass transition temperature (Tg) shift in response to plasticization with low water uptake (≈6% swelling). The resulting networks are stiff prior to implantation and chronically softer with moduli nearer to those of surrounding tissue. An initial attempt focused on the synthesis of hybrid thiol-ene/thiol-isocyanate networks using IPDI and TATATO. DMA of these networks is shown in the Supporting Information, Figure S1a. The thiourethane content in the network raised the Tg and degree of plasticization, but the rapid reaction of thiols with isocyanates (once catalyzed) led to a significant increase in the viscosity of the monomer/oligomer mixture and prohibited formation of uniform, thin (35 μm) substrates. To combat this issue and form more uniform networks, the thiol-ene and thiol-epoxy reactions, which are more temporally controllable, were utilized: two alkene monomers (DAU and TATATO) and one epoxy monomer (BADGE) were selected to be copolymerized with two multifunctional thiols (3Ti and PETMP). The resulting polymers were characterized by DMA and DSC and the results are shown in Figure 1.
Figure 1.
In order to evaluate each of the monomers selected for this study both DMA and DSC were used. DMA of the dry samples polymerized with both a,c) 3Ti and b,d) PETMP reveal that each composition yielded a polymer network with a Tg at or above 37 °C. d,e) DSC before and after exposure to simulated physiological conditions indicates plasticization with a drop in Tg of between (≈18 and 44 °C).
Notably each sample formed is an amorphous polymer network with the onset of the Tg above room temperature. For each stoichiometric alkene/epoxide and thiol copolymer, the crosslink density for samples made with the tetra-functional PETMP is higher than the corresponding crosslink densities for samples made with tri-functional 3Ti. Interestingly the increase in crosslink density (≈2× greater) associated with replacing 3Ti with PETMP is not associated with a large increase in Tg, as denoted by the peak of tan delta. Similarly, the crosslink density increases as the molecular weight per functionality decreases for the alkene/epoxide monomers with both thiol comonomers (TATATO > DAU > BADGE). It should be noted that using either thiol, the networks containing BADGE and TATATO both have the onset of the glass transition sufficiently high to allow implantation into neural tissue.
To understand the response of these materials to simulated physiological conditions, DSC was performed on as-prepared polymer networks and after immersion for 24 h at 37 °C in PBS. Each network exhibited a significant decrease in Tg caused by fluid uptake. TATATO containing networks exhibit a degree of plasticization (change in Tg) of approximately 18 °C; BADGE containing networks plasticize approximately 30 °C; and DAU containing networks plasticize approximately 42 °C. This drastic increase in the degree of plasticization observed for the BADGE and DAU networks, which both swell ≈5% over 3 weeks, is accompanied by only a slight increase of swelling from the TATATO networks, which swells ≈2%, Supporting Information, Figure S1b. Tunability of network structure is further demonstrated in Supporting Information, Figure S1b,c. By utilizing the versatility of thiol-click reactions, a wide range of properties can be obtained by polymers readily synthesized with facile reaction conditions.
The fact that there was no large difference in the degree of plasticization of the polymer networks induced by changing the thiol comonomer suggests the degree of plasticization is primarily dictated by the introduction of polar groups in the network and not the degree of crosslinking or initial Tg. Since the higher functionality PETMP increases crosslink density as compared to 3Ti but does not increase plasticization or lead to significantly higher glass transition, PETMP-containing polymers are eliminated from further comparison.
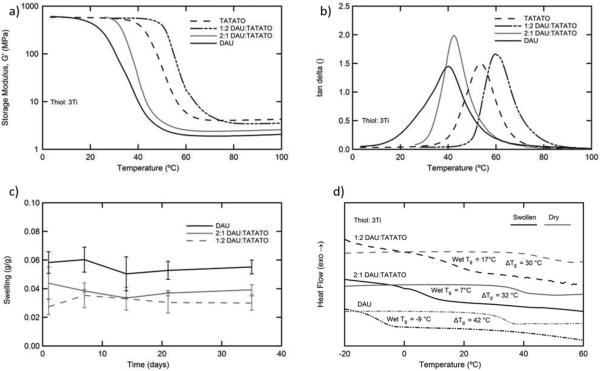
While 3Ti copolymerized with BADGE and TATATO both have the sufficient modulus to enable devices similar in size to those that are currently utilized that can be implanted into soft tissue, DAU-3Ti does not. In an attempt to raise the Tg to achieve a glassy substrate at 37 °C, TATATO was added as a comonomer, and Figure 2a,b shows the thermomechanical analysis of this series of polymer networks. The crosslink density of the networks increases with the addition of the tri-functional TATATO. The Tg of the 3Ti-based network consisting of a 2:1 ratio of DAU:TATATO is higher than the Tg resulting from copolymerization with DAU alone. Interestingly the 1:2 of DAU:TATATO mixture of alkenes exhibits a higher Tg than that of the TATATO network composition. This is likely due to the competing effects of strong hydrogen bonding introduced to the system through incorporation of the urea linkage but a corresponding decrease in covalent crosslink density. In Figure 2c, the swelling of each DAU containing 3Ti-network is monitored over 5 weeks, and water uptake is shown to decrease slightly from 5.5%±0.5% to 3.0%±0.5% as DAU is replaced with TATATO. This swelling is comparable to the swelling for thiol-ene/acrylate substrates reported earlier by our group as viable for neural recording.[9] In addition to the increase in water uptake observed with increased DAU, Figure 2d demonstrates that the change in Tg associated with plasticization increases with increasing DAU content from 30 to 42 °C after 1 d. Similar behavior is also observed after exposure to physiological conditions over one week (Supporting Information, Figure S2). Of these samples, the 1:2 DAU: TATATO-3Ti sample meets the criteria of being glassy at 37 °C and was selected to be compared to the BADGE-3Ti and TATATO-3Ti samples.
