Abstract
Background
Congenital cytomegalovirus (cCMV) infection is common among infants born to HIV-infected women. Nelfinavir (NFV), an antiretroviral drug that is safe during pregnancy, inhibits CMV replication in vitro at concentrations that standard doses achieve in plasma. We hypothesized that infants born to women receiving NFV for prevention of mother-to-child transmission of HIV (PMTCT) would have a reduced prevalence of cCMV infection.
Methods
The prevalence of cCMV infection was compared among HIV-uninfected infants whose HIV-infected mothers either received NFV for ≥4 weeks during pregnancy (NFV-exposed) or did not receive any NFV in pregnancy (NFV-unexposed). CMV PCR was performed on infant blood samples collected at <3 weeks from birth.
Results
Of the 1,255 women included, 314 received NFV for ≥4 weeks during pregnancy and 941 did not receive any NFV during pregnancy. The overall prevalence of cCMV infection in the infants was 2.2%, which did not differ by maternal NFV use. Maternal CD4 T cell counts were inversely correlated with risk of cCMV infection, independent of the time NFV was initiated during gestation. Infants with cCMV infection were born 0.7 weeks earlier (p=0.010) and weighed 170 grams less (p=0.009) than uninfected infants.
Conclusion
Among HIV-exposed uninfected infants, cCMV infection was associated with adverse perinatal outcomes. NFV use in pregnancy was not associated with protection against cCMV. Safe and effective strategies to prevent cCMV infection are needed.
Keywords: congenital cytomegalovirus infection, nelfinavir, HIV-exposed uninfected infants
Introduction
Congenital cytomegalovirus (cCMV) infection occurs in 0.2 – 2% of all live births in the United States, and is a major cause of deafness and intellectual disability.(1) Studies have reported high rates of cCMV infection among infants born to HIV-infected women,(2–8) which is likely due in part to the impaired control of CMV replication that results from HIV-related immune deficiencies.(4) Maternal antiretroviral use during pregnancy is associated with protection against cCMV infection; however, even with combination antiretroviral regimens, studies suggest that the risk of cCMV is relatively higher compared to rates for HIV-uninfected women.(5, 7) Given the prevalence and morbidity of cCMV, effective preventive strategies would be of substantial clinical benefit to infants’ health.
The use of antiviral drugs to prevent cCMV infection is limited by the toxicity of available agents. Ganciclovir and its pro-drug valganciclovir are currently the only oral medications approved for treatment of CMV infection, and have carcinogenic, teratogenic, and gonadotoxic effects that preclude their routine use in pregnancy.(9) Non-randomized studies of intravenous immunoglobulin given to pregnant women with primary CMV infection initially showed protective effects, but these results were not confirmed by a randomized controlled trial.(10) The HIV protease inhibitor nelfinavir (NFV) has been shown in vitro to inhibit replication of CMV and other herpesviruses at concentrations achieved clinically.(11) This anti-herpesvirus activity was unique to NFV among the broad panel of antiretrovirals tested. The in vitro inhibitory concentrations of NFV against CMV were found to be in the low micromolar range (IC50=4.4 uM), and comparable to ganciclovir against susceptible CMV isolates.(12) The anti-CMV activity of NFV raises the possibility that antiretroviral regimens that contain NFV may reduce cCMV infection. NFV was deemed particularly attractive as a potential preventive agent for cCMV infection given that it is already approved for the treatment of HIV infection and it has been used extensively for the prevention of perinatal HIV transmission.(13) NFV has an excellent safety profile, and is relatively inexpensive. Therefore, we tested the hypothesis that NFV can reduce the risk of in utero CMV infection.
Methods
Cohort
Data and specimens were obtained from 2 prospective cohorts studied by the Pediatric AIDS Clinical Trials Group (PACTG) and International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) Network; PACTG 316(14) and P1025 studies.(15) PACTG 316 was a multicenter, randomized, double-blind trial of 2-doses of intrapartum/newborn nevirapine compared with placebo to reduce perinatal transmission of HIV from women on standard ARV therapy between 1997 and 2000. Of the 1,270 women-infant pairs recruited in the US, Europe, Brazil and the Bahamas between 1997 and 2000,(14) the current study included only the 661 women-infant pairs from the US. Another 594 women-infant pairs were drawn from the P1025 study, a US-based observational study designed to assess the use and safety of antiretroviral drugs and other interventions for HIV-infected pregnant women and their infants. In both studies NFV was prescribed as part of clinical care, and not part of a randomized regimen. All procedures followed were in accordance with the ethical standards of the responsible human subjects protection committees and with the Helsinki Declaration of 1975, as revised in 2000.
