Abstract
ISG15 is an ubiquitin-like protein induced by type I interferon associated with antiviral activity. ISG15 is also secreted and known to function as an immunomodulatory molecule. However, ISG15's role in influencing the adaptive CD8 T-cell responses has not been studied. Here, we demonstrate the efficacy of ISG15 as a vaccine adjuvant, inducing human papilloma virus (HPV) E7-specific IFNγ responses as well as the percentage of polyfunctional, cytolytic, and effector CD8 T-cell responses. Vaccination with ISG15 conferred remarkable control and/or regression of established HPV-associated tumor-bearing mice. T-cell depletion coupled with adoptive transfer experiments revealed that ISG15 protective efficacy was CD8 T-cell mediated. Importantly, we demonstrate that ISG15 vaccine-induced responses could be generated independent of ISGylation, suggesting that responses were mostly influenced by free ISG15. Our results provide more insight into the immunomodulatory properties of ISG15 and its potential to serve as an effective immune adjuvant in a therapeutic tumor or infectious disease setting.
Introduction
The induction of cytotoxic CD8 T-cells is believed to be essential in tumor control, and, thus, a necessary goal for any therapeutic cancer vaccine. Nevertheless, insufficient generation of CD8 effector T-cells has led to the failure of several therapeutic cancer vaccines to produce clinical regression of solid tumors.1,2,3 For such vaccines, the incorporation of adjuvants can assist in generating potent and durable tumor immunity,4,5 but most of the effects of adjuvants have been limited to Th1 CD4 expansion with poor CD8 T-cell killing function induced. Thus identifying adjuvants capable of amplifying CD8 T-cell antitumor immunity is very important for therapeutic antitumor vaccines.
Interferon-stimulating gene 15 (ISG15) is one of the first and most abundant proteins induced by type I interferon stimulation.6 ISG15 is an ubiquitin-like protein, which plays a major role in antiviral defense.6 Its ubiquitin-like C-terminal (LRLRGG) motif is necessary for its conjugation to a variety of intracellular proteins in a process known as ISGylation6 producing “conjugated” ISG15. When not in its conjugated form, free or “unconjugated” ISG15 can exist intracellularly or extracellularly. For decades, free ISG15 has been implicated in the production of IFNγ.7,8,9 Recently, a new study confirmed this cytokine-like (a molecule that can exert cytokine activity) role for ISG15 by demonstrating that ISG15 deficiency was associated with a loss of IFNγ, which in turn led to increased susceptibility to mycobacterial disease in both mice and humans.10 Although these studies have established the ability of ISG15 to function as an immunomodulatory molecule, its ability to influence CD8 T-cell immune responses and act as a vaccine adjuvant remains unknown. Here, we sought to investigate the role of ISG15 as an adjuvant to enhance tumor-specific CD8 T-cell immunity using a human papilloma virus (HPV)-associated tumor murine therapeutic model.
Here, we report that ISG15 can act as an effective CD8 T-cell-mediated adjuvant when codelivered with a HPV16 DNA vaccine via in vivo electroporation (EP). The inclusion of ISG15 substantially increased E7-specific IFNγ responses as well as the percentage of polyfunctional, cytolytic, and effector CD8 T-cells. Importantly, we report that the augmentation of ISG15's functional CD8-mediated tumor immunity achieved control and/or regression of tumors in established HPV-associated tumor-bearing mice. We also show that the therapeutic efficacy of ISG15 correlates with the increase in magnitude and phenotype of tetramer-specific, effector CD8 T-cells. Finally, we demonstrate that ISG15 delivered as an immunoadjuvant generates responses independent of conjugation as an LRLRGG-mutant ISG15 also induced potent CD8 T-cell responses. We conclude that ISG15 may be a valuable tool to improve the immunogenicity of vaccines against cancer as well as to treat persistent infections.
Results
Design and expression of ISG15 constructs
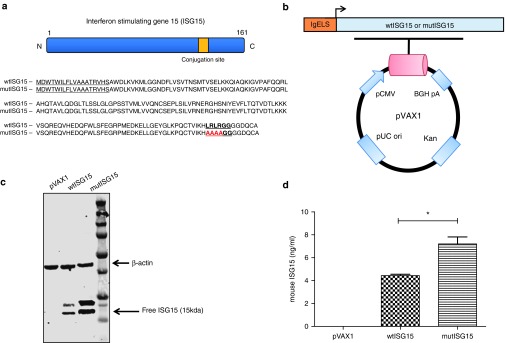
The wild-type ISG15 (wtISG15) adjuvant construct was generated using the mouse ISG15 sequence retrieved from GenBank (accession number: Q64339) with several modifications (Figure 1a). ISG15 contains a C-terminal LRLRGG motif that is necessary for its conjugation to a variety of target proteins in a process referred to as ISGylation.11,12,13 In order to determine if conjugation was necessary for ISG15-mediated immunomodulation, the ISG15 conjugation sequence site was mutated (LRLRGG to AAAAGG) to generate the mutant ISG15 (mutISG15), incapable of conjugation (Figure 1a). Both ISG15 constructs were genetically optimized and subcloned into a modified pVAX1 mammalian expression vector (Figure 1b).5,14 To verify the expression of both ISG15 encoding constructs, human rhabdomyosarcoma (RD) cells were transfected separately with each vector and examined by western blot analysis. As shown in Figure 1c, an ~15 kDa free ISG15 was observed for each in cell lysates harvested 48 hours after transfection using anti-ISG15 monoclonal antibody (mAb) for detection. ISG15 expression was not detected in the negative control pVAX1 group. Next, via an enzyme-linked immunosorbent assay, the secretion of free ISG15 was monitored from the cell supernatants that were obtained 48 hours after transfection of RD cells. As projected, supernatants from mutISG15-transfected RD cells exhibited a higher concentration of detectable secreted free ISG15 (7.2 ng/ml), compared to wtISG15 (4.4 ng/ml) (Figure 1d). This supports the concept that through mutating ISG15's conjugation motif, more unconjugated ISG15 would be available and secreted to the extracellular environment.
Figure 1.
Generation and expression of ISG15 encoding DNA vaccine plasmids. (a) Schematic illustration of ISG15 protein and the amino acid sequences of wild-type ISG15 (wtISG15) and mutated ISG15 (mutISG15). The IgE leader sequences are underlined. The C-terminal ubiquitin-like conjugation site is bold and underlined. The mutation sites introduced into the conjugation motif for mutISG15 (unconjugated form) are in red. (b) Map of ISG15 constructs. (c) Expression of ISG15 constructs examined by western blot analysis. The lowest band represents free ISG15. (d) Detection of secreted wtISG15 and mutISG15 from transfected RD cells were confirmed via enzyme-linked immunosorbent assay. Data represent the means with standard error of the mean for two replicate assays. RD, human rhabdomyosarcoma.