Figure 2.
A series of copolymers or varying mixtures of DAU and TATATO polymerized with 3Ti. The a) storage modulus and b) tan delta were obtained for each of the polymer networks by DMA. The response of each composition to exposure to physiological conditions was observed c) by monitoring mass change and d) by comparing the Tg obtained by DSC in the dry state and after swelling for 1 d.
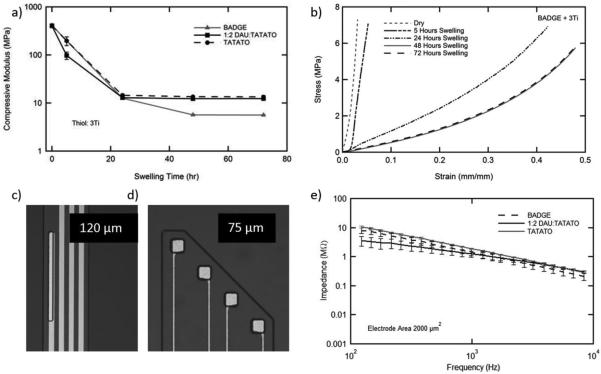
The quasi-static stress–strain response in simulated physiological conditions explains the effect of network plasticization on the modulus. This change in compressive modulus is quantified in Figure 3a; each composition exhibits a decrease in modulus from ≈500 MPa to below 15 MPa after plasticization. After immersion in physiological conditions, modulus drops rapidly over a day for each sample. Little change in compressive modulus is observed beyond 48 h for any of the three compositions. This behavior is consistent with diffusion-based plasticization as opposed to chemical degradation in these hydrophobic polyesters. A representative stress–strain response is shown in Figure 3b for BADGE-3Ti over time, indicating a significant change in modulus. The changes in stress–strain response for each composition are evident in Supportiong Information, Figure S3: the material progresses from exhibiting largely glassy behavior to viscoelastic behavior over hours to days. The BPADGE-3Ti composition reaches the lowest modulus, 5.6±0.15 MPa. It is important to note that this modulus, while significantly lower than the modulus of the inserted polymer is still approximately two orders of magnitude higher than the modulus of cortical tissue. Future work to enable substrates that soften even further may be of significant interest to the community. Additionally, other polymeric materials that take advantage of the strong Au-thiol interactions may provide another organic platform to improve the performance of neural interfaces.[19]
Figure 3.
a) Compressive modulus for three select compositions was measured in physiological conditions. b) A representative stress–strain response for the BPADGE-3Ti network at each time point is shown indicating a transition from glassy-rubbery behavior. c) Intrafascicular and d) intracortical electrode arrays were fabricated on each substrate representative. e) Impedance spectroscopy of gold intrafascicular electrodes fabricated on each substrate indicating no difference in the initial impedance response.
Each of the networks meeting the requirement of being glassy at 37 °C, BPADGE-3Ti, TATATO-3Ti, and 1:2 DAU:TATATO-3Ti, was also used as a substrate (35 μm thick) for patterning of two types of high electrode density softening neural interfaces, intracortical (Figure 3c) and intrafascicular (Figure 3d and Supportiong Information, Figure S4) electrodes. Each device consists of the responsive substrate and patterned gold electrodes and conductors, with a demonstrated minimum feature size of 5 μm. The device is insulated, except at the electrodes and at the connector, by Parylene-C. After patterning Parylene-C by photolithography, the devices were laser micromachined, connected to a zero-insertion force connector and the electrochemical impedance measured, Figure 3e. The impedance of the 2000 μm2 gold electrodes was approximately 1.4 MΩ at 1 kHz. The magnitude of the impedance, phase angle and cyclic voltammetry (Supportiong Information, Figure S4) also indicates similar behavior of these gold electrodes, namely largely capacitative, high impedance behavior, to others that have been previously published in the literature.[13]
4. Conclusion
“Thiol-click” reactions are highly useful in the design of responsive polymer networks. These networks have been successfully used as substrates for both intra-cortical and intrafascicular electrode arrays that undergo a transition from a glassy state to a lower modulus rubbery state when exposed to physiological conditions. Increasing hydrogen bonding moieties in these substrates moderately increases fluid uptake but drastically increases the degree of plasticization. The described thiol-click substrates provide enhanced versatility in the design of responsive neural interfaces. Future work regarding in vitro and in vivo testing of the cellular response to these materials is required to determine suitability of these materials for neural interfaces.
