Abstract
Ligands for the NKG2D receptor are overexpressed on tumors, making them interesting immunotherapy targets. To assess the tumoricidal properties of T cells directed to attack NKG2D ligands, we engineered murine T cells with two distinct NKG2D-based chimeric antigen receptors (CARs): (i) a fusion between the NKG2D receptor and the CD3ζ chain and (ii) a conventional second-generation CAR, where the extracellular domain of NKG2D was fused to CD28 and CD3ζ. To enhance the CAR surface expression, we also engineered T cells to coexpress DAP10. In vitro functionality and surface expression levels of all three CARs was greater in BALB/c T cells than C57BL/6 T cells, indicating strain-specific differences. Upon adoptive transfer of NKG2D-CAR-T cells into syngeneic animals, we observed significant clinical toxicity resulting in morbidity and mortality. The severity of these toxicities varied between the CAR configurations and paralleled their in vitro NKG2D surface expression. BALB/c mice were more sensitive to these toxicities than C57BL/6 mice, consistent with the higher in vitro functionality of BALB/c T cells. Treatment with cyclophosphamide prior to adoptive transfer exacerbated the toxicity. We conclude that while NKG2D ligands may be useful targets for immunotherapy, the pursuit of NKG2D-based CAR-T cell therapies should be undertaken with caution.
Introduction
Treating patients with T cells that are engineered to express tumor-specific receptors has proven to be a clinically efficacious form of immunotherapy. In particular, the use of chimeric antigen receptors (CARs) to direct T cells to attack tumors has shown significant promise in clinical trials.1,2,3,4 These receptors aim to target surface-expressed antigens that are either restricted to, or overexpressed on, tumor cells, eliminating the conventional T cell receptor requirement for antigen presentation on MHC molecules. One method of generating CARs fuses native proteins, which naturally ligate proteins on the surface of tumor cells, with the intracellular signaling domains required to induce T cell activation. Ligands for the natural killer group 2 member D (NKG2D) receptor are numerous and are frequently upregulated on many cancer types.5,6,7 Additionally, NKG2D ligand (NKG2DL) expression can be upregulated on tumor cells through the use of already approved drugs such as spironolactone, allowing for further target enhancement.8
Using a CAR comprised of NKG2D fused to the CD3ζ TCR signaling domain enables T cells to recognize any of the several natural NKG2DL, and exert their cytolytic functions.1,2,3,4,9–11 While NKG2D is an activating receptor on natural killer (NK) cells, it functions primarily as a costimulatory receptor on activated CD8+ T cells.5,6,7,12–15 In both murine and human T cells, signaling through the NKG2D receptor is mediated through an adaptor protein, DAP10 (ref. 8,13). This adaptor protein activates the PI3-K and Grb-2 pathways, much like the T cell costimulatory molecule, CD28 (ref. 14,16). Research has revealed that the inclusion of costimulatory domains in CARs enhances T cell efficacy and persistence postadoptive transfer.17,18,19,20 In that regard, fusion of full-length NKG2D with CD3ζ may provide costimulatory signals via the NKG2D portion of the receptor, in addition to the activation signal delivered through CD3ζ. In this manuscript, we investigated two distinct CARs based on the NKG2D receptor: (i) a fusion of NKG2D with CD3ζ (NKz) and (ii) a fusion of the NKG2D extracellular domain to signaling domains from a conventional second-generation CAR composed of CD28 fused to CD3ζ (NK28z). Since surface expression of full-length NKG2D is dependent upon the DAP10 molecule,9,21 we also investigated whether coexpression of DAP10 along with the NKz fusion protein (NKz10) could further augment CAR activity.
Our results revealed that the functionality of the CARs was strain-dependent in murine T cells. Further, T cells expressing NKG2D-based CARs displayed in vivo toxicity, which was exacerbated when T cell infusion was combined with chemotherapeutic lymphodepletion. The NKz-CAR-T cells displayed the lowest toxicity in vivo, which suggests that this configuration may be amenable to clinical evaluation. These results revealed that NKG2D-based CAR-T cells can be highly toxic when delivered systemically and indicate that further research is required to better understand how to deploy these CAR-T cells safely in the clinic.
Results
Inclusion of DAP10 in the retrovirus significantly enhances surface expression of NKz
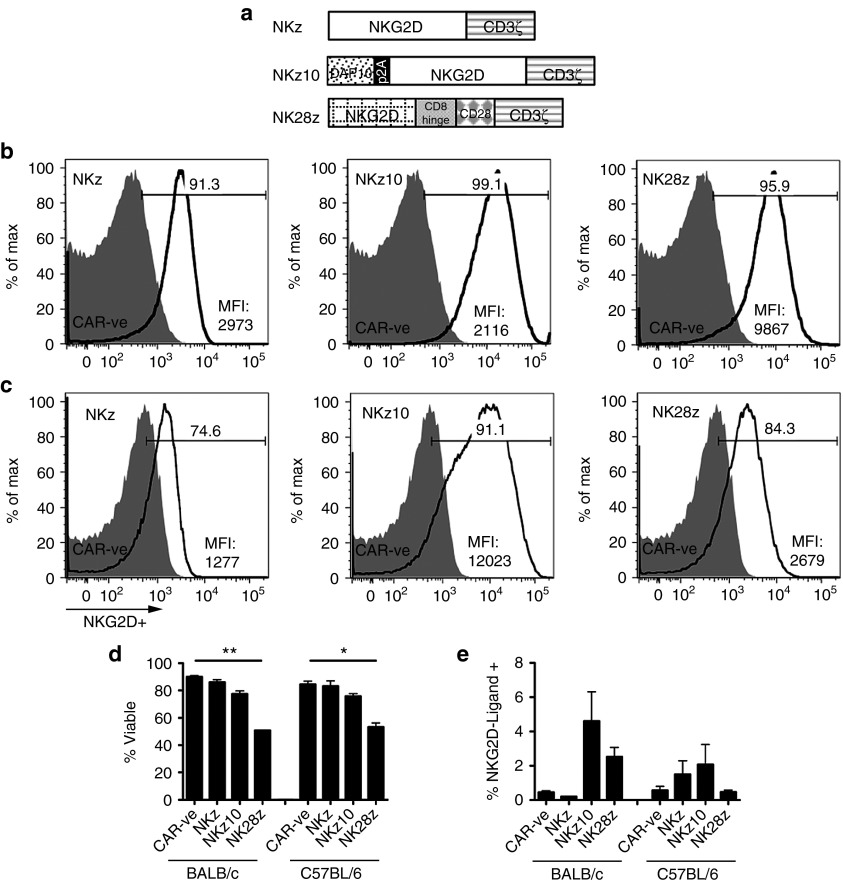
To produce T cells that would be activated by NKG2D-ligands, we engineered murine T cells with one of three different NKG2D-based CAR retrovirus (RV) constructs: full-length NKG2D fused to cytoplasmic CD3ζ (NKz), the same NKz-CAR with the addition of adaptor protein DAP10 to the RV construct (NKz10), or a conventional second-generation CAR that fuses the extracellular domain of NKG2D to a CD8-hinge region, CD28 transmembrane and cytoplasmic domains, and the cytoplasmic domain of CD3ζ (NK28z; Figure 1a). We utilized NKG2D cell surface staining as an indicator of NKG2D-CAR expression, as CAR -ve T cells show very low levels of endogenous NKG2D expression (Figure 1b,c). Engineering T cells with any of the three NKG2D-CAR RVs resulted in over 90% of both CD8+ (Figure 1b) and CD4+ (Supplementary Figure S1, top panels) T cells staining positive for NKG2D within three days of transduction with BALB/c-derived T cells. Frequencies of CAR+ T cells were generally lower using C57BL/6 T cells for all three CARs tested, with only NKz10 reaching NKG2D+ frequencies above 90% on either CD8+ or CD4+ T cells (Figure 1c; Supplementary Figure S1, bottom panels) On a per-cell basis, the NKz10-CAR-T cells showed over 7- to 10-fold higher expression of NKG2D compared to the NKz construct, indicating that the endogenous availability of DAP10 can limit surface expression of the NKz-CAR (Figure 1b,c). The NK28z-CAR showed an intermediate level of NKG2D surface expression, with twofold lower expression compared to NKz10 in BALB/c T cells (Figure 1b) and fivefold lower expression in C57BL/6 T cells (Figure 1c).