Inclusion criteria for the current study included all women-infant pairs with a cryopreserved newborn blood specimen (plasma or peripheral blood mononuclear cells (PBMC)) collected at <3 weeks from birth and a history of maternal ARV use. Infants whose mothers received NFV for ≥4 weeks during pregnancy were in the NFV-exposed group and those whose mother did not receive NFV during pregnancy were in the unexposed group. HIV-infected infants were excluded due to the potential interaction with risk of CMV infection.(3)
Demographic and treatment data were analyzed: maternal data included age, race and ethnicity, stage of HIV disease,(16) antiretroviral use, gestational week at initiation of NFV and other antiretrovirals, earliest and latest maternal CD4 T cell counts and HIV plasma RNA loads in pregnancy, and mode of delivery. Infant data included sex, gestational age, weight at delivery, and infant HIV infection status. For both PACTG 316 and P1025, infant gestational age was estimated at the baseline pregnancy visit, and modified based on ultrasound data and physical exam at delivery.
CMV PCR testing
cCMV infection was determined by detection of CMV DNA in newborn blood specimen using real-time PCR at the University of Washington Virology Laboratory.(17) DNA was extracted from 0.4 mL of plasma or ≥106 PBMC. Detection of ≥50 CMV genome copies per mL of plasma or ≥5 CMV genome copies per reaction for PBMC was considered positive. All runs of the assay included positive and negative controls, and each reaction was spiked with an internal control to detect inhibition of PCR. Study personnel were blinded to the NFV exposure group assignment until all testing was completed.
Statistical analyses
The proportion of infants with cCMV infection in the NFV-exposed and the NFV-unexposed groups was compared using Fisher’s exact test. Among infants with cCMV infection, we compared CMV plasma viral load by NFV exposure using the Wilcoxon test. Other predictors of cCMV infection were also explored using Fisher’s exact test and Wilcoxon tests as appropriate. Multivariable logistic regression models were constructed to explore the independent associations of selected covariates with cCMV infection. Due to the low number of events, we used exact methods to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CI).
Results
Characteristics of study subjects
A total of 314 infant subjects from the PACTG 316 and P1025 studies were identified who had a specimen available for CMV testing and whose mothers received NFV for at least 4 weeks during pregnancy (Table 1). Among them, 69 (22%) initiated NFV prior to pregnancy, 36 (11%) during the first trimester, 163 (52%) during the second trimester and 46 (15%) during the third trimester. 85% continued NFV through the time of delivery. There were 941 NFV-unexposed infants whose mothers by design did not receive any NFV. Women not exposed to NFV were more likely to have been diagnosed with HIV prior to the pregnancy (p=0.018), initiated antiretrovirals earlier (p=0.007), and have higher CD4 T cell counts during pregnancy (p=0.043) and at delivery (p=0.006), compared to women who received NFV. NFV-exposed and unexposed women did not differ in HIV viral load during pregnancy or at delivery, gestational age at delivery, delivery mode or their infant’s birth weight.
Table 1.
Maternal, obstetrical and neonatal characteristics by nelfinavir exposure
| Characteristics | Total (N=1255) | Missing data | Nelfinavir exposure group
|
||
|---|---|---|---|---|---|
| Unexposed (N=941) | Exposed (N=314) | P value* | |||
| cCMV status of infant | |||||
| CMV PCR positive | 27 (2%) | 0 | 19 (2%) | 8 (3%) | 0.653 |
| Maternal characteristics | |||||
| Maternal age at time of delivery (IQR) | 27.4 (23.1, 32.2) | 0 | 27.5 (23.0, 32.2) | 27.3 (23.8, 32.3) | 0.848 |
| Race/ethnicity | 8 | 0.175 | |||
| Non-Hispanic Black | 734 (59%) | 542 (58%) | 192 (62%) | ||
