Abstract
The continuous rise in obesity is a major concern for future healthcare management. Many strategies to control body weight focus on a permanent modification of food intake with limited success in the long term. Metabolism or energy expenditure is the other side of the coin for the regulation of body weight, and strategies to enhance energy expenditure are a current focus for obesity treatment, especially since the (re)-discovery of the energy depleting brown adipose tissue in adult humans. Conversely, several human illnesses like neurodegenerative diseases, cancer, or autoimmune deficiency syndrome suffer from increased energy expenditure and severe weight loss. Thus, strategies to modulate energy expenditure to target weight gain or loss would improve life expectancies and quality of life in many human patients. The aim of this book chapter is to give an overview of our current understanding and recent progress in energy expenditure control with specific emphasis on central control mechanisms.
Keywords: Neuronal circuits, Hypothalamus, Hormones, Leptin, FGF21, Dorsomedial hypothalamus, Body weight
1 Introduction
The nervous system has evolved to regulate and coordinate bodily functions and behavior in a changing environment. The mammalian brain thus extensively communicates not only with the external world, but also with all aspects of internal physiology. While the neural controls of cardiovascular and gastrointestinal functions have been most intensively studied, the neural control of metabolism is less well understood and appreciated, probably owing to its complexity. Neural control of metabolism includes energy production, storage, mobilization, conversion, and utilization, as accomplished by the coordinated actions of the gastrointestinal tract, liver, pancreas, muscle, white adipose tissue (WAT), and brown adipose tissue (BAT).
The neural control of metabolism can be conceived as consisting of sensory inputs, central integrative circuits, and motor outputs (sympathetic and parasympathetic), allowing for typical feedback regulation of specific functions (Fig. 1). Sensory input to the brain is accomplished by primary afferents innervating and by humoral factors secreted from relevant peripheral organs (Cechetto 1987; Craig 2002; Fealey 2013; Janig 1996). In turn, the brain controls specific functions of these organs through the autonomic nervous system and endocrine outflow. Most of these sensory and motor neural pathways have been well documented since the inception of neural tract tracing methods some 40 years ago (Ricardo 1983) and particularly the ascent of trans-synaptic viral tracing techniques (Card and Enquist 2014; Loewy 1998). Much less is known about the organization of integrative circuits in the brain. This is probably due to the complexity of these circuits and lack of appropriate methodology to untangle them. However, the recent availability of neuron-specific stimulation and recording techniques in animals and functional neuroimaging techniques in humans is starting to provide exciting new insights (Rezai-Zadeh and Munzberg 2013; Williams and Elmquist 2012).
Fig. 1.
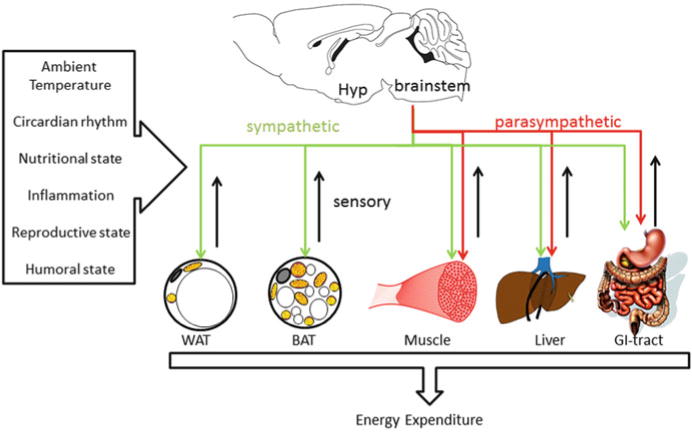
Schematic view of the complex interaction of brain, peripheral tissues, and environment
One of the first accounts demonstrating brain-evoked changes in a metabolic parameter can be traced back almost 150 years to Claude Bernard’s “piqûre diabetique,” in which he showed a slow rise in blood glucose following lesions in the caudal brainstem (Bernard 1957). More systematic investigations followed much later in the context of the classical studies on the hypothalamic control of energy balance and body weight of the mid-1900’s (Brobeck 1946; Kennedy 1951; Mayer and Barrnett 1955). A key observation was the increase in body weight and adiposity in rats with ventromedial hypothalamic (VMH) lesions, even when food intake was restricted to sham-operated rats (Cox and Powley 1981); it is a clear evidence that the hypothalamus not only controls energy intake but also energy expenditure to achieve energy balance. This led to the discovery of BAT and its role in heat production and body weight regulation (Rothwell and Stock 1979). The discovery of leptin some 20 years ago provided the final push toward identification of neural circuitry in the hypothalamus and beyond, responsible for the regulation of energy balance and control of metabolism (Halaas et al. 1995). In this chapter, we review recent progress in the identification of brain circuits and pathways that control energy expenditure via peripheral organs.
2 Input and Output Systems for Energy Expenditure Control
Energy expenditure depends on several external and internal factors such as ambient temperature, nutritional or reproductive state, circadian rhythms, and levels of circulating hormones (Fig. 1). These external and internal modulators have sometimes opposing physiological effects and need to be integrated and translated via the brain to allow appropriate physiological changes and ensure homeostasis. Cold exposure is an excellent example that demonstrates the quick increase in energy expenditure within minutes after such an external challenge (Fig. 2a). Conversely, increased ambient temperature results in decreased energy expenditure (Fig. 2b).
Fig. 2.
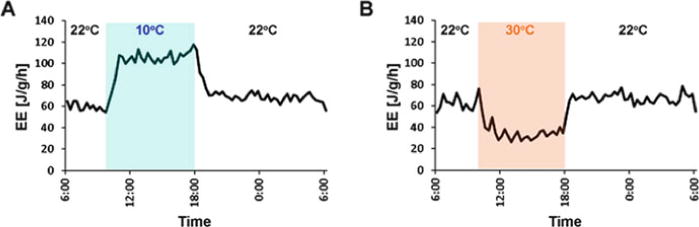
Temperature changes induce robust adaptations in energy expenditure. (a) Acute decreases in ambient temperature quickly and robustly increase energy expenditure. (b) Acute increase in ambient temperature results in adaptive decrease in energy expenditure
Three components of whole-body energy expenditure can be distinguished: basal metabolic rate (BMR), adaptive thermogenesis, and physical activity (Fig. 3). BMR is the energy expenditure that is measured at thermoneutrality (no extra energy needed for cold- or warm-defensive adaptations), postprandially (after active meal digestion), and at rest (minimal muscle movement) and defines the oxygen consumed for ATP production. The coupling of oxidative processes to ATP production is not perfect, and some energy is lost by proton leaks (basal leak) across the mitochondrial membrane (Brand et al. 1999). Thus, BMR also includes such basal leaks and is also called obligatory metabolism as it is required to maintain minimal bodily functions.
