Abstract
OBJECTIVES
Visit-to-visit variability of blood pressure is an important independent risk factor for premature death and cardiovascular events, but relatively little is known about this phenomenon in patients with chronic kidney disease not yet on dialysis.
METHODS
We conducted a retrospective study in a community-based cohort of 114,900 adults with chronic kidney disease stages 3–4 (estimated glomerular filtration rate 15–59 mL/min per 1.73 m2). We hypothesized that visit-to-visit variability of blood pressure would be independently associated with higher risks of death, incident treated end-stage renal disease, and cardiovascular events. We defined systolic visit-to-visit variability of blood pressure using three metrics: (1) coefficient of variation (2) standard deviation of the mean systolic blood pressure, and (3) average real variability.
RESULTS
The highest versus the lowest quintile of the coefficient of variation was associated with higher adjusted rates of death (hazard ratio 1.22; 95% confidence interval 1.11–1.34) and hemorrhagic stroke (hazard ratio 1.91, confidence interval 1.36–2.68). Visit-to-visit variability of blood pressure was inconsistently associated with heart failure, and was not significantly associated with acute coronary syndrome and ischemic stroke. Results were similar when using the other two visit-to-visit variability of blood pressure. Visit-to-visit variability of blood pressure had inconsistent associations with end-stage renal disease, perhaps due to the relatively low incidences of this outcome.
CONCLUSIONS
Higher visit-to-visit variability of blood pressure is independently associated with higher rates of death and hemorrhagic stroke in patients with moderate to advanced chronic kidney disease not yet on dialysis.
Keywords: renal insufficiency, hypertension, heart disease, mortality, epidemiology
INTRODUCTION
There is increasing evidence that visit-to-visit variability of blood pressure (VVV of BP), defined as variation in blood pressure that occurs over days to months, is an important risk factor for premature death and cardiovascular events, independent of mean BP level [1]. Chronic kidney disease (CKD) is a known risk factor for cardiovascular disease, and patients with CKD are among the highest risk populations for cardiovascular morbidity and mortality [2]. However, relatively little is known about VVV of BP in patients with CKD not yet on dialysis, because most previous studies were focused on highly selected cohorts from randomized clinical trials [3, 4] or on patients with end-stage renal disease (ESRD) receiving maintenance hemodialysis [5–11]. The few studies that were conducted in “real-world” CKD cohorts were limited by relatively small sample sizes [12–14].
To address some of the limitations of these previous studies, we conducted a retrospective study of VVV of BP in a large, community-based cohort of adults with CKD stages 3–4 (estimated glomerular filtration rate [GFR] 15–59 mL/min per 1.73m2). We hypothesized that VVV of BP would be independently associated with higher risks of death, incident treated ESRD, and cardiovascular events in patients with CKD who were not yet on dialysis.
METHODS
Cohort Assembly
Kaiser Permanente Northern California is a large integrated healthcare delivery system providing comprehensive care to >3.5 million persons in the San Francisco and greater Bay Area. The Kaiser Permanente Northern California population is highly representative of the surrounding population except for slightly lower representation at the extremes of age and income [15, 16].
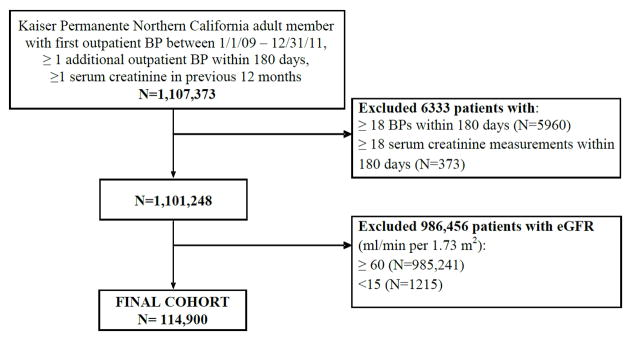
The study sample included all adults aged ≥18 years with an outpatient, non-emergency department BP measurement between January 1, 2009 and December 31, 2011, and at least one more BP measurement within 6 months of the initial measurement. We chose these inclusion dates due to improved data quality, uniformity, and BP control for Kaiser Permanente as a whole during this time frame [17]. The initial BP measurement was set as the study index date. We excluded persons with <6 months of continuous membership after the index date, or who received maintenance dialysis or organ transplant prior to and within 6 months after index date. Persons without at least one ambulatory serum creatinine measurement within 12 months prior, or with ≥18 BP measurements or ambulatory serum creatinine measurements within 180 days after their index date were also excluded (Figure 1). As a sensitivity analysis, we restricted the cohort to patients with ≥4 BP measurements available. Finally, because we wanted to focus on patients with stable CKD stages 3–4, we relied only on outpatient, non-emergency department values of serum creatinine and excluded patients with any estimated GFR (using the CKD-EPI equation [18]) of <15 or ≥60 mL/min per 1.73m2 in the 365 days before the index date. For patients with more than one serum creatinine determination during this time period, the baseline estimated GFR was calculated from the serum creatinine value measured closest to the index date.
Figure 1.
Cohort assembly of patients with chronic kidney disease stage 3–4 and at least 2 but no more than 18 outpatient blood pressure measurements within 180 days.
Visit-to-Visit Variability of Blood Pressure
All outpatient clinics used automated sphygmomanometers operated by trained medical assistants, with repeat measurements performed as needed by physicians using aneroid sphygmomanometers[17]. We analyzed BP measurements during a 6-month period after the initial outpatient measurement to characterize VVV of BP (Figure 2). Because systolic BP is generally considered to be a stronger risk factor for cardiovascular disease and other outcomes than diastolic BP, we focused on systolic VVV of BP [19]. We used three metrics to define VVV of BP[1]: (1) the coefficient of variation (standard deviation of mean systolic BP divided by mean systolic BP); (2) the standard deviation of the mean systolic BP and (3) the average real variability, which takes an average of the absolute differences in BP over consecutive visits, thereby accounting for the order in which the measurements were made [1, 20]. We categorized the cohort into quintiles of VVV of BP based on each metric.