| Hispanic | 354 (28%) | 278 (30%) | 76 (24%) | ||
| Non-Hispanic White/Other | 159 (13%) | 115 (12%) | 44 (14%) | ||
| CDC HIV Classification | 0 | 0.099 | |||
| A | 833 (80%) | 675 (81%) | 158 (75%) | ||
| B | 93 (9%) | 68 (8%) | 25 (12%) | ||
| C | 119 (11%) | 90 (11%) | 29 (14%) | ||
| Study | 0 | ||||
| PACTG 316 | 661 (53%) | 447 (48%) | 214 (68%) | <0.001 | |
| P1025 | 594 (47%) | 494 (52%) | 100 (32%) | ||
| Maternal HIV infection | |||||
| Timing of HIV diagnosis | 2 | ||||
| >2 years prior to pregnancy | 597 (48%) | 467 (50%) | 130 (41%) | 0.018 | |
| <2 years prior to pregnancy | 233 (19%) | 174 (19%) | 59 (19%) | ||
| During pregnancy | 423 (34%) | 298 (32%) | 125 (40%) | ||
| 1st CD4 count <200 cell/uL during pregnancy | 164 (13%) | 19 | 112 (12%) | 52 (17%) | 0.043 |
| Near delivery CD4 count <200 cells/uL | 114 (11%) | 235 | 74 (10%) | 40 (15%) | 0.006 |
| 1st HIV RNA during pregnancy ≤400c/mL | 489 (45%) | 171 | 355 (43%) | 134 (50%) | 0.170 |
| Near delivery HIV RNA ≤400c/mL | 725 (66%) | 156 | 545 (67%) | 180 (64%) | 0.482 |
| Antiretroviral exposure | |||||
| GA at 1st ARV use during pregnancy, weeks | 14.4 (0.0, 21.0) | 0 | 14.0 (0.0, 20.5) | 16.1 (0.0, 22.9) | 0.007 |
| ARV, used for longest duration in pregnancy | 0 | <0.001 | |||
| No ARV | 5 (0%) | 5 (1%) | 0 (0%) | ||
| Monotherapy | 160 (13%) | 149 (16%) | 11 (4%) | ||
| Other | 244 (19%) | 225 (24%) | 19 (6%) | ||
| 3 NRTIs | 48 (4%) | 46 (5%) | 2 (1%) | ||
| ART (≥3 drugs, ≥2 classes) | 798 (64%) | 516 (55%) | 282 (90%) | ||
| Total duration of ARV during pregnancy (weeks) | 24.1 (17.7, 37.1) | 0 | 24.3 (18.1, 37.1) | 23.4 (17.1, 37.0) | 0.531 |
| Obstetrical characteristics | |||||
| GA at delivery, weeks | 38.6 (37.7, 39.7) | 0 | 38.6 (37.7, 39.6) | 38.7 (38.0, 39.7) | 0.207 |
| Mode of delivery | 6 | ||||
| Vaginal delivery | 679 (54%) | 510 (54%) | 169 (54%) | 0.886 | |
| Cesarean after labor/membrane rupture | 238 (19%) | 175 (19%) | 63 (20%) | ||
| Elective Cesarean section | 332 (26%) | 252 (27%) | 80 (25%) | ||
| Infant characteristics | |||||
| Birth weight (kg) | 3.0 (2.7, 3.4) | 0 | 3.0 (2.7, 3.4) | 3.1 (2.8, 3.4) | 0.675 |
| Infant birth size category | 4 | ||||
| Small for GA | 67 (5%) | 0 | 50 (5%) | 17 (5%) | 0.840 |
| Appropriate for GA | 1,108 (89%) | 830 (88%) | 278 (89%) | ||
| Large for GA | 74 (6%) | 57 (6%) | 17 (5%) | ||
| Intrauterine growth retardation | 2 (0%) | 2 (0%) | 0 (0%) | ||
| Infant’s age at specimen collection, weeks | 1.1 (0.1, 2.1) | 0 | 1.3 (0.1, 2.1) | 0.9 (0.1, 2.0) | 0.003 |
| Specimen type: PBMC (vs. plasma) | 491 (39%) | 0 | 374 (40%) | 117 (37%) | 0.435 |
Data are presented as absolute frequency (%) or median (interquartile range).
Wilcoxon Test was used to compare medians and Chi-Square Test was used to compare frequencies.
ARV: antiretroviral
ART: combination antiretroviral therapy using ≥3 drugs from ≥2 classes
GA: gestational age
NRTI: nucleoside reverse transcriptase inhibitor
c/mL: copies per milliliter
Prevalence of cCMV infection by NFV exposure
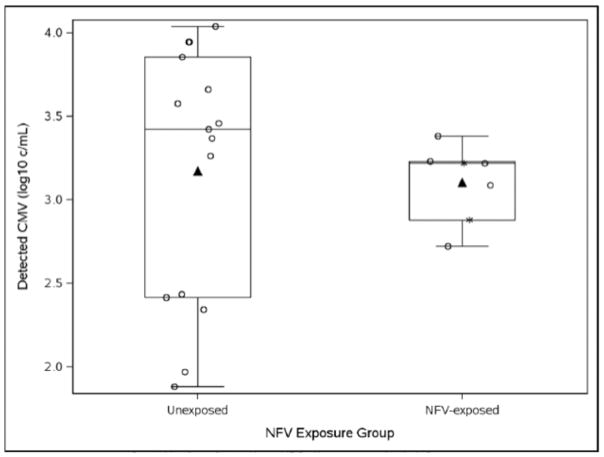
A total of 27 infants (2.2%) had cCMV infection (Table 1). The proportion of infants with cCMV infection did not differ significantly between the NFV-exposed and unexposed groups (8/314 (2.5%) vs. 19/941 (2.0%); p=0.653). CMV was detected in plasma specimens significantly more often than in PBMC (22/764 (2.9%) vs. 5/491 (1.0%); p=0.028). The proportion of infant PBMC samples tested for CMV did not differ significantly between NFV exposure groups (exposed, 117/314 (37%) vs. unexposed, 374/941 (40%); p=0.435). Of those with CMV detected in plasma (N=22), there was no significant difference in the median number of genome copies/mL between NFV exposure groups (Figure 1; PBMC were excluded given the different units and small number of PBMC specimens in which CMV was detected).
Figure 1. CMV DNA plasma viral load by nelfinavir exposure groups among CMV congenitally infected newborns (N=22).