Fig. 3.
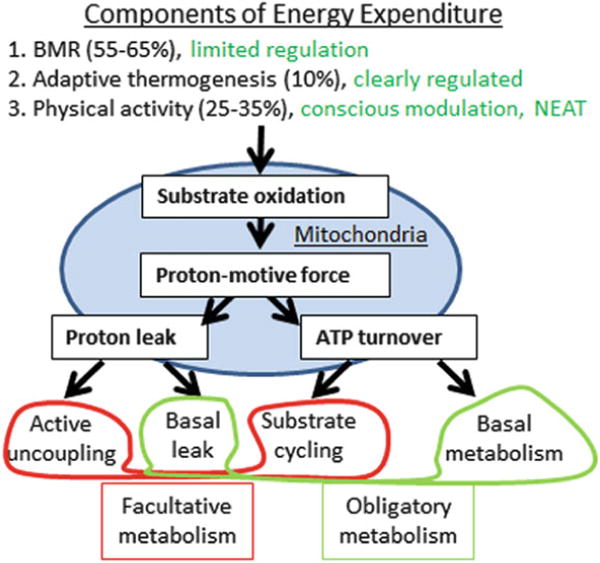
Components of energy expenditure. Oxidative processes result in a proton-motive force in the mitochondrion used to generate ATP, even though basal proton leaks are observed that “wastes” energy. ATP production and basal proton leaks together account for obligatory metabolism, required for minimal bodily functions. The active uncoupling of proton-motive force from ATP production is used to generate heat, e.g., in the brown adipose tissue. And uncoupling protein 1 (UCP1) is a well-studied example for active uncoupling. Substrate cycling also actively contributes to heat production. Together these mechanisms account for facultative metabolism, which is optional and not used for baseline maintenance of bodily functions, e.g., at thermoneutral conditions
During external challenges such as cold exposure, additional systems are activated and increase energy expenditure beyond BMR. This can be conscious and voluntary exercise, and involuntary muscle shivering (Rowland et al. 2014). In addition, adaptive systems are activated – specifically during chronic cold exposure – that are known as cold-induced thermogenesis or adaptive thermogenesis. These processes use active uncoupling of the mitochondrial proton-motive force from ATP production or futile cycling to “waste” energy and to release energy as heat (Rowland et al. 2014). This active energy wasting is also known as facultative metabolism, because it is optional and not required to maintain minimal bodily functions under thermoneutral conditions. BAT is an invention of euthermic animals such as birds and mammals and is a heat-generating tissue that is specialized in adaptive thermogenesis.
2.1 BAT and Adaptive Thermogenesis
In rodents, the interscapular BAT is the largest depot, with smaller depots in the mediastinum, along the cervical and thoracic aorta, and around the kidney (Giordano et al. 2004). In humans, BAT is less centralized than in rodents, with significant depots in supraclavicular, neck, and paraspinal regions (Cypess et al. 2009; Lidell et al. 2013; Saito et al. 2009). Based on numerous experiments with denervation of the interscapular pads in rodents, as well as pharmacological studies using ß3-adrenergic agonists and blockers, the main driver of BAT thermogenesis seems to be its noradrenergic sympathetic innervation (Andrews et al. 1985; Bartness and Wade 1984; Himms-Hagen et al. 1990; Takahashi et al. 1992; Tsukazaki et al. 1995). Retrograde tracing and other studies in rats and Siberian hamsters have identified postganglionic perikarya innervating BAT in the stellate ganglia (Grkovic and Anderson 1997; Oldfield et al. 2002), known to receive input from preganglionic neurons in the intermediolateral cell column of the cervical and thoracic spinal cord (Nozdrachev et al. 2002; Tanche and Therminarias 1967) (Fig. 4).
Fig. 4.
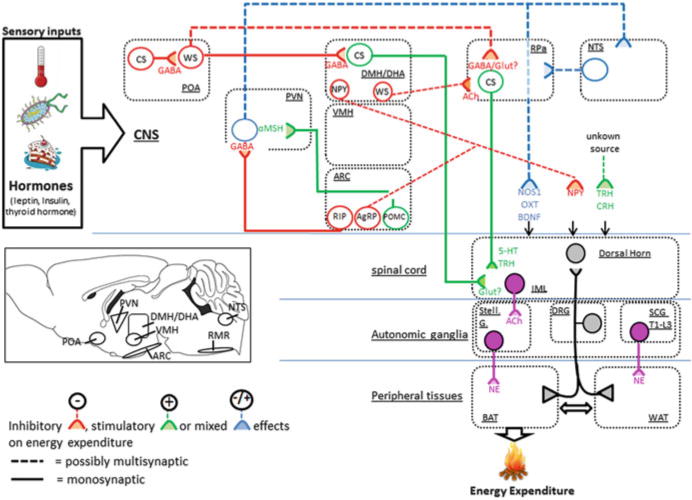
Schematic overview of central circuits that modulate energy expenditure
BAT is also innervated by dorsal root sensory nerve fibers (De et al. 1998; Himms-Hagen et al. 1990; Vaughan et al. 2014), but based on the lack of cholinergic markers, BAT is not innervated by the parasympathetic nervous system (Norman et al. 1988). Sympathetic activation of BAT leads to the activation and gene expression of the uncoupling protein-1 (UCP1), which is well accepted as a true uncoupler with heat-producing properties (Shabalina and Nedergaard 2011). The idea to burn excess calories by activating BAT in the fight against obesity flared up 35 years ago (Rothwell and Stock 1979) and, after a long hiatus, returned only recently because the existence of functional and inducible BAT was convincingly demonstrated in adult humans (Cypess et al. 2009; Saito et al. 2009; van Marken Lichtenbelt et al. 2009; Virtanen et al. 2009).
2.2 Muscle, Energy Expenditure, and Thermogenesis
Physical activity greatly contributes to whole-body energy expenditure and can be distinguished into exercise and non-exercise activity thermogenesis (NEAT). NEAT involves occupational activities, walking, sitting, standing, fidgeting, talking, leisure activities, etc., but excluding voluntary exercise, sleeping, or eating (Levine 2004) and can substantially contribute to total energy expenditure (15–50%). Therefore, NEAT has been studied as a malleable variable for body weight regulation through increasing energy expenditure.