Figure 2.
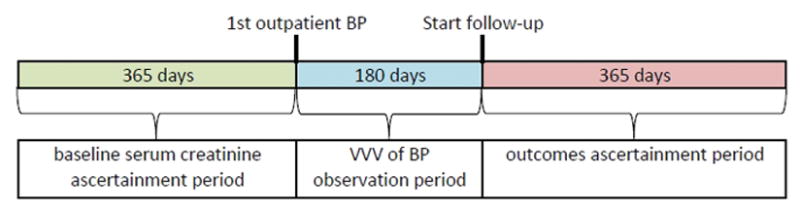
Study design showing periods of kidney function ascertainment, visit- to-visit variability of blood pressure ascertainment, and outcomes ascertainment.
Outcomes
Follow-up occurred after the initial 6-month window used to characterize VVV of BP, and all patients were followed for the subsequent 12 months (Figure 2). Censoring during this follow-up period occurred at the end of follow-up or disenrollment from the health plan. Outcomes of interest included death from any cause, development of treated ESRD, and cardiovascular events. Deaths were comprehensively identified from hospital and billing claims databases, administrative health plan databases, state death certificate registries, and Social Security Administration vital status files. Occurrence of treated ESRD, defined as initiation of maintenance dialysis or receipt of kidney transplant, was determined based on a comprehensive ESRD treatment registry [21, 22]. Cardiovascular events included hospitalization for acute coronary syndrome (defined as myocardial infarction or unstable angina), heart failure, ischemic stroke, and hemorrhagic stroke. Cardiovascular events were ascertained based on relevant diagnostic International Classification of Disease, Ninth Edition (ICD-9) codes found in hospitalization and billing claims databases using previously validated algorithms (specific codes available on request) [23–25]. Cardiovascular events of interest that occurred during the initial 6-month observation period to define VVV of BP were excluded from the analyses.
Covariates
We ascertained information on comorbid conditions during a five-year period prior to the index date, based on relevant ICD-9 diagnosis and procedure codes as previously described [23–27]. We collected baseline data on diagnoses of: acute myocardial infarction, heart failure, intracranial hemorrhage, ischemic stroke or transient ischemic attack, ventricular tachycardia or fibrillation, peripheral arterial disease, mitral and/or aortic valvular disease, atrial flutter and or fibrillation, diabetes mellitus, hypertension, dyslipidemia, smoking, dementia, depression, chronic liver disease, chronic lung disease, systemic cancer, and hospitalized bleed. We ascertained baseline medication use for cardiovascular and diabetes medications from outpatient pharmacy dispensing records in the 120 days prior to the index date.
We ascertained baseline diastolic BP, baseline and time-updated systolic BP, estimated GFR, proteinuria based on urine dipstick results[28], serum low-density lipoprotein (LDL) and high-density lipoprotein (HDL) cholesterol levels and hemoglobin from health plan laboratory databases. Baseline laboratory results were obtained during the 12 months before the index date.
Statistical Approach
Analyses were conducted using SAS statistical software, version 9.3 (Cary, N.C.). A two-sided P value <0.05 was considered significant. All analyses were performed separately for each metric of VVV of BP. We performed multivariable linear regression to identify independent correlates of VVV of BP and considered all variables listed in Table 1. To examine the association among quintiles of VVV of BP and clinical outcomes and to avoid assumptions of linearity, we calculated rates (per 100 person-years) and associated 95% confidence limits for each outcome. We conducted proportional hazard regression to examine the association between each quintile of VVV of BP with the lowest quintile as the referent (i.e. 1st quintile) and outcomes after adjustment for potential confounding factors, and present results as an adjusted hazard ratio (HR) and 95% confidence intervals (CI).
Table 1.
Baseline characteristics by quintile of visit-to-visit variability of systolic blood pressure using coefficient of variation among 114,900 adults with chronic kidney disease stage 3–4
| Visit-to-Visit Variability in Blood Pressure Using Coefficient of Variation | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Characteristic | 1st Quintile (N=22,983) | 2nd Quintile (N=22,977) | 3rd Quintile (N=22,980) | 4th Quintile (N=22,980) | 5th Quintile (N=22,980) | P Value |
| Age, yr, mean (SD) | 73.8 (11.3) | 73.7 (11.3) | 73.9 (11.1) | 74.8 (10.8) | 75.8 (10.8) | <0.001 |
| Women, % | 58.5 | 58.5 | 58.5 | 58.7 | 59.2 | 0.56 |