Figure 1.
Retrovirus construction and in vitro phenotypic profiles of NKG2D-ligand-specific chimeric antigen receptor (CAR)-engineered T cells. (a) Schematic diagram of the retrovirus (RV) constructs used to engineer murine T cells. The chimeric NKG2D-CD3ζ (NKz) CAR contains the full-length murine NKG2D gene fused to the cytoplasmic portion of CD3ζ. The NKz10 CAR bears the same CAR as NKz with the addition of adaptor protein DAP10 to the retrovirus, separated by a self-cleaving 2A peptide. The NK28z CAR combines the extracellular domain of murine NKG2D, fused to the CD8 hinge region, CD28 transmembrane and endodomains, and cytoplasmic CD3ζ. NKG2D expression on (b) BALB/c or (c) C57BL/6 CD8+ T cells was evaluated 3 days after transduction with the indicated CAR-containing retroviruses. Surface expression was determined using a fluorescence-minus one control of the anti-NKG2D – APC antibody and compared to basal expression on control CAR -ve T cells (shaded peaks). Mean fluorescence intensity and percentage of NKG2D+ CD8+ cells are shown. Data is representative of at least three independent experiments. (d) Viability of NKG2D-CAR-T cells from BALB/c and C57BL/6 mice was determined by flow cytometry using Molecular Probes LIVE/DEAD staining. (e) NKG2D-CAR cells were analyzed for NKG2D-ligand expression, as indicated by staining using an NKG2D-IgG-Fc chimeric protein and detected with an anti-human IgG secondary antibody to detect ligand expression.
NKG2D-CARs show strain-specific differences
We evaluated differences in CAR surface expression, T-cell viability, and NKG2DL expression on the NKG2D-CAR-T cells between the three NKG2D-CAR constructs, as well as between two mouse strains. Interestingly, both BALB/c and C57BL/6 T cells showed the same changes in cell viability across NKG2D-CAR constructs; NKz-engineered T cells showed no reduction in viability compared to CAR –‘ve T cells, NKz10-CAR-T cells showed a slight reduction in viability, and NK28z-CAR-T cells had a considerably decreased viability (Figure 1d; Supplementary Figure S2a). We observed variable levels of NKG2DL on CAR-engineered T cells across CAR type and T-cell origin (Figure 1e; Supplementary Figure S2b). While NKG2DL tended to be higher on NKz10-CAR T cells of either mouse strain, these data did not achieve statistical significance (Figure 1e).
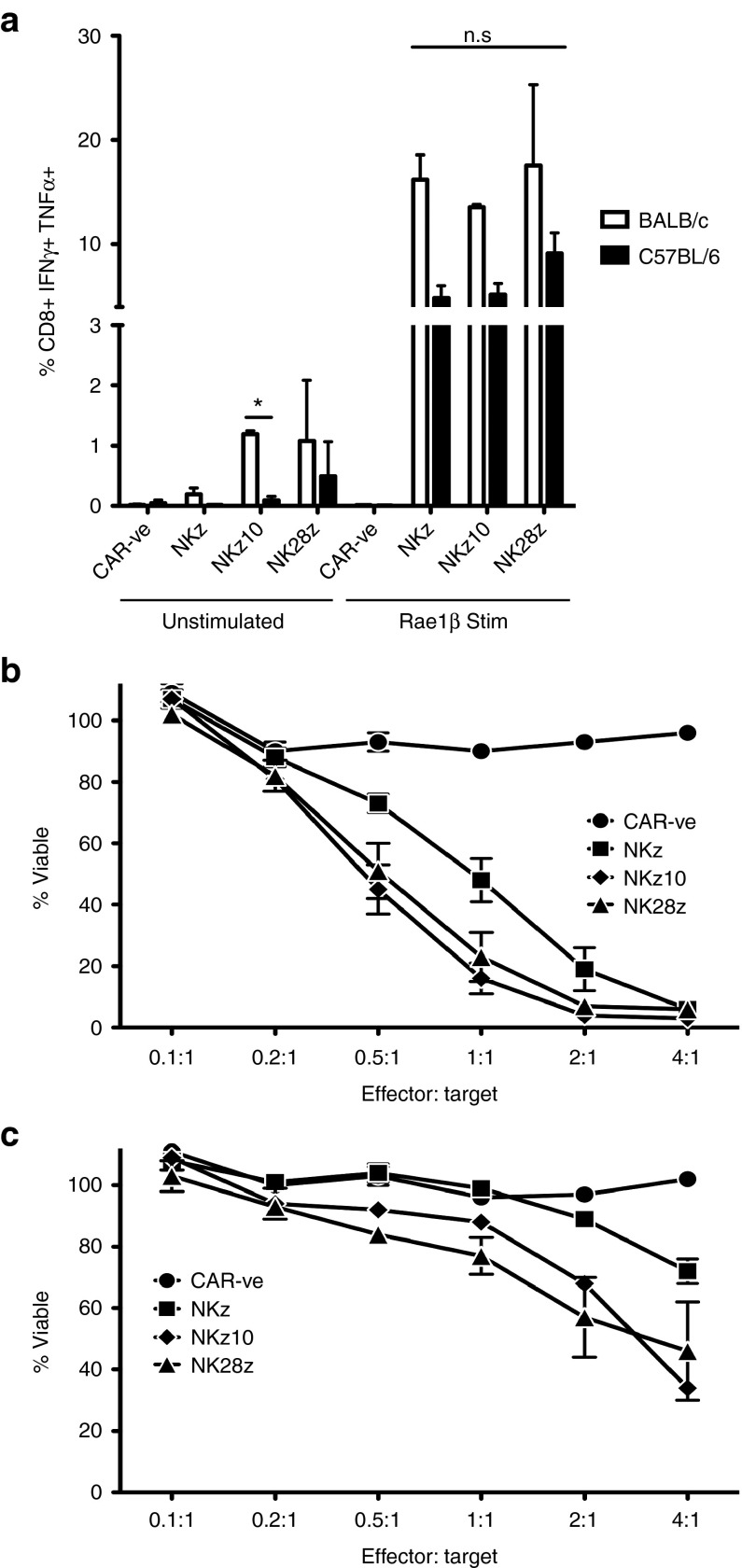
Despite the higher per-cell expression, NKz10-CAR did not demonstrate an equivalent enhancement of in vitro functionality in BALB/c T cells. In BALB/c T cells, all three NKG2D-CARs were similarly capable of producing the activation cytokines IFNγ and TNFα upon stimulation with recombinant Rae-1β, a well-defined NKG2D ligand (Figure 2a; Supplementary Figure S3). This was not directly attributed to differences in baseline cytokine production, as only the BALB/c NKz10-CAR T cells showed an increase in cytokine production over their C57BL/6 counterparts without stimulation (Figure 2a). The levels of background cytokine were very low in all cases (<2%; Figure 2a). In addition, all three NKG2D-CARs were able to induce robust killing of murine breast tumor cells in vitro, although NKz10 and NK28z BALB/c-derived NKG2D-CAR-T cells demonstrated moderately increased cytotoxicity compared to NKz counterparts at intermediate effector to target ratios. For example, NKz10 and NK28z were able to kill ~80% of tumor targets compared to only ~50% by NKz after 6 hours of coincubation at a 1:1 T-cell to tumor cell ratio (Figure 2b). Most of the tumor targets were killed after coculture of BALB/c NKG2D-CAR-T cells and tumor cells at a 2:1 ratio, illustrating the strong cytotoxic potential of these NKG2D-CAR-T cells (Figure 2b).
Figure 2.
In vitro functional profiles of NKG2D-CAR-T cells. (a,b) BALB/c or (a,c) C57BL/6 T cells were engineered with the indicated CARs. (a) NKG2D-CAR T cells were stimulated with plate-bound recombinant Rae1β-Fc fusion protein and stained for production of IFNγ and TNFα, and analyzed by flow cytometry. Data is expressed as mean frequency ± SEM normalized to background levels from three independent experiments. In vitro CAR-T cell-mediated cytotoxicity was assayed using 4T1.2 tumor cells using a 6-hour AlamarBlue assay at the indicated effector: target ratios of BALB/c (b) or C57BL/6 (c) T cells. Mean frequency of viable tumor cells ± SEM from 3–4 independent experiments of triplicate wells is presented.