Immunization with ISG15 adjuvant induced strong HPV E7-specific CD8 T-cell immune responses
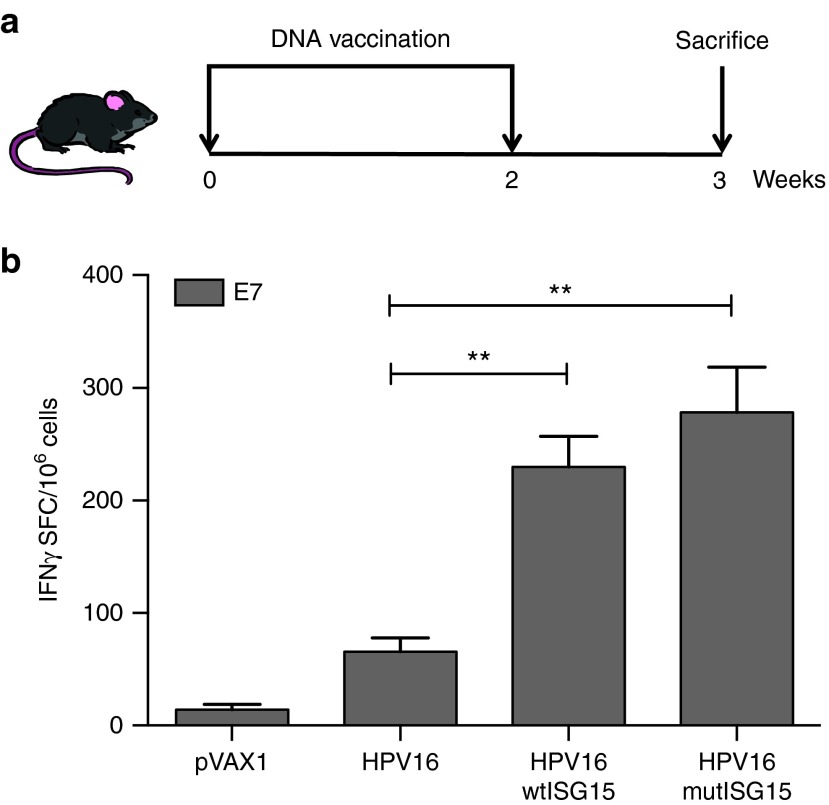
To assess the immunogenic properties of ISG15, an quantitative IFNγ ELISpot assay was performed to determine the number of vaccine-induced E7-specific IFNγ secreting cells in response to E7 pooled peptides containing the immunodominant CTL epitope H-2-DbE749-57 (E7) from adjuvanted or unadjuvanted vaccine groups. The immunization regimen is shown in Figure 2a. Briefly, groups of B6 mice (n = 4–5/group) were vaccinated twice at 2-week intervals as follows: (i) HPV16 DNA/EP; (ii) HPV16/wtISG15 DNA/EP; (iii) HPV16/mutISG15 DNA/EP; and (iv) pVAX1/EP (pVAX1 copy plasmid was used to keep DNA mass consistent in each group). The co-administration of HPV16 with wtISG15 resulted in a substantial 3.5-fold increase in E7-specific IFNγ responses (~230 spot forming colony (SFC)/million splenocytes) compared with HPV16 alone-immunized group (~66 SFC/million splenocytes) (P = 0.0016). ISG15 is a ubiquitin-like protein that conjugates to target proteins and is critical for control of certain viral and bacterial infections.6 In addition to the conjugated form of ISG15, it is known, that ISG15 is present in an unconjugated form (free ISG15) and can also play an important role in immunomodulation or during infection.6 Thus, in the same experiment, we examined if vaccine-induced responses were independent of conjugation by immunizing mice with a mutated form of ISG15 lacking a functional C-terminal LRLRGG conjugating motif. Interestingly, similar to wtISG15, the mutISG15 vaccinated-group demonstrated an increase (~4-fold) in total E7-specific cells compared with HPV16-only group, suggesting ISG15 can induce its effects (influencing T-cell immunity) independent of conjugation. We did not find clear evidence of higher induced levels of E6-specific vaccine-induced responses (data not shown). Together, these data suggest that ISG15 can act as an adjuvant to enhance and stimulate E7-specific CD8 T-cell responses. Moreover, these data demonstrated that the elevated antigen (Ag)-specific responses maybe likely attributed to free ISG15.
Figure 2.
Codelivery of ISG15 DNA vaccination promoted E7-specific CD8 T-cell immune responses secreting IFNγ production. (a) Immunization schedule for DNA vaccine adjuvant study. C57BL/6 mice (n = 4–5/group) were immunized twice at 2-week intervals with HPV16 construct with or without wtISG15 or mutISG15 adjuvant constructs via IM/EP delivery. One week after last vaccination, spleens were harvested to analyze the Ag-specific CD8 T-cell responses. (b) The frequency of E7-specific IFNγ (spot forming cells/106 splenocytes) responses induced after each vaccination was determined by IFNγ ELISpot assay in response to E7 pooled peptide containing the specific CD8 HPV16 E7 epitope (RAHYNIVTF). Data represent two independent experiments with four to five mice per group. *P < 0.05; **P < 0.01. Error bars indicate standard error of the mean.
ISG15-mediated augmentation of polyfunctional HPV E7-specific cell-mediated responses
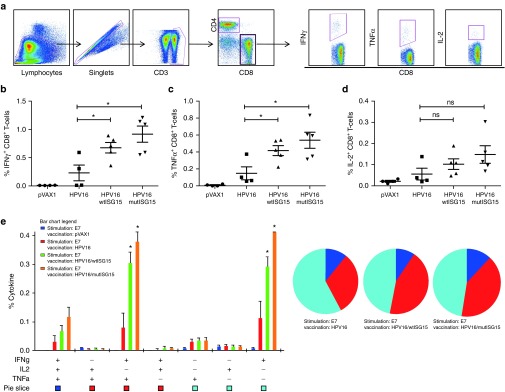
Considering that CD8 T-cell immune responses are essential in prevention of tumorigenesis and elimination of tumors,1,15,16,17,18 we further examined the functional profile of E7-specific CD8 T-cell populations from vaccinated mice to secrete IFNγ, TNFα, and IL-2 in response to DbE749-57 peptide stimulation. Our gating strategy for intracellular cytokine multiparametric flow cytomtery analysis is shown in Figure 3a. One week after final vaccination all tested vaccination regimens induced detectable CD8 T-cell responses producing all three effector cytokines (Figure 3). Compared to the antigen alone group, both ISG15 vaccine regimens induced significant E7-specific CD8 T-cells producing total IFNγ (wtISG15, 0.68%; mutISG15, 0.92%) (Figure 3b) and total TNFα (wtISG15, 0.42%; mutISG15, 0.54%) (Figure 3c). However, ISG15 only induced a minor increase of Ag-specific CD8 T-cells secreting IL-2 (Figure 3d). Importantly, the ISG15-immunized groups elicited significantly higher frequencies of E7-specific CD8 T-cells producing either IFNγ alone or dual IFNγ+TNFα+ in the spleens 7 days postvaccinations (Figure 3e), also a modest increase in the triple-positive IFNγ+TNFα+IL-2+ CD8 secreting cells in the ISG15-treated groups. Since ISG15 can have an effect on NK cells, we monitored vaccine-induced NK responses, but no significant changes were seen after vaccination with ISG15 (Supplementary Figure S1a).9 Furthermore, the administration of ISG15 did not increase vaccine-induced CD4 T-cell responses as measured by ex vivo E6 and E7 pooled peptide stimulation (Supplementary Figure S1b).