The distribution of CMV DNA levels in plasma is not different between congenitally-infected infants (open-circles) whose mothers did or did not receive NFV during pregnancy. Box plots show the medians and interquartile ranges of the CMV DNA copies/ml in plasma for NFV-unexposed (n=15; median 3.42; IQR 2.41, 3.85) and NFV-exposed infants (n=7; median 3.22; IQR 2.88, 3.23; p=0.290); means shown by triangles. Asterisks indicate the values of two NFV-exposed infants whose mothers were no longer receiving NFV at the time of delivery.
Clinical characteristics by cCMV infection status
Maternal and infant characteristics by cCMV infection status are shown in Table 2. In the univariable analysis, mothers whose first CD4 T cell counts in pregnancy was <200 cells/mm3 were more likely to have infants with cCMV infection than mothers with higher CD4 T cell counts. Mothers of infants with cCMV infection initiated antiretroviral therapy significantly later during gestation compared to mothers of CMV-uninfected infants (p=0.037). However, significant differences were not observed in the time that maternal NFV was initiated or duration of fetal exposure to NFV by cCMV infection status. Infants with cCMV infection had a lower median birth weight (2.87 vs. 3.04 kg; p=0.009) and were born a median gestation that was 5 days shorter compared to uninfected infants (p=0.010). When adjusted for first CD4 count <200 cells/μL in pregnancy, the odds of preterm birth was not higher in those with cCMV infection compared to those without cCMV infection (aOR 2.32, 95% CI 0.81, 5.81; p=0.116). In the univariable analysis, NFV exposure was not associated with cCMV infection by (OR 1.27; 95% CI 0.48, 3.07; p=0.710; Table 3). Furthermore, there was no association between NFV exposure and cCMV infection in two multivariable models that adjusted for timing of antiretroviral initiation plus either specimen type or timing of antiretroviral initiation (Table 3).
Table 2.
Maternal and infant characteristics by infant cCMV DNA PCR results from 0 to ≤3 weeks of age
| Characteristics | Negative CMV PCR (N=1228) | Positive CMV PCR (N=27) | P-Value* |
|---|---|---|---|
| Demographics | |||
| Maternal age at time of delivery | 27.4 (23.1, 32.2) | 27.3 (22.4, 33.3) | 0.924 |
| Race/ethnicity | |||
| Black | 714 (58%) | 20 (74%) | 0.110 |
| Hispanic | 351 (29%) | 3 (11%) | |
| White/Other | 155 (13%) | 4 (15%) | |
| Study | |||
| PACTG 316 | 648 (53%) | 13 (48%) | 0.699 |
| P1025 | 580 (47%) | 14 (52%) | |
| Maternal HIV infection | |||
| First CD4 count <200 cells/uL during pregnancy | 155 (13%) | 9 (33%) | 0.006 |
| CD4 count <200 cells/uL near delivery | 107 (9%) | 7 (26%) | 0.016 |
| First viral load during pregnancy <=400c/mL | 477 (39%) | 12 (44%) | 0.681 |
| Viral load near delivery <=400c/mL | 710 (58%) | 15 (56%) | 0.828 |
| Antiretroviral exposure | |||
| GA at first use of any ARV, weeks | 14.3 (0.0, 21.0) | 17.86 (10.9, 26.7) | 0.037 |
| Any ARV initiation prior to pregnancy (vs during) | 406 (33%) | 5 (19%) | 0.146 |
| Longest ARV regimen used during pregnancy | |||
| Non-ART or no ARV | 447 (36%) | 10 (37%) | 1.000 |
| ART (≥3 drugs, ≥2 classes) | 781 (64%) | 17 (63%) | |
| Weeks of any ARV use during pregnancy | 24.1 (17.7, 37.1) | 21.9 (13.0, 35.4) | 0.120 |
| NFV-exposed (versus unexposed) | 306 (25%) | 8 (30%) | 0.650 |
| Weeks of NFV use during pregnancy | 19.00 (12.6, 26.9) | 19.50 (12.9, 24.4) | 0.657 |
| GA in weeks at first NFV use | 17.86 (7.00, 24.29) | 18.36 (8.43, 25.21) | 0.789 |
| Obstetrical characteristics | |||
| Mode of delivery | 0.465 | ||
| Vaginal delivery | 666 (54%) | 13 (48%) | |
| Cesarean section after labor/membrane rupture | 230 (19%) | 8 (30%) | |
| Elective Cesarean section | 326 (27%) | 6 (22%) | |
| GA in weeks at delivery | 38.7 (37.7, 39.7) | 38.0 (36.9, 38.7) | 0.010 |
| Preterm delivery (<37 weeks) | 161 (13%) | 7 (4%) | 0.078 |
| Infant characteristics | |||
| Birth weight, kg | 3.04 (2.74, 3.40) | 2.87 (2.41, 3.12) | 0.009 |
| Small for GA/IUGR | 68 (6%) | 1 (4%) | 1.000 |
| Infant sex: male | 615 (50%) | 13 (48%) | 0.846 |
| Infant age at specimen collection, weeks | 1.1 (0.1, 2.1) | 1.71 (0.3, 2.3) | 0.298 |
| Specimen type: PBMC (versus plasma) | 486 (40%) | 5 (19%) | 0.028 |
Data are presented as absolute frequency (%) or median (25th, 75th centile).