Physical activity is at least in part genetically encoded, because selective breeding for physical activity in rats resulted in the genetic distinction of high- and low-capacity runners (HCR, LCR, respectively) with low and high incidences of obesity, respectively (Wisloff et al. 2005). HCR had higher total energy expenditure, even though resting energy expenditure was similar between HCR and LCR, suggesting that increased locomotion largely accounts for changes in total energy expenditure. However, HCR rats had increased mitochondria content and increased sympathetic drive in their skeletal muscle, and the existence of skeletal muscle thermogenesis, not exercise per se, has been suggested as a contributing factor for weight gain resistance on high-fat diets (Gavini et al. 2014; Wisloff et al. 2005). HCR also have increased expression of uncoupling protein 2 and 3 (UCP2 and UCP3). However, in contrast to UCP1, UCP2 and UCP3 do not have true uncoupling functions (Brand and Esteves 2005; Shabalina and Nedergaard 2011). Another potential mechanism to mediate muscle non-shivering thermogenesis is sarco-/endoplasmic reticulum Ca2+-ATPase (SERCA) by uncoupling ATP hydrolysis from Ca2+ transport (Bal et al. 2012). Whether SERCA uncoupling is also controlled by sympathetic nerve activity (SNA) similar to UCP1 is unknown. Thus, muscle thermogenesis and its regulatory properties, e.g., during diet-induced obesity, remains ill defined and is an urgent future topic.
2.3 Sensory System
The skin is a very important sensory organ for thermoregulatory control of energy expenditure. It works as a feed-forward system, so that ambient temperature changes are communicated to the brain for temperature defensive adaptations, even though core temperature is not immediately compromised. Temperature-sensing receptors (superfamily of transient receptor potential (TRP) channels) are located in the nerve endings of sensory cells throughout the skin. The cell bodies of these bipolar sensory neurons are found in trigeminal and dorsal root ganglia from where they further communicate with central structures in the spinal cord (dorsal horn lamina) and brain (Damann et al. 2008; Julius and Nathans 2012). Temperature changes in the skin cause the opening of TRP channels and promote the activation of sensory neurons (Voets 2014). This is further communicated to the CNS to modulate SNA in peripheral tissues (e.g., BAT, WAT), which is further addressed in Sect. 3.
BAT and WAT also send feedback information to the CNS via sensory nerves that connect adipocytes via dorsal root ganglia with the brain (Bartness et al. 2010a; b; De et al. 1998; Himms-Hagen et al. 1990; Vaughan et al. 2014). Incoming (afferent) sympathetic nerves can be distinguished from outgoing (efferent) sensory nerves with multisynaptic anterograde (Herpes virus) and retrograde (pseudorabies virus) viral tracers that are injected into BAT or WAT. Surprisingly, many CNS sites showed both sympathetic and sensory connectivity, so that an extensive feedback system for incoming and outgoing signals is likely (Ryu et al. 2015; Ryu and Bartness 2014). Adipose tissue sensory nerves are responsive to metabolic changes (e.g., lipolysis) (Song et al. 2009) and the adipokine leptin (Murphy et al. 2013). In the BAT, sensory input may specifically influence the trophic adaptations to changes in ambient temperature (Himms-Hagen et al. 1990). However, we still do not have a firm understanding of the extent and how sensory feedback loops influence physiological function.
2.4 Endocrine Systems and Energy Expenditure Regulation
The modulation of energy expenditure in response to external and internal challenges involves neuronal input to and outputs from the brain to perform energy expenditure changes in peripheral tissues (e.g., BAT). In addition to this neural loop, various endocrine hormones are important communicators between peripheral tissues and the central sites to regulate energy expenditure.
2.4.1 Cold-Induced Endocrine Hormones
The thyroid hormone is perhaps the most important humoral regulator of metabolism and energy expenditure. Its production is regulated by the brain via the hypothalamic-pituitary-thyroid (HPT) axis, in which the activation of thyrotropin-releasing hormone (TRH) neurons within the hypothalamus ultimately leads to increased thyroid hormone (T4/T3) signaling at peripheral tissues (for review, see Fekete and Lechan 2014; Joseph-Bravo et al. 2015). Thyroid hormone acts on many tissues to promote cellular metabolism and energy expenditure, including effects on heart function, skeletal muscle, and BAT, and as such thyroid hormone is a critical positive regulator of BMR. Changes in ambient temperature or nutritional state influence the activity of TRH neurons within the paraventricular hypothalamus (PVH), resulting in increased release of thyroid hormone from the thyroid gland (Bianco et al. 2002; Zoeller et al. 1990). Leptin also directly stimulates TRH neurons while fasting inhibits these neurons (Huo et al. 2004; Legradi et al. 1997, 1998). Thus, TRH neurons and the HPT axis are critically involved in the regulation of whole-body energy expenditure in response to changes in the external and internal milieu. The thyroid axis may also modulate BAT thermogenesis via central thermoregulatory circuits as discussed in Sect. 3.
Ongoing efforts aim to discover additional “peripheral” endocrine modulators of energy expenditure that could promote weight loss. The muscle-derived hormone irisin (produced by the fndc5 gene) has received considerable attention. Irisin is increased by exercise to promote the transition of lipid-storing WAT to energy expending BAT-like properties, also known as “browning” of WAT, and is also induced by cold exposure (Bostrom et al. 2012; Lee et al. 2014). Another notable metabolic hormone is fibroblast growth factor 21(FGF21) (Lee et al. 2014). FGF21 is mainly secreted from the liver (Markan et al. 2014) but is also robustly induced by cold exposure in the BAT (Chartoumpekis et al. 2011). Whether FGF21 in BAT is solely induced by cold exposure or instead requires additional metabolic stressors as observed in UCP1-deficient mice (Keipert et al. 2015) remains to be clarified. Also, it is unclear if cold-induced production and secretion of irisin (from muscle) or FGF21 (e.g., BAT) depends on increased sympathetic outflow to skeletal muscle and BAT, respectively.