Figure 3.
ISG15 induces polyfunctional HPV16 E7-specific CD8 T-cells. (a) Schematic diagram of gating strategy used to identify Ag-specific CD8 T-cell populations. (b–d) Column graphs show the percentages of HPV16 E7-specific CD8 T-cells releasing total cytokines IFNγ (b), TNFα (c), and IL-2 (d) after stimulation with DbE749-57-specific peptide. (e) Column chart show polyfunctional subpopulations of single-, double-, or triple-positive CD8 T-cells releasing effector cytokines: IFNγ, TNFα, and IL-2 to E749-57-specific stimulation. Pie charts represent proportion of each cytokine population. Experiments were performed at least two times with similar results with four to five mice per group. All cell counts are relative and not absolute. *P < 0.05 compared with HPV16 group. Error bars indicate standard error of the mean.
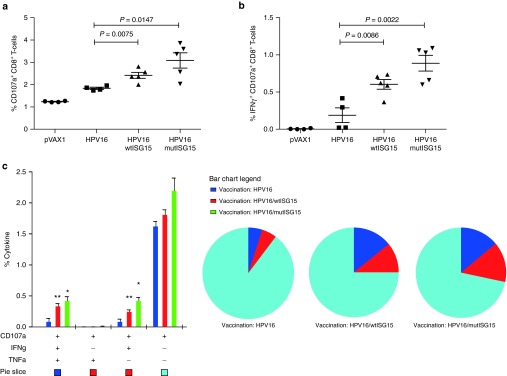
Given that cytotoxic CD8 T-lymphocytes (CTL) are critical components in protection,1,5,17,18 we assessed the cytolytic properties of the adjuvant-induced CTL responses to undergo degranulation and secrete effector cytokines simultaneously (Figure 4). The groups vaccinated with immunoadjuvant ISG15 showed higher percentages of the degranulation marker, CD107a (wtISG15, 2.4%; mutISG15, 3.1%), compared with HPV16-alone group (Figure 4a). More interestingly, the ISG15-adjuvanted vaccines elicited substantially higher frequencies of polyfunctional CTLs, with a substantial representation of cells showing one, two, and three immunological functions (Figure 4b,c). Notably, compared to HPV16 administered alone, the ISG15-treated groups showed significantly higher frequencies of CD8 T-cells coexpressing CD107a+IFNγ+TNFα+ (wtISG15, 0.35%; mutISG15, 0.43%) (Figure 4c). Collectively, the high frequencies of effector cells secreting proinflammatory cytokines are indicative of the ISG15 cytokine-like properties and its adjuvant effects to enhance vaccine potency by diving potent functional effector CTL immunity. Overall, an important observation here was that a DNA plasmid expressing the mutISG15, incapable of conjugation, maintained the adjuvant effects displayed by wild-type form, suggesting that ISGylation is likely not required for immunomodulation of CD8 T-cells.
Figure 4.
ISG15 induces HPV16 E7-specific CD8 T-cells undergoing cytotoxic degranulation following immunization. E7-specific CD8 T-cell responses measured by intracellular cytokine and CD107a staining after stimulation of splenocytes with DbE749-57 restricted (CD8) peptide were examined in all groups of animals 1 week after final immunization. (a) Ag-specific cytolytic degranulation of CD8 T-cells measured by staining for degranulation marker expression, CD107a. (b and c) Column graph shows the frequency of cytolytic CD8 T-cells simultaneously expressing only IFNγ (b) or the frequency of polyfunctional cytokine producing and/or CD107a expressing CD8 T-cells (c). Experiments were performed at least twice with similar results with four to five mice per group. All cell counts are relative and not absolute. *P < 0.05; **P < 0.01 compared with HPV16 group. Error bars indicate standard error of the mean.
ISG15 adjuvant amplifies robust Ag-specific effector CD8 T-cell responses
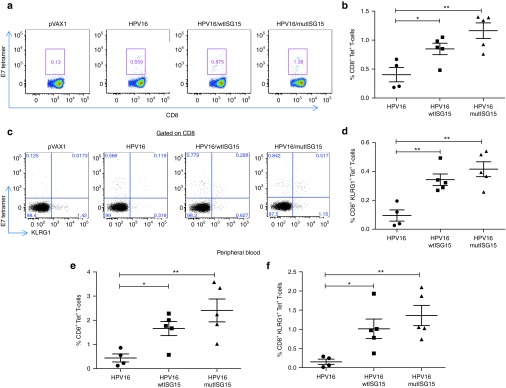
Since it has been demonstrated that magnitude and quality of E7-specific CD8 T-cell responses correlates with the therapeutic efficacy of HPV vaccine against established tumors,17,19 we next investigated tetramer-specific CD8 T-cell responses as a correlate with vaccine-induced HPV-associated tumor control. To this end, nontumor-bearing B6 mice were immunized with the aforementioned vaccination formulations and schedule in Figure 2a. One week after final immunization, the magnitude and subset differentiation of Ag-specific CD8 T-cell responses were examined using the CD8 epitope specificity of HPV16 E749-57 H2-Db-RAHYNIVTF tetramer in the spleen and blood (Figure 5). Both wtISG15 and mutISG15 constructs were able to significantly increase the DbE7 tetramer-specific CD8 T-cell responses in the spleen compared to HPV16 group alone (Figure 5a,b). In addition, the delivery of both ISG15 plasmids also significantly amplified the number of DbE7 tetramer-specific CD8 T-cells in the peripheral blood, suggesting trafficking of tumor target-specific CTL's into the periphery (Figure 5e).19 The frequency of E7-tetramer T-cells in the blood within the wtISG15 and mutISG15 groups were 4- and 5.5-fold higher compared with the nonadjuvanted group, respectively. These data confirmed that immunoadjuvant ISG15 could amplify the Ag-specific CD8 T-cells.
