Abstract
Cytokines are central components of the mucosal inflammatory responses that take place during the development of Crohn's disease. Cell-specific combination therapies against cytokines may lead to increased efficacy and even reduced side effects. Therefore, a colonic macrophage-specific therapy using miR-16 precursors that can target both TNF-α and IL-12p40 was tested for its efficacy in experimental colitic mice. Galactosylated low molecular weight chitosan (G-LMWC) associated with miR-16 precursors were intracolonically injected into mice. The cellular localization of miR-16 precursors was determined. The therapeutic effects and possible mechanism were further studied in 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced colitic mice. The results show that specific upregulation of miR-16 level in colonic macrophages significantly reduces TNF-α and IL-12p40 expression, which could suppress the associated mucosal inflammation and ultimately result in the relief of colitic symptoms. This strategy, based on the dual silencing of colonic macrophage-specific cytokines, represents a potential therapeutic approach that may be valuable for colitis therapy.
Introduction
Crohn's disease (CD), one of the two main subtypes of inflammatory bowel disease (IBD), is a chronic, relapsing, and remitting inflammatory disorder in the gastrointestinal tract.1 Substantial evidences show that aberrantly expressed cytokines in the colon tissue play important roles during the progression of CD.2 Particularly, during the initiative phase of inflammation process, defect recognition of pathogenic microflora by pattern recognition leads to overproduction of several proinflammatory cytokines such as TNF-α, IL-12, and IL-23. Large amounts of TNF-α directly drive colonic inflammation via causing damage to colonic epithelial cells and inducing influx and activation of leukocytes. IL-12p70 and IL-23, which share the same subunit IL-12p40, favor differentiation and activation of mucosal Th1/Th17 cells in CD patients. T-cell-mediated immune responses contribute to the adaptive immunity and are parts of second wave of alimentary tract inflammation. Therefore, a number of therapeutic approaches based on cytokine blockades have been developed for the treatment of CD.3 However, few therapeutic approaches are present to exert protective effect throughout different stages of colonic inflammation via attenuating both innate responses and adaptive cellular responses.
MicroRNA (miRNA) is a class of noncoding single-stranded RNA molecules, 21–25 nucleotides in length. Recent studies have found that miRNAs can influence a series of IBD-related gene expression and promote the progression of IBD, indicating that miRNAs could be targets or potential drugs for the treatment of IBD.4,5,6,7 It is reported that miR-16 can bind to the AU-rich region localized at the 3′-untranslated region (3′UTR) of TNF-α and induce the degradation of TNF-α mRNA.8 We also observed a similar binding site of miR-16 in the 3′UTR of IL-12p40 and further proved the regulation effect of miR-16 on IL-12p40 expression in present study. Due to dual targeting ability of miR-16 on TNF-α and IL-12p40, miR-16 is supposed to serve as a therapeutic target for CD treatment.
However, as it can regulate a series of genes, miR-16 may exert distinct functions on different colonic cells. As a consequence, systemic upregulation of colonic miR-16 level for long term may interfere with the normal physiological functions and will not achieve the expected therapeutic effects on colonic inflammation. The activated colonic macrophages in the inflamed mucosal have been proven to be a main source of TNF-α and IL-12p40 in experimental colitis and CD patients, and we have already developed a colonic macrophage-targeted delivery system based on galactosylated low molecular weight chitosan (G-LMWC).9,10 As a result, macrophage-specific delivery of colonic miR-16 is proposed as a potential strategy for CD treatment in present study. G-LMWC can associate with nucleic acid to form a stable nano-complex. In addition, its galactose residues have high affinity for macrophage galactose-type lectin which is highly expressed on the surface of macrophage and is responsible for receptor-mediated endocytosis. In this study, we investigate macrophage-targeting ability of G-LMWC&miR-16 precursors (pre-miR-16) and evaluate whether the upregulation of miR-16 level in colonic macrophages could protect against TNBS-induced colitis.
Results
Identification of TNF-α and IL-12p40 as targets of miR-16
The binding sites of miR-16 in mouse/human TNF-α and IL-12p40 are shown in Figure 1a. The correlation between miR-16 and TNF-α/IL-12p40 was examined by evaluating the expression of TNF-α/IL-12p40 in mouse peritoneal macrophages (PMCs) under lipopolysaccharide (LPS) stimulation while changing the miR-16 level. The level of miR-16 in PMCs was not significantly changed after LPS stimulation, while the transfection of pre-miR-16 into PMCs greatly increased the miR-16 level compared with scramble pre-miRNA treatment (Figure 1b). The upregulation of miR-16 significantly reduced the mRNA level of TNF-α and IL-12p40 in PMCs, as well as the concentration of TNF-α and IL-12p40 in the supernatant of PMCs (Figure 1c,d). Meanwhile, anti-miR-16 reduced the miR-16 level in LPS-stimulated PMCs and upregulated the expression of TNF-α and IL-12p40 in LPS-stimulated PMCs (Figure 1c,d). Luciferase assays were performed in unstimulated RAW 264.7 cells via cotransfection of miR-16 precursors/inhibitors and luciferase plasmid to determine whether miR-16 regulated TNF-α or IL-12p40 by directly binding to its 3'UTR binding site. The activity of luciferase reporter containing 3'UTR of TNF-α had 35% reduction and the activity of that containing 3'UTR of IL-12p40 had 40% reduction after transfection with pre-miR-16 compared to scrambled precursors (Figure 1e). On the other hand, the activity of luciferase reporter containing 3'UTR of TNF-α appeared a nearly 40% increase and the activity of that containing 3'UTR of IL-12p40 appeared 50% increase after treated with anti-miR-16 (Figure 1e). Mutant of the seed binding sequences of miR-16 with 3'UTR of TNF-α/IL-12p40 restored luciferase expression (Figure 1e).
Figure 1.
Identification of TNF-α and IL-12p40 as targets of miR-16. (a) Schematic description of conserved binding sites for miR-16 and the seed-recognizing sites are marked. The levels of miR-16 (b), TNF-α (c), and IL-12p40 (d) from primary peritoneal macrophages after transfection with pre/anti-miR-16 or scrambled pre/anti-miR-16. (e) Luciferase activity containing full length 3'UTR of mouse TNF-α/IL-12p40 in Raw 264.7 cells was assayed after transfection with pre/anti-miR-16 or scrambled pre/anti-miR-16. The parental luciferase plasmid and the binding site mutant plasmid (Mut) were also used. Values are expressed as the means (mean ± SEM). Five samples were analyzed per condition, and experiments were performed in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001.