NKG2D-CAR-engineered C57BL/6 T cells showed slightly reduced cytokine production upon CAR-stimulation compared to BALB/c-derived CAR-T cells (Figure 2a; Supplementary Figure S2). Interestingly, while NKz10-CAR-T cells had the highest level of CAR expression in C57BL/6-derived cells, the NK28z-CAR-T cells showed the greatest cytokine production (Figure 2a). This cytokine production did not translate to killing potential, as the C57BL/6 NK28z-CAR-T cells displayed weak cytotoxicity in vitro (Figure 2c). Similarly, the C57BL/6-derived NKz-CAR T cells also exhibited weak cytotoxicity (Figure 2c). While C57BL/6 NKz10-CAR-T cells were capable of killing tumor targets at higher E:T ratios, their activity was considerably diminished when compared to their BALB/c counterparts (Figure 2b,c). Taken together, our data reveal striking strain-dependent differences in the functionality of the various NKG2D-based CARs.
NKG2D-CAR-T cells can induce significant, acute toxicity upon adoptive transfer
We next investigated the functionality of NKG2D-CAR-T cells in vivo. We employed the 4T1.2 breast tumor model in BALB/c mice. Mice bearing established tumors were treated with cyclophosphamide (CTX), followed by infusion of NKG2D-CAR T cells. Strikingly, we observed dramatic clinical toxicity symptoms within just a few hours of adoptive transfer. To understand whether this toxicity was related to an over-exuberant antitumor immune response resulting from the NKG2D–CAR-T cell infusion, we adoptively transferred NKG2D-CAR-T cells into naive, tumor-free animals. Tumor-free mice still exhibited significant clinical symptoms, including lethargy, hunched body posture, ruffled fur, and a lack of grooming, indicating that the NKG2D-CAR-T cells were producing off-tumor toxicities in vivo.
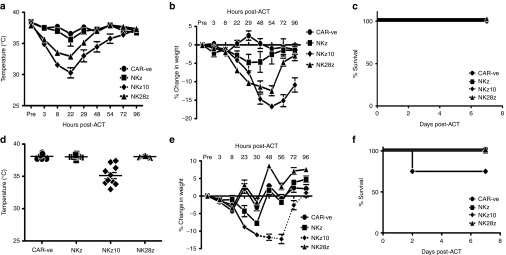
To better understand these off-tumor toxicities, naive BALB/c mice were infused with 107 NKz, NKz10, or NK28z CAR-T cells, and toxicity was evaluated via changes in core body temperature, body weight, and overall survival (Figure 3a–c). Despite similar in vitro functionality, the BALB/c-derived NKG2D-CAR-T cells displayed a distinct hierarchy of disease severity between the different CAR-T cells in vivo. The NKz10-CAR-T cells elicited the most significant toxicity, with core body temperatures dropping as low as 30°C within 24 hours of adoptive cell transfer (ACT) (Figure 3a). Additionally, these mice lost up to 17% of their body weight in less than 3 days post-ACT (Figure 3b). The NKz-CAR treated animals conversely showed no significant hypothermia, and only slight weight loss over the course of one week post-ACT (Figure 3a,b). The NK28z-CAR-T cells induced an intermediate level of toxicity with respect to both temperature changes and weight loss (Figure 3a,b). These toxicities were accompanied by significant clinical symptoms such as lethargy, ruffled fur, hunched posture, and labored breathing that corresponded to the severity of hypothermia and weight loss (Supplementary Table S1). Interestingly, these data parallel our observations of the differences in per-cell NKG2D-CAR expression in vitro (Figure 1b), with the greatest expression and most severe toxicities being observed with NKz10-CAR-T cells. Despite these significant, acute toxicities, all mice survived the treatment, and recovered within 7 days of ACT (Figure 3c). We evaluated whether similar toxicities were observed in C57BL/6 mice. Interestingly, C57BL/6 mice did not display any overt physical clinical symptoms such as changes in posture, fur or body condition (Figure 3d,e, data not shown). In addition, only the NKz10-CAR T cells caused measurable toxicities in the form of hypothermia, weight loss, and morbidity (Figure 3d–f). The temperature changes and weight loss in these animals were considerably more modest compared to those observed in BALB/c mice. NKz10-CAR-T cell–treated mice displayed variable temperature changes, with some mice showing only mild hypothermia and others dropping to 33°C within 8 hours of ACT (Figure 3d). Weight loss by NKz10-CAR-T cell–treated mice was consistent; over the course of 3 days post-ACT, C57BL/6 mice lost up to 12% of their body mass (Figure 3e). Despite the reduced severity of hypothermia and weight loss in C57BL/6 mice, ACT of NKz10-CAR-T cells was lethal in 25% of the treated animals within 48 hours of treatment (n = 8; Figure 3f). The NKz and NK28z CAR-T cells showed no significant toxicity or lethality in C57BL/6 mice. These data reveal a notable difference in severity of NKG2D-CAR off-tumor toxicity between the two strains of mice tested, with BALB/c mice exhibiting greater toxicity than their C57BL/6 counterparts.
Figure 3.
NKG2D-CAR-T cells induce differing levels of toxicity in mice. (a–c) Naive BALB/c mice were treated with 107 NK-CAR-T cells intravenously as indicated (n = 6). (a) Temperatures, (b) weight loss, and (c) survival were monitored over the course of 7 days post-adoptive cell transfer (ACT). (d–f) Naive C57BL/6 mice were treated as in a (n = 5–10). (d) Temperatures are shown at the peak drop of 8 hours post ACT. (e) Weight loss and (f) survival were monitored over 7 days post-ACT. Temperature and weight loss data are presented as mean ± SEM. Dotted lines indicate data from surviving animals.
Preconditioning cyclophosphamide exacerbates NKG2D-CAR toxicity
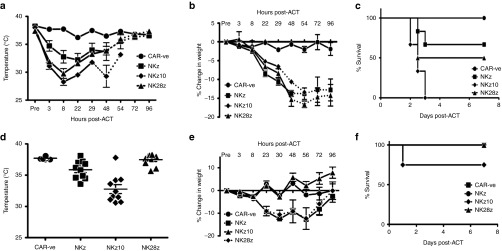
In both preclinical models and clinical trials of CAR-T cells, regimes inducing lymphopenia have proven to enhance the engraftment and persistence of CAR-T cells following adoptive transfer.22,23 As chemotherapeutic agents cause DNA damage and cell stress, which can upregulate NKG2DL expression, we tested whether pretreatment with CTX would influence toxicity following adoptive transfer of NKG2D-CAR-T cells. Strikingly, CTX pretreatment significantly exacerbated the toxicity induced by all three NKG2D-CARs (Figure 4).
Figure 4.
NKG2D-CAR-T cell toxicity is exacerbated by pre-conditioning with chemotherapy. Mice were treated with 150 mg/kg cyclophosphamide (CTX) intraperitoneally 24 hours prior to adoptive transfer. (a–c) BALB/c/c mice were treated with 107 NK-CAR-T cells intravenously as indicated (n = 6). (a) Temperatures, (b) weight loss, and (c) survival were monitored over the course of 7 days post adoptive cell transfer (ACT). (d–f) Naive C57BL/6 mice were treated as in a (n = 5–10). (d) Temperatures are shown at the peak drop of 8 hours post ACT. (e) Weight loss and (f) survival were monitored over 7 days post-ACT. Temperature and weight loss data are presented as mean ± SEM. Dotted lines indicate data from surviving animals.