Wilcoxon Test was used to compare medians and Fisher’s exact Test was used to compare frequencies.
ARV: antiretroviral
ART: combination antiretroviral therapy using ≥3 drugs from ≥2 classes
GA: gestational age
NFV : nelfinavir
PBMC: peripheral blood mononuclear cells
cCMV – congenital cytomegalovirus infection
IUGR – intrauterine growth retardation
Table 3.
Association of NFV exposure on cCMV infection in infants adjusted for potential confounders
| Covariates | Unadjusted* | Adjusted* | ||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
|
|
||||||
| OR (95% CI) | P value | OR (95%CI) | P value | OR (95%CI) | P value | |
| NFV exposure (versus unexposed) | 1.27 (0.48, 3.07) | 0.710 | 1.20 (0.45, 2.92) | 0.981 | 1.10 (0.41, 2.68) | 0.980 |
| First maternal CD4 count (<200 versus ≥200) | 3.40 (1.32, 8.13) | 0.011 | 3.56 (1.37, 8.61) | 0.009 | ||
| Specimen type PBMC vs. plasma | 0.35 (0.10, 0.95) | 0.036 | 0.36 (0.11, 0.99) | 0.046 | ||
| ARV initiation prior to pregnancy vs. during | 2.19 (0.80, 7.44) | 0.153 | 2.07 (0.76, 7.08) | 0.192 | 2.34 (0.85, 8.02) | 0.116 |
Exact logistic regression analysis. Each multivariable models include adjustment for all the covariates for which odds ratios and P values are shown (Model 1 includes NFV exposure, specimen type and ARV initiation prior to pregnancy; Model 2: includes NFV exposure, first maternal CD4 count, and ARV initiation prior to pregnancy).
OR (95% CI): odds ratio (95% confidence interval)
ARV: antiretroviral
Discussion
In this observational cohort study, maternal NFV during gestation was not associated with a reduction in the probability of cCMV infection, nor with the quantity of CMV detected in plasma of infected infants. While NFV inhibits CMV replication in vitro,(11) the lack of a detectable protective effect of NFV may have been due to low plasma concentrations of NFV during the pregnancy(18) or insufficient transport of NFV across the placenta.(19) We hypothesized that NFV might suppress maternal CMV viremia and viral replication in the placental cytotrophoblasts, thereby reducing the likelihood of virus crossing the placenta to the fetus. However, it is possible that transplacental passage of the antiviral is required for effective antiviral prophylaxis against cCMV infection. NFV-resistant CMV is also possible, though there may be a high barrier to resistance as NFV appears to inhibit herpesvirus replication by targeting the host cell rather than the virus.(20, 21)
Given that NFV use was not randomly assigned to women in this study, the highly variable timing in initiation of NFV and duration of NFV exposure may have confounded our findings. The absence of significant protection of the infant in association with maternal NFV therapy in this study might be due to suboptimal timing of NFV use, since 15% of women in the NFV-exposed group only began receiving NFV during the third trimester and 15% stopped treatment before delivery. CMV transplacental transmission rates from maternal primary infection increase with advancing gestational age from approximately 20% in the first trimester, to approximately 75% in the third trimester.(22, 23) Multivariable models were constructed to adjust for potential confounders but had limited power given the relatively small number of cCMV infections. Thus, we cannot rule out that NFV may have a modest effect on the risk of cCMV infection, especially if given throughout pregnancy at optimal doses. However, determining this would require a large randomized clinical trial.
Notably, the prevalence of cCMV infection found in our study (2.2%) – although high compared to the general population(1) – was in the lower range when compared to previously published rates of congenital CMV (range 1% – 10%) in studies of HIV-infected women,(2–8) including a French cohort study during the same time period that reported a prevalence of cCMV infection of 3 – 4%.(5) The total rate of cCMV infection in this study was likely underestimated by using blood, especially PBMC, to test for CMV, rather than saliva or urine samples. Numerous studies have reported that PCR of dried blood spots, serum, or whole blood had low sensitivity compared with urine or saliva rapid culture or PCR.(24–28) Although CMV can readily be detected in PBMC of viremic transplant recipients by PCR,(29–31) our significantly lower rate of cCMV among infants with PBMC compared to plasma suggest that PBMC may be inferior to detection of cCMV infection. The type of samples tested did not influence the effect of nelfinavir on cCMV infection that was measured, based on the relatively equal numbers of PBMC and plasma samples in each group and because sample type was adjusted for in the multivariable analysis.