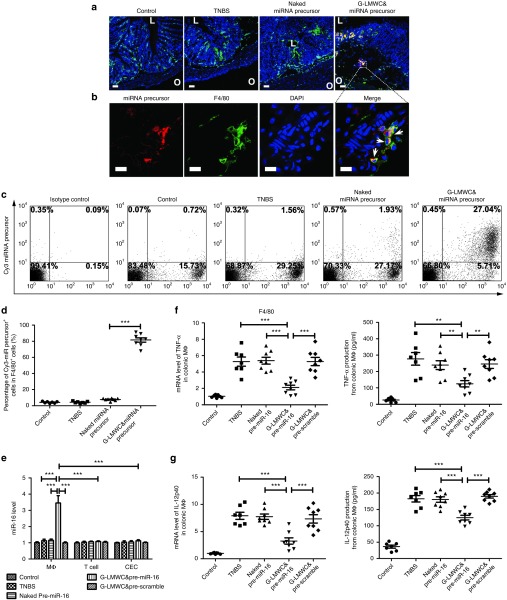
Colon uptake of miRNA precursors and the level of TNF-α and IL-12p40 in colonic macrophages after G-LMWC&pre-miR-16 administration
As shown in Figure 2a, the significant accumulation of miRNA precursors can be observed in the colon of colitic mice treated with G-LMWC&Cy3-miRNA precursors. Their high magnification images are also presented in Figure 2b. Most of the miRNA precursors were predominantly taken up by colonic macrophages (F4/80+ cells). Flow cytometry analysis of lamina propria mononuclear cells (LPMCs) was further used to examine the targeting ability of G-LMWC&Cy3-miRNA precursors. In consistent with the result of fluoresce image, nearly 80% macrophages (F4/80+ cells) were positive of Cy3-miRNA precursors after G-LMWC&Cy3-miRNA treatment and only about 0.45% Cy3-miRNA precursors + LPMCs were F4/80− (Figure 2c,d). In contrast, few F4/80+ cells were Cy3-miRNA precursors + for mice treated with naked Cy3-miRNA precursors (Figure 2c,d).
Figure 2.
Cellular distribution of miRNA precursors and cellular quantification of miR-16, TNF-α, and IL-12p40 after G-LMWC&Cy3-miRNA precursors or G-LMWC&pre-miR-16 administration. (a) Frozen sections of the colons from healthy mice, colitic mice without any treatment, colitic mice with naked Cy3 miRNA precursors or colitic mice with G-LMWC&Cy3-miRNA precursors were stained with colonic macrophages markers (red, Cy3 miRNA precursor; green, F4/80; blue, 4′,6-diamidino-2-phenylindole (DAPI) nuclear staining). Scale bar, 50 μm. The inner lumen (abbreviated as L) and outer wall (abbreviated as O) was marked on the image to indicate the orientation of colon section. (b) Local magnification of the frozen colon sections from colitic mice with G-LMWC&Cy3-miRNA precursors (red, Cy3 miRNA precursor; green, F4/80; blue, DAPI nuclear staining). Scale bar, 10 μm. (c) Flow cytometry used to analysis lamina propria mononuclear cells from mice with different treatments. (d) The ratio of Cy3 miRNA precursor+ cells in F4/80+ cells. (e) The level of miR-16 in different types of colonic cells after naked pre-miR-16, G-LMWC&pre-miR-16, or G-LMWC&pre-scramble administration at the dose of 5 mg miRNA precursors/kg body. (f,g) The mRNA levels of TNF-α/IL-12p40 in isolated colonic macrophages and the concentrations of TNF-α/IL-12p40 in the supernatants of colonic macrophages with different treatments. Values are expressed as the means (mean ± SEM). n = 7–8 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001.
Colonic macrophages, T cells, and colonic epithelial cells (CEC) were isolated for quantitative real-time PCR (qRT-PCR) analysis 12 hours after intracolonic administration with G-LMWC&pre-miR-16. The significant upregulation of miR-16 was observed in colonic macrophages, but not in T cells and CECs (Figure 2e). G-LMWC&pre-miR-16 also significantly downregulated the level of TNF-α and IL-12p40 in colonic macrophages as shown in Figure 2f,g. As macrophages were not the only source of TNF-α and IL-12p40 during the colonic inflammation, the level of TNF-α and IL-12p40 in colonic T cells and CECs were also measured. The treatment with G-LMWC&pre-miR-16 could decrease the level of TNF-α both in T cells (Supplementary Figure S1a) and in CECs (Supplementary Figure S1b) and the level of IL-12p40 (Supplementary Figure S1d) in CECs. The level of IL-12p40 in colonic T cells showed no significant change (Supplementary Figure S1c).
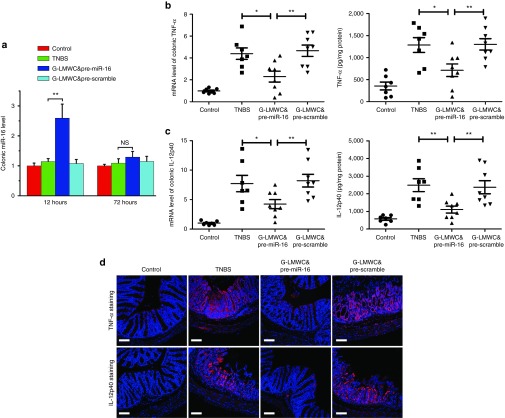
Reduction of mucosal TNF-α and IL-12p40 administrated by G-LMWC&pre-miR-16 complex
G-LMWC&pre-miR-16 significantly upregulated the level of colonic miR-16 at 12 hours and slightly increased miR-16 at day 3 after administration (Figure 3a), while still significantly decreased mucosal TNF-α (Figure 3b) and IL-12p40 (Figure 3c) at day 3 after administration. Immunofluoresence staining of TNF-α and IL-12p40 in the lamina propria showed that little TNF-α or IL-12p40 was detected after colitis mice were treated with G-LMWC&pre-miR-16 complex (Figure 3d)
Figure 3.