2.4.2 Endocrine Signals and Adaptive Responses to Energy Restriction
Changes in energy availability (e.g., during fasting) also induce adaptive changes in energy expenditure. This process of energy homeostasis requires the CNS to detect and respond to endocrine hormones (and possibly sensory inputs from peripheral tissues) that are triggered by negative or positive energy balances (Morrison and Berthoud 2007). Such a decrease in energy expenditure typically accompanies fasting and starvation (Dulloo and Jacquet 1998; Leibel et al. 1995), even though acute fasting may initially rather trigger an increased sympathetic tone to mobilize fat stores in WAT (Goodner et al. 1973; Havel 1968; Koerker et al. 1975). Fasting-induced hypometabolism involves a variety of circulating hormones with central actions, including the adipose-derived hormone leptin. Circulating leptin levels rapidly fall with negative energy balance, and the resulting hypometabolism can be prevented by restoring serum or central leptin levels (Ahima et al. 1996; Rosenbaum et al. 2002, 2005). Taken together, falling leptin levels during starvation are detected by the CNS to change the motivation to eat and to reduce energy expenditure.
The gut hormone ghrelin also contributes to starvation-induced adaptive responses. Ghrelin release is increased during starvation and suppresses energy expenditure (Muller et al. 2015). Also insulin and glucagon are highly regulated by energy intake and contribute substantially to the starvation response, e.g., induction of lipolysis. Considering the variety of hormones that act in the brain to suppress food intake and energy expenditure simultaneously, it is suggested that a precise interaction of feeding and thermoregulatory neuronal circuits exist. However, comprehensive knowledge of how these systems are coordinated is missing and a key goal for the future.
2.4.3 Overfeeding and Energy Expenditure: Diet-Induced Thermogenesis
A negative energy balance (e.g., during fasting) is associated with a reduction in energy expenditure, while increased food intake (e.g., during high-fat feeding) induces thermogenic responses, also known as diet-induced thermogenesis (DIT) (Rothwell et al. 1983). Rothwell and Stock also demonstrated that low-protein diet increased energy expenditure, suggesting that both overfeeding and protein restriction triggered DIT (Rothwell et al. 1983). The circulating hormone FGF21 is well known to increase energy expenditure and promote the browning of WAT (Douris et al. 2015; Fisher et al. 2012), but only recent work showed that FGF21 is required for the low protein-induced energy expenditure (Laeger et al. 2014; Morrison and Laeger 2015). Whether FGF21 promotes these effects within the periphery and/or through the brain remains unclear (Kharitonenkov and Adams 2014; Owen et al. 2015).
In summary, the maintenance of body weight and thermoregulation in response changes in external temperature and food availability are mediated by an intricate neural and endocrine network.
3 Neural Circuits That Modulate Energy Expenditure
The brain network that regulates adaptive thermogenesis receives inputs from temperature- and energy-sensing neurons through hypothalamic and brain stem areas such as the preoptic area (POA), arcuate nucleus (ARC), and nucleus of the solitary tract (NTS) (Morrison et al. 2014). Naturally, many physiological states such as fever, stress-induced hyperthermia, and diurnal fluctuation of body temperature require attention from these central thermoregulatory circuits. Some physiological states may require opposing adaptations of energy expenditure, e.g., cold exposure increases energy expenditure, but fasting requires energy preservation and decreased energy expenditure. Thus, if cold exposure and fasting challenges are combined, this conflict needs to be solved by the brain for an appropriate modulation of energy expenditure, manifested in a change of SNA and secretion of neurohormones.
The anatomical location of BAT-related CNS neurons that control BAT thermogenesis stems from multisynaptic, retrograde tracing studies with PRV infections of the BAT (Cano et al. 2003). Another tool to identify thermoregulatory neurons is to track which neurons are activated by changes in ambient temperature. The early response gene cFos is rapidly induced by neuronal firing and is a reliable and efficient marker for neurons that are activated in response to temperature changes. (Bamshad et al. 1999; Cano et al. 2003; Oldfield et al. 2002). However, molecular identities and synaptic connections of these neurons are not entirely understood. In this section, we focus on central circuits that govern BAT SNA. We also briefly discuss neural circuits that modulate the release of neurohormones that affect adaptive thermogenesis.
3.1 Hypothalamus
Much of the literature that defines central sites that control BAT thermogenesis stems from research on pyrogenic stimuli and cold-defense behavior. The POA has received specific attention in the control of such thermoregulatory processes (Nakamura 2011). Thermoregulatory neurons in the POA receive thermosensory neuronal inputs from the skin, but many POA neurons are internally temperature sensitive. They change their firing activity with local temperature changes, thus enabling the POA to detect both peripheral and brain temperature changes (Boulant and Dean 1986; Nakamura and Morrison 2008, 2010). These neurons are proposed to be mostly warm-sensitive GABAergic (inhibitory) neurons that directly inhibit BAT sympathetic premotor neurons in the rostral medullary raphe (RMR) or indirectly through the dorsomedial hypothalamus/dorsal hypothalamic area (DMH/DHA) (Yoshida et al. 2009). Therefore, during a cold exposure, warm-sensitive POA neurons are inhibited and enable thermogenic neurons in the DMH/DHA and RMR to increase BAT SNA.
Some warm-sensitive GABAergic POA neurons express prostaglandin E receptor subtype EP3 and mediate febrile responses by using the same POA > DMH/DHA > RMR circuits to BAT (Lazarus et al. 2007; Nakamura et al. 2009; Scammell et al. 1996; Ushikubi et al. 1998). However, stimulatory glutamatergic inputs to the DMH/DHA have been proposed as well (Madden and Morrison 2004), and cold-sensitive glutamatergic POA neurons may provide these inputs (Dimitrov et al. 2011). Because there are also warm-activated cholinergic neurons in the DMH that directly inhibit thermogenic RMR neurons (Jeong et al. 2015), the POA is very likely to contain warm-sensitive glutamatergic neurons that directly innervate these DMH neurons.
Other lines of research that are more concerned with body weight regulation have focused on additional neuronal sites and their effect on energy expenditure and body weight regulation. These energy homeostatic sites have not been well characterized for their responsiveness to thermal inputs, but clearly modulate BAT SNA. The ARC is highly responsive to changes in energy/nutritional state (e.g., fasting) and mediates changes in BAT SNA. Pro-opiomelanocortin (POMC)-expressing neurons in the ARC are anorexigenic neurons that increase BAT thermogenesis. The secretion of ∝-melanocyte-stimulating hormone, a byproduct of POMC, or melanotan II (MTII), an MC4R agonist, activates melanocortin 4 receptors (MC4R) to increase energy expenditure and UCP1 expression via BAT SNA, while loss of MC4R decreases energy expenditure and promotes weight gain (Chen et al. 2000; Haynes et al. 1999; Ste Marie et al. 2000). Although the exact sites of MC4R-mediated BAT activation is not completely understood, MC4Rs in the PVH are not involved in the regulation of energy expenditure, while cholinergic neurons in the intermediolateral nucleus (IML) within the spinal cord are sufficient to restore energy expenditure in whole-body MC4R-deficient mice (Berglund et al. 2014; Rossi et al. 2011; Sohn et al. 2013).