| Race, % | <0.001 | |||||
| White | 74.6 | 75.5 | 75.4 | 76.4 | 75.6 | |
| Black | 12.0 | 12.0 | 12.5 | 11.7 | 11.9 | |
| Asian/Pacific Islander | 10.7 | 10.1 | 10.0 | 9.8 | 10.4 | |
| Other/Unknown | 2.6 | 2.4 | 2.1 | 2.1 | 2.1 | |
| Hispanic ethnicity, % | 9.2 | 9.4 | 9.4 | 9.7 | 10.0 | <0.05 |
| Current or former smoker, % | 43.4 | 45.4 | 46.9 | 48.7 | 49.1 | <0.001 |
| Cardiovascular history, % | ||||||
| Myocardial infarction | 2.1 | 2.5 | 2.8 | 3.0 | 3.6 | <0.001 |
| Heart failure | 8.3 | 10.9 | 12.3 | 13.2 | 14.3 | <0.001 |
| Intracranial hemorrhage | 0.6 | 0.6 | 0.5 | 0.6 | 0.7 | <0.05 |
| Ischemic stroke or transient ischemic attack | 2.9 | 3.1 | 3.3 | 3.6 | 4.3 | <0.001 |
| Peripheral arterial disease | 1.1 | 1.2 | 1.6 | 1.7 | 2.3 | <0.001 |
| Mitral and/or aortic valvular disease | 5.2 | 5.8 | 6.6 | 7.1 | 7.2 | <0.001 |
| Atrial fibrillation and/or flutter | 9.5 | 10.7 | 11.6 | 12.1 | 12.5 | <0.001 |
| Medical history, % | ||||||
| Diabetes mellitus | 27.1 | 28.2 | 29.7 | 31.3 | 32.7 | <0.001 |
| Hypertension | 83.4 | 83.9 | 85.7 | 88.0 | 90.4 | <0.001 |
| Dyslipidemia | 69.9 | 70.1 | 71.4 | 72.9 | 74.3 | <0.001 |
| Diagnosed dementia | 5.4 | 5.0 | 4.9 | 5.5 | 7.0 | <0.001 |
| Diagnosed depression | 15.4 | 17.7 | 18.4 | 18.2 | 18.2 | <0.001 |
| Chronic liver disease | 1.4 | 1.8 | 1.8 | 1.8 | 1.6 | <0.01 |
| Chronic lung disease | 20.2 | 22.6 | 23.0 | 23.6 | 23.3 | <0.001 |
| Systemic cancer | 14.1 | 15.7 | 16.4 | 17.5 | 16.6 | <0.001 |
| Hospitalized bleed | 2.3 | 2.6 | 2.7 | 2.8 | 3.3 | <0.001 |
| Baseline lab values, % | ||||||
| Estimated GFR, mL/min/1.73m2 | <0.001 | |||||
| 45–59 | 69.9 | 67.2 | 65.2 | 63.1 | 59.7 | |
| 30–44 | 25.6 | 26.8 | 27.7 | 29.7 | 32.0 | |
| 15–29 | 4.6 | 6.1 | 7.2 | 7.2 | 8.3 | |
| Hemoglobin, g/dL | <0.001 | |||||
| ≥13.0 | 47.6 | 46.6 | 45.2 | 43.1 | 41.3 | |
| 12.0–12.9 | 16.5 | 17.0 | 17.4 | 18.0 | 18.7 | |
| 11.0–11.9 | 8.5 | 9.9 | 11.0 | 11.6 | 12.3 | |
| < 11.0 | 4.4 | 5.4 | 6.9 | 7.7 | 8.0 | |
| Unknown | 22.9 | 21.1 | 19.5 | 19.6 | 19.6 | |
| Documented Proteinuria | 15.0 | 17.2 | 19.1 | 20.3 | 22.2 | <0.001 |
| Baseline medication use, % | ||||||
| ACE inhibitor/ Angiotensin II receptor blocker | 50.9 | 50.6 | 52.6 | 55.4 | 57.6 | <0.001 |
| Aldosterone receptor antagonist | 1.4 | 1.7 | 1.8 | 1.9 | 1.7 | <0.001 |
| Beta blocker | 43.4 | 45.0 | 47.2 | 49.3 | 51.9 | <0.001 |
| Minoxidil | 0.1 | 0.1 | 0.2 | 0.2 | 0.2 | <0.01 |
| Calcium channel blocker | 24.6 | 26.1 | 26.3 | 26.3 | 26.1 | <0.001 |
| Diuretic | 50.6 | 51.3 | 52.8 | 53.3 | 52.5 | <0.001 |
| Hydralazine | 2.4 | 3.0 | 3.5 | 4.0 | 4.6 | <0.001 |
| Nitrates | 3.4 | 4.2 | 4.9 | 5.5 | 6.3 | <0.001 |
| Digoxin | 2.5 | 2.7 | 3.0 | 3.0 | 2.9 | <0.01 |
| Statin | 54.3 | 54.6 | 55.8 | 56.9 | 57.6 | <0.001 |
| Other lipid-lowering agent | 4.8 | 5.3 | 5.2 | 5.4 | 5.1 | <0.05 |
| Non-aspirin antiplatelet agent | 4.2 | 4.7 | 4.9 | 5.3 | 6.2 | <0.001 |
| Diabetic therapy | 20.3 | 21.4 | 22.9 | 23.6 | 24.4 | <0.001 |
| Number of blood pressure measurements, median (IQR) | 2 (2–3) | 3 (2–5) | 4 (3–6) | 4 (3–6) | 3 (2––5) | <0.001 |
| Systolic blood pressure, mm Hg, mean (SD) | 129 (12) | 130 (14) | 131 (16) | 133 (20) | 136 (26) | <0.001 |
| Diastolic blood pressure, mm Hg, mean (SD) | 71 (10) | 71 (10) | 71 (11) | 71 (11) | 72 (13) | <0.001 |
Approval for the study was obtained from the institutional review boards of Kaiser Permanente Northern California and Stanford University. A waiver of informed consent was obtained due to the nature of the study.
RESULTS
Our cohort consisted of 114,900 patients with CKD stages 3–4 (Figure 1). The average VVV of BP defined as the coefficient of variation was 9.0%, the standard deviation was 11.9 mm Hg, and the average real variability was 14.6 mm Hg. There was a higher prevalence of most comorbid conditions in the higher quintiles of VVV of BP (Table 1, Supplemental tables 1 & 2). In multivariable-adjusted models, older age, higher mean systolic BP, and more BP measurements were associated with higher VVV of BP, as were lower estimated GFR and proteinuria (Supplemental Table 3). Baseline use of angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers, beta-blockers and nitrates was associated with higher VVV of BP, while use of calcium channel blockers and diuretics was associated with lower VVV of BP. (Supplemental Table 3).