G-LMWC&pre-miR-16 complexes effectively reduced the levels of colonic TNF-α/IL-12p40. G-LMWC&pre-miR-16 or G-LMWC&pre-scramble were injected into TNBS-induced colitic mice by intracolonic administration at the dose of 5 mg miRNA precursors/kg body. The levels of colonic miR-16 (a), TNF-α/IL-12p40 (b,c) in colitis mice were examined by qRT-PCR. The expressions of colonic TNF-α/IL-12p40 were determined by enzyme-linked immunosorbent assay kits (b,c) or by immunofluorescence staining (d). Scale bar, 100 μm. n = 7–8 mice per group; values are expressed as the means (mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001. NS, no significant change.
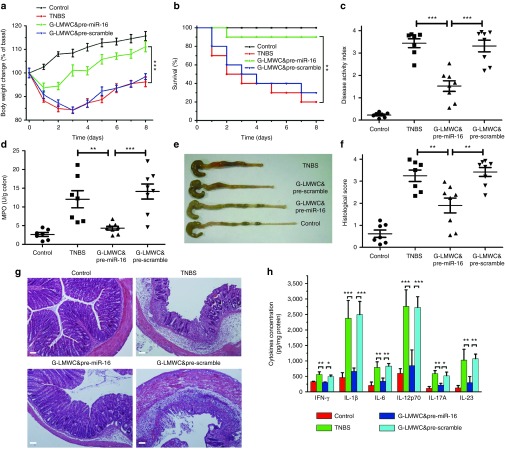
G-LMWC&pre-miR-16 treatment protects against the development of TNBS-induced colitis
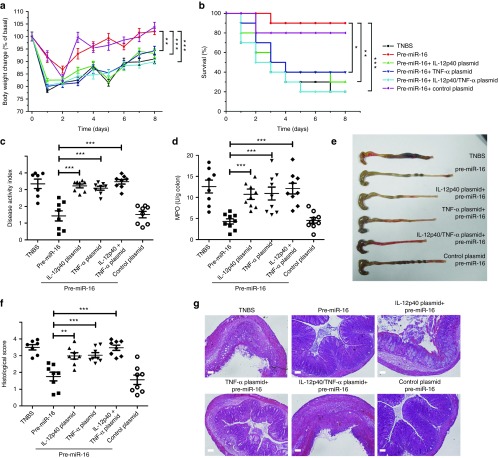
The effects of G-LMWC&pre-miR-16 are shown in Figure 4. Mice rapidly regained weight (Figure 4a) and had a significant reduction of mortality, disease activity index (DAI) and macroscopic damage (Figure 4b,c,e). Increased colonic myeloperoxidase (MPO) activity of colitic mice was significantly reduced by treatment with G-LMWC&pre-miR-16 (Figure 4d).
Figure 4.
G-LMWC&pre-miR-16 complexes ameliorated TNBS-induced colitis. The therapeutic effect of pre-miR-16 in TNBS colitis mice was observed through body weight changes (a), survival analysis (b), disease activity index (c), myeloperoxidase activity determination (d), and colon photographs (e). Colon sections at day 3 from TNBS colitic mice that received pre-miR-16 were examined by H&E staining and histopathological scoring (f,g). Scale bar, 100 μm. The levels of colonic inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-12p70, IL-17A, and IL-23) from colitis mice with G-LMWC&pre-miR-16 treatment was determined (h). n = 7–8 mice per group; values are expressed as the means (mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
Colon sections form colitic mice were stained by H&E and exhibited transmural inflammation involving all layers of the bowel wall with increased muscle layer thickness, patchy ulceration, epithelial cell loss, pronounced depletion of mucin-producing goblet cells, reduction of the density of the tubular glands, disseminated fibrosis, and focal loss of crypts. In contrast, treatment with G-LMWC&pre-miR-16 resulted in significant histological improvements (Figure 4f,g). TNBS-induced colitis mice also exhibited the high level of proinflammatory cytokines, Th1 cytokines, Th17 cytokines, and cytokines containing an IL-12p40 subunit (IL-12p70 and IL-23). All of these cytokines (INF-γ, IL-1β, IL-6, IL-12p70, IL-17A, and IL-23) were dramatically reduced after treatment with G-LMWC&pre-miR-16 (Figure 4h).
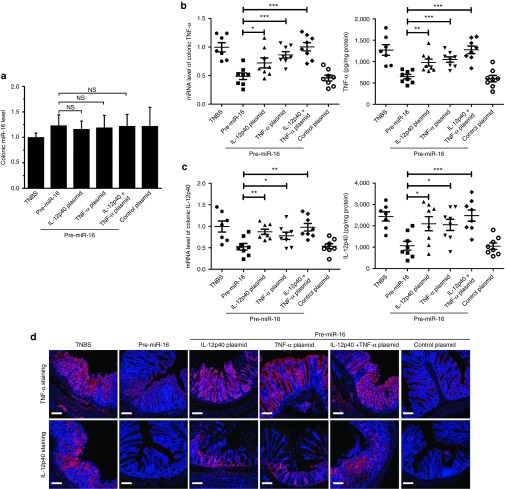
The therapeutic effect of pre-miR-16 on TNBS-induced colitis is mediated by both IL-12p40 and TNF-α
We found that the overexpression of IL-12p40/TNF-α induce by IL-12p40/TNF-α 3'UTR-deficient plasmids could not be attenuated by miR-16 in macrophages (Supplementary Figure S2). The plasmids were then associated with G-LMWC to form complexes, which were intracolonically administered to mice at 3 days prior to the induction of TNBS. G-LMWC&pre-miR-16 was intracolonically given to mice 12 hours after the induction of TNBS. The level of colonic miR-16, IL-12p40, and TNF-α was evaluated at 3 days after treated by G-LMWC&pre-miR-16. The plasmids pretreatment showed no influence on colonic miR-16 (Figure 5a). The injection alone of IL-12p40 plasmid/TNF-α plasmid or the combination administration of two plasmids could significantly improve colonic IL-12p40 and TNF-α compared to treated only by pre-miR-16 (Figure 5b,c). The immunofluoresence staining of TNF-α/IL-12p40 indicated that the plasmids enhanced the expression of TNF-α/IL-12p40 (Figure 5d).
Figure 5.