Figure 5.
ISG15 augments the formation of the E7-specific effector CD8 T-cell population. Groups B6 mice (n = 4–5) were immunized twice with HPV16, HPV16/wtISG15 or HPV16/mutISG15 at two-week intervals. One week after last immunization, both splenocytes and peripheral blood mononuclear cells were strained for CD8, DbE749-57 tetramer, and the effector KLRG1 marker. (a) Representative flow plot showing H2-Db-RAHYNIVTF-restrticted HPV16 E7-specific CD8 T-cells in the spleen 1 week after final immunization, or (b) in data represented as a scatter plot graph. (c,d) Representative dot plots (c) and compiled data of the percentages of E7 tetramer-specific KLRG1+CD8+ effector phenotype population in the spleen (d). (e,f) The percentages of total DbE749-57 tetramer-binding CD8 T-cells from the peripheral blood (e) and tetramer-specific effector CD8 T-cells (f). Data are representative of at least two experiments. All cell counts are relative and not absolute. *P < 0.05; **P < 0.01. Error bars indicate standard error of the mean.
It has been suggested that effector CD8 T-cells are optimal subsets for protective immunity and may predict therapeutic efficacy against tumors.4,5,20,21 Effector T-cells are the focus of cancer vaccines as they can initiate rapid effector function and migrate quickly to the infected- or tumor-site.1,22,23,24 In this study, we measured the DbE7 MHC class I tetramer vaccine-induced effector/effector-memory CD8 T-cell subset based on expression marker of KLRG1 (effector—Teff)5,20,25 (Figure 5). The administration of wtISG15 resulted in a ~3-fold increase in the percentages of Teff cells in the spleen, compared with the HPV16-only vaccinated group (Figure 5c,d). Similarly, the inclusion of mutISG15 also significantly enhanced the Teff responses in the spleen (Figure 5d). In addition, as shown in Figure 5f, the percentages of Teff cells in the blood were significantly higher in the adjuvant groups. These data suggest that immunoadjuvant ISG15 can enhance the magnitude and quality of E7-specific CD8 T-cell responses.
ISG15 acted as an effective CD8 T-cell immunoadjuvant inducing antitumor immunity
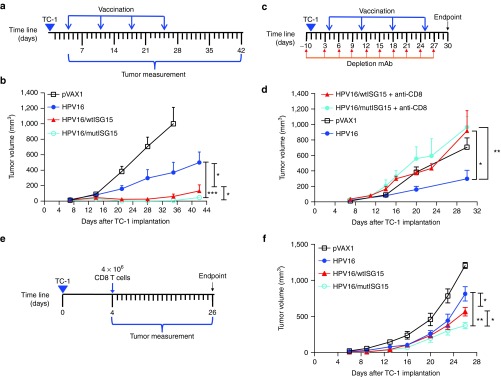
We next determined the therapeutic efficacy of ISG15 in a TC-1 tumor-bearing mice model. Naive recipient B6 mice (n = 10/group) were first inoculated subcutaneously with TC-1 tumor (5 × 104) cells followed by HPV16, HPV16/wtISG15, HPV16/mutISG15, or pVAX1 vaccination 7 days after tumor implantation (tumors had reached an average size of 3 mm), followed with three boosts at 1-week intervals (Figure 6a). Tumors in mice immunized with the mixture of HPV16/wtISG15 grew significantly slower than HPV16-vaccinated group alone (Figure 6b). In contrast, pVAX1 control group failed to show any therapeutic effect with all mice dying by day 35. Interestingly, mice given the HPV16/mutISG15 had significantly better tumor control than mice given HPV16/wtISG15 by day 42 post-tumor implantation (Figure 6b), likely due to greater induction of tumor-specific CTL responses. At day 42, post-tumor implantation, 6/10 mice in the HPV16-mutISG15 were tumor free, compared with either HPV16 (1/10) or HPV16-wtISG15 (2/10). Taken together, the adjuvant properties of ISG15 demonstrated effective control and therapeutic cure of HPV-associated tumor-bearing mice.
Figure 6.
The therapeutic effects induced by ISG15 in tumor-bearing mice. (a) Schematic representation for therapeutic study. (b) Tumor growth measurement after therapeutic DNA/electroporation vaccination (n = 10). (c) Schematic representation for CD8 T-cell depletion with therapeutic vaccination. (d) Tumor growth curve of vaccinated groups (n = 5) without CD8 T-cells. (e and f) Schematic representation for T-cell adoptive transfer study (e). Approximately 4 × 106 CD8 T-cells from vaccinated mice were purified from splenocytes and adoptively transferred into tumor-bearing T-cell immunodeficient B6 Rag1 KO mice (n = 5) and assessed for tumor growth (f). All tumor-bearing mice were injected subcutaneously with 5 × 104 TC-1 cells. *P < 0.05; **P < 0.01; ***P < 0.001. Error bars indicate standard error of the mean.
Given ISG15 adjuvant properties to enhance E7-specific-CTL responses that are essential to target established pre-existing HPV infections,15,16 we investigated the role of ISG15-elicited CD8 T-cells for HPV-associated tumor elimination. Therefore, in a therapeutic setting, CD8 T-cells were depleted by intraperitoneally injection of anti-CD8 antibody, beginning 1-day before tumor inoculation and repeated every 3 days post-tumor implantation (Figure 6c). Our results revealed that CD8 depletion significantly abrogates the therapeutic effects of ISG15 adjuvancy as no mice survived to 30 days postimplantation (Figure 6d). To confirm these findings, we performed CD8 T-cell adoptive transfer experiments in T-cell-immunodeficient B6 Rag1 KO mice.26 4 × 106 CD8 T-cells purified from splenocytes of HPV16, HPV16/wtISG15, and HPV16/mutISG15 immunized mice (Figure 2a) were injected intravenously 4 days postinoculation of TC-1 cells (Figure 6e). As compared to HPV16 and naive controls, mice that received either wtISG15 or mutISG15 vaccine-induced CD8 T-cells had significantly slower tumor growth (Figure 6f), likely owing to their functional CTL phenotype (Figures 3 and 4). Taken together, the results suggest that ISG15-elicited CD8 T-cells are essential to prolonging survival and controlling tumor growth in the TC-1 therapeutic tumor model.