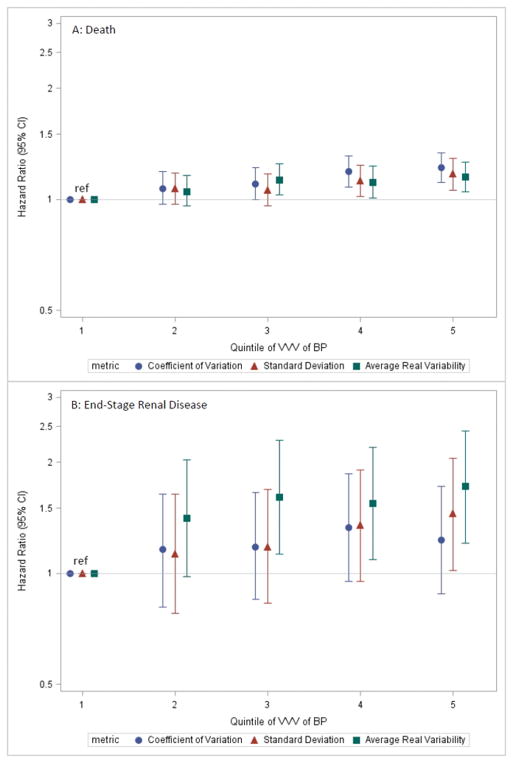
During the 12-month follow-up period, there were 5114 deaths (4.6 per 100 person-years). Event rates were generally higher in higher quintiles of VVV of BP (Table 2 & Supplemental Table 4), an association which persisted in fully adjusted models (Figure 3A). Patients in the highest quintile of coefficient of variation of BP had a 22% (CI 11% to 34%) higher rate of death compared with patients in the lowest quintile. Similarly, patients in the highest versus lowest quintiles of standard deviation and average real variability had 17% (CI 6% to 29%) and 15% (CI 5% to 26%) higher rates of death, respectively.
Table 2.
Crude event rates by quintile of visit-to-visit variability of systolic blood pressure defined as the coefficient of variation or average real variability among 114,900 adults with chronic kidney disease stage 3–4.
| Visit-to-Visit Variability in Blood Pressure Using Coefficient of Variation | |||||
|---|---|---|---|---|---|
|
| |||||
| 1st Quintile | 2nd Quintile | 3rd Quintile | 4th Quintile | 5th Quintile | |
| Death | |||||
| Events, N | 721 | 878 | 992 | 1194 | 1329 |
| Rate per 100 p-y (95% CI) | 3.23 (2.99, 3.47) | 3.95 (3.69, 4.21) | 4.48 (4.20, 4.76) | 5.42 (5.11, 5.73) | 6.05 (5.73, 6.38) |
| End-stage renal disease | |||||
| Events, N | 50 | 90 | 133 | 162 | 147 |
| Rate per 100 p-y (95% CI) | 0.22 (0.16, 0.29) | 0.41 (0.32, 0.49) | 0.60 (0.50, 0.70) | 0.74 (0.62, 0.85) | 0.67 (0.56, 0.78) |
| Acute coronary syndrome | |||||
| Events, N | 194 | 214 | 249 | 292 | 340 |
| Rate per 100 p-y (95% CI) | 0.87 (0.75, 0.99) | 0.96 (0.83, 1.09) | 1.12 (0.98, 1.26) | 1.33 (1.17, 1.48) | 1.55 (1.38, 1.71) |
| Heart failure | |||||
| Events, N | 270 | 423 | 520 | 660 | 655 |
| Rate per 100 p-y (95% CI) | 1.21 (1.07, 1.35) | 1.90 (1.72, 2.09) | 2.35 (2.15, 2.55) | 3.00 (2.77, 3.22) | 2.98 (2.76, 3.21) |
| Ischemic Stroke | |||||
| Events, N | 138 | 133 | 144 | 193 | 209 |
| Rate per 100 p-y (95% CI) | 0.62 (0.52, 0.72) | 0.60 (0.50, 0.70) | 0.65 (0.54, 0.76) | 0.88 (0.75, 1.00) | 0.95 (0.82, 1.08) |
| Hemorrhagic Stroke | |||||
| Events, N | 96 | 146 | 187 | 185 | 213 |
| Rate per 100 p-y (95% CI) | 0.43 (0.34, 0.52) | 0.66 (0.55, 0.76) | 0.84 (0.72, 0.97) | 0.84 (0.72, 0.96) | 0.97 (0.84, 1.10) |
| Visit-to-Visit Variability in Blood Pressure Using Average Real Variability | |||||
|---|---|---|---|---|---|
|
| |||||
| 1st Quintile | 2nd Quintile | 3rd Quintile | 4th Quintile | 5th Quintile | |
| Death | |||||
| Events, N | 761 | 1009 | 1070 | 1108 | 1166 |
| Rate per 100 p-y (95% CI) | 3.45 (3.21–3.70) | 4.54 (4.26–4.82) | 5.08 (4.77–5.38) | 4.98 (4.69–5.28) | 5.05 (4.76–5.34) |
| End-stage renal disease | |||||
| Events, N | 42 | 99 | 129 | 154 | 158 |
| Rate per 100 p-y (95% CI) | 0.19 (0.13–0.25) | 0.45 (0.36–0.53) | 0.61 (0.51–0.72) | 0.69 (0.58–0.80) | 0.69 (0.58–0.79) |
| Acute coronary syndrome | |||||
| Events, N | 191 | 227 | 239 | 295 | 337 |
| Rate per 100 p-y (95% CI) | 0.87 (0.74–0.99) | 1.02 (0.89–1.15) | 1.13 (0.99–1.28) | 1.33 (1.18–1.48) | 1.46 (1.30–1.62) |
| Heart failure | |||||
| Events, N | 307 | 485 | 534 | 602 | 600 |
| Rate per 100 p-y (95% CI) | 1.39 (1.24–1.55) | 2.18 (1.99–2.38) | 2.53 (2.32–2.75) | 2.71 (2.49–2.92) | 2.60 (2.39–2.81) |
| Ischemic Stroke | |||||
| Events, N | 133 | 143 | 142 | 173 | 226 |
| Rate per 100 p-y (95% CI) | 0.60 (0.50–0.71) | 0.64 (0.54–0.75) | 0.67 (0.56–0.78) | 0.78 (0.66–0.89) | 0.98 (0.85–1.11) |
| Hemorrhagic Stroke | |||||
| Events, N | 130 | 105 | 213 | 174 | 205 |
| Rate per 100 p-y (95% CI) | 0.59 (0.49–0.69) | 0.47 (0.38–0.56) | 1.01 (0.87–1.15) | 0.78 (0.67–0.90) | 0.89 (0.77–1.01) |
Figure 3. Adjusted hazard ratios (error bars = 95% CI) showing the association of quintile of VVV of BP defined using the specified metrics with (A) death and (B) end-stage renal disease.
