Viruses exploit diverse strategies to evade host immunity and to facilitate their own replication. Some of these strategies use viral-derived or cellular noncoding RNAs to influence host immune responses.1 As reported in this issue, Deng et al. have demonstrated that respiratory syncytial virus (RSV) employs a novel mechanism to suppress antiviral responses that involves RNA fragments derived from host transfer RNA (so-called tRNA-derived fragments, or tRFs).2 Specifically, they show that RSV infection induces an abundant production of specific tRFs that enhance viral replication and counteract the host immune response by modulating gene expression at the posttranscriptional level.2,3 This report adds to the growing list of tRF functions in human health and disease, and suggests that tRFs may be targets for the development of new drugs for the treatment of respiratory tract infections caused by RSV.
tRFs, a novel class of noncoding RNAs, have recently gained significant attention. tRFs are not products of random tRNA cleavage or degradation but are produced by the endonucleolytic cleavage of tRNA at specific cleavage sites by cellular ribonucleases. tRFs are heterogeneous in size (10–50 nucleotides), nucleotide composition, biogenesis, and function. Distinct subclasses of tRFs are defined by their tRNA cleavage sites and whether they are produced from precursor or mature tRNA molecules (reviewed in refs. 4,5,6). Although the abundance of tRFs varies in different cell types and tissues, the levels of specific tRNA fragments are similar to the levels of abundant microRNAs. Moreover, production of selected tRFs is tightly regulated, and some tRFs are only expressed in proliferating cells or cells exposed to adverse conditions.4,5,6
Although the biogenesis and function of most tRFs are unknown, biogenesis of the selected subgroup of tRNA fragments called tRNA-derived stress-induced RNAs (tiRNAs) is well understood. Under stress conditions, the ribonuclease angiogenin (ANG) is activated to cleave cytoplasmic mature tRNAs in the anticodon loop to produce 5′- and 3′-tRNA halves, which are designated as 5′-tiRNAs and 3′-tiRNAs, respectively.7 ANG targets a minor fraction (2–5%) of the tRNA pool, and consequently does not significantly change levels of functional tRNAs or cause abrupt translational arrest in cells.7,8 However, because cellular tRNA levels are significantly higher than those of many other transcripts, e.g., messenger RNAs (mRNAs), even limited ANG-mediated tRNA cleavage leads to the release of high levels of tiRNAs that can impact cell physiology.
What are the functions of ANG-induced tiRNAs in stressed cells? Studies from several groups have implicated selected tiRNAs in stress response programs and the regulation of cell survival. We showed that selected 5′-tiRNAs derived from tRNAAla and tRNACys are efficient inhibitors of protein synthesis in cultured cells. These tiRNAs target mRNA translation by impeding translation initiation, the first step in protein synthesis.8,9,10 Transcripts targeted by 5′-tiRNAAla/Cys are then packed/compartmentalized into stress granules, dynamic cytoplasmic RNA granules with pro-survival and anti-apoptotic functions.11 As a consequence of stress granule assembly, cells reprogram gene expression to adapt to stress and to repair stress-induced injuries. Moreover, work from Hatzoglou's lab showed that a subpopulation of 5′- and 3′-tiRNAs directly inhibits stress-induced apoptosis during hyperosmotic stress.12,13 In response to stress, cytochrome c (Cyt c) is released from mitochondria to trigger apoptosome formation and subsequent caspase activation. Selected tiRNAs directly bind to Cyt c to prevent efficient apoptosome formation and thereby promote cell survival.12 Future studies will characterize further molecular details about the roles of diverse tRNA fragments in cell survival and stress response programs.
Several reports have also implicated tRNA fragments in the pathogenesis of human diseases such as cancer, neurodegenerative disease, neurodevelopmental disorders, and other pathological conditions (discussed in detail in ref. 4). For example, tissue damage (as a consequence of toxic injuries, ischemia/reperfusion injury, or γ-irradiation) in human patients and animal models leads to the ANG-dependent accumulation of circulating tRNA fragments in blood. These circulating tRFs can serve as early biomarkers of stress and tissue damage. In neurons, excessive accumulation of 5′-tiRNAs that are derived from a specific subset of tRNAs (Asp, Glu, Gly, His, Val, and Lys) triggers a sustained stress response leading to neuronal loss and links aberrant tRNA metabolism to the development of certain forms of intellectual disability.