The overexpression of IL-12p40/TNF-α 3'UTR-deficient plasmids abrogated the regulating ability of miR-16 on the colonic IL-12p40/TNF-α. G-LMWC&pre-miR-16, G-LMWC&pre-scramble, G-LMWC&IL-12p40 plasmids, or G-LMWC&TNF-α plasmids were injected into mice 3 days prior to TNBS induction at the dose of 5 mg plasmid/kg body. At day 3 in colitis mice, the levels of colonic miR-16 (a) and TNF-α/IL-12p40 (b,c) were examined by qRT-PCR. The expressions of colonic TNF-α/IL-12p40 were determined by enzyme-linked immunosorbent assay kits (b,c) or by immunofluorescence staining (d). Scale bar, 100 μm. n = 7–8 mice per group; values are expressed as the means (mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001. NS, no significant change.
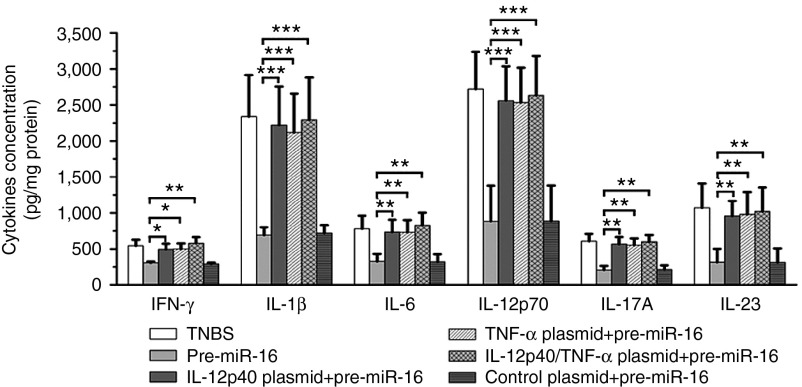
The intracolonic administration alone of TNF-α/IL-12p40 plasmids partially abolished the anti-inflammatory effects of pre-miR-16 on TNBS-induced colitis, such as causing more body weight loss (Figure 6a), decreased survival rate (Figure 6b), increased DAI index (Figure 6c), and elevated MPO activity (Figure 6d). The colon macroscopic observation and histopathological examination also approved that the plasmids pretreatment led to severer tissue inflammation symptoms and pathological injury (Figure 6e–g). In contrast, the cotreatment of IL-12p40 and TNF-α plasmids by intracolonic administration completely abrogated the therapeutic effect of pre-miR-16 on colitic mice (Figure 6a–g). Moreover, pre-miR-16 administration could decrease the level of proinflammatory cytokines (including IFN-γ, IL-1β, IL-6, IL-12p70, IL-17A, and IL-23). However, the pretreatment alone of TNF-α/IL-12p40 plasmid or the combined application of two plasmids could significantly abolish the suppression effect of pre-miR-16 on colonic proinflammatory cytokines (Figure 7).
Figure 6.
G-LMWC&pre-miR-16 complexes protected against TNBS-induced colitis by inhibiting IL-12p40 and TNF-α expression. The effects of administering IL-12p40/TNF-α plasmids to colitic mice were observed by body weight changes (a), survival analysis (b), disease activity index (c), myeloperoxidase activity determination (d), and colon photographs (e). Colon sections were examined by H&E staining and histopathological scoring at day 3 from colitic mice that received G-LMWC&pre-miR-16 treatment (f,g). Scale bar, 100 μm. n = 7–8 mice per group; values are expressed as the means (mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.01. NS, no significant change.
Figure 7.
The influence of administration of IL-12p40/TNF-α plasmids on the expressions of colonic cytokines. The levels of colonic inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-12p70, IL-17A, and IL-23) were determined from colitis mice with G-LMWC&pre-miR-16 treatment. n = 7–8 mice per group; values are expressed as the means (mean ± SEM). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
In this study, we applied G-LMWC to specifically transfer pre-miR-16 into colonic macrophages to suppress TNF-α and IL-12p40 production, which finally resulted in the alleviation of TNBS-induced experimental colitis.
Mucosal inflammation is promoted by a complex interplay of multiple pathological genes.11 Nucleic acid drugs have been widely applied in experimental colitic models by knocking down these disease-causing genes.12,13,14 However, due to the complexity of CD's pathogenesis, a single application of the traditional nucleic acid drug, which was designed to specially target a single gene, may not achieve a better therapeutic effect than the combined use of them against different pathogenic genes. It was proved that a combination of siRNA duplexes specifically targeted against TNF-α and cyclin D1 to exert more potent therapeutic effects than the treatment with a single siRNA in a DSS-bearing colitic mouse model.15 Moreover, artificial siRNA may induce the potent interferon responses and disturb the endogenous mRNA biogenesis. These adverse effects of siRNA may greatly hamper its clinical application. Therefore, seeking natural nucleic acid drugs that can target multiple genes may provide promising alternative therapeutic strategies for the treatment of CD. Compared with siRNA, the synthesis process and its regulating mechanism keep miRNA away from these side effects. Besides this, as it can produce global effects on a series of genes, miRNAs are ideal reagents for combined therapies in the treatment of CD.
MiR-16, which belongs to the miR-15 family, is highly expressed in a variety of cell types such as monocytes, neutrophils, and lymphocytes.16 Studies have indicated that miR-16 is involved in the regulation of immunity. After binding to the AU-rich region localized in the 3'UTR, miR-16 can lead to the rapid degradation of several inflammatory mediators, including TNF-α.8 Commonly used anti-TNF-α agents are antibodies and soluble receptors, some of which have proven to be effective in treating both CD-like murine colitis models and CD patients.17,18 Our target validation study indicated that miR-16 could also regulate IL-12p40 via the same mechanism. IL-12p40 is a common subunit of IL-12p70 and IL-23, which are representative cytokines that can induce Th1 or Th17 cell expansion, ultimately leading to the destruction of the mucosa.19,20 Antibodies against IL-12p40 have achieved satisfactory therapeutic effects in TNBS colitic mice, cumulating in the remission of CD patients; these findings imply that IL-12p40 may be another promising therapeutic target for the treatment of CD.21,22 As our study affirmed the suppressive effects of miR-16 precursors on TNF-α or IL-12p40 secretion in LPS-activated macrophages, miR-16 may both attenuate the direct damage effect of TNF-α and suppress the pathogenic Th1/Th17 cells responses which are initiated by IL-12/IL-23. The dual targeting ability of miR-16 allows it to exert protective effect in both the acute inflammation phases and the following adaptive immune responses and reduce the inconveniences of replacing drugs against different stages.