Discussion
ISG15 is known to play a major role in antiviral defense. In addition, it has also been reported to function as an immunoregulatory molecule.6 The existence of a secreted unconjugated form of ISG15 has been reported to have cytokine-like activity, with evidence supporting its ability to induce IFNγ responses.9,10,14,27,28 Thus, this study builds on these findings and extends ISG15 research in a therapeutic tumor model system.
Herein, we first report the therapeutic efficacy of ISG15 immunoadjuvant properties to augment Ag-specific CD8 T-cell tumor immunity. We used a well-established preclinical HPV therapeutic challenge model to test the adjuvant effects of ISG15 in a DNA vaccine setting. The main results of this study are that inclusion of ISG15 can (i) increase the polyfunctional Ag-specific CTL responses; (ii) induce effector CD8 T-cell subset differentiation; (iii) have antitumor therapeutic effects; and (iv) elicit vaccine-induced protective immunity independent of conjugation, further establishing free ISG15 immunomodulatory properties.
In this study, we report that the inclusion of ISG15-induced robust Ag-specific IFN-γ responses. We find this in accordance with previous studies indicating that ISG15 can induce IFNγ secretion by lymphocytes.7,8,9,10 However, we include new evidence that ISG15 delivered as a separate molecule in an adjuvant function, can drive CD8 T-cells to enhance their secretion of IFNγ and TNFα, further adding to the pool of information regarding ISG15 immunomodulatory properties on T-cells. It is noteworthy to report that in this model or approach, ISG15 was not involved in induction of NK cells, as previously shown.8 Furthermore, given that CTL functionality represents an important correlate of protective capacity against HPV16 established tumors,17,18,29 we report that ISG15 promoted the expansion and Ag-specific cytolytic function of CD8 T-cells by augmenting the expression of IFNγ, TNFα, and the degranulation maker CD107a in various combinations. We also demonstrated that ISG15 delivered as a vaccine adjuvant, amplified E7 tetramer-specific CD8 vaccine-induced T-cell responses. The reasons behind ISG15 ability to enhance the frequency of Ag-specific CD8 T-cell responses are unknown. However, a study by Casanova and colleagues have shown that ISG15 may work in synergy with IL-12, suggesting that ISG15 likely promoted enhanced CD8 T-cells induction and expansion synergistically with IL-12.10 In addition, the ability of ISG15 to induce IFNγ secretion by lymphocytes, may also suggest that ISG15 might bind to a cell surface receptor to modulate immune responses.6 The identity of a cell surface receptor for ISG15 has yet to be discovered. In addition, ISG15 may also amplify cell-mediated adaptive immunity by eliciting antigen-presenting cell number and function. However, further studies will be needed to elucidate the mechanism(s) underlying the adjuvant effects of ISG15 including soluble ISG15.
The administration of immunoadjuvants in vaccines has long been studied as an important method of improving their efficacy, or potency. Here, we explored the antitumor role of ISG15. Prior work in the TC-1 tumor challenge model, has demonstrated that this model is CD8 T-cell dependent for protection.27,29,30,31 As expected, the results revealed that accordant with the enhance polyfunctional CTL responses, administration of ISG15 led to strong inhibition of tumor growth, regression, and prolonged survival. Moreover, depletion of CD8 T-cells in mice nullified the antitumor effects of ISG15 and supported the tumors to grow larger compared to the nontreated CD8 mAb HPV16 group. These data support that the antitumor activity of ISG15 was dependent on CD8 T-cells. Subsequently, as demonstrated in our adoptive transfer experiments, CD8 T-cells alone can reduce tumor volume, suggesting that the ISG15 vaccine-induced CD8 T-cell responses must be more functional at clearing HPV-infected cells. In this experiment setting, the enhanced induction of E7-specific effector T-cells may have correlated with the therapeutic efficacy of ISG15-treated groups against established tumors. This notion is supported by the enhanced CTL activity (Figure 4) and E7 tetramer-positive Teff cells in both the spleen and blood of mice vaccinated with ISG15 (Figure 5). Notably, there were more detectable CD8 Teff cells responses in the periphery, suggesting trafficking of target specific CD8 T-cells to the site of malignancy and initiating immediate effector function.19,24 Our studies appear to be supportive of previous work demonstrating that vaccines eliciting higher Teff correlated with superior protective immunity against inhibiting tumor growth.1,5,25 Therefore, the magnitude and quality of E7-specific CD8 T-cell memory population correlated with the efficacy of ISG15-treated groups to control or resolve tumors during the first 3 weeks of treatment. These potential correlates of immunity may represent a major tool for continued development of future tumor vaccines. Consistent with these are recent reports indicating that vaccine-induced effector memory may be the best prognostic factor for therapeutic vaccines targeting established tumors or latently infected pathogens.1,5,20,21 Central memory CD8 T-cells elicited by ISG15 may have also been important, as central memory T-cells are essential features by which vaccines can mediate protective immunity.22,23,24,32 This is an area of further investigation. Overall, on the basis of these findings, the improved therapeutic effect by ISG15 is associated with Ag-specific CTL responses.
In the same experimental setting, a highlight of this study was demonstrating that the protection afforded by ISG15 was most likely not dependent on its conjugated form, but rather on free ISG15. Our results indicated that mutISG15 was able to induce a similar trend of robust Ag-specific antitumor T-cell responses compared to wtISG15, suggesting that this activity was independent of ISGylation. The immunomodulatory properties of soluble free ISG15 is in agreement with several studies suggesting that ISG15 acts as an immune activating cytokine.33 Both ISG15 constructs did not differ enormously in their effectiveness at eliciting E7-specific tumor immunity. Thus, given that both forms of the ISG15 constructs exerted similar immunostimulatory effects, the phenotypes in CD8 T-cells were not dependent on the motif.34 Interestingly, mutISG15 was significantly better able to control the progression of established tumors compared to wtISG15-adjuvanted treated mice. As more mutISG15 is secreted from transfected cells in our experiments (Figure 1d), it may orchestrate a more effective adaptive response; thus, leading to better control of tumor growth and pathogen clearance. The modified ISG15 protein inducing slightly more stimulatory properties than the wtISG15 reflects its dedication to secreted function or that its altered form is seen as foreign, thus eliciting heightened immune responses. However, a recent report by Zhang et al.35 has demonstrated that a similar altered ISG15 can reverse IFNγ production of patient's cells with ISG15 deficiency. However, the manner in which unconjugated secreted form of ISG15 (mutISG15) is able to induce better antitumor responses compared to wtISG15 is still not yet clear. Nevertheless, the ability of the mutISG15 form to induce superior antitumor responses highlights its potential to serve as an alternative potent ISG15 adjuvant. In addition, it emphasizes that developing new ways to increase the levels of free ISG15 may be a novel approach to treat cancer and other infectious diseases.