Risk factors for cCMV infection in HIV-exposed uninfected children have been previously described. Maternal CMV serostatus was not available from these cohorts; however, most HIV-1-infected pregnant women are co-infected with CMV even in resource-rich countries,(32) and maternal serostatus is a poor predictor of the risk of congenital CMV transmission.(33) Our findings are consistent with reports that type of maternal antiretroviral therapy is not associated with the rate of cCMV infection,(5, 7) whereas maternal CD4 T cell count <200 cells/mm3 is independently associated with cCMV infection.(5) Similarly, earlier initiation of antiretrovirals in pregnancy appeared to be protective against cCMV infection risk in our cohort, supporting the findings of Guibert et al. that beginning antiretroviral treatment before or during the first trimester of pregnancy decreases the likelihood of infection compared to initiation during the second trimester,(5, 34) presumably due to improved immunologic control of maternal CMV replication.
cCMV infection has been associated with preterm delivery in both HIV-infected and uninfected women.(35, 36) Although the mechanism is unclear, placental infection with CMV often results in chronic villitis(37) and local cytokine modulation(38, 39) that is thought to cause preterm labor. Infants with cCMV infection were born at slightly earlier gestational ages compared to uninfected infants in this cohort, but there was no significant association with preterm birth.
Despite previous findings of in vitro activity against CMV replication,(11) in this study NFV did not protect HIV-exposed uninfected infants from cCMV infection. Other strategies to reduce congenital CMV infection, including valacyclovir(8) and passive immunization during pregnancy,(10) also did not appear to prevent cCMV infection. Given the profound morbidity that can result from cCMV infection, these failures highlight the need to better understand CMV transmission to the fetus, as well as for additional trials of pharmacologic interventions and maternal CMV vaccines. The high incidence of cCMV infection in infants of HIV-infected women argues for studies to determine if other antiretrovirals may provide dual protection by targeting sites shared by HIV and CMV.(40)
Acknowledgments
Funding
This work was supported by NIH funds through an International Maternal Pediatric Adolescent AIDS Clinical Trials Group (IMPAACT) Virology Developmental Laboratory award (U01 AI068632) to LMF, the Clinical Research and Retrovirology Core of the Seattle Centers for AIDS Research (P30 AI 027757), and the IMPAACT Statistical and Data Management Center (U01 AI068616). Overall support for IMPAACT was provided by the National Institute of Allergy and Infectious Diseases (NIAID) of the National Institutes of Health (NIH) under Award Numbers UM1AI068632 (IMPAACT LOC), UM1AI068616 (IMPAACT SDMC) and UM1AI106716 (IMPAACT LC), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) and the National Institute of Mental Health (NIMH). SG was supported by an investigator award from the Child & Family Research Institute.
The authors would like to thank Amy Gonzalez and Stacy Selke for data and specimen management, as well as Drs. Marc Lallemant, the PACTG 316 and P1025 study investigators, teams, and participants.
Footnotes
Conflicts of interest
No authors declare a conflict of interest.
References
- 1.Cannon MJ, Griffiths PD, Aston V, Rawlinson WD. Universal newborn screening for congenital CMV infection: what is the evidence of potential benefit? Rev Med Virol. 2014 doi: 10.1002/rmv.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovacs A, Schluchter M, Easley K, Demmler G, Shearer W, La Russa P, Pitt J, et al. Cytomegalovirus infection and HIV-1 disease progression in infants born to HIV-1-infected women. Pediatric Pulmonary and Cardiovascular Complications of Vertically Transmitted HIV Infection Study Group. N Engl J Med. 1999;341:77–84. doi: 10.1056/NEJM199907083410203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doyle M, Atkins JT, Rivera-Matos IR. Congenital cytomegalovirus infection in infants infected with human immunodeficiency virus type 1. Pediatr Infect Dis J. 1996;15:1102–1106. doi: 10.1097/00006454-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Slyker JA, Lohman-Payne BL, John-Stewart GC, Maleche-Obimbo E, Emery S, Richardson B, Dong T, et al. Acute cytomegalovirus infection in Kenyan HIV-infected infants. Aids. 2009 doi: 10.1097/QAD.0b013e32833016e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guibert G, Warszawski J, Le Chenadec J, Blanche S, Benmebarek Y, Mandelbrot L, Tubiana R, et al. Decreased risk of congenital cytomegalovirus infection in children born to HIV-1-infected mothers in the era of highly active antiretroviral therapy. Clin Infect Dis. 2009;48:1516–1525. doi: 10.1086/598934. [DOI] [PubMed] [Google Scholar]