Recently, miR-16 has been shown to be increased in the sigmoid colon biopsies of active UC patients and the terminal ileal biopsies of chronically active CD patients, but did not show any significant difference in the sigmoid colon biopsies of chronically active CD patients.4,5,6 In line with the clinical data of CD patients, we did not observe any significant change of colonic miR-16 level in both TNBS-induced colitis and IL-10 knockout chronic colitis model.7 The increased miR-16 level in UC patients or unchanged miR-16 in CD patients may not sufficiently suppress the highly expressed TNF-α and IL-12p40 during the mucosal inflammation process. Therefore, exogenous administration of miR-16 precursors to further improve colonic miR-16 level may effectively inhibit TNF-α and IL-12p40 production and reduce the colonic inflammatory responses.
However, as a pleiotropic molecule with multiple targets, miR-16 could also play distinct roles in different cell types. For example, miR-16 is involved in cell cycle progression via its suppression of several survival-associated genes, including BCL-2 and WNT3A.22,23,24 It has been identified that colonic epithelial-specific BCL-2 overexpression protected IL-10 KO mice from chronic colitis via the suppression of epithelial cell death.25 The systemic administration of miR-16 precursors may lead to the upregulation of miR-16 levels in all types of colon cells, including colonic epithelial cells, which may abrogate the therapeutic effects. Therefore, a cell-specific therapy strategy is needed to precisely regulate miR-16 levels in pathologically dysfunctional cells. During colonic inflammation, TNF-α produced by myeloid cells, mostly macrophages, has been found to be essential for the development of colitis.26 Evidences have shown that the depletion of macrophages in IL-10 knock-out mice protects against the spontaneous colitis that would otherwise occur due to the unregulated secretion of IL-12 and IL-23 by macrophages.27 Thus, the position of activated macrophage at the top of the inflammatory cascade makes it an attractive target for drug treatment.
Our previous study indicated that colonic macrophages have macrophage galactose-type lectin receptors that mediate the internalization of G-LMWC-coated antisense oligonucleotide (ASO).10 As the essences of ASO and miRNA precursors are nucleic acids, we supposed that G-LMWC could also specifically delivery miRNA precursors into colonic macrophages. Indeed, we observed that G-LMWC&miR-16 precursors accumulated in the colonic lamina propria and were predominantly taken up by colonic macrophages. The cellular localization study further approved that G-LMWC could specially delivery miR-16 precursors into colonic macrophages but not into colonic T cells and epithelial cells. The results also revealed that G-LMWC&pre-miR-16 did not change the level of miR-16 in colonic T cells and epithelial cells but decreased IL-12p40 and TNF-α in these two types of cells, suggesting that the expression of TNF-α and IL-12p40 in T cells and colonic epithelial cells were indirect regulated by miR-16. A possible explanation is provided here: aberrantly activated macrophages play an essential role in the initiation and maintenance of immune responses in colonic mucosa, by triggering a cytokine cascade, which in turn stimulate the release of additional proinflammatory mediators by T cells and colonic epithelial cells. It is reported that both TNF-α and IL-12p40 could activate macrophages in a feedback autocrine loop that amplifies local inflammatory responses.28,29 TNF-α and IL-12p40 blockade by pre-miR-16 can impair the activation of macrophages and lead to decreased level of inflammatory mediators. As a consequence, T cells and colonic epithelial cells may be less activated. The study in vivo further affirmed that pre-miR-16 administration led to the decrease of TNF-α/IL-12p40 in colonic tissue nearly the same as that in colonic macrophages.
In this study, G-LMWC&pre-miR-16 complexes were given into TNBS-induced colitic mice via intracolonic administration. As the length of adult colon is about 1 m, intracolonic administration cannot guarantee the drug to spread all over the colon, which may reduce the therapeutic efficacy. Moreover, intracolonic administration brings inconvenience to the patients and the complexity of its operation is a limiting obstacle to its clinical application. Therefore, we try to develop a novel oral administration system based on G-LMC&pre-miR-16 to resolve the problems brought by intracolonic administration in the future.
Materials and Methods
Complex preparation of G-LMWC&miR-16 precursors, G-LMWC&miR-16 inhibitors, and G-LMWC&miR-resistant plasmids. MiR-16 precursors/inhibitors and the corresponding scramble controls were purchased from Life Technologies (Grand Island, NY). MiR-resistant plasmids containing TNF-α/IL-12p40 cDNA lacking their 3'UTR were obtained from FulenGen Corporation (Guangzhou, China). Cy3-labeled miRNA precursors (Cy3-miRNA precursors, Life Technologies) were used for in vivo cellular localization assays. G-LMWC was prepared according to our previous description.10 The complexes of G-LMWC&miRNA precursors, G-LMWC&miRNA inhibitor, and G-LMWC&plasmids were prepared by mixing an aqueous saline solution of G-LMWC (6 mg/ml) with an equal volume of miRNA precursors, inhibitors, or plasmids (2 mg/ml). The mixture was vortexed rapidly for 3–5 seconds and left for 1 hour at room temperature to allow the complexes to form completely.
Peritoneal macrophage isolation and treatment. Resting peritoneal macrophages were obtained from 6–8-week-old female BALB/C mice. Mice were sacrificed by neck dislocation, and peritoneal exudate cells were harvested by lavage with 5 ml ice-cold PBS and seeded in six-well plates at the density of 3 × 106 cells/well in 1 ml complete medium (RPMI1640 containing 10% bovine serum, Life Technologies) for 2 hours to remove nonbound cells. The macrophage isolates (purity > 95%) was determined by immunofluorescent staining for F4/80 (Abcam, Cambridge, MA). Trypan blue staining demonstrated that the viability of isolated peritoneal macrophages was greater than 90%.