In BALB/c mice, the NKz-CAR-T cells, which showed minimal toxicity in naive mice, were highly toxic when combined with CTX, inducing hypothermia and weight loss comparable to those induced by the NKz10-CAR-T cells (Figure 4a,b). Further, infusion of NKz-CAR-T cells in CTX pretreated BALB/c mice resulted in 33% mortality (Figure 4c). Toxicities produced by NKz10-CAR-T cells were also dramatically exacerbated, with body temperatures dropping below 29°C within as little as 8 hours post-ACT (Figure 4a). Other clinical symptoms were likewise exacerbated; mice demonstrated lethargy, a complete lack of grooming and the appearance of an ocular discharge. Most alarmingly, all mice treated with CTX and NKz10-CAR-T cells succumbed to the toxicities within 72 hours of ACT (Figure 4c). As observed in naive animals, NK28z-CAR-T cells showed an intermediate level of toxicity that was similarly worsened by CTX; 50% of animals in this treatment group succumbed to T-cell–mediated toxicity (Figure 4c).
The preconditioning chemotherapy also enhanced the toxicity of NKG2D-CAR-T cells in C57BL/6 mice (Figure 4d–f). Mice treated with NKz-CAR-T cells showed significant weight loss of over 12% of the pretreatment weight (Figure 4e), with average core body temperatures dropping below 35 °C within 8 hours of T-cell infusion (Figure 4d). In the case of NKz10-CAR-T cells, 25% of the treated animals succumbed to toxicities in less than 24 hours following ACT (Figure 4f). Surviving animals varied in their weight loss, with some losing more than 18% of their body weight before recovering (Figure 4e). Core body temperatures were also variable, with some of the survivors exhibiting little change, while others dropped below 31°C within 8 hours of ACT (Figure 4d). While C57BL/6 mice failed to show any of the physical symptoms of toxicity observed in BALB/c mice, chemotherapeutic pretreatment prior to ACT of NKz10-CAR-T cells resulted in an observable lethargy (noted upon handling the animals) in the C57BL/6 mice (data not shown). The NK28z-CAR-T cells showed no signs of toxicity, even when combined with CTX in C57BL/6 mice. These data reveal that the toxicities are dependent on both the strain and the CAR structure.
Adoptive transfer of NKG2D-CAR-T cells results in an acute cytokine storm in vivo
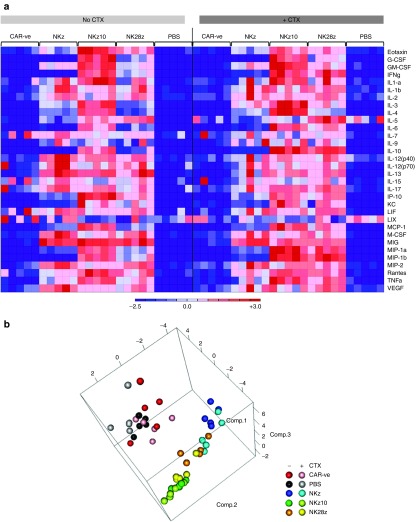
We next sought to investigate possible causes of this in vivo toxicity. Using a 32-plex cytokine array, we examined serum cytokine levels in BALB/c mice at 8 hours post-ACT, both with and without preconditioning cyclophosphamide. Strikingly, the majority of the cytokines evaluated were upregulated in the serum of mice receiving any of the NKG2D-CAR-T cells (Figure 5a). Mice treated with NKz10-CAR-T cells showed the most dramatic upregulation, with all but four analytes increased compared to CAR -ve controls (Figure 5a; Supplementary Tables S2 and S3). These mice showed serum concentrations of over 4 ng/ml of IFNγ, among others, indicating severe immune responses were occurring (Figure 5a; Supplementary Tables S2 and S3).
Figure 5.
NKz10-CAR-T cells induce severe cytokine storm in BALB/c mice. Mice with or without pre-conditioning CTX were treated with 107 engineered T cells as indicated (n = 5). Serum was collected at 8 hours post-ACT and sent for Luminex analysis. (a) Heat map displaying the relative changes in serum cytokine concentrations from mice treated with CAR -ve, NKz, NKz10 or NK28z CAR-T cells, or PBS control. Data was normalized by row. (b) Principal component analysis of serum cytokine concentrations indicating clustering of treatment groups.
Our previous observations indicated that NKz and NKz10 represented the lowest and highest observed toxicity and CAR-expression, respectively, with NK28z exhibiting an intermediate outcome. This pattern was reinforced through principal component analysis of serum cytokine and chemokine levels. While each NKG2D-CAR-T cell treatment clustered tightly regardless of CTX pretreatment, each NKG2D-CAR-T cell cluster was separate from the others, with NK28z falling between NKz and NKz10 (Figure 5b).
CTX pretreatment enhanced the serum concentration of many cytokines/chemokines (Supplementary Tables S2 and S3). The observed serum concentration increases following CTX pretreatment were NKG2D-CAR-T cell specific, as we did not observe any measureable changes between CAR -ve ± CTX or PBS ± CTX control groups (Figure 5a,b). The most dramatic effects of CTX were observed in mice treated with NKz-CAR-T cells and NK28z-CAR-T cells, where the concentrations of many cytokines were more than doubled by CTX pretreatment (Supplementary Tables S2 and S3). Overall, our data are indicative of a severe cytokine storm induced by ACT of NKG2D-CAR-T cells that is exacerbated by pre-conditioning with CTX.
NKz10 CAR-T cells induce severe lung immunopathology
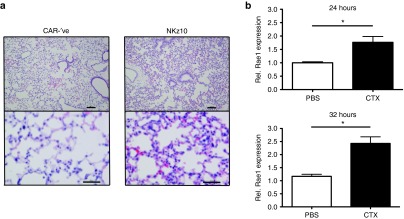
Given that the most substantial toxicities were observed in BALB/c mice treated with CTX and NKz10-CAR-T cells, we sought to further investigate the pathology elicited by this treatment. BALB/c mice were treated with CTX followed by adoptive transfer of 107 CAR -ve or NKz10-CAR-T cells and subjected to a comprehensive necropsy performed by a veterinary pathologist in a blinded fashion. NKz10-CAR-T cell-treated animals exhibited severe necrotizing pneumonitis, which was deemed to have been likely fatal in these animals (Figure 6a). No tissue pathology was observed in CAR -ve control animals. The lungs of NKz10-CAR-treated mice displayed severe perivascular edema, diffuse thickening of the alveolar septae, as well as heavy infiltration by neutrophils and mononuclear cells (Figure 6a).
Figure 6.
NKG2D-CAR toxicity is mediated via pulmonary inflammation. (a) BALB/c mice were treated with 150 mg/kg cyclophosphamide, followed by intravenous injection of 107 CAR-′ve or NKz10 CAR-T cells. Animals were sacrificed for complete veterinary necropsy 24 hours later. Hematoxylin and eosin–stained cross-sections of lung tissues from representative mice are shown (n = 5). Top panel bars = 100 μm, bottom panel bars =50 μm. (b) NKG2D ligand expression was evaluated by qRT-PCR in the lungs of either naive or CTX-treated mice at 24 or 32 hours post-CTX (n = 5).
Lungs from BALB/c mice treated with either PBS or CTX were harvested at 24 and 36 hours posttreatment and NKG2D ligand expression was measured by qRT-PCR. H60a, H60b, and Mult-1 were detected in all samples, but the expression was not impacted by CTX treatment (Supplementary Figure S4b–d). In contrast, CTX treatment resulted in a significant upregulation of Rae1 gene transcription at both time points (Figure 6b). Taken together, our data suggests that NKG2D-CAR toxicity is driven by severe systemic pro-inflammatory changes including pneumonitis.
Discussion
On-target, off-tumor toxicity remains a primary concern for all immunotherapy approaches, especially given that the vast majority of tumor targets are not tumor-restricted in their expression.24 Here, we examined the preclinical in vitro and in vivo functionality of three CARs based on the NKG2D receptor. Unexpectedly, we observed severe off-tumor toxicity upon in vivo testing of these NKG2D-CARs in multiple mouse strains. We have treated both C57BL/6 and BALB/c mice with CAR-T cells specific for a variety of antigens, including HER-2 and GPNMB, and have never observed toxicity following treatment (unpublished observations). Thus, the toxicity observed with the NKG2D-CARs is not simply reflective of toxicity associated with the infusion of engineered T cells. These previously unreported findings provide insight into considerations that must be taken into account prior to the clinical application of NKG2D-CARs.