- 6.Khamduang W, Jourdain G, Sirirungsi W, Layangool P, Kanjanavanit S, Krittigamas P, Pagdi K, et al. The interrelated transmission of HIV-1 and cytomegalovirus during gestation and delivery in the offspring of HIV-infected mothers. J Acquir Immune Defic Syndr. 2011;58:188–192. doi: 10.1097/QAI.0b013e31822d0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frederick T, Homans J, Spencer L, Kramer F, Stek A, Operskalski E, Kovacs A. The effect of prenatal highly active antiretroviral therapy on the transmission of congenital and perinatal/early postnatal cytomegalovirus among HIV-infected and HIV-exposed infants. Clin Infect Dis. 2012;55:877–884. doi: 10.1093/cid/cis535. [DOI] [PubMed] [Google Scholar]
- 8.Roxby AC, Atkinson C, Asbjornsdottir K, Farquhar C, Kiarie JN, Drake AL, Wald A, et al. Maternal valacyclovir and infant cytomegalovirus acquisition: a randomized controlled trial among HIV-infected women. PLoS One. 2014;9:e87855. doi: 10.1371/journal.pone.0087855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schleiss MR. Antiviral therapy of congenital cytomegalovirus infection. Semin Pediatr Infect Dis. 2005;16:50–59. doi: 10.1053/j.spid.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Revello MG, Lazzarotto T, Guerra B, Spinillo A, Ferrazzi E, Kustermann A, Guaschino S, et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med. 2014;370:1316–1326. doi: 10.1056/NEJMoa1310214. [DOI] [PubMed] [Google Scholar]
- 11.Gantt S, Carlsson J, Ikoma M, Gachelet E, Gray M, Geballe AP, Corey L, et al. The HIV protease inhibitor nelfinavir inhibits Kaposi’s sarcoma-associated herpesvirus replication in vitro. Antimicrobial agents and chemotherapy. 2011;55:2696–2703. doi: 10.1128/AAC.01295-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erice A, Borrell N, Li W, Miller WJ, Balfour HH., Jr Ganciclovir susceptibilities and analysis of UL97 region in cytomegalovirus (CMV) isolates from bone marrow recipients with CMV disease after antiviral prophylaxis. J Infect Dis. 1998;178:531–534. doi: 10.1086/517467. [DOI] [PubMed] [Google Scholar]
- 13.Kilewo C, Karlsson K, Ngarina M, Massawe A, Lyamuya E, Swai A, Lipyoga R, et al. Prevention of mother-to-child transmission of HIV-1 through breastfeeding by treating mothers with triple antiretroviral therapy in Dar es Salaam, Tanzania: the Mitra Plus study. J Acquir Immune Defic Syndr. 2009;52:406–416. doi: 10.1097/QAI.0b013e3181b323ff. [DOI] [PubMed] [Google Scholar]
- 14.Dorenbaum A, Cunningham CK, Gelber RD, Culnane M, Mofenson L, Britto P, Rekacewicz C, et al. Two-dose intrapartum/newborn nevirapine and standard antiretroviral therapy to reduce perinatal HIV transmission: a randomized trial. Jama. 2002;288:189–198. doi: 10.1001/jama.288.2.189. [DOI] [PubMed] [Google Scholar]
- 15.Brogly S, Read JS, Shapiro D, Stek A, Tuomala R. Participation of HIV-infected pregnant women in research in the United States. AIDS Res Hum Retroviruses. 2007;23:51–53. doi: 10.1089/aid.2006.0045. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease C and Prevention. Revised surveillance case definition for HIV infection--United States, 2014. MMWR Recomm Rep. 2014;63:1–10. [PubMed] [Google Scholar]
- 17.Boeckh M, Huang M, Ferrenberg J, Stevens-Ayers T, Stensland L, Nichols WG, Corey L. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. J Clin Microbiol. 2004;42:1142–1148. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bryson YJ, Mirochnick M, Stek A, Mofenson LM, Connor J, Capparelli E, Watts DH, et al. Pharmacokinetics and safety of nelfinavir when used in combination with zidovudine and lamivudine in HIV-infected pregnant women: Pediatric AIDS Clinical Trials Group (PACTG) Protocol 353. HIV clinical trials. 2008;9:115–125. doi: 10.1310/hct0902-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack SA, Best BM. Protecting the Fetus Against HIV Infection: A Systematic Review of Placental Transfer of Antiretrovirals. Clin Pharmacokinet. 2014;53:989–1004. doi: 10.1007/s40262-014-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalu NN, Desai PJ, Shirley CM, Gibson W, Dennis PA, Ambinder RF. Nelfinavir inhibits maturation and export of herpes simplex virus 1. J Virol. 2014;88:5455–5461. doi: 10.1128/JVI.03790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gantt S, Gachelet E, Carlsson J, Barcy S, Casper C, Lagunoff M. Nelfinavir impairs glycosylation of herpes simplex virus 1 envelope proteins and blocks virus maturation. Adv Virol. 2015;2015:687162. doi: 10.1155/2015/687162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gindes L, Teperberg-Oikawa M, Sherman D, Pardo J, Rahav G. Congenital cytomegalovirus infection following primary maternal infection in the third trimester. BJOG. 2008;115:830–835. doi: 10.1111/j.1471-0528.2007.01651.x. [DOI] [PubMed] [Google Scholar]