Similarly, accumulation of specific tRNA fragments processed from intron-containing tRNATyr is observed in neurons derived from patients with pontocerebellar hypoplasia, a group of inherited neurodegenerative disorders. Mutations in the ANG gene are found in patients with amyotrophic lateral sclerosis and Parkinson's disease, neurodegenerative diseases that cause selective death of neurons. These mutations diminish the ribonuclease activity of ANG and thus its ability to produce tiRNAs required for motor neuron survival. Administration of recombinant ANG to cultured neurons protects them from stress-induced apoptosis and promotes the lifespan and motor function of SOD1(G93A) mice, a laboratory amyotrophic lateral sclerosis model (reviewed in ref. 4). Finally, diverse tRNA fragments are overexpressed in different cancer cell lines, where they regulate cancer cell proliferation and are involved in DNA replication and repair (reviewed in refs. 4 and 14).
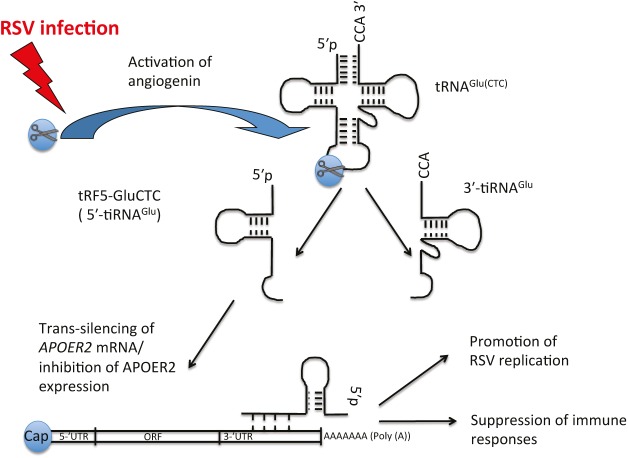
Deng et al. have revealed another link of tRFs to human health and disease.2 Previous work from this lab showed that RSV infection in airway epithelial cells leads to activation of ANG with subsequent abundant production of tRNA fragments that resemble classic 5′-tiRNAs. These tRNA fragments (which they called tRF5s) were derived from various tRNAs (e.g., Cys, Gln, Glu, Gly, His, Leu, Lys, Met, Phe, Ser, Val).3 At least one of the fragments, called tRF5-GluCTC (derived from tRNAGlu), repressed expression of a luciferase mRNA reporter at the posttranscriptional level. Importantly, targeted suppression of tRF5-GluCTC downregulates RSV replication and significantly decreases the yield of RSV particles produced by infected cells. These data imply a stimulatory role for tRF5-GluCTC in RSV replication.3
Using an elegant biochemical approach to capture tRF5-GluCTC–containing ribonucleoprotein complexes followed by bioinformatics analysis, Deng et al. have identified a number of potential cellular targets of tRF5-GluCTC.2 One of the candidate mRNAs encoding apolipoprotein E receptor 2 (APOER2) has been shown to be a direct target of tRF5-GluCTC. Mechanistically, tRF5-GluCTC recognizes a target site in the 3′-untranslated region of APOER2 mRNA and suppresses its expression by an unknown mechanism. Further analysis identified a previously unappreciated role for APOER2 protein in antiviral defense against RSV infection2 (see Figure 1). In summary, this study illuminates roles of tRNA fragments in host–virus interactions and describes a novel molecular mechanism for posttranscriptional regulation of immune responses (reviewed in ref. 15)).
Figure 1.
Respiratory syncytial virus (RSV)-induced transfer RNA (tRNA) fragment suppresses host immune responses. Upon infection, RSV activates the ribonuclease angiogenin (ANG). ANG cleaves mature cytoplasmic tRNAGlu(CTC) in its anticodon loop to produce two halves: 5′-half (tRF5-GluCTC or 5′-tiRNAGlu) and 3′-half (3′-tiRNAGlu). Although the function of 3′-tiRNAGlu remains unknown, tRF5-GluCTC targets several cellular transcripts on the posttranscriptional level through a poorly understood mechanism involving base-pairing between the 3′-end of the tRNA fragment and a 3′-untranslated region (3′-UTR) of target messenger RNA (“trans-silencing”). One such target is APOER2 mRNA encoding antiviral protein APOER2. RSV-induced tRF5-GluCTC inhibits expression of APOER2, thus suppressing host immune responses and promoting RSV replication. ORF, open reading frame.