In summary, the results of the current preclinical study provide more insight into the immunomodulatory properties of free ISG15 and its potential to serve as a promising vaccine adjuvant in cancer immunotherapy. The results also confirm the notion that ISG15 can function as an immunomodulatory molecule. Overall, these results highlight ISG15 as a new feasible CD8 T-cell-driven adjuvant for therapeutic cancer vaccination, like other potential effective tumor vaccine adjuvants such as poly IC, CpG-ODN and other immunopotentiators.36,37,38 Moreover, the evidence that ISG15 can be an effective adjuvant to drive potent CD8 T-cell responses, support future studies to evaluate its application in TB- or HIV-infection models.
Materials and Methods
DNA construction and expression. The GenBank accession no. Q64339 for mouse ISG15 was used to synthesize the DNA construct encoding wild-type ISG15 (wtISG15). Mutated ISG15 (mutISG15) is a variant of wtISG15 with point mutations at its C-terminal conjugation site (LRLRGG to AAAAGG). All constructs contained highly efficient immunoglobulin E (IgE) leader sequence inserted at the 5' end of the gene. The constructs were commercially synthesized and optimized as described previously.5,14 HPV16 plasmid containing the E6 and E7 antigens was prepared as previously described.39 In vitro expression of both ISG15 constructs was confirmed by using western blot analysis. Human RD cells were maintained in Dulbecco's modified Eagle's medium (Life Technologies, Grand Island, NY) and supplemented with 10% heat-inactivated fetal calf serum as well as penicillin and streptomycin. After plating 3.0 × 105 cells per well, transfection was performed using Neofectin (NeoBiolab, Cambridge, MA) following the manufacture's protocol. Cell was transfected with 2 μg of each DNA construct including pVAX1 empty vector backbone as a negative control. Following 48-hour incubation, cell supernatants were collected and cells were washed with cold PBS. After centrifugation, cells were lysed using cell lysing buffer (Cell Signaling Technology, Danvers, MA) and ethylenediaminetetraacetic acid-free protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO). Cell lysate was run on a 10% Tris-Acetate gel with MES buffer (Life Technologies) and transferred onto a polyvinylidene fluoride membrane (Millipore, Darmstadt, Germany). The membrane was blocked using Odyssey blocking buffer (Licor, Lincoln, Nebraska) for 3 hours at room temperature followed by probing with rabbit anti-mouse ISG15 (Cell Signaling, Technology, Danvers, MA) and mouse anti-human β-actin (Sigma-Aldrich) as a loading control at 4° overnight. After washing with PBS-Tween, secondary goat anti-mouse IRDye 680RD and goat anti-rabbit IRDye 800 CW (Licor, Lincoln, NE) were added for 1 hour at room temperature. The membrane was then washed and imaged on the Odyssey CLX (Licor). In addition, supernatants were also collected at 48 hours after transfection and cytokine secretion was examined by using a CircuLex mouse ISG15 ELISA kit (MBL International, Woburn, MA), according to manufacturer's protocol. Optical density was measured at 450 nm using a Bioteck EL312e Bio-Kinetics reader (Biotek US, Winooski, VT). All supernatants were tested in duplicate with two separate supernatant samples per a plasmid.
Animals. All animals were conducted and maintained in accordance with the NIH and the University of Pennsylvania Institutional Animal Care and Use Committee guidelines. Female C57BL/6 (H-2b) 8-week-old mice and H2b B6.129S7-Rag1tm1Mom/J mice (Rag1 KO) were purchased from Jackson Laboratory.
Animal immunizations. All mice were immunized intramuscularly (i.m.) in the tibialis anterior muscle. In vivo EP was delivered, with the CELLECTRA adaptive constant current EP device (Inovio Pharmaceuticals), at the same site immediately following immunization as previously described.14 The mice were immunized with either 5 μg pVAX1 and 5 μg of HPV16 construct with or without 11 μg of wtISG15 and mutISG15. All studies were performed at least twice. The pVAX1 empty plasmid was used to keep the DNA vaccination amount consistent in each group.
ELISPOT assay. Spleens were harvested and processed 7 days following the final immunization as previously described.5,14 After spleens were harvested and processed, an IFNγ ELISpot assay was performed to determine antigen-specific cytokine secretion from immunized mice as described previously in detail.5,14,27 HPV16 Ag-specific T-cell responses were measured by stimulating splenocytes with E6 or E7 pooled overlapping peptides (2.5 μg/ml final concentration of peptide). The E7 overlapping pooled peptides contained the CD8 T-cell immunodominant HPV16 DbE749-57 epitope (RAHYNIVTF).
Flow cytometry. Lymphocytes were isolated and processed from the spleen and peripheral blood as previously described.5,14,28 2 × 106 Lymphocytes were stained with CD8, KLRG1, and MHC class I peptide tetramer to HPV16 H-2DbE749-57 (RAHYNIVTF) (MBL International) as described previously.5,25 Intracellular cytokine staining was performed after 5 hours of ex vivo stimulation with the HPV16 E7 peptide DbE7 (RAHYNIVTF) (2.5 μg/ml final concentration of peptide) or E7 pooled overlapping peptides to assess CD4 T-cell responses.27 In cultures being used to measure degranulation, anti-CD107a (FITC; clone 1D4B; Biolegend) was added during time of stimulation to capture the degranulation induced by exposure to stimulation by Ag-specific cells.5 The cells were then fixed and stained as described elsewhere.5,39 The following antibodies were used for surface staining: LIVE/DEAD Fixable Violet Dead Cell stain kit (Invitrogen, Grand Island, NY), CD4 (FITC; clone RM4-5; eBioscience, San Diego, CA), CD8 (APC-Cy7; clone 53–6.7; BD Biosciences), NK1.1 (FITC; clone PK136; Biolegend); CD49b (FITC; clone DX5; eBioscience). For intracellular staining, the following antibodies were used: IFNγ (APC; clone XMG1.2; Biolegend, San Diego, CA), TNFα (PE; clone MP6-XT22; eBioscience), CD3 (PerCP/Cy5.5; clone 145-2C11; Biolegend); IL-2 (PeCy7; clone JES6-SH4; eBioscience). All data were collected using a LSRII flow cytometer (BD Biosciences, San Jose, CA) and analyzed using FlowJo software (Tree Star, Ashland, OR) and SPICE v5.3 (free available from http://exon.niaid.nih.gov/spice/). Boolean gating was performed using Flowjo v9.7.7 software (Ashland, OR) to examine the polyfunctionality of the T-cells from vaccinated animals.