GABAergic ARC neurons agouti-related peptide/neuropeptide Y (AgRP/NPY) neurons and RIP-Cre neurons both affect BAT SNA. NPY derived from ARC AgRP/NPY neurons inhibits BAT SNA via activation of Y1 receptor in unknown target neurons (Shi et al. 2013). Similarly, DMH NPY neurons also inhibit BAT sympathetic control (Bi et al. 2003; Chao et al. 2011), and the central administration of NPY induces torpor-like hypothermia (Dark and Pelz 2008), suggesting overall sympathoinhibitory NPY function in the brain. Another set of sympathoinhibitory neurons exist in the DMH that are warm-activated neurons and project to the RMR (Jeong et al. 2015). RIP-Cre neurons are a distinct population of GABAergic neurons within the ARC that inhibit PVH neurons to enhance BAT activation (Kong et al. 2012).
Interestingly, several central thermoregulatory and energy homeostatic neurons express leptin receptors or are controlled by LepRb neurons (Bachman et al. 2002). Leptin action within the DMH has a clear sympathostimulatory effect on BAT thermogenesis and associated cardiovascular responses, which are largely independent of anorexic leptin effects (Enriori et al. 2011; Rezai-Zadeh et al. 2014). Some of these leptin-mediated effects on energy expenditure requires glutamate signaling (Xu et al. 2013), even though leptin likely exerts its effects via complex inhibition and stimulation of several neuronal populations, including its interaction with insulin via POMC neurons to promote browning of WAT (Dodd et al. 2015).
The PVH is an essential output center for neuronal and humoral signals and has been rediscovered as an important thermoregulatory site. General PVH activation prevents cold- and prostaglandin E2-increased BAT SNA by increasing GABAergic inputs to the RMR (Madden and Morrison 2009). Because the PVH consists of mainly glutamatergic neurons, this may involve multisynaptic circuits, possibly involving identified PVH > NTS > RMR circuits that are modulated by ARC RIP-Cre neurons (Kong et al. 2012). With the use of the modern genetic toolbox, some thermoregulatory PVH subpopulations have been further identified as nitric oxide synthase 1 (NOS1), oxytocin (OXT), or brain-derived neurotrophic factor (BDNF)-expressing neurons that project directly to sympathetic preganglionic neurons in the spinal cord to increase BAT activity (An et al. 2015; Sutton et al. 2014).
PVH OXT neurons are a subpopulation of NOS1 neurons, and activation of either population activates BAT thermogenesis. Interestingly, simple-minded homolog 1 expressing PVH neurons, which marks most PVH neurons, also increase BAT thermogenesis when activated (Sutton et al. 2014), contradicting earlier findings mentioned above. Similarly, posterior PVH BDNF neurons seem to regulate BAT thermogenesis because PVH-specific BDNF deletion reduced energy expenditure and increased body weight (An et al. 2015). Whether PVH BDNF neurons also express NOS1 is not known. These findings are in line with earlier studies showing that cFos in the PVH is induced by both cold and warm exposures (Cano et al. 2003; Yoshida et al. 2002). Taken together, the PVH seems to be involved in both directional controls of BAT sympathetic activity, and future research needs to identify the neurochemical properties of sympathoinhibitory PVH neurons.
In addition to the neural control of sympathetic BAT inputs, the PVH also modulates humoral effectors of BAT thermogenesis. Cold exposure increases while warm exposure decreases thyroid hormone levels via TRH neurons to stimulate BAT activity and BMR (Andersson et al. 1963; Eastman et al. 1974; Kim 2008). Interestingly, in addition to the intensely studied peripheral effects of thyroid hormone, more recent data also indicate a central function of thyroid hormone to increase BAT SNA (Coppola et al. 2007; Lopez et al. 2010). Furthermore, TRH neurons are found outside the PVH in important thermoregulatory sites like the DMH and RMR (based on Allen Brain Atlas data, http://mouse.brain-map.org/), and compelling functional data support thermoregulatory synergistic effects of TRH and leptin via brainstem circuits (Hermann et al. 2006; Rogers et al. 2009, 2011).
Another thermoregulatory PVH neurohormone is corticotropin-releasing hormone (CRH), which is increased by low glucose levels or other stressor. CRH increased pituitary adrenocorticotropic hormone release to induce stress hormones like glucocorticoids that upon other functions inhibit BAT activity peripherally (Moriscot et al. 1993). Like TRH neurons, CRH neurons are also found outside the PVH (based on Allen Brain Atlas data) and may function within thermoregulatory central circuitries, e.g., central CRH infusions into the POA and other hypothalamic sites stimulate BAT SNA output (Egawa et al. 1990a,b).
The VMH has long been implicated in the control of BAT SNA and energy expenditure even though the pathways leading to BAT sympathetic neurons have not been identified (Perkins et al. 1981), due to the typical lack of PRV labeling. Nonetheless, insulin, thyroid hormone, and estrogen affect BAT SNA through the VMH (Klockener et al. 2011; Lopez et al. 2010; Musatov et al. 2007). Estrogen signals via its receptor ER∝ and promotes different aspects of thermogenesis via distinct ER∝-expressing VMH neurons (Correa et al. 2015; Xu et al. 2011). Future studies are needed to explore the involved downstream targets within these thermoregulatory circuitries.
Finally, a subpopulation of orexin/hypocretin neurons in the lateral hypothalamic area (LHA) project to BAT sympathetic premotor neurons in the RMR, and the secretion of orexin seems to potentiate already existing BAT sympathostimulatory signals onto the RMR (Berthoud et al. 2005; Tupone et al. 2011). Interestingly, LHA orexin neurons are not involved in cold- or pyrogen-induced BAT thermogenesis (Nakamura et al. 2005) but are rather critical for stress-induced BAT thermogenesis (Zhang et al. 2010), even though the DMH may be more dominantly involved in stress-induced thermogenesis (Kataoka et al. 2014).