The range of homeostatic NKG2DL expression at both the transcriptional and protein levels is currently unclear in both mice and humans. Multiple reports have established NKG2DL expression on various healthy, noncancerous/infected tissues, and these may serve as off-tumor ligand sources for NKG2D-CAR-T cells in vivo. At a transcriptional level, several NKG2DL are expressed across diverse tissues such as the spleen, lungs, gut and bronchial epithelia, cardiac and skeletal muscle, and the skin.25,26,27,28 Data from others suggest epithelial, endothelial, and antigen-presenting cells may constitutively express NKG2DL such as UL16-binding proteins (ULBPs).29 We have found that systemic treatment with CTX leads to upregulation of NKG2DL in the lungs, which provides an explanation of the enhanced toxicity of NKG2D-CAR-T cells in hosts that were pretreated with CTX and suggests that caution should be employed when combining chemotherapy with cytotoxic therapeutics that target NKG2DLs.
There are known expression differences in the NKG2DLs, H60and Rae-1, between BALB/c and C57BL/6 mice, which may explain observed strain-specific differences in NKG2D-CAR-T cell toxicity.25,30 In particular, H60a has been shown to be predominantly expressed in BALB/c mice and not in C57BL/6 mice due to a truncated genomic sequence,31 which results in a lack of functional H60a protein in C57BL/6 mice.30,32 Conversely, C57BL/6 mice show elevated levels of expression of H60b and H60c when compared to BALB/c mice.33 Expression of Rae-1 family members also differs across mouse strains; BALB/c mice express Rae-1α, Rae-1β, and Rae1γ, while C57BL/6 mice express Rae-1δ and Rae-1ɛ.30,34 Overall, BALB/c mice show elevated levels of expression of Rae-1 proteins and pan-NKG2D-ligands (detected using an NKG2D-Fc fusion protein) on bone marrow isolates compared to C57BL/6 mice,30 which may explain the heightened toxicity of NKG2D-CAR T cells in BALB/c mice. The diversity of expression patterns of NKG2DL in humans (or mice), coupled with our data indicating the potential for toxicity, cautions strongly against the use of NKG2D-CAR-T cells for therapeutic applications in humans without considerable care and monitoring.
Commensal microbiota may also underpin the strain-specific toxicities of NKG2D-CAR T as C57Bl/6 mice and BALB/c have distinct microbiomes.35 Commensal microbial composition in the gut has been shown to influence respiratory immune responses,36 so it is possible that such differences influence the lethal pneumonitis observed following intravenous infusion of NKG2D-CAR-T cells. CTX may further compound the influence of the microbiome as treatment with CTX has been found to alter the microbial composition within the gut and subsequent immune responses.37 Thus, the differences in toxicity may result from both genetic and microbial differences between the mouse strains.
Endogenous NKG2D receptors recognize NKG2DLs on healthy cells without inducing toxicity. We hypothesize that inherent differences between these receptors and NKG2D-CARs explain our toxicity observations. NKG2D-CARs bind NKG2DL, which induce T-cell activation and cytolytic functions.9 Similarly, in NK cells, NKG2D acts as a positive signal to induce cytolytic functions.38 However, in NK cells these positive signals are balanced by inhibitory signals from other NK cell surface receptors that act in concert to determine the fate of the target cell.38 Under homeostatic conditions, low levels of NKG2DL and concomitant NKG2D signaling would occur in concert with inhibitory signals, such as the presence of MHC-1, preventing NK cell reactivity.39 Removing the contributions of the inhibitory signals, as in NKG2D-based CARs, effectively takes the brakes off NKG2D-mediated cytotoxicity. Coupling this with a highly expressed, highly activating chimeric NKG2D receptor can therefore have potentially serious consequences, as revealed by the severe toxicity of NKz10-CAR-T cells.
One of our most striking observations was the varied manifestation of toxicity between the two different strains of mice tested. Whereas both NKz10- and NK28z-CAR-T cells were markedly toxic to BALB/c mice, these CAR-T cells manifested little toxicity in C57BL/6 mice. Likewise, although the NKz-CAR-T cells alone were minimally toxic in both strains, when combined with CTX, the NKz-CAR only caused significant morbidity and mortality in BALB/c mice. Our findings are consistent with previous reports describing NKz-CAR-T cells as a tumor therapy in the absence of overt toxicity using C57BL/6 mice, even when combined with CTX.7,9,10,11 These data demonstrate that a therapeutic window may exist for treatment with NKz-CAR T cells; however, many factors (as described above) must be taken into consideration prior to treatment to avoid unwanted severe toxicities. The NKz10-CAR, which demonstrated the most severe toxicity in our studies, has only been previously tested on human NK cells using immunodeficient animals, which precludes the ability to assess toxicity due to a lack of cross-reactivity.40,41 Likewise, CARs composed of the NKG2D extracellular domain fused to a conventional CAR scaffold have thus far only been tested using in vitro assays, and so possible toxicities were not yet evaluated.42,43 The results of this work conclude that NKG2D-CAR-T cells can be highly toxic and that the toxicity is influenced by both the host and the conditioning regimen. As such, our findings suggest that preclinical studies in C57BL/6 mice may underestimate the toxicity associated with NKG2D-CAR-T cells.
While a hierarchy in T-cell surface expression of our NKG2D-CARs (NKz < NK28z < NKz10) was consistent between T cells from BALB/c and C57BL/6 donors, we also observed startling differences when comparing CAR-T cell functionality between mouse strains. While all three NKG2D-CARs exhibited similar in vitro functionality in BALB/c T cells, the CARs displayed reduced overall functionality when expressed in C57BL/6 T cells, including capacity to induce both cytokine production and cytotoxicity. In both cases, functional abilities did not appear to correlate with the level of receptor surface expression. However, our data also suggests that measuring CAR-T-cell functionality in vitro does not equate to toxicity in vivo. Interestingly, the intensity of NKG2D staining on engineered T cells did correspond to the level of toxicity observed, with NKz showing the lowest NK28z an intermediate level, and NKz10 showing the highest in both NKG2D mean fluorescence intensity, as well as toxicity in both BALB/c and C57BL/6 mice. The NKz10-CAR was the only toxic CAR in C57BL/6 mice, consistent with the highest level of surface expression of the three NKG2D-CARs. This hierarchical toxicity was also observed in the magnitude of serum cytokine elevations in BALB/c mice. The high levels of inflammatory cytokines likely contributed to systemic toxicity, similar to those observed in some clinical trials of T-cell therapies.44 These data suggests that the level of NKG2D-CAR surface expression on each cell may be predictive of their in vivo functionality. Together, these studies reveal previously unappreciated strain-specific differences in CAR-T cell functionality following NKG2D-CAR engineering, indicating an important role for the recipient T cells in CAR function.
Overall, our data shows that NKG2D-based CARs have the potential to induce significant toxicities in vivo, especially if delivered subsequent to lymphodepletion regimens, like cyclophosphamide. NKz-CAR-T cells revealed similar functionality to the other NKG2D-CAR-T cells in vitro while demonstrating the lowest levels of observed toxicity in both BALB/c and C57BL/6 hosts. Thus, the NKz-CAR-T cells may be suitable for further clinical development. However, given the potential toxicities of these T cells, clinical translation should be undertaken with caution. Our study reveals important new information regarding potential toxicity of therapies targeting NKG2DLs and accentuates the need to identify tumor-restricted antigens, or antigens with limited expression off-tumor (on non-vital organs), for targeting with CAR-T cell therapy to avoid the potential for off-tumor toxicity.
Materials and Methods
Mice. Six- to eight-week-old female BALB/c or C57BL/6 mice were purchased from Charles River Breeding Laboratories (Wilmington, MA). All animal studies have received approval by the McMaster University Animal Research Ethics Board.