For target validation, each well was transfected with 100 pmol of either pre/anti-miR-16 or their corresponding scramble controls using Lipofectamine 2000 (Life Technologies), according to the manufacturer's instructions. Control experiments were performed in the same manner but used mock-transfected cells. Fourty-eight hours after transfection, the cells were stimulated with 100 ng/ml LPS (Sigma, St Louis, MO). The supernatants were collected for TNF-α and IL-12p40 quantification by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, MN), and the total cellular RNA was isolated using TRIzol (Life Technologies) to measure miR-16, TNF-α, and IL-12p40 6 hours after LPS stimulation.
To test the role of miR-16 in regulating TNF-α/IL-12p40, peritoneal macrophages were pretransfected with miR-resistant plasmids encoding TNF-α or IL-12p40 for 72 hours before their transfection with pre/anti-miR-16 or their corresponding scramble controls (100 pmol for one well in six-well plates). The supernatants were collected for TNF-α/IL-12p40 quantification by ELISA, and the total cellular RNA was isolated using TRIzol to measure the miR-16 and TNF-α/IL-12p40 levels 24 hours after transfection.
Plasmid constructs and luciferase reporter assay. To examine the direct regulating role of miR-16 on TNF-α/IL-12p40, the luciferase assay was performed in resting Raw 264.7 cells, a murine macrophage cell line. The whole 3'-UTR sequence of mouse TNF-α and IL-12p40, which contained a presumed miR-16 binding site, was obtained from the GenBank database and a segment with one wild-type or mutant binding sites of miR-16 was cloned, respectively, into the Spe I/Hind III sites of a luciferase gene in the pMIR-REPORT luciferase vector (Life Technologies). This construct was cotransfected into nonstimulated Raw 264.7 cells with pre/anti-miR-16 or their corresponding scramble controls. The parental luciferase plasmid and binding site mutant luciferase (AATATTTA replaced by TTATAAAT) were also transfected as a control. Cells were lysed to measure luciferase activity 24 hours later. The plasmid encoding β-gal was cotransfected and used for normalization.
Establishment of TNBS-induced colitis. All of the animals received care according to Chinese legal requirements. TNBS-induced acute colitis was established in 6–8-week-old female BALB/c mice, as reported previously.21 Briefly, 2.5 mg of TNBS (Sigma) in 100 μl 50% ethanol was slowly administered into the lumen of the colon via a catheter inserted 4 cm into the colon through the anus. Mice were then kept in a vertical position for 30 seconds. Control mice received 50% ethanol alone.
Cellular localization of miRNA precursors and cellular determination of miR-16. Cy3-miRNA precursors were used to determine the cellular localization. G-LMWC&Cy3-miRNA precursors or naked Cy3-miRNA precursors at a dose of 5 mg miRNA precursor/kg body weight was injected into TNBS-mice via intracolonic administration. Normal saline-treated mice were used as control. Then, frozen sections of the colons, harvested 12 hours after the administration, were stained by rat anti-mouse F4/80-antibody at 4 °C overnight. The secondary antibody, Alexa Flour 488-labeled goat anti-rabbit (Life Technologies), was applied at 37 °C for 30 minutes, followed by the staining of nuclei with 4, 6-diamidino-2-phenylindole (Sigma). Frozen sections were photographed using Nikon confocal microscope (C2+, Nikon, Tokyo, Japan) and analyzed using Nis-element advanced research software (Nikon). To examine the targeting ability of G-LMWC&Cy3-miRNA precursors, LPMCs were collected 12 hours after miRNA precursors treatment according to our previous report, stained with APC labeled rat anti-mouse F4/80 antibody (Biolegend, San Diego, CA) and analyzed via flow cytometer (BD Biosciences, San Jose, CA).10
To quantify the cellular level of miR-16, colonic macrophages, T cells, and colonic epithelial cells were isolated from freshly obtained colonic specimens 12 hours after pre-miR-16 injection. CECs were isolated using chelating agents as described previously.7 Colonic macrophages were purified from LPMCs by FACS (BD FACSAria) using APC-labeled rat anti-mouse F4/80 antibody. Colonic T cells were sorted from LPMCs through the Pan T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany) according the manufacture's instruction. The purity of three types of cells was analyzed by flow cytometry. Isolated colonic macrophages, T cells and CECs (purity > 95%, respectively) were cultured in RPMI1640 containing 10% bovine serum. Twelve hours later, ELISA assay was used to determine the concentration of TNF-α/IL-12p40 in the supernatants, and the total cellular RNA was isolated to examine the levels of miR-16, TNF-α, and IL-12p40.
Treatment of TNBS-induced colitis mice. Mice were treated with G-LMWC&pre-miR-16 or G-LMWC &scrambled pre-miR-16 at the dose of 5 mg miRNA precursor/kg body weight 12 hours after TNBS injection. The solution was injected into the mice by intracolonic administration at a total volume of 100 μl.
To examine the influence of TNF-α/IL-12p40 overexpression on the therapeutic effects of pre-miR-16, mice were treated with G-LMWC&IL-12p40 plasmids, G-LMWC&TNF-α plasmids, G-LMWC&IL-12p40+TNF-α plasmids, control plasmid or PBS in a 150 μl rectal infusion under sedation 3 days prior to a 2.5 mg TNBS injection. The dose was 5 mg plasmid/kg body weight. Then, 12 hours after the induction of TNBS, G-LMWC&pre-miR-16 (5 mg/kg body weight) was intracolonically administered.
The mice were monitored daily for weight loss and pathological features. To investigate the therapeutic effects, mice were sacrificed at day 3 after the induction of colitis. The colons were excised for macroscopic observation. Standardized colon samples were harvested precisely 2 cm above the anal canal. Part of the colon tissue were fixed in Bouis buffer (for H&E staining) or in 4% paraformaldehyde solution (for immunofluorescence analysis). The other parts of standardized colon samples were snapping freezed in liquid nitrogen for miRNA and mRNA quantification, cytokine determination, and MPO activity measurement. For weight and survival observation, the mice were euthanized 8 days after the establishment of colitis.
DAI evaluation. The DAI used to evaluate intestinal inflammation was based on a previously published grading system.30 The scores ranged from 0 to 4 based on the following parameters: change in weight (0, ≤1%; 1, 1–5%; 2, 5–10%; 3, 10–15%; 4, >15%), rectal bleeding (0, no blood using hemoccult (Beckman Coulter, Palo Alto, CA); 2, positive hemoccult; 4, gross bleeding) and stool consistency (0, normal; 2, loose stools; 4, diarrhea). The combined scores were then averaged to obtain the final DAI score.