- 23.Griffiths PD, Baboonian C. A prospective study of primary cytomegalovirus infection during pregnancy: final report. Br J Obstet Gynaecol. 1984;91:307–315. doi: 10.1111/j.1471-0528.1984.tb05915.x. [DOI] [PubMed] [Google Scholar]
- 24.Boppana SB, Ross SA, Novak Z, Shimamura M, Tolan RW, Jr, Palmer AL, Ahmed A, et al. Dried blood spot real-time polymerase chain reaction assays to screen newborns for congenital cytomegalovirus infection. JAMA. 2010;303:1375–1382. doi: 10.1001/jama.2010.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson CT, Istas AS, Wilkerson MK, Demmler GJ. PCR detection of cytomegalovirus DNA in serum as a diagnostic test for congenital cytomegalovirus infection. J Clin Microbiol. 1995;33:3317–3318. doi: 10.1128/jcm.33.12.3317-3318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford RD, Cloud G, Lakeman AD, Boppana S, Kimberlin DW, Jacobs R, Demmler G, et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J Infect Dis. 2005;191:227–233. doi: 10.1086/426456. [DOI] [PubMed] [Google Scholar]
- 27.Kimberlin DW, Acosta EP, Sanchez PJ, Sood S, Agrawal V, Homans J, Jacobs RF, et al. Pharmacokinetic and pharmacodynamic assessment of oral valganciclovir in the treatment of symptomatic congenital cytomegalovirus disease. J Infect Dis. 2008;197:836–845. doi: 10.1086/528376. [DOI] [PubMed] [Google Scholar]
- 28.Ross SA, Novak Z, Fowler KB, Arora N, Britt WJ, Boppana SB. Cytomegalovirus blood viral load and hearing loss in young children with congenital infection. Pediatr Infect Dis J. 2009;28:588–592. doi: 10.1097/INF.0b013e3181979a27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hernando S, Folgueira L, Lumbreras C, San Juan R, Maldonado S, Prieto C, Babiano MJ, et al. Comparison of cytomegalovirus viral load measure by real-time PCR with pp65 antigenemia for the diagnosis of cytomegalovirus disease in solid organ transplant patients. Transplant Proc. 2005;37:4094–4096. doi: 10.1016/j.transproceed.2005.10.087. [DOI] [PubMed] [Google Scholar]
- 30.Razonable RR. Rare, unusual, and less common virus infections after organ transplantation. Current opinion in organ transplantation. 2011;16:580–587. doi: 10.1097/MOT.0b013e32834cdaf2. [DOI] [PubMed] [Google Scholar]
- 31.Schafer P, Tenschert W, Cremaschi L, Gutensohn K, Laufs R. Utility of major leukocyte subpopulations for monitoring secondary cytomegalovirus infections in renal-allograft recipients by PCR. J Clin Microbiol. 1998;36:1008–1014. doi: 10.1128/jcm.36.4.1008-1014.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Reitter A, Buxmann H, Haberl AE, Schlosser R, Kreibich M, Keppler OT, Berger A. Incidence of CMV co-infection in HIV-positive women and their neonates in a tertiary referral centre: a cohort study. Med Microbiol Immunol. 2015 doi: 10.1007/s00430-015-0427-9. [DOI] [PubMed] [Google Scholar]
- 33.Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol. 2015;204:263–271. doi: 10.1007/s00430-015-0399-9. [DOI] [PubMed] [Google Scholar]
- 34.Transmission PoToH-IPWaPoP. Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-1-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. 2014. [Google Scholar]
- 35.Lorenzoni F, Lunardi S, Liumbruno A, Ferri G, Madrigali V, Fiorentini E, Forli F, et al. Neonatal screening for congenital cytomegalovirus infection in preterm and small for gestational age infants. J Matern Fetal Neonatal Med. 2014;27:1589–1593. doi: 10.3109/14767058.2013.871253. [DOI] [PubMed] [Google Scholar]
- 36.Duryea EL, Sanchez PJ, Sheffield JS, Jackson GL, Wendel GD, McElwee BS, Boney LF, et al. Maternal human immunodeficiency virus infection and congenital transmission of cytomegalovirus. Pediatr Infect Dis J. 2010;29:915–918. doi: 10.1097/INF.0b013e3181e0ce05. [DOI] [PubMed] [Google Scholar]
- 37.Nakamura Y, Sakuma S, Ohta Y, Kawano K, Hashimoto T. Detection of the human cytomegalovirus gene in placental chronic villitis by polymerase chain reaction. Hum Pathol. 1994;25:815–818. doi: 10.1016/0046-8177(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 38.Hamilton ST, Scott G, Naing Z, Iwasenko J, Hall B, Graf N, Arbuckle S, et al. Human cytomegalovirus-induces cytokine changes in the placenta with implications for adverse pregnancy outcomes. PLoS One. 2012;7:e52899. doi: 10.1371/journal.pone.0052899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chaudhuri S, Lowen B, Chan G, Davey A, Riddell M, Guilbert LJ. Human cytomegalovirus interacts with toll-like receptor 2 and CD14 on syncytiotrophoblasts to stimulate expression of TNFalpha mRNA and apoptosis. Placenta. 2009;30:994–1001. doi: 10.1016/j.placenta.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Yan Z, Bryant KF, Gregory SM, Angelova M, Dreyfus DH, Zhao XZ, Coen DM, et al. HIV integrase inhibitors block replication of alpha-, beta-, and gammaherpesviruses. MBio. 2014;5:e01318–01314. doi: 10.1128/mBio.01318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]