Generation of CAR retroviral vectors. For cloning CAR retroviruses, the shuttle plasmid pDONR222 oTK-P2A-oFL-T2A-eGFP, and retroviral vectors pRV2011-oFL and pRV100G eGFP were used.45 The NKz-CAR construct was generated according to previous reports,9 where the cytoplasmic region of murine CD3ζ was fused to full-length murine NKG2D via DNA synthesis (GenScript, Piscataway, NJ). The NKz-CAR was synthesized and inserted into the shuttle plasmid pDONR222 oTK-P2A-oFL-T2A-eGFP by deleting oTK-P2A and replacing eGFP with NKz, resulting in pDONR222 oFL-T2A-NKz (oFL = firefly luciferase,45 T2A = Thosea asigna virus self-cleaving 2A peptide 46). The oFL-T2A-NKz expression cassette was transferred into the retroviral vector pRV100G by LR recombination (Gateway LR Clonase II Enzyme Mix; Life Technologies, Grand Island, NY). Production of the NKz10-CAR construct was similar; pDONR222 oTK-P2A-oFL-T2A-eGFP was modified by replacing oTK with full-length murine DAP10 (accessed from NM_011827.3, codon optimized for murine expression, and synthesized (GenScript)) and eGFP with the synthesized NKz sequence, resulting in pDONR222 DAP10-P2A-oFL-T2A-NKz (P2A = porcine teschovirus-1 self-cleaving 2A peptide46). LR recombination transferred DAP10-P2A-oFL-T2A-NKz into pRV100G. The NK28z-CAR was constructed by modifying an existing second generation CAR vector (HER2scFv28z). The extracellular portion of NKG2D was amplified out of the NKz10-CAR (using the primers NKDG2DF: 5′-GTTCAAGGAGACATTTCAGCCTGTG-3′ and NKG2DR: 5′-ACAGCTCTCTTCATACAAATATAGGTATTC-3′) and inserted into the HER2scFv28z vector in place of the scFv and c-myc. The HER2-CAR used as the backbone for the NK28z-CAR was described previously, with the human sequences replaced by murine equivalents.47 In short, an scFv specific for HER2, a marker epitope from c-myc, the membrane proximal hinge region of murine CD8, the transmembrane and cytoplasmic regions of murine CD28, and the cytoplasmic region of murine CD3ζ were fused together and cloned into the retroviral vector pRV2011 oFL45 in place of firefly luciferase, leaving the IRES and Thy1.1 sequences intact.
Retroviral supernatants were generated by transient transfection of CAR retroviral vectors (10 μg) and the packaging plasmid pCL-Eco (10 μg) into Platinum-E (PLAT-E) cells48 using Lipofectamine 2000 (Life Technologies). After 24 hours of culture, media was changed. Retrovirus containing supernatants were collected 48 hours later and concentrated 10-fold using an Amicon Ultra 100K centrifugal filter (EMD Millipore, Billerica, MA).
Murine T-cell transduction. Freshly isolated splenocytes from BALB/C or C57BL/6 mice were cultured in 24-well plates in T-cell growth media (RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum (FBS), 10 mmol/l HEPES, 2 mmol/l l-glutamine, 0.1 mmol/l nonessential amino acids (Gibco, Life Technologies), 0.1 mg/ml normocin (Invivogen, San Diego, CA), 1 mmol/l sodium pyruvate, and 55 nmol/l β-mercaptoethanol). Splenocytes were activated with 0.1 μg/ml each hamster anti-mouse CD3 (clone 2C11; BD Biosciences, San Jose, CA) and hamster anti-mouse CD28 (clone 37.51; BD Biosciences), and cultured in the presence of 60 IU rhIL-2 (Peprotech, Rocky Hill, NJ). After 24 hours, T cells were transduced via spinfection, whereby 100 μl of concentrated retroviral supernatant was added to 3 × 106 cells in the presence of 1.6 μg/ml Polybrene (Sigma-Aldrich, St. Louis, MO) and 2 μg/ml Lipofectamine 2000. Cultures were centrifuged at 2,000 rpm at 32 °C for 90 minutes. Cells were incubated at 37 °C for 2–4 hours and supplemented with fresh medium and IL-2. Cells were expanded in T-cell growth media supplemented with rhIL-2. Four days after activation, T cells were stained for CAR expression and used for both in vitro and in vivo experiments.
Intracellular cytokine staining. T cells were stimulated in round-bottom 96-well plates coated with 2,000 ng/ml recombinant mouse Rae1β-Fc chimera (R&D Systems, Minneapolis, MN) or 1,000 ng/ml recombinant human HER2-Fc chimera (R&D Systems). Plates were coated 24–72 hours prior to stimulation at 4 °C. T cells were stimulated for 4 hours at 37 °C in the presence of brefeldin A (BD Pharmingen, San Jose, CA). After stimulation, cells were resuspended in PBS containing 5% FBS and stored at 4 °C overnight. Production of activation cytokines was determined by flow cytometry.
Flow cytometry antibodies and analytical instruments. The following antibodies used for flow cytometry were purchased from BD Biosciences: mouse Fc-Block, anti-CD4-PerCpCy5.5 (clone RM4-5), anti-CD8a-FITC (clone 53–6.7), anti-IFNγ (clone XMG1.2), and anti-TNFα (clone MP6-XT22). The following antibodies were purchased from eBiosciences (San Diego, CA): anti-CD8a-AlexaFluor 700 (clone 53–6.7) and anti-NKG2D-APC (clone CX5). Recombinant murine NKG2D-Fc (R&D Systems) was used to stain for NKG2D ligands, and detected with goat-anti-human IgG PE (Jackson ImmunoResearch). Viability staining was performed using the Molecular Probes LIVE/DEAD Fixable Near-IR kit (Life Technologies). For intracellular cytokine staining, CAR-T cells were fixed and permeabilized according to Cytofix/Cytoperm Fixation/Permeabilization protocol (BD Biosciences). Data were acquired on a FACSCanto or LSRII (BD Biosciences) and analyzed using FlowJo software (FlowJo LLC, Ashland, OR).
In vitro cytotoxicity assay. The NKG2DL+ murine 4T1.2 breast tumor line was used for in vitro cytotoxicity assays. We confirmed NKG2DL expression by flow cytometry following expansion from recently thawed vials. Varying ratios of transduced T cells were cocultured with 1.25 × 104 target cells (adhered overnight) per well in triplicate in 96-well flat-bottom plates in a 200 μl volume. After 6 hours of coculture, plates were washed three times with PBS, and 100 μl of a 10% alamarBlue (Life Technologies) in T-cell media was added. Three hours later, fluorescence was measured with excitation at 530 nm and emission at 590 nm using a Safire plate reader (Tecan, Maennedorf, Switzerland). Viability was calculated as the loss of fluorescence between experimental wells compared to untreated target cells.
Adoptive transfer and in vivo monitoring. For ACT studies, 107 viable CAR-T cells were administered i.v. in 200 μl of sterile PBS. In experiments using preconditioning CTX treatment, CTX (Sigma-Aldrich) was reconstituted in sterile PBS and administered i.p. at 150 mg/kg 24 hours prior to ACT. Temperatures were assessed by rectal probe at the indicated time points.
Multiplex cytokine analysis. We quantified 32 murine chemokines and cytokines using the Mouse Discovery Assay (Eve Technologies, Calgary, Canada). The 32-plex panel included: Eotaxin, G-CSF, GM-CSF, IFNγ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12 (p40), IL-12 (p70), IL-13, IL-15, IL-17, IP-10, KC, LIF, LIX, MCP-1, M-CSF, MIG, MIP-1α, MIP-1β, MIP-2, RANTES, TNFα, and VEGF. The assay sensitivities of these markers range from 0.3–33.3 pg/ml. Individual analyte values can be found in the Milliplex protocol. Serum samples were derived from terminal retro-orbital blood samples processed as per Eve Technologies recommendations. The multiplex assay was performed by Eve Technologies using the Bio-Plex 200 system and the Milliplex Mouse Cytokine/Chemokine Magnetic Bead Panel Kit according to their protocol. Heat maps were created using the HeatMapImage (version 6) module available on Gene Pattern (http://genepattern.broadinstitute.org/gp/pages/index.jsf). Luminex data were preprocessed using the “affy” package in R, with RMA background adjustment and quantile normalization procedures.49 Resulting cytokine expression values were transformed to the log2 scale. Linear models were fit for each cytokine using the “limma” package in R to test for differential expression for pre-specified contrasts.50 P values for each contrast were obtained for each cytokine and adjusted for multiple comparisons using the Benjamini–Hochberg procedure.51 After preprocessing, we confirmed that samples were separated into homogeneous groups matching experimental groups and performed principal component analysis (princomp function from “stats” package R) with all 32 cytokines.