Histopathological analysis. For histopathological examination, the obtained colonic sample were fixed in Bouis buffer, embedded in paraffin, sectioned and stained with H&E. Inflammation was blindly scored from 0 to 4, as follows: 0, no signs of inflammation; 1, low leukocyte infiltration; 2, moderate leukocyte infiltration; 3, high leukocyte infiltration, moderate fibrosis, high vascular density, thickening of the colon wall, moderate goblet cell loss, and focal loss of crypts; and 4, transmural infiltration, massive loss of goblet cells, extensive fibrosis, and diffuse loss of crypts.
Cytokine and MPO analysis. For cytokine determination and MPO activity measurement in the colon mucosa, protein extracts were prepared by homogenizing colonic segments (50 mg of tissue per ml) in 50 mmol/l Tris-HCl (pH 7.4)/0.5 mmol/l DTT/10 mg/ml proteinase inhibitor mixture (Sigma). Samples were centrifuged at 3,000*rpm for 20 minutes and stored at −70 °C until analysis. The concentrations of IFN-γ, IL-1β, IL-6, IL-12p70, IL-17A, and IL-23 in the colonic mucosa were evaluated by ELISA kit. MPO activity was determined by the kit from Jiancheng Biotech (Nanjing, China).
The mRNA levels of colonic TNF-α/IL-12p40 were determined by qRT-PCR. Immunofluorescence staining was also used to detect the expression of TNF-α and IL-12p40 in the inflamed colon.
QRT-PCR assay. Total RNA from cells (including macrophages, T cells, and CECs) were prepared with TRIzol reagent according to the manufacture's protocol. For RNA isolated from the colon, the tissue sample was homogenized via liquid nitrogen grinding and then TRIzol reagent was used for the following isolation process. Two micrograms of total RNA were converted to cDNA using avian myeloblastosis virus reverse transcriptase (Takara Bio, Shiga, Japan) and a stem-loop RT primer (miRNA assay)/Oligo(dT)18 Primer (gene assay) under the following conditions: 16 °C for 30 minutes, 42 °C for 30 minutes, and 85 °C for 5 minutes. Real-time qPCR was performed on a 7300 Sequence Detection System (Applied Biosystems, Foster City, CA) with LightCycler FastStart DNA Master SYBR Green I (Roche Diagnostics, Indianapolis, IN) to determine the gene expressions or with the NCode SYBR Green miRNA qRT-PCR Kit (Roche Diagnostics) to examine miRNA levels, according to the manufacturer's protocol. Primers for β-actin were used as internal controls for gene expression, and the expression of miR-16 in cells and tissues was calculated relative to U6, a ubiquitously expressed small nuclear RNA. The primer sequences were as follows: TNF-α sense: 5′-GAACTGGCAGAAGAGGCACT-3′; TNF-α antisense: 5′-CTCCGCAAAGTCTAAG-3′; IL-12p40 sense: 5′-GGGTGTCCAGGCACATCAGA-3′; IL-12p40 antisense: 5′-GGAGTCCAGTCCACCTCTACAAC-3′; β-actin sense: 5′-GGTGTGATGGTGGGAATGGG-3′; β-actin antisense: 5′-ACGGTTGGCCTTAGGGTTCAG-3′; miR-16 sense: ACACTCCAGCTGGGTAGCAGCACGTAAATA; miR-16 antisense: UAGCAGCACGUAAAUAUUGGCG; U6 sense: CTCGCTTCGGCAGCACA; U6 antisense: AACGCTTCACGAATTTGCGT.
Immunofluorescence staining. For TNF-α and IL-12p40 expression studies, paraffin-embedded colon sections were deparaffinized and hydrated, then slides were boiled in 1× citrate buffer for 10min for epitope retrieval. Afterwards the sections were stained with a goat anti-mouse TNF-α or IL-12p40 antibody (Santa Cruz Biotechnology, Santa Cruz, CA) at 4 °C overnight. An Alexa Flour 546-labeled goat anti-rabbit secondary antibody (Life Technologies) was administered at room temperature for 45 minutes, followed by nuclear staining with 4, 6-diamidino-2-phenylindole. Sections were imaged using C2+ Nikon confocal microscope. Colon sections stained with matched isotype controls were used as negative controls to eliminate the nonspecific binding of antibodies.
Statistics. The results are expressed as the means ± standard error (mean ± SEM). The differences between groups were analyzed by the Mann–Whitney U-test and, if appropriate, by the Kruskal–Wallis analysis of variance test. A value of P ≤ 0.05 was considered significant. The survival curves were analyzed by the Kaplan–Meyer log-rank test.
SUPPLEMENTARY MATERIAL Figure S1. The level of TNF-α and IL-12p40 in colonic T cells and CECs after intracolonic administration of pre-miR-16 or pre-scramble. Figure S2. miR-16 regulated TNF-α/IL-12p40 level via binding to its 3′UTR element.
Acknowledgments
This work was supported by the National Basic Research Program of China (2012CB517603), the National High Technology Research and Development Program of China (2014AA020707), the National Natural Science Foundation of China (J1103512, J1210026, 31170751, 31071232, 31271013, 31200695, 31400671 and 51173076), the Ph.D. Programs Foundation of the Ministry of Education of China (20130091110037), the Program for New Century Excellent Talents in University (NCET-13–0272), Jiangsu Planned Projects for Postdoctoral Research Funds (1302009B), China Postdoctoral Science Foundation funded project (2014M551555, 2015T80536). Competing interests: None.