Supplementary Material
Acknowledgements
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship #1114211 and #2011113646 and by the National Institutes of Health/National Institute of Biomedical Imaging and Bioengineering Grant R01EB000462. This work is partly supported by the Defense Advanced Research Projects Agency (DARPA)/MTO Young Faculty Award (YFA), D13AP00049. The content expressed here does not necessarily reflect the position of these funding agencies.
Footnotes
Supporting Information is available from the Wiley Online Library or from the author.
Author Declaration: T.W. and W.V. have a significant financial interest in Syzygy Memory Plastics, Inc. This financial interest has been disclosed to UT Dallas and a conflict of interest management plan is in place to manage the potential conflict of interest associated with this research program.
References
- [1].Someya T, Sekitani T, Iba S, Kato Y, Kawaguchi H, Sakurai T. Proc. Natl. Acad. Sci. U. S. A. 2004;101:9966. doi: 10.1073/pnas.0401918101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hassler C, Boretius T, Stieglitz T. J. Polym. Sci., Part B: Polym. Phys. 2011;49:18. [Google Scholar]
- [3].Ware T, Simon D, Rennaker RL, Voit W. Polym. Rev. 2013;53:108. [Google Scholar]
- [4].Lacour S, Benmerah S, Tarte E, FitzGerald J, Serra J, McMahon S, Fawcett J, Graudejus O, Yu Z, Morrison B. Med. Biol. Eng. Comput. 2010;48:945. doi: 10.1007/s11517-010-0644-8. [DOI] [PubMed] [Google Scholar]
- [5].Seymour J, Langhals N, Anderson D, Kipke D. Biomed. Microdev. 2011;13:441. doi: 10.1007/s10544-011-9512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Polikov V, Tresco P, Reichert W. J. Neurosci. Methods. 2005;148:1. doi: 10.1016/j.jneumeth.2005.08.015. [DOI] [PubMed] [Google Scholar]
- [7].Abidian MR, Martin DC. Adv. Funct. Mater. 2009;19:573. [Google Scholar]
- [8].Fan W, Maesoon I, Euisik Y. A flexible fish-bone-shaped neural probe strengthened by biodegradable silk coating for enhanced biocompatibility. presented at Solid-State Sensors, Actuators and Microsystems Conference (TRANSDUCERS), 2011 16th International; 5–9 June 2011; 2011. [Google Scholar]
- [9].Harris JP, Capadona JR, Miller RH, Healy BC, Shanmuganathan K, Rowan SJ, Weder C, Tyler DJ. J. Neural Eng. 2011;8:066011. doi: 10.1088/1741-2560/8/6/066011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Subbaroyan J, Martin DC, Kipke DR. J. Neural Eng. 2005;2:103. doi: 10.1088/1741-2560/2/4/006. [DOI] [PubMed] [Google Scholar]
- [11].Prasad A, Xue Q-S, Sankar V, Nishida T, Shaw G, Streit WJ, Sanchez JC. J. Neural Eng. 2012;9:056015. doi: 10.1088/1741-2560/9/5/056015. [DOI] [PubMed] [Google Scholar]
- [12].Green RA, Hassarati RT, Goding JA, Baek S, Lovell NH, Martens PJ, Poole-Warren LA. Macromol. Biosci. 2012;12:494. doi: 10.1002/mabi.201100490. [DOI] [PubMed] [Google Scholar]
- [13].Abidian MR, Ludwig KA, Marzullo TC, Martin DC, Kipke DR. Adv. Mater. 2009;21:3764. doi: 10.1002/adma.200900887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ware T, Simon D, Liu C, Musa T, Vasudevan S, Sloan A, Keefer EW, Rennaker RL, Voit W. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2013 doi: 10.1002/jbmb.32946. doi: 10.1002/jbmb.32946. [DOI] [PubMed] [Google Scholar]
- [15].Marvel C, Chambers R. J. Am. Chem. Soc. 1948;70:993. [Google Scholar]
- [16].Kade MJ, Burke DJ, Hawker CJ. J. Polym. Sci., Part A: Polym. Chem. 2010;48:743. [Google Scholar]
- [17].Hoyle CE, Lowe AB, Bowman CN. Chem. Soc. Rev. 2010;39:1355. doi: 10.1039/b901979k. [DOI] [PubMed] [Google Scholar]
- [18].Small IVW, Singhal P, Wilson TS, Maitland DJ. J. Mater. Chem. 2010;20:3356. doi: 10.1039/B923717H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Baek S, Green R, Granville A, Martens P, Poole-Warren L. J. Mater. Chem. B. 2013;1:3803. doi: 10.1039/c3tb20152j. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.