Covariates adjusted for included age, gender, race, known Hispanic ethnicity, current or former smoker, systolic blood pressure, number of blood pressure measurements, hospitalized acute myocardial infarction, heart failure, hospitalized intracranial hemorrhage, hospitalized ischemic stroke or transient ischemic attack, peripheral arterial disease, Mitral and/or aortic valvular disease, atrial fibrillation and/or flutter, diabetes mellitus, hypertension, dyslipidemia, diagnosed dementia, diagnosed depression, chronic liver disease, chronic lung disease, systemic cancer, hospitalized bleed, documented proteinuria, estimated glomerular filtration rate, hemoglobin, angiotensin-converting enzyme inhibitor / angiotensin II receptor blocker, aldosterone receptor antagonist, beta blocker, minoxidil, calcium channel blocker, diuretic, hydralazine, nitrates, digoxin, statin, other lipid-lowering agent, non-aspirin antiplatelet agent, and diabetic therapy. Abbreviations: VVV of BP = visit-to-visit variability of blood pressure; CI=confidence interval.
Treated ESRD developed in 582 patients during follow-up (0.5 per 100 person-years) and event rates were generally higher in higher quintiles of VVV of BP (Table 2 & Supplemental Table 4). In fully adjusted models, higher quintiles of the coefficient of variation had no significant associations with ESRD (Figure 3B). In contrast, the highest quintile of the standard deviation was associated with a 1.45-fold (CI 1.02–2.05) higher risk of ESRD, and higher average real variability was associated with a significantly higher rate of ESRD (HR 1.55, CI 1.09–2.20 for 4th quintile; HR 1.72, CI 1.21–2.43 for 5th quintile versus 1st quintile; Figure 3B).
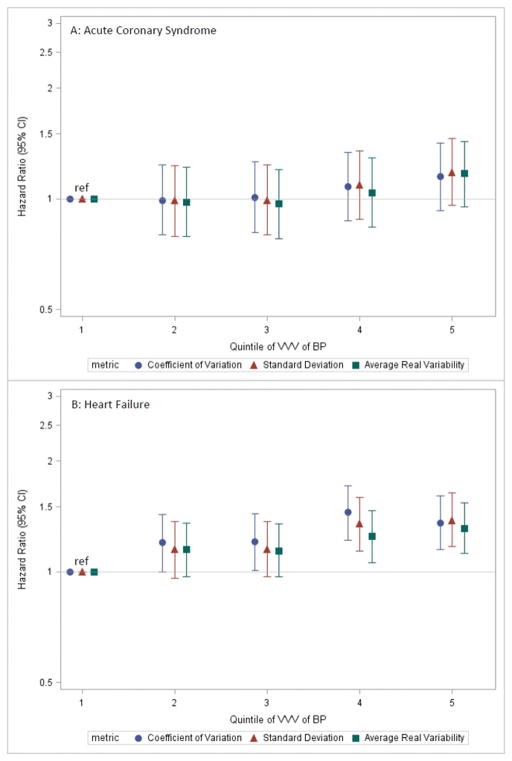
Cardiac events were relatively common during follow-up: 1289 hospitalizations for acute coronary syndrome (1.2 per 100 person-years) and 2528 for heart failure (2.3 per 100 person-years). Event rates were generally higher in higher quintiles of VVV of BP (Table 2 & Supplemental Table 4). In fully adjusted models, there was no significant association with rate of acute coronary syndrome, but patients in the higher quintiles of the coefficient of variation of systolic BP had higher rates of heart failure (Figure 4). Results were consistent regardless of the metric of VVV of BP used.
Figure 4. Adjusted hazard ratios (error bars = 95% CI) showing the association of quintile of VVV of BP defined using the specified metrics with (A) acute coronary syndrome and (B) heart failure.
Covariates adjusted for are the same as for Figure 3. Abbreviations: VVV of BP = visit-to-visit variability of blood pressure; CI=confidence interval.
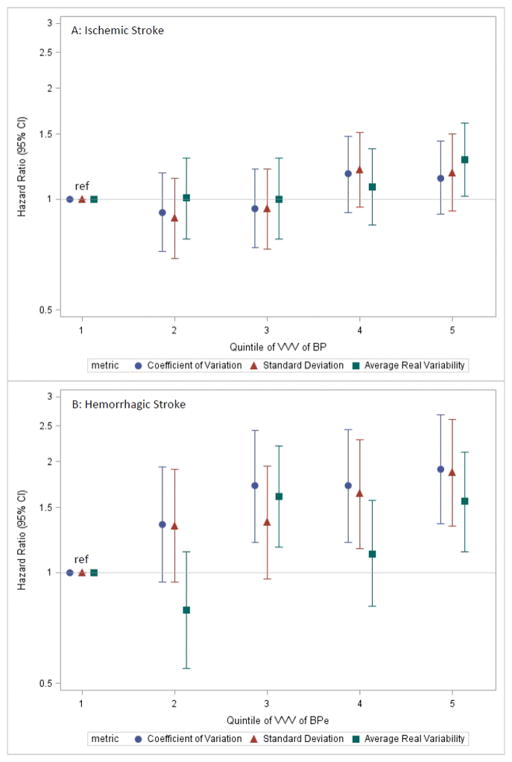
The incidence of stroke was relatively low: 817 ischemic strokes (0.7 per 100 person-years), and 827 hemorrhagic strokes (0.8 per 100 person-years), and event rates were generally higher in higher quintiles of VVV of BP using all three metrics (Table 2 & Supplemental Table 4). In fully adjusted models, higher VVV of BP was generally not associated with a higher rates of ischemic stroke (Figure 5A), but was associated with higher rates of hemorrhagic stroke (Figure 5B).