Tumor cell line. The TC-1 cell line was a graciously given gift from Dr. Yvonne Paterson of the University of Pennsylvania, Philadelphia, PA. TC-1 cell line is a well-characterized lung epithelial immortalized cell line, constitutively expresses E6 and E7, and is highly tumorigenic.40,41 The TC-1 cells were purchased from American Type Culture Collection and cultured as previously described.5
In vivo therapeutic study. B6 mice were separated into four groups of 10 mice each and 5 × 104 TC-1 cells were subcutaneously implanted into the right flank of each mouse. On day 7, after tumor implantation, each group of mice was immunized by intramuscular EP with pVAX1, HPV16, HPV16/wtISG15, or HPV16/mutISG15 and boosted on days 14, 21, and 28. Tumor size was measured twice a week using electronic calipers and tumor volume calculated as described previously (½ (length × width2)). Mice were monitored twice a week for tumor growth and were measured as described previously.5,27 Under Penn Institutional Animal Care guidelines, mice were sacrificed when tumor size reached 18–20 mm.
In vivo CD8 T-cell depletion study. During therapeutic vaccination, B6 mice were injected intraperitoneally with 200 μg of anti-CD8 (53–6.72, Bio X cell) 1 day before tumor inoculation and repeated every 3 days post-tumor implantation. Successful T-cell depletion was confirmed by flow cytometric analysis of peripheral blood mononuclear cells.
T-cell purification and adoptive transfer. CD8 T-cells were isolated from splenocytes of vaccinated B6 mice 1 week after final immunization in nonbearing tumor mice (Figure 2a). CD8 T-cells were purified from splenocytes using negative selection to deplete CD4 T-cells, B-cells, and myeloid cells. Briefly, following RBC lysis, splenocytes were incubated with rat IgG anti-CD4 (GK1.5), anti-B220 (RA3), anti-CD11b (M170.13), anti-MHC-II (M5/114), and anti-CD16/32 (2.4G2). Antibody-bound cells were removed using anti-rat IgG magnetic beads.42 For adoptive transfer, ~4 × 106 CD8 T-cells in 200 μl PBS were injected intravenously via tail vein into each H2b B6.129S7-Rag1tm1Mom/J mouse.
Statistical analysis. Group analyses were completed by matched, two-tailed, unpaired student's t-tests to analyze statistical significance of all quantitative data produced in this study. A P value <0.05 was considered statistically significant. Error bars indicate standard error of the mean and all tests were performed using the Prism 6 Software (La Jolla, CA) (*P < 0.05; **P < 0.01; ***P < 0.001 compared with HPV16 immunization) (Supplementary Material).
SUPPLEMENTARY MATERIAL Figure S1. ISG15 had no profound influence on the NK or CD4 T cells.
Acknowledgments
The authors thank Jewell Walters and Nikos Svoronos for their technical help. This work was supported by Inovio Pharmaceuticals, as well as by a Basser grant to D.B.W. The authors also thank Penn CFAR and ACC core facilities for their support. D.B.W. discloses grant funding, industry collaborations, speaking honoraria, and fees for consulting. His service includes serving on scientific review committees and advisory boards. Remuneration includes direct payments, stock, or stock options. In the interest of disclosure, he therefore notes potential conflicts associated with his work with Pfizer, Bristol Myers Squibb, Inovio, Touchlight, Oncosec, Merck, VGXI, and others. Licensing of technology from his laboratory has created over 100 jobs in the private sector of the biotech/pharma industry. The other authors declare no competing financial interests. No writing assistance was utilized in the production of this manuscript.
Supplementary Material
ISG15 had no profound influence on the NK or CD4 T cells.
References
- Slaney, CY, Kershaw, MH and Darcy, PK (2014). Trafficking of T cells into tumors. Cancer Res 74: 7168–7174. [DOI] [PubMed] [Google Scholar]
- Mellman, I, Coukos, G and Dranoff, G (2011). Cancer immunotherapy comes of age. Nature 480: 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski, TF, Meng, Y, Blank, C, Brown, I, Kacha, A, Kline, J et al. (2006). Immune resistance orchestrated by the tumor microenvironment. Immunol Rev 213: 131–145. [DOI] [PubMed] [Google Scholar]
- Jensen, BA, Steffensen, MA, Nielsen, KN, Christensen, JP, Thomsen, AR and Holst, PJ (2014). Co-expression of tumor antigen and interleukin-2 from an adenoviral vector augments the efficiency of therapeutic tumor vaccination. Mol Ther 22: 2107–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal, DO, Wise, MC, Walters, JN, Reuschel, EL, Choi, MJ, Obeng-Adjei, N et al. (2014). Alarmin IL-33 acts as an immunoadjuvant to enhance antigen-specific tumor immunity. Cancer Res 74: 1789–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, JA and Lenschow, DJ (2013). Emerging roles for immunomodulatory functions of free ISG15. J Interferon Cytokine Res 33: 728–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight, E Jr and Cordova, B (1991). IFN-induced 15-kDa protein is released from human lymphocytes and monocytes. J Immunol 146: 2280–2284. [PubMed] [Google Scholar]
- Recht, M, Borden, EC and Knight, E Jr (1991). A human 15-kDa IFN-induced protein induces the secretion of IFN-gamma. J Immunol 147: 2617–2623. [PubMed] [Google Scholar]
- D'Cunha, J, Knight, E Jr, Haas, AL, Truitt, RL and Borden, EC (1996). Immunoregulatory properties of ISG15, an interferon-induced cytokine. Proc Natl Acad Sci USA 93: 211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic, D, Byun, M, Durfee, LA, Abhyankar, A, Sanal, O, Mansouri, D et al. (2012). Mycobacterial disease and impaired IFN-γ immunity in humans with inherited ISG15 deficiency. Science 337: 1684–1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb, KR and Haas, AL (1992). The interferon-inducible 15-kDa ubiquitin homolog conjugates to intracellular proteins. J Biol Chem 267: 7806–7813. [PubMed] [Google Scholar]
- Lenschow, DJ, Giannakopoulos, NV, Gunn, LJ, Johnston, C, O'Guin, AK, Schmidt, RE et al. (2005). Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J Virol 79: 13974–13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloper-Mould, KE, Jemc, JC, Pickart, CM and Hicke, L (2001). Distinct functional surface regions on ubiquitin. J Biol Chem 276: 30483–30489. [DOI] [PubMed] [Google Scholar]