Why is it important to learn about specific roles of tRFs in RSV infection? Although respiratory tract infections caused by RSV are the second leading cause of death worldwide in children under five years old, no vaccine exists for RSV. Although the study of Deng et al. is at a very early stage, it identifies a new class of targets for the development of therapies to treat RSV infections. Therapies that interfere with the function of a specific tRF (such as tRF5-GluCTC) or inhibit ANG could interfere with RSV replication. Further studies on the functions of tRFs in viral infections are likely to provide valuable insights into details of virus–host interactions that could lead to the design of new therapeutic strategies to prevent/treat viral infections.
References
- Li, F and Ding, SW (2006). Virus counterdefense: diverse strategies for evading the RNA-silencing immunity. Ann Rev Microbiol 60: 503–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, J, Ptashkin, RN, Chen, Y, Cheng, Z, Liu, G, Phan, T et al. (2015). Respiratory syncytial virus utilizes a tRNA fragment to suppress antiviral responses through a novel targeting mechanism. Mol Ther 23: 1622–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q, Lee, I, Ren, J, Ajay, SS, Lee, YS and Bao, X (2013). Identification and functional characterization of tRNA-derived RNA fragments (tRFs) in respiratory syncytial virus infection. Mol Ther 21: 368–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, P and Ivanov, P (2014). tRNA fragments in human health and disease. FEBS Lett 588: 4297–4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebetsberger, J and Polacek, N (2013). Slicing tRNAs to boost functional ncRNA diversity. RNA Biol 10: 1798–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobala, A and Hutvagner, G (2011). Transfer RNA–derived fragments: origins, processing, and functions. Wiley Interdiscip Rev RNA 2: 853–862. [DOI] [PubMed] [Google Scholar]
- Yamasaki, S, Ivanov, P, Hu, GF and Anderson, P (2009). Angiogenin cleaves tRNA and promotes stress-induced translational repression. J Cell Biol 185: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emara, MM, Ivanov, P, Hickman, T, Dawra, N, Tisdale, S, Kedersha, Net al. (2010). Angiogenin-induced tRNA-derived stress-induced RNAs promote stress-induced stress granule assembly. J Biol Chem 285: 10959–10968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, P, Emara, MM, Villen, J, Gygi, SP and Anderson, P (2011). Angiogenin-induced tRNA fragments inhibit translation initiation. Mol Cell 43: 613–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, P, O'Day, E, Emara, MM, Wagner, G, Lieberman, J and Anderson, P (2014). G-quadruplex structures contribute to the neuroprotective effects of angiogenin-induced tRNA fragments. Proc Natl Acad Sci USA 111: 18201–18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha, N, Ivanov, P and Anderson, P (2013). Stress granules and cell signaling: more than just a passing phase? Trends Biochem Sci 38: 494–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia, M, Jobava, R, Parisien, M, Putnam, A, Krokowski, D, Gao, XH et al. (2014). Angiogenin-cleaved tRNA halves interact with cytochrome c, protecting cells from apoptosis during osmotic stress. Mol Cell Biol 34: 2450–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saikia, M, Krokowski, D, Guan, BJ, Ivanov, P, Parisien, M, Hu, GF et al. (2012). Genome-wide identification and quantitative analysis of cleaved tRNA fragments induced by cellular stress. J Biol Chem 287: 42708–42725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mleczko, AM, Celichowski, P and Bakowska-Zywicka, K (2014). Ex-translational function of tRNAs and their fragments in cancer. Acta Biochim Pol 61: 211–216. [PubMed] [Google Scholar]
- Ivanov, P and Anderson, P (2013). Posttranscriptional regulatory networks in immunity. Immunol Rev 253: 253–272. [DOI] [PMC free article] [PubMed] [Google Scholar]