3.2 Brainstem
Hypothalamic areas that receive BAT-related inputs send efferent fibers to sympathetic premotor neurons in the RMR or project directly to spinal preganglionic neurons as mentioned above for the PVH. The RMR includes the rostral raphe pallidus (rRPa), raphe magnus, parapyramidal area, and ventrolateral medulla (VLM) and contains main sympathetic premotor neurons for BAT, vasculature, and heart (Nakamura 2011). The rRPa is especially important for BAT thermogenesis and innervated by many excitatory and inhibitory neuronal fibers that are originated from the hypothalamus and brainstem. rRPa neurons receive tonic inhibitory inputs at neutral conditions, most notably by warm-sensitive GABAergic POA neurons, and disinhibition of rRPa neurons by various thermogenic signals increases BAT SNA. Catecholaminergic neurons in the VLM including the A1/C1 neurons inhibit rRPa BAT premotor neurons through the activation of ∝2 adrenergic receptor, possibly explaining the systemic ∝2 adrenergic agonist-mediated hypothermia (Cao et al. 2010; Madden et al. 2013). C1 neurons have been proposed to respond to emergencies such as hypoxia and glucoprivation (Guyenet et al. 2013), and this VLM > rRPa pathway may account for inhibition of BAT during those situations (Madden 2012; Madden and Morrison 2005).
Neurons in the NTS receive viscerosensory information and inhibit BAT SNA when activated (Cao et al. 2010). The NTS also regulates BAT activity independent of hypothalamic inputs via hindbrain leptin/TRH signaling (Rogers et al. 2009) and ARC RIP-Cre neurons (Kong et al. 2012), implying it as a potential integrative site of viscerosensory and metabolic signals.
Other brainstem areas such as the lateral parabrachial nucleus, periaqueductal gray, and locus coeruleus have been associated with BAT sympathetic control as sensory afferent or effector efferent relay stations, but more studies are required to precisely identify their involvement in specific conditions (Almeida et al. 2004; Chen et al. 2002; Nakamura and Morrison 2008, 2010; Rathner and Morrison 2006; Yoshida et al. 2005).
Overall, many central sites possess a mixture of sympathostimulatory and sympathoinhibitory sets of neurons that may interact with the RMR as a master modulator for sympathetic BAT inputs. The hypothalamus is central for regulation of adaptive thermogenesis. Recent technical advancements of various neural mapping, recording, and manipulation tools at a spatially, genetically, and temporally controlled manner have accelerated our understanding of how brain works. The next step of understanding how the brain regulates energy expenditure would be deciphering where and how various environmental and internal signals are integrated and ramified to different thermoeffectors.
4 Remaining Questions and Conclusion
Metabolic diseases like obesity and diabetes are still increasing and remain a serious healthcare problem. Despite considerable efforts to treat obesity, it has been widely recognized that any treatment is flawed by the powerful ability of the body to adapt to dietary changes. A considerable part of the population is affected by the opposite problem: the failure to maintain healthy body weight and excessive weight loss due to enhanced energy expenditure in cachexic patients. This state is observed in neurodegenerative diseases and cancer and in patients with progressing acquired immune deficiency syndrome (AIDS) (Argiles et al. 2015; Dupuis et al. 2011; Salomon et al. 2002). Thus, targeting the central or peripheral nervous system to modulate energy expenditure is a realistic goal that would benefit many human patients and is currently under intense investigation.
One major roadblock is that the molecular basis of defended homeostatic levels (e.g., body weight and body temperature) remains unclear. Also, how changes in the defended levels (e.g., during obesity) are realized at the molecular level. Specifically, the interplay of energy expenditure and food intake is important to defend homeostatic levels. The reviewed work in this chapter compiles research from the thermoregulation and body weight regulation fields. However, more studies are required with emphasis on the interaction of thermoregulatory and food intake regulating neuronal circuits. Only if we are able to modulate the defended homeostatic body weight, we a will be able to achieve sustainable corrections in body weight.
References
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Almeida MC, Steiner AA, Coimbra NC, Branco LG. Thermoeffector neuronal pathways in fever: a study in rats showing a new role of the locus coeruleus. J Physiol. 2004;558:283–294. doi: 10.1113/jphysiol.2004.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An JJ, Liao GY, Kinney CE, Sahibzada N, Xu B. Discrete BDNF neurons in the paraventricular hypothalamus control feeding and energy expenditure. Cell Metab. 2015;22:175–88. doi: 10.1016/j.cmet.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson B, Ekman L, Gale CC, Sundsten JW. Control of thyrotrophic hormone (Tsh) secretion by the “Heat Loss Center”. Acta Physiol Scand. 1963;59:12–33. doi: 10.1111/j.1748-1716.1963.tb02719.x. [DOI] [PubMed] [Google Scholar]
- Andrews PL, Rothwell NJ, Stock MJ. Influence of subdiaphragmatic vagotomy and brown fat sympathectomy on thermogenesis in rats. Am J Physiol. 1985;249:E239–E243. doi: 10.1152/ajpendo.1985.249.3.E239. [DOI] [PubMed] [Google Scholar]
- Argiles JM, Lopez-Soriano FJ, Busquets S. Muscle wasting in cancer: the role of mitochondria. Curr Opin Clin Nutr Metab Care. 2015;18:221–225. doi: 10.1097/MCO.0000000000000164. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang CY, Cinti S, Bianco AC, Kobilka BK, Lowell BB. betaAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am J Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Wade GN. Effects of interscapular brown adipose tissue denervation on body weight and energy metabolism in ovariectomized and estradiol-treated rats. Behav Neurosci. 1984;98:674–685. doi: 10.1037//0735-7044.98.4.674. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010a;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int J Obes (Lond) 2010b;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berglund ED, Liu T, Kong X, Sohn JW, Vong L, Deng Z, Lee CE, Lee S, Williams KW, Olson DP, Scherer PE, Lowell BB, Elmquist JK. Melanocortin 4 receptors in autonomic neurons regulate thermogenesis and glycemia. Nat Neurosci. 2014;17:911–913. doi: 10.1038/nn.3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C. An introduction to the study of experimental medicine. Dover; New York: 1957. Originally published in 1865; first English translation by Henry Copley Greene, published by Macmillan & Co., Ltd., 1927 Reprint Edn. Dover edn. [Google Scholar]