- Shedlock, DJ, Aviles, J, Talbott, KT, Wong, G, Wu, SJ, Villarreal, DO et al. (2013). Induction of broad cytotoxic T cells by protective DNA vaccination against Marburg and Ebola. Mol Ther 21: 1432–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendron, KB, Rodriguez, A and Sewell, DA (2006). Vaccination with human papillomavirus type 16 E7 peptide with CpG oligonucleotides for prevention of tumor growth in mice. Arch Otolaryngol Head Neck Surg 132: 327–332. [DOI] [PubMed] [Google Scholar]
- Peng, S, Trimble, C, Alvarez, RD, Huh, WK, Lin, Z, Monie, A et al. (2008). Cluster intradermal DNA vaccination rapidly induces E7-specific CD8+ T-cell immune responses leading to therapeutic antitumor effects. Gene Ther 15: 1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow, MP, Yan, J and Sardesai, NY (2013). Human papillomavirus therapeutic vaccines: targeting viral antigens as immunotherapy for precancerous disease and cancer. Expert Rev Vaccines 12: 271–283. [DOI] [PubMed] [Google Scholar]
- Bagarazzi, ML, Yan, J, Morrow, MP, Shen, X, Parker, RL, Lee, JC et al. (2012). Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Sci Transl Med 4: 155ra138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldonado, L, Teague, JE, Morrow, MP, Jotova, I, Wu, TC, Wang, C et al. (2014). Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Sci Transl Med 6: 221ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson, JA, McDonald-Hyman, C, Jameson, SC and Hamilton, SE (2013). Effector-like CD8⁺ T cells in the memory population mediate potent protective immunity. Immunity 38: 1250–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, SG, Ford, JC, Lewis, MS, Ventura, AB, Hughes, CM, Coyne-Johnson, L et al. (2011). Profound early control of highly pathogenic SIV by an effector memory T-cell vaccine. Nature 473: 523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto, F, Geginat, J and Lanzavecchia, A (2004). Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol 22: 745–763. [DOI] [PubMed] [Google Scholar]
- Huster, KM, Stemberger, C and Busch, DH (2006). Protective immunity towards intracellular pathogens. Curr Opin Immunol 18: 458–464. [DOI] [PubMed] [Google Scholar]
- Seder, RA and Ahmed, R (2003). Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol 4: 835–842. [DOI] [PubMed] [Google Scholar]
- van Duikeren, S, Fransen, MF, Redeker, A, Wieles, B, Platenburg, G, Krebber, WJ et al. (2012). Vaccine-induced effector-memory CD8+ T cell responses predict therapeutic efficacy against tumors. J Immunol 189: 3397–3403. [DOI] [PubMed] [Google Scholar]
- Bracci, L, Moschella, F, Sestili, P, La Sorsa, V, Valentini, M, Canini, I et al. (2007). Cyclophosphamide enhances the antitumor efficacy of adoptively transferred immune cells through the induction of cytokine expression, B-cell and T-cell homeostatic proliferation, and specific tumor infiltration. Clin Cancer Res 13(2 Pt 1): 644–653. [DOI] [PubMed] [Google Scholar]
- Yan, J, Reichenbach, DK, Corbitt, N, Hokey, DA, Ramanathan, MP, McKinney, KA et al. (2009). Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine 27: 431–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelosanto, JM, Blackburn, SD, Crawford, A and Wherry, EJ (2012). Progressive loss of memory T cell potential and commitment to exhaustion during chronic viral infection. J Virol 86: 8161–8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamikanra, A, Pan, ZK, Isaacs, SN, Wu, TC and Paterson, Y (2001). Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol 75: 9654–9664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indrová, M, Bieblová, J, Bubeník, J and Reinis, M (2008). IL-12 immunotherapy of minimal residual disease in murine models of HPV16-associated tumours: induction of immune responses, cytokine production and kinetics of immune cell subsets. Int J Oncol 32: 499–507. [PubMed] [Google Scholar]
- Khairuddin, N, Blake, SJ, Firdaus, F, Steptoe, RJ, Behlke, MA, Hertzog, PJ et al. (2014). In vivo comparison of local versus systemic delivery of immunostimulating siRNA in HPV-driven tumours. Immunol Cell Biol 92: 156–163. [DOI] [PubMed] [Google Scholar]
- Plotkin, SA (2010). Correlates of protection induced by vaccination. Clin Vaccine Immunol 17: 1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogunovic, D, Boisson-Dupuis, S and Casanova, JL (2013). ISG15: leading a double life as a secreted molecule. Exp Mol Med 45: e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, W, Zhang, M, Xiao, ZZ and Sun, L (2012). Cynoglossus semilaevis ISG15: a secreted cytokine-like protein that stimulates antiviral immune response in a LRGG motif-dependent manner. PLoS One 7: e44884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X, Bogunovic, D, Payelle-Brogard, B, Francois-Newton, V, Speer, SD, Yuan, C et al. (2015). Human intracellular ISG15 prevents interferon-α/β over-amplification and auto-inflammation. Nature 517: 89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingos-Pereira, S, Decrausaz, L, Derré, L, Bobst, M, Romero, P, Schiller, JT et al. (2013). Intravaginal TLR agonists increase local vaccine-specific CD8 T cells and human papillomavirus-associated genital-tumor regression in mice. Mucosal Immunol 6: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimian, S, Fransen, MF, Kleinovink, JW, Christensen, JR, Amidi, M, Hennink, WE et al. (2015). Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J Control Release 203: 16–22. [DOI] [PubMed] [Google Scholar]
- Song, YC, Cheng, HY, Leng, CH, Chiang, SK, Lin, CW, Chong, P et al. (2014). A novel emulsion-type adjuvant containing CpG oligodeoxynucleotides enhances CD8+ T-cell-mediated anti-tumor immunity. J Control Release 173: 158–165. [DOI] [PubMed] [Google Scholar]
- Villarreal, DO, Walters, J, Laddy, DJ, Yan, J and Weiner, DB (2014). Multivalent TB vaccines targeting the esx gene family generate potent and broad cell-mediated immune responses superior to BCG. Hum Vaccin Immunother 10: 2188–2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, KY, Guarnieri, FG, Staveley-O'Carroll, KF, Levitsky, HI, August, JT, Pardoll, DM et al. (1996). Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res 56: 21–26. [PubMed] [Google Scholar]
- Gunn, GR, Zubair, A, Peters, C, Pan, ZK, Wu, TC and Paterson, Y (2001). Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol 167: 6471–6479. [DOI] [PubMed] [Google Scholar]
- Stephen, TL, Rutkowski, MR, Allegrezza, MJ, Perales-Puchalt, A, Tesone, AJ, Svoronos, N et al. (2014). Transforming growth factor β-mediated suppression of antitumor T cells requires FoxP1 transcription factor expression. Immunity 41: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ISG15 had no profound influence on the NK or CD4 T cells.