Figure 5. Adjusted hazard ratios (error bars = 95% CI) showing the association of quintile of VVV of BP defined using the specified metrics with (A) ischemic stroke and (B) hemorrhagic stroke.
Covariates adjusted for are the same as for Figure 3. Abbreviations: VVV of BP = visit-to-visit variability of blood pressure; CI=confidence interval.
For sensitivity analyses, we had 52,604 patients (46% of the original cohort) with ≥ 4 BP measurements available to calculate the VVV of BP. Overall, the results were not materially changed, with the one notable exception that higher VVV of BP was no longer consistently associated with higher rates of hospitalized heart failure (Supplemental Figure).
DISCUSSION
In this large, community-based cohort of 114,900 adults with CKD stages 3–4 not yet on dialysis, higher VVV of BP was associated with significantly higher rates of death and hemorrhagic stroke, even after controlling for mean systolic BP, severity of kidney disease, and other comorbid conditions. However, VVV of BP had no significant association with rates of hospitalization for acute coronary syndrome or ischemic stroke. Results were similar when VVV of BP was defined as the coefficient of variation, standard deviation or average real variability. We did not find consistent associations of VVV of BP with rates of treated ESRD or heart failure.
While numerous reports indicate an association between higher VVV of BP and death in patients on hemodialysis [5–11], there have been few studies that focus on patients with CKD not yet on dialysis. McMullan et al. conducted two secondary analyses of clinical trials in patients with CKD, both of which showed an association of higher VVV of BP with death. The first analyzed data from 908 participants in the African American Study of Kidney Diseases and Hypertension (AASK), and showed that the highest tertile of VVV of BP was associated with 2.8-fold higher adjusted risk of death compared with the lowest tertile [3]. The second combined data from two diabetic nephropathy trials, and showed that each standard deviation increase in VVV of BP was association with a 16% (CI 4% to 29%) higher risk of death [4]. An observational study of 374 patients from Italian nephrology clinics found a 6% (CI 2% to 9%) increased risk of death associated with each 1% increase in VVV of BP [12]. Results from our analysis, which showed a 7%, 10%, 19% and 22% higher risk of death associated with the 2nd, 3rd, 4th, and 5th quintiles (versus 1st quintile) of VVV of BP, respectively, extend the findings of those previous studies to a much larger cohort of real-world patients with moderate to advanced CKD.
In our study, the association of VVV of BP with the development of treated ESRD differed depending on the metric used. The few previous studies to examine this outcome have yielded similarly varied results. For example, in the secondary analysis of the two diabetic nephropathy trials discussed above[4], VVV of BP had no significant association with a composite endpoint of ESRD, reduction in GFR, or death (HR 1.05, CI 0.67–1.62). In contrast, the secondary analysis of AASK observed that each standard deviation increase in VVV of BP was associated with a 12% (CI 2% to 22%) higher adjusted risk of ESRD [3]. Differences among the studies may stem in part from the fact that development of ESRD is relatively uncommon, leading to uncertainty in the estimates. In addition, differences in cohort characteristics may also account for at least some of the discrepancies (e.g. African Americans only, diabetic versus hypertensive nephropathy). Larger studies over longer periods of time are needed to provide a more definitive answer as to whether VVV of BP is a risk factor for ESRD.
Although studies in non-CKD populations have shown associations of VVV of BP with higher risks of acute coronary syndrome and heart failure [29, 30], we saw no significant associations with acute coronary syndrome. While we found that higher VVV of BP was associated with a 20–45% higher rate of heart failure, the association was no longer significant in sensitivity analyses that restricted the cohort to patients with at least four BP measurements available. Our results are consistent with studies in patients with CKD not yet on dialysis that have also failed to show an association [3, 4]. Perhaps in patients with CKD, the combined impact of traditional (e.g. hypertension, diabetes, dyslipidemia) and non-traditional risk factors (uremic milieu, disordered bone and mineral metabolism, inflammation) on heart failure and acute coronary syndrome outweighs any theoretical risk conferred by higher VVV of BP.
We found that higher VVV of BP was associated with a 35 to 90% higher rate of hemorrhagic stroke, but found no associations with ischemic stroke. Our results are in direct contrast to the findings of Rothwell et al., who showed that higher VVV of BP was more strongly associated with ischemic versus hemorrhagic stroke[29]. However, in that study, a secondary analysis of a trial of patients with hypertension and other cardiovascular risk factors, there were relatively few hemorrhagic strokes (N=74 total), leading to wide confidence limits. In a large study of 58,228 postmenopausal women enrolled in the Women’s Health Initiative, women in the highest quartile of VVV of BP, had a significant 1.5–fold higher risk of stroke, which did not differ by stroke type[31]. However, that cohort differed significantly from our study population, in that they were relatively healthy participants with few comorbid conditions.
Taken together, the association of VVV of BP and cardiovascular outcomes is mixed, reflecting in part the heterogeneity of the studies themselves. Moreover, the precise mechanisms underlying the putative effect of VVV of BP on cardiovascular outcomes remain to be elucidated and cannot be answered in observational analyses such as ours. Previous work showed that higher VVV of BP is associated with endothelial damage[32], increased arterial stiffness[33], progression of coronary artery calcification[34], and left ventricular diastolic dysfunction[35], all of which may contribute to higher risks of death and cardiovascular outcomes. The interplay of these factors with CKD and other comorbid conditions need further clarification.