Supplementary Material
References
- Abraham, C and Cho, JH (2009). Inflammatory bowel disease. N Engl J Med 361: 2066–2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neurath, MF (2014). Cytokines in inflammatory bowel disease. Nat Rev Immunol 14: 329–342. [DOI] [PubMed] [Google Scholar]
- Pizarro, TT and Cominelli, F (2007). Cytokine therapy for Crohn's disease: advances in translational research. Annu Rev Med 58: 433–444. [DOI] [PubMed] [Google Scholar]
- Wu, F, Zhang, S, Dassopoulos, T, Harris, ML, Bayless, TM, Meltzer, SJ et al. (2010). Identification of microRNAs associated with ileal and colonic Crohn's disease. Inflamm Bowel Dis 16: 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, F, Zikusoka, M, Trindade, A, Dassopoulos, T, Harris, ML, Bayless, TM et al. (2008). MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology 135: 1624–1635.e24. [DOI] [PubMed] [Google Scholar]
- Fasseu, M, Tréton, X, Guichard, C, Pedruzzi, E, Cazals-Hatem, D, Richard, C et al. (2010). Identification of restricted subsets of mature microRNA abnormally expressed in inactive colonic mucosa of patients with inflammatory bowel disease. PLoS One 5: pii: e13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, Z, Shi, T, Zhou, Q, Shi, S, Zhao, R, Shi, H et al. (2014). miR-141 Regulates colonic leukocytic trafficking by targeting CXCL12β during murine colitis and human Crohn's disease. Gut 63: 1247–1257. [DOI] [PubMed] [Google Scholar]
- Jing, Q, Huang, S, Guth, S, Zarubin, T, Motoyama, A, Chen, J et al. (2005). Involvement of microRNA in AU-rich element-mediated mRNA instability. Cell 120: 623–634. [DOI] [PubMed] [Google Scholar]
- Heinsbroek, SE and Gordon, S (2009). The role of macrophages in inflammatory bowel diseases. Expert Rev Mol Med 11: e14. [DOI] [PubMed] [Google Scholar]
- Zuo, L, Huang, Z, Dong, L, Xu, L, Zhu, Y, Zeng, K et al. (2010). Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut 59: 470–479. [DOI] [PubMed] [Google Scholar]
- Strober, W, Zhang, F, Kitani, A, Fuss, I and Fichtner-Feigl, S (2010). Proinflammatory cytokines underlying the inflammation of Crohn's disease. Curr Opin Gastroenterol 26: 310–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laroui, H, Geem, D, Xiao, B, Viennois, E, Rakhya, P, Denning, T et al. (2014). Targeting intestinal inflammation with CD98 siRNA/PEI-loaded nanoparticles. Mol Ther 22: 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocampo, SM, Romero, C, Aviñó, A, Burgueño, J, Gassull, MA, Bermúdez, J et al. (2012). Functionally enhanced siRNA targeting TNFα attenuates DSS-induced colitis and TLR-mediated immunostimulation in mice. Mol Ther 20: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boirivant, M, Pallone, F, Di Giacinto, C, Fina, D, Monteleone, I, Marinaro, M et al. (2006). Inhibition of Smad7 with a specific antisense oligonucleotide facilitates TGF-beta1-mediated suppression of colitis. Gastroenterology 131: 1786–1798. [DOI] [PubMed] [Google Scholar]
- Kriegel, C and Amiji, MM (2011). Dual TNF-α/Cyclin D1 gene silencing with an oral polymeric microparticle system as a novel strategy for the treatment of inflammatory bowel disease. Clin Transl Gastroenterol 2: e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay, MA (2008). microRNAs and the immune response. Trends Immunol 29: 343–351. [DOI] [PubMed] [Google Scholar]
- Van Assche, G and Rutgeerts, P (2000). Anti-TNF agents in Crohn's disease. Expert Opin Investig Drugs 9: 103–111. [DOI] [PubMed] [Google Scholar]
- Shen, C, de Hertogh, G, Bullens, DM, Van Assche, G, Geboes, K, Rutgeerts, P et al. (2007). Remission-inducing effect of anti-TNF monoclonal antibody in TNBS colitis: mechanisms beyond neutralization? Inflamm Bowel Dis 13: 308–316. [DOI] [PubMed] [Google Scholar]
- Pallone, F and Monteleone, G (1998). Interleukin 12 and Th1 responses in inflammatory bowel disease. Gut 43: 735–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T, Okamoto, S, Hisamatsu, T, Kamada, N, Chinen, H, Saito, R et al. (2008). IL23 differentially regulates the Th1/Th17 balance in ulcerative colitis and Crohn's disease. Gut 57: 1682–1689. [DOI] [PubMed] [Google Scholar]
- Neurath, MF, Fuss, I, Kelsall, BL, Stüber, E and Strober, W (1995). Antibodies to interleukin 12 abrogate established experimental colitis in mice. J Exp Med 182: 1281–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannon, PJ, Fuss, IJ, Mayer, L, Elson, CO, Sandborn, WJ, Present, D et al.; Anti-IL-12 Crohn's Disease Study Group. (2004). Anti-interleukin-12 antibody for active Crohn's disease. N Engl J Med 351: 2069–2079. [DOI] [PubMed] [Google Scholar]
- Bonci, D, Coppola, V, Musumeci, M, Addario, A, Giuffrida, R, Memeo, L et al. (2008). The miR-15a-miR-16-1 cluster controls prostate cancer by targeting multiple oncogenic activities. Nat Med 14: 1271–1277. [DOI] [PubMed] [Google Scholar]
- Cimmino, A, Calin, GA, Fabbri, M, Iorio, MV, Ferracin, M, Shimizu, M et al. (2005). miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A 102: 13944–13949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima, T, Arakawa, S, Sanada, Y, Yoshino, I, Miyazaki, D, Urushima, H et al. (2013). Inhibition of epithelial cell death by Bcl-2 improved chronic colitis in IL-10 KO mice. Am J Pathol 183: 1936–1944. [DOI] [PubMed] [Google Scholar]
- Williams, AM, Whiting, CV, Bonhagen, K, Reimann, J, Bregenholt, S, Claesson, MH et al. (1999). Tumour necrosis factor-alpha (TNF-alpha) transcription and translation in the CD4+ T cell-transplanted scid mouse model of colitis. Clin Exp Immunol 116: 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada, N, Hisamatsu, T, Okamoto, S, Sato, T, Matsuoka, K, Arai, K et al. (2005). Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol 175: 6900–6908. [DOI] [PubMed] [Google Scholar]
- Mosser, DM and Edwards, JP (2008). Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8: 958–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos, KR, Marinho, CR, Barboza, R, Russo, M, Alvarez, JM and D'Império Lima, MR (2004). What kind of message does IL-12/IL-23 bring to macrophages and dendritic cells? Microbes Infect 6: 630–636. [DOI] [PubMed] [Google Scholar]
- Cooper, HS, Murthy, SN, Shah, RS and Sedergran, DJ (1993). Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest 69: 238–249. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.