Immunohistochemistry. Tissues were prepared for veterinary necropsy via whole body formalin perfusion as described previously.52 After fixation in 10% neutral buffered formalin, tissues were paraffin embedded, sectioned, and stained using hematoxylin and eosin at the Core Histology Facility, McMaster Immunology Research Centre.
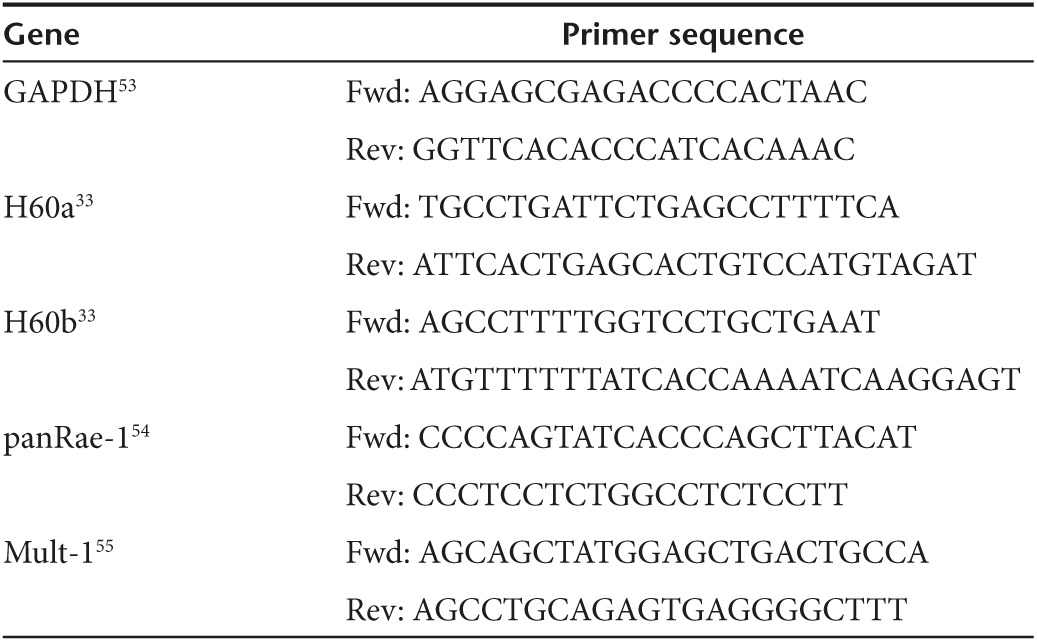
RNA extraction from lungs and quantitative real-time PCR. Lungs were perfused with PBS, excised, and snap-frozen in liquid nitrogen prior to storage at −80 °C. Tissues were homogenized in Trizol (Life Technologies) using a Polytron PT1200c (Kinematica, Bohemia, NY), with total RNA extracted as per the manufacturer's specifications. RNA samples were then treated with the DNA-Free kit (Ambion, Austin, TX), followed by reverse transcription using Superscript III First-Strand (Invitrogen, Life Technologies) according to the manufacturer's directions. Quantitative PCR was performed on a StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA) using Perfecta SYBR Green SuperMix with ROX (Quanta Biosciences, Gaithersburg, MD). Data for the indicated target genes (primer sequences found in Table 1) were analyzed via ΔΔCT method using GAPDH as the endogenous control. Analysis was performed using the StepOne Software.
Table 1. Primer sequences used for gene detection.
Statistical analysis. Student's t-tests were used to compare data between two groups. One and two-way ANOVA were used for analysis of more than two groups, with Bonferroni posttests used to evaluate significance between groups. Results were prepared using GraphPad Prism 5. Significant differences between means were defined as: *P < 0.05; **P < 0.01; ***P < 0.001.
SUPPLEMENTARY MATERIAL Figure S1. NKG2DCARs are well expressed on murine CD4+ T cells. Figure S2. Phenotypic profile of NKG2DCAR T cells in vitro. Figure S3. In vitro cytokine production from CAR-T cells. Figure S4. NKG2D ligand expression in the lungs of BALB/c mice. Table S1. Clinical observations following ACT into naive BALB/c mice. Table S2. Serum cytokine concentrations 8h post ACT. Table S3. Serum cytokine concentrations 8h post ACT into CTX-pretreated mice.
Acknowledgments
This work was supported by funds from the Terry Fox Foundation and the Canadian Institutes for Health Research. H.V. and J.A.H. were supported by scholarships from the Canadian Institutes for Health Research, the Government of Ontario and the Canadian Breast Cancer Foundation. J.L.B. holds the Canada Research Chair in Translational Cancer Immunology and the John Bienenstock Chair in Molecular Medicine. John Stagg (Institut du Cancer de Montreal, Montreal, QC) kindly provided the murine 4T1.2 breast tumor line.
Supplementary Material
References
- Grupp, SA, Kalos, M, Barrett, D, Aplenc, R, Porter, DL, Rheingold, SR et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med 368: 1509–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentjens, RJ, Davila, ML, Riviere, I, Park, J, Wang, X, Cowell, LG et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 5: 177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maus, MV, Grupp, SA, Porter, DL and June, CH (2014). Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood 123: 2625–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda, F, Vacchelli, E, Obrist, F, Eggermont, A, Galon, J, Hervé Fridman, W et al. (2014). Trial watch: adoptive cell transfer for anticancer immunotherapy. Oncoimmunology 3: e28344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear, P, Barber, A, Rynda-Apple, A and Sentman, CL (2013). NKG2D CAR T-cell therapy inhibits the growth of NKG2D ligand heterogeneous tumors. Immunol Cell Biol 91: 435–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nausch, N and Cerwenka, A (2008). NKG2D ligands in tumor immunity. Oncogene 27: 5944–5958. [DOI] [PubMed] [Google Scholar]
- Barber, A, Meehan, KR and Sentman, CL (2011). Treatment of multiple myeloma with adoptively transferred chimeric NKG2D receptor-expressing T cells. Gene Ther 18: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, WH, Vong, QP, Lin, W, Janke, L, Chen, T and Leung, W (2013). Modulation of NKG2D ligand expression and metastasis in tumors by spironolactone via RXRγ activation. J Exp Med 210: 2675–2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T, Lemoi, BA and Sentman, CL (2005). Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood 106: 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, T and Sentman, CL (2013). Mouse tumor vasculature expresses NKG2D ligands and can be targeted by chimeric NKG2D-modified T cells. J Immunol 190: 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber, A, Zhang, T, DeMars, LR, Conejo-Garcia, J, Roby, KF and Sentman, CL (2007). Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res 67: 5003–5008. [DOI] [PubMed] [Google Scholar]
- Jamieson, AM, Diefenbach, A, McMahon, CW, Xiong, N, Carlyle, JR and Raulet, DH (2002). The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity 17: 19–29. [DOI] [PubMed] [Google Scholar]
- Long, EO (2002). Versatile signaling through NKG2D. Nat Immunol 3: 1119–1120. [DOI] [PubMed] [Google Scholar]
- Groh, V, Rhinehart, R, Randolph-Habecker, J, Topp, MS, Riddell, SR and Spies, T (2001). Costimulation of CD8alphabeta T cells by NKG2D via engagement by MIC induced on virus-infected cells. Nat Immunol 2: 255–260. [DOI] [PubMed] [Google Scholar]
- Verneris, MR, Karimi, M, Karami, M, Baker, J, Jayaswal, A and Negrin, RS (2004). Role of NKG2D signaling in the cytotoxicity of activated and expanded CD8+ T cells. Blood 103: 3065–3072. [DOI] [PubMed] [Google Scholar]
- Upshaw, JL and Leibson, PJ (2006). NKG2D-mediated activation of cytotoxic lymphocytes: unique signaling pathways and distinct functional outcomes. Semin Immunol 18: 167–175. [DOI] [PubMed] [Google Scholar]
- Savoldo, B, Ramos, CA, Liu, E, Mims, MP, Keating, MJ, Carrum, G et al. (2011). CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 121: 1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, DG, Ye, Q, Carpenito, C, Poussin, M, Wang, LP, Ji, C et al. (2011). In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res 71: 4617–4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milone, MC, Fish, JD, Carpenito, C, Carroll, RG, Binder, GK, Teachey, D et al. (2009). Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol Ther 17: 1453–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till, BG, Jensen, MC, Wang, J, Qian, X, Gopal, AK, Maloney, DG et al. (2012). CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119: 3940–3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, J, Song, Y, Bakker, AB, Bauer, S, Spies, T, Lanier, LL et al. (1999). An activating immunoreceptor complex formed by NKG2D and DAP10. Science 285: 730–732. [DOI] [PubMed] [Google Scholar]
- Brentjens, RJ, Rivière, I, Park, JH, Davila, ML, Wang, X, Stefanski, J et al. (2011). Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118: 4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheadle, EJ, Gilham, DE and Hawkins, RE (2008). The combination of cyclophosphamide and human T cells genetically engineered to target CD19 can eradicate established B-cell lymphoma. Br J Haematol 142: 65–68. [DOI] [PubMed] [Google Scholar]