Our study has several strengths, including its large size with detailed information on comorbid conditions and laboratory values, and the relatively dense number of BP measurements. However there are several limitations. First, our cohort had a mean age of 74 years, and so our results may not be generalizable to younger patients with CKD. We suspect the older age of our cohort may stem from our selection of patients with CKD stage 3–4, as younger patients are less likely to have had creatinine measured, and of those, are less likely to have moderate to severe CKD [2]. Second, our databases did not contain specific information on longitudinal medication adherence, which is associated with VVV of BP [36, 37]. We also did not have information on timing of medication administration or on adjustments to the antihypertensive medication regimen, which could also have affected VVV of BP. Third, our follow-up period was limited to 12 months, whereas a recent study showed that in an elderly cohort of primary care patients with 12 years of follow-up, BP change over time was a more important predictor of mortality and cardiovascular risk than VVV of BP[38]. Finally, although KPNC instituted rigorous BP measurement training to all outpatient clinics including repeat BP measurement where indicated [17], in most cases only one BP measurement was made at each clinic visit which may have introduced measurement error. We also did not have access to home BP measurements, which can provide additional prognostic information [39].
CONCLUSIONS
In our study of 114,900 patients with CKD stage 3–4, we found that higher VVV of BP was associated with higher risks of death and hemorrhagic stroke, independent of mean BP and after adjustment for comorbid conditions and other potential confounding factors. We did not find consistent associations with risks of ESRD, acute coronary syndrome, heart failure or ischemic stroke. Our results suggest a possible role for using VVV of BP to identify a subgroup of very high risk patients within a population already at high risk for adverse outcomes (i.e. patients with moderate-to-advanced CKD) to target for closer clinical follow-up, evaluation of medication adherence, or other interventions. However, the optimal number and interval of BP measurements, and the best metric to use to define VVV of BP still need to be clarified. Finally, whether reducing VVV of BP will ultimately improve outcomes requires future investigation in prospective clinical trials.
Supplementary Material
Acknowledgments
Sources of Funding: Dr. Chang is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (5K23DK095914).
Dr. Go received a research grant from Astra-Zeneca.
Footnotes
Conflicts of Interest: The other authors report no potential relevant conflicts of interest.
References
- 1.Diaz KM, Tanner RM, Falzon L, Levitan EB, Reynolds K, Shimbo D, Muntner P. Visit-to-visit variability of blood pressure and cardiovascular disease and all-cause mortality: a systematic review and meta-analysis. Hypertension. 2014;64(5):965–82. doi: 10.1161/HYPERTENSIONAHA.114.03903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.U.S. Renal Data System. USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; Bethesda, MD: 2013. [Google Scholar]
- 3.McMullan CJ, Bakris GL, Phillips RA, Forman JP. Association of BP variability with mortality among African Americans with CKD. Clin J Am Soc Nephrol. 2013;8(5):731–8. doi: 10.2215/CJN.10131012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMullan CJ, Lambers Heerspink HJ, Parving HH, Dwyer JP, Forman JP, de Zeeuw D. Visit-to-visit variability in blood pressure and kidney and cardiovascular outcomes in patients with type 2 diabetes and nephropathy: a post hoc analysis from the RENAAL study and the Irbesartan Diabetic Nephropathy Trial. Am J Kidney Dis. 2014;64(5):714–22. doi: 10.1053/j.ajkd.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Rossignol P, Cridlig J, Lehert P, Kessler M, Zannad F. Visit-to-visit blood pressure variability is a strong predictor of cardiovascular events in hemodialysis: insights from FOSIDIAL. Hypertension. 2012;60(2):339–46. doi: 10.1161/HYPERTENSIONAHA.111.190397. [DOI] [PubMed] [Google Scholar]
- 6.Tozawa M, Iseki K, Yoshi S, Fukiyama K. Blood pressure variability as an adverse prognostic risk factor in end-stage renal disease. Nephrol Dial Transplant. 1999;14(8):1976–81. doi: 10.1093/ndt/14.8.1976. [DOI] [PubMed] [Google Scholar]
- 7.Shafi T, Sozio SM, Bandeen-Roche KJ, Ephraim PL, Luly JR, St Peter WL, et al. Predialysis systolic BP variability and outcomes in hemodialysis patients. J Am Soc Nephrol. 2014;25(4):799–809. doi: 10.1681/ASN.2013060667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang TI, Flythe JE, Brunelli SM, Muntner P, Greene T, Cheung AK, Chertow GM. Visit-to-visit systolic blood pressure variability and outcomes in hemodialysis. J Hum Hypertens. 2014;28(1):18–24. doi: 10.1038/jhh.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim HY, Kang YU, Kim CS, Choi JS, Bae EH, Ma SK, Kim SW. Association of age and BP variability with long-term mortality in hemodialysis patients. Kidney Blood Press Res. 2013;38(2–3):172–80. doi: 10.1159/000355765. [DOI] [PubMed] [Google Scholar]
- 10.Brunelli SM, Thadhani RI, Lynch KE, Ankers ED, Joffe MM, Boston R, et al. Association between long-term blood pressure variability and mortality among incident hemodialysis patients. Am J Kidney Dis. 2008;52(4):716–26. doi: 10.1053/j.ajkd.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 11.Di Iorio B, Di Micco L, Torraca S, Sirico ML, Guastaferro P, Chiuchiolo L, et al. Variability of blood pressure in dialysis patients: a new marker of cardiovascular risk. J Nephrol. 2013;26(1):173–82. doi: 10.5301/jn.5000108. [DOI] [PubMed] [Google Scholar]
- 12.Di Iorio B, Pota A, Sirico ML, Torraca S, Di Micco L, Rubino R, et al. Blood pressure variability and outcomes in chronic kidney disease. Nephrol Dial Transplant. 2012;27(12):4404–10. doi: 10.1093/ndt/gfs328. [DOI] [PubMed] [Google Scholar]