- Berthoud HR, Patterson LM, Sutton GM, Morrison C, Zheng H. Orexin inputs to caudal raphe neurons involved in thermal, cardiovascular, and gastrointestinal regulation. Histochem Cell Biol. 2005;123:147–156. doi: 10.1007/s00418-005-0761-x. [DOI] [PubMed] [Google Scholar]
- Bi S, Robinson BM, Moran TH. Acute food deprivation and chronic food restriction differentially affect hypothalamic NPY mRNA expression. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1030–R1036. doi: 10.1152/ajpregu.00734.2002. [DOI] [PubMed] [Google Scholar]
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23:38–89. doi: 10.1210/edrv.23.1.0455. [DOI] [PubMed] [Google Scholar]
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Bostrom EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Hojlund K, Gygi SP, Spiegelman BM. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulant JA, Dean JB. Temperature receptors in the central nervous system. Annu Rev Physiol. 1986;48:639–654. doi: 10.1146/annurev.ph.48.030186.003231. [DOI] [PubMed] [Google Scholar]
- Brand MD, Esteves TC. Physiological functions of the mitochondrial uncoupling proteins UCP2 and UCP3. Cell Metab. 2005;2:85–93. doi: 10.1016/j.cmet.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Brand MD, Brindle KM, Buckingham JA, Harper JA, Rolfe DF, Stuart JA. The significance and mechanism of mitochondrial proton conductance. Int J Obes Relat Metab Disord. 1999;23(Suppl 6):S4–11. doi: 10.1038/sj.ijo.0800936. [DOI] [PubMed] [Google Scholar]
- Brobeck JR. Mechanism of the development of obesity in animals with hypothalamic lesions. Physiol Rev. 1946;26:541–559. doi: 10.1152/physrev.1946.26.4.541. [DOI] [PubMed] [Google Scholar]
- Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am J Physiol Regul Integr Comp Physiol. 2010;299:R277–R290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Card JP, Enquist LW. Transneuronal circuit analysis with pseudorabies viruses. Curr Protoc Neurosci. 2014;68:1. doi: 10.1002/0471142301.ns0105s68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cechetto DF. Central representation of visceral function. Fed Proc. 1987;46:17–23. [PubMed] [Google Scholar]
- Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartoumpekis DV, Habeos IG, Ziros PG, Psyrogiannis AI, Kyriazopoulou VE, Papavassiliou AG. Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Mol Med. 2011;17:736–740. doi: 10.2119/molmed.2011.00075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen AS, Metzger JM, Trumbauer ME, Guan XM, Yu H, Frazier EG, Marsh DJ, Forrest MJ, Gopal-Truter S, Fisher J, Camacho RE, Strack AM, Mellin TN, MacIntyre DE, Chen HY, Van der Ploeg LH. Role of the melanocortin-4 receptor in metabolic rate and food intake in mice. Transgenic Res. 2000;9:145–154. doi: 10.1023/a:1008983615045. [DOI] [PubMed] [Google Scholar]
- Chen XM, Nishi M, Taniguchi A, Nagashima K, Shibata M, Kanosue K. The caudal periaqueductal gray participates in the activation of brown adipose tissue in rats. Neurosci Lett. 2002;331:17–20. doi: 10.1016/s0304-3940(02)00757-7. [DOI] [PubMed] [Google Scholar]
- Coppola A, Liu ZW, Andrews ZB, Paradis E, Roy MC, Friedman JM, Ricquier D, Richard D, Horvath TL, Gao XB, Diano S. A central thermogenic-like mechanism in feeding regulation: an interplay between arcuate nucleus T3 and UCP2. Cell Metab. 2007;5:21–33. doi: 10.1016/j.cmet.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa SM, Newstrom DW, Warne JP, Flandin P, Cheung CC, Lin-Moore AT, Pierce AA, Xu AW, Rubenstein JL, Ingraham HA. An estrogen-responsive module in the ventromedial hypothalamus selectively drives sex-specific activity in females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JE, Powley TL. Intragastric pair feeding fails to prevent VMH obesity or hyperinsulinemia. Am J Physiol. 1981;240:E566–E572. doi: 10.1152/ajpendo.1981.240.5.E566. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, Kolodny GM, Kahn CR. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damann N, Voets T, Nilius B. TRPs in our senses. Curr Biol. 2008;18:R880–R889. doi: 10.1016/j.cub.2008.07.063. [DOI] [PubMed] [Google Scholar]
- Dark J, Pelz KM. NPY Y1 receptor antagonist prevents NPY-induced torpor-like hypothermia in cold-acclimated Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R236–R245. doi: 10.1152/ajpregu.00587.2007. [DOI] [PubMed] [Google Scholar]
- De MR, Ricquier D, Cinti S. TH-, NPY-, SP-, and CGRP-immunoreactive nerves in interscapular brown adipose tissue of adult rats acclimated at different temperatures: an immunohistochemical study. J Neurocytol. 1998;27:877–886. doi: 10.1023/a:1006996922657. [DOI] [PubMed] [Google Scholar]
- Dimitrov EL, Kim YY, Usdin TB. Regulation of hypothalamic signaling by tuberoin-fundibular peptide of 39 residues is critical for the response to cold: a novel peptidergic mechanism of thermoregulation. J Neurosci. 2011;31:18166–18179. doi: 10.1523/JNEUROSCI.2619-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd GT, Decherf S, Loh K, Simonds SE, Wiede F, Balland E, Merry TL, Munzberg H, Zhang ZY, Kahn BB, Neel BG, Bence KK, Andrews ZB, Cowley MA, Tiganis T. Leptin and insulin act on POMC neurons to promote the browning of white fat. Cell. 2015;160:88–104. doi: 10.1016/j.cell.2014.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douris N, Stevanovic DM, Fisher FM, Cisu TI, Chee MJ, Nguyen NL, Zarebidaki E, Adams AC, Kharitonenkov A, Flier JS, Bartness TJ, Maratos-Flier E. Central fibroblast growth factor 21 browns white fat via sympathetic action in male mice. Endocrinology. 2015;156:2470–2481. doi: 10.1210/en.2014-2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulloo AG, Jacquet J. Adaptive reduction in basal metabolic rate in response to food deprivation in humans: a role for feedback signals from fat stores. Am J Clin Nutr. 1998;68:599–606. doi: 10.1093/ajcn/68.3.599. [DOI] [PubMed] [Google Scholar]