- Dotti, G, Gottschalk, S, Savoldo, B and Brenner, MK (2014). Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 257: 107–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champsaur, M and Lanier, LL (2010). Effect of NKG2D ligand expression on host immune responses. Immunol Rev 235: 267–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eagle, RA, Jafferji, I and Barrow, AD (2009). Beyond stressed self: evidence for NKG2D ligand expression on healthy cells. Curr Immunol Rev 5: 22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchers, MT, Harris, NL, Wesselkamper, SC, Vitucci, M and Cosman, D (2006). NKG2D ligands are expressed on stressed human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol 291: L222–L231. [DOI] [PubMed] [Google Scholar]
- Kraetzel, K, Stoelcker, B, Eissner, G, Multhoff, G, Pfeifer, M, Holler, E et al. (2008). NKG2D-dependent effector function of bronchial epithelium-activated alloreactive T-cells. Eur Respir J 32: 563–570. [DOI] [PubMed] [Google Scholar]
- González, S, López-Soto, A, Suarez-Alvarez, B, López-Vázquez, A and López-Larrea, C (2008). NKG2D ligands: key targets of the immune response. Trends Immunol 29: 397–403. [DOI] [PubMed] [Google Scholar]
- Ogasawara, K, Benjamin, J, Takaki, R, Phillips, JH and Lanier, LL (2005). Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol 6: 938–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H, Hardamon, C, Sagoe, B, Ngolab, J and Bui, JD (2011). Studies of the H60a locus in C57BL/6 and 129/Sv mouse strains identify the H60a 3'UTR as a regulator of H60a expression. Mol Immunol 48: 539–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkannan, S, Shih, PP, Eden, PA, Horng, T, Zuberi, AR, Christianson, G et al. (1998). The molecular and functional characterization of a dominant minor H antigen, H60. J Immunol 161: 3501–3509. [PubMed] [Google Scholar]
- Takada, A, Yoshida, S, Kajikawa, M, Miyatake, Y, Tomaru, U, Sakai, M et al. (2008). Two novel NKG2D ligands of the mouse H60 family with differential expression patterns and binding affinities to NKG2D. J Immunol 180: 1678–1685. [DOI] [PubMed] [Google Scholar]
- Cerwenka, A and Lanier, LL (2003). NKG2D ligands: unconventional MHC class I-like molecules exploited by viruses and cancer. Tissue Antigens 61: 335–343. [DOI] [PubMed] [Google Scholar]
- Ericsson, AC, Davis, JW, Spollen, W, Bivens, N, Givan, S, Hagan, CE et al. (2015). Effects of vendor and genetic background on the composition of the fecal microbiota of inbred mice. PLoS One 10: e0116704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinohe, T, Pang, IK, Kumamoto, Y, Peaper, DR, Ho, JH, Murray, TS et al. (2011). Microbiota regulates immune defense against respiratory tract influenza A virus infection. Proc Natl Acad Sci USA 108: 5354–5359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud, S, Saccheri, F, Mignot, G, Yamazaki, T, Daillère, R, Hannani, D et al. (2013). The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science 342: 971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretta, L and Moretta, A (2004). Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J 23: 255–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, EO, Kim, HS, Liu, D, Peterson, ME and Rajagopalan, S (2013). Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol 31: 227–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, YH, Connolly, J, Shimasaki, N, Mimura, K, Kono, K and Campana, D (2013). A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res 73: 1777–1786. [DOI] [PubMed] [Google Scholar]
- Zhang, T and Sentman, CL (2011). Cancer immunotherapy using a bispecific NK receptor fusion protein that engages both T cells and tumor cells. Cancer Res 71: 2066–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner, M, Götz, G, Proff, J, Schaft, N, Dörrie, J, Full, F et al. (2012). Redirecting T cells to Ewing's sarcoma family of tumors by a chimeric NKG2D receptor expressed by lentiviral transduction or mRNA transfection. PLoS One 7: e31210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song, DG, Ye, Q, Santoro, S, Fang, C, Best, A and Powell, DJ Jr (2013). Chimeric NKG2D CAR-expressing T cell-mediated attack of human ovarian cancer is enhanced by histone deacetylase inhibition. Hum Gene Ther 24: 295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauss, HJ and Morris, EC (2013). Immunotherapy with gene-modified T cells: limiting side effects provides new challenges. Gene Ther 20: 1029–1032. [DOI] [PubMed] [Google Scholar]
- Rabinovich, BA, Ye, Y, Etto, T, Chen, JQ, Levitsky, HI, Overwijk, WW et al. (2008). Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA 105: 14342–14346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szymczak, AL, Workman, CJ, Wang, Y, Vignali, KM, Dilioglou, S, Vanin, EF et al. (2004). Correction of multi-gene deficiency in vivo using a single ‘self-cleaving' 2A peptide-based retroviral vector. Nat Biotechnol 22: 589–594. [DOI] [PubMed] [Google Scholar]
- Haynes, NM, Trapani, JA, Teng, MW, Jackson, JT, Cerruti, L, Jane, SM et al. (2002). Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 100: 3155–3163. [DOI] [PubMed] [Google Scholar]
- Morita, S, Kojima, T and Kitamura, T (2000). Plat-E: an efficient and stable system for transient packaging of retroviruses. Gene Ther 7: 1063–1066. [DOI] [PubMed] [Google Scholar]
- Irizarry, RA, Hobbs, B, Collin, F, Beazer-Barclay, YD, Antonellis, KJ, Scherf, U et al. (2003). Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 4: 249–264. [DOI] [PubMed] [Google Scholar]
- Smyth, GK (2005). limma: Linear Models for Microarray Data. In: Gentleman, R, Carey, V, Dudoit, R, Irizarry, RA and Huber, W (eds.) Bioinformatics and Computational Biology Solutions using R and Bioconductor, Chapter 23. Springer-Verlag: New York. pp. 397–420. <http://link.springer.com>. [Google Scholar]
- Benjamini, Y (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B 57: 289–300. [Google Scholar]
- Kwiecien, JM, Blanco, M, Fox, JG, Delaney, KH and Fletch, AL (2000). Neuropathology of bouncer Long Evans, a novel dysmyelinated rat. Comp Med 50: 503–510. [PubMed] [Google Scholar]
- McGray, AJ, Hallett, R, Bernard, D, Swift, SL, Zhu, Z, Teoderascu, F et al. (2014). Immunotherapy-induced CD8+ T cells instigate immune suppression in the tumor. Mol Ther 22: 206–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa, N, Cedile, O, Pollet-Villard, X, Bagnis, C, Durbec, P and Boucraut, J (2011). RAE-1 is expressed in the adult subventricular zone and controls cell proliferation of neurospheres. Glia 59: 35–44. [DOI] [PubMed] [Google Scholar]
- Hansen, CH, Holm, TL, Krych, Ł, Andresen, L, Nielsen, DS, Rune, I et al. (2013). Gut microbiota regulates NKG2D ligand expression on intestinal epithelial cells. Eur J Immunol 43: 447–457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.