- 13.Yokota K, Fukuda M, Matsui Y, Kario K, Kimura K. Visit-to-visit variability of blood pressure and renal function decline in patients with diabetic chronic kidney disease. J Clin Hypertens (Greenwich) 2014;16(5):362–6. doi: 10.1111/jch.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mallamaci F, Minutolo R, Leonardis D, D'Arrigo G, Tripepi G, Rapisarda F, et al. Long-term visit-to-visit office blood pressure variability increases the risk of adverse cardiovascular outcomes in patients with chronic kidney disease. Kidney Int. 2013;84(2):381–9. doi: 10.1038/ki.2013.132. [DOI] [PubMed] [Google Scholar]
- 15.Gordon NP. Characteristics of Adult Health Plan Members in Kaiser Permanente's Northern California Region, as Estimated from the 2011 Member Health Survey. 2013 Available from: www.dor.kaiser.org/external/mhs11reg/
- 16.Krieger N. Overcoming the absence of socioeconomic data in medical records: validation and application of a census-based methodology. Am J Public Health. 1992;82(5):703–10. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe MG, Lee GA, Young JD, Sidney S, Go AS. IMproved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310(7):699–705. doi: 10.1001/jama.2013.108769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franklin SS, Larson MG, Khan SA, Wong ND, Leip EP, Kannel WB, Levy D. Does the Relation of Blood Pressure to Coronary Heart Disease Risk Change With Aging? : The Framingham Heart Study. Circulation. 2001;103(9):1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 20.Mena L, Pintos S, Queipo NV, Aizpurua JA, Maestre G, Sulbaran T. A reliable index for the prognostic significance of blood pressure variability. J Hypertens. 2005;23(3):505–11. doi: 10.1097/01.hjh.0000160205.81652.5a. [DOI] [PubMed] [Google Scholar]
- 21.Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008;74(1):101–7. doi: 10.1038/ki.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hsu C-y, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, Go AS. Nonrecovery of Kidney Function and Death after Acute on Chronic Renal Failure. Clinical Journal of the American Society of Nephrology. 2009;4(5):891–898. doi: 10.2215/CJN.05571008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, et al. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290(20):2685–92. doi: 10.1001/jama.290.20.2685. [DOI] [PubMed] [Google Scholar]
- 24.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart failure. JAMA. 2006;296(17):2105–11. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 25.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–23. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 26.Smith DH, Thorp ML, Gurwitz JH, McManus DD, Goldberg RJ, Allen LA, et al. Chronic kidney disease and outcomes in heart failure with preserved versus reduced ejection fraction: the Cardiovascular Research Network PRESERVE Study. Circ Cardiovasc Qual Outcomes. 2013;6(3):333–42. doi: 10.1161/CIRCOUTCOMES.113.000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20(9):1396–402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 29.Rothwell PM, Howard SC, Dolan E, O'Brien E, Dobson JE, Dahlöf B, et al. Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. The Lancet. 2010;375(9718):895–905. doi: 10.1016/S0140-6736(10)60308-X. [DOI] [PubMed] [Google Scholar]
- 30.Hata J, Arima H, Rothwell PM, Woodward M, Zoungas S, Anderson C, et al. Effects of Visit-to-Visit Variability in Systolic Blood Pressure on Macrovascular and Microvascular Complications in Patients With Type 2 Diabetes Mellitus: The ADVANCE Trial. Circulation. 2013;128(12):1325–1334. doi: 10.1161/CIRCULATIONAHA.113.002717. [DOI] [PubMed] [Google Scholar]
- 31.Shimbo D, Newman JD, Aragaki AK, LaMonte MJ, Bavry AA, Allison M, et al. Association between annual visit-to-visit blood pressure variability and stroke in postmenopausal women: data from the Women's Health Initiative. Hypertension. 2012;60(3):625–30. doi: 10.1161/HYPERTENSIONAHA.112.193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, et al. Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 2012;35(1):55–61. doi: 10.1038/hr.2011.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Okada H, Fukui M, Tanaka M, Inada S, Mineoka Y, Nakanishi N, et al. Visit- to-visit variability in systolic blood pressure is correlated with diabetic nephropathy and atherosclerosis in patients with type 2 diabetes. Atherosclerosis. 2012;220(1):155–9. doi: 10.1016/j.atherosclerosis.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 34.Okada H, Fukui M, Tanaka M, Matsumoto S, Mineoka Y, Nakanishi N, et al. Visit-to-visit variability in systolic blood pressure is a novel risk factor for the progression of coronary artery calcification. Hypertens Res. 2013;36(11):996–9. doi: 10.1038/hr.2013.66. [DOI] [PubMed] [Google Scholar]
- 35.Masugata H, Senda S, Murao K, Inukai M, Hosomi N, Iwado Y, et al. Visit-to-visit variability in blood pressure over a 1-year period is a marker of left ventricular diastolic dysfunction in treated hypertensive patients. Hypertens Res. 2011;34(7):846–50. doi: 10.1038/hr.2011.54. [DOI] [PubMed] [Google Scholar]
- 36.Hong K, Muntner P, Kronish I, Shilane D, Chang TI. Medication adherence and visit-to-visit variability of systolic blood pressure in African Americans with chronic kidney disease in the AASK trial. J Hum Hypertens. 2015 doi: 10.1038/jhh.2015.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Muntner P, Levitan EB, Joyce C, Holt E, Mann D, Oparil S, Krousel-Wood M. Association between antihypertensive medication adherence and visit-to-visit variability of blood pressure. J Clin Hypertens (Greenwich) 2013;15(2):112–7. doi: 10.1111/jch.12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gao S, Hendrie HC, Wang C, Stump TE, Stewart JC, Kesterson J, et al. Redefined blood pressure variability measure and its association with mortality in elderly primary care patients. Hypertension. 2014;64(1):45–52. doi: 10.1161/HYPERTENSIONAHA.114.03576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stergiou GS, Ntineri A, Kollias A, Ohkubo T, Imai Y, Parati G. Blood pressure variability assessed by home measurements: a systematic review. Hypertens Res. 2014;37(6):565–72. doi: 10.1038/hr.2014.2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.