- Dupuis L, Pradat PF, Ludolph AC, Loeffler JP. Energy metabolism in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:75–82. doi: 10.1016/S1474-4422(10)70224-6. [DOI] [PubMed] [Google Scholar]
- Eastman CJ, Ekins RP, Leith IM, Williams ES. Thyroid hormone response to prolonged cold exposure in man. J Physiol. 1974;241:175–181. doi: 10.1113/jphysiol.1974.sp010647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Effect of corticotropin releasing hormone and neuropeptide Y on electrophysiological activity of sympathetic nerves to interscapular brown adipose tissue. Neuroscience. 1990a;34:771–775. doi: 10.1016/0306-4522(90)90181-3. [DOI] [PubMed] [Google Scholar]
- Egawa M, Yoshimatsu H, Bray GA. Preoptic area injection of corticotropin-releasing hormone stimulates sympathetic activity. Am J Physiol. 1990b;259:R799–R806. doi: 10.1152/ajpregu.1990.259.4.R799. [DOI] [PubMed] [Google Scholar]
- Enriori P, Sinnayah P, Simonds S, Garcia Rudaz C, Cowley M. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fealey RD. Interoception and autonomic nervous system reflexes thermoregulation. Handb Clin Neurol. 2013;117:79–88. doi: 10.1016/B978-0-444-53491-0.00007-9. [DOI] [PubMed] [Google Scholar]
- Fekete C, Lechan RM. Central regulation of hypothalamic-pituitary-thyroid axis under physiological and pathophysiological conditions. Endocr Rev. 2014;35:159–194. doi: 10.1210/er.2013-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavini CK, Mukherjee S, Shukla C, Britton SL, Koch LG, Shi H, Novak CM. Leanness and heightened nonresting energy expenditure: role of skeletal muscle activity thermogenesis. Am J Physiol Endocrinol Metab. 2014;306:E635–E647. doi: 10.1152/ajpendo.00555.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano A, Frontini A, Castellucci M, Cinti S. Presence and distribution of cholinergic nerves in rat mediastinal brown adipose tissue. J Histochem Cytochem. 2004;52:923–930. doi: 10.1369/jhc.3A6246.2004. [DOI] [PubMed] [Google Scholar]
- Goodner CJ, Koerker DJ, Werbach JH, Toivola P, Gale CC. Adrenergic regulation of lipolysis and insulin secretion in the fasted baboon. Am J Physiol. 1973;224:534–539. doi: 10.1152/ajplegacy.1973.224.3.534. [DOI] [PubMed] [Google Scholar]
- Grkovic I, Anderson CR. Calbindin D28K-immunoreactivity identifies distinct subpopulations of sympathetic pre- and postganglionic neurons in the rat. J Comp Neurol. 1997;386:245–259. [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB. C1 neurons: the body’s EMTs. Am J Physiol Regul Integr Comp Physiol. 2013;305:R187–R204. doi: 10.1152/ajpregu.00054.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas JL, Gajiwala KS, Maffei M, Cohen SL, Chait BT, Rabinowitz D, Lallone RL, Burley SK, Friedman JM. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995;269:543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Havel RJ. The autonomic nervous system and intermediary carbohydrate and fat metabolism. Anesthesiology. 1968;29:702–713. doi: 10.1097/00000542-196807000-00014. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Morgan DA, Djalali A, Sivitz WI, Mark AL. Interactions between the melanocortin system and leptin in control of sympathetic nerve traffic. Hypertension. 1999;33:542–547. doi: 10.1161/01.hyp.33.1.542. [DOI] [PubMed] [Google Scholar]
- Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res. 2006;1117:118–124. doi: 10.1016/j.brainres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- Himms-Hagen J, Cui J, Lynn SS. Sympathetic and sensory nerves in control of growth of brown adipose tissue: Effects of denervation and of capsaicin. Neurochem Int. 1990;17:271–279. doi: 10.1016/0197-0186(90)90149-n. [DOI] [PubMed] [Google Scholar]
- Huo L, Munzberg H, Nillni EA, Bjorbaek C. Role of signal transducer and activator of transcription 3 in regulation of hypothalamic trh gene expression by leptin. Endocrinology. 2004;145:2516–2523. doi: 10.1210/en.2003-1242. [DOI] [PubMed] [Google Scholar]
- Janig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensations. Biol Psychol. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Jeong JH, Lee DK, Blouet C, Ruiz HH, Buettner C, Chua S, Jr, Schwartz GJ, Jo YH. Cholinergic neurons in the dorsomedial hypothalamus regulate mouse brown adipose tissue metabolism. Mol Metab. 2015;4:483–492. doi: 10.1016/j.molmet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Bravo P, Jaimes-Hoy L, Maria UR, Charli JL. 60 YEARS OF NEUROENDOCRINOLOGY: TRH, the first hypophysiotropic releasing hormone isolated: control of the pituitary-thyroid axis. J Endocrinol. 2015;226:T85–T100. doi: 10.1530/JOE-15-0124. [DOI] [PubMed] [Google Scholar]
- Julius D, Nathans J. Signaling by sensory receptors. Cold Spring Harb Perspect Biol. 2012;4:a005991. doi: 10.1101/cshperspect.a005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N, Hioki H, Kaneko T, Nakamura K. Psychological stress activates a dorsomedial hypothalamus-medullary raphe circuit driving brown adipose tissue thermogenesis and hyperthermia. Cell Metab. 2014;20:346–358. doi: 10.1016/j.cmet.2014.05.018. [DOI] [PubMed] [Google Scholar]
- Keipert S, Kutschke M, Lamp D, Brachthauser L, Neff F, Meyer CW, Oelkrug R, Kharitonenkov A, Jastroch M. Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Mol Metab. 2015;4:537–542. doi: 10.1016/j.molmet.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy GC. Experimental hypothalamic obesity. Proc R Soc Med. 1951;44:899–902. doi: 10.1177/003591575104401017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharitonenkov A, Adams AC. Inventing new medicines: The FGF21 story. Mol Meta. 2014;3:221–229. doi: 10.1016/j.molmet.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid. 2008;18:141–144. doi: 10.1089/thy.2007.0266. [DOI] [PubMed] [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koerker DJ, Goodner CJ, Chideckel EW, Ensinck JW. Adaptation to fasting in baboon. II. Regulation of lipolysis early and late in fasting. Am J Physiol. 1975;229:350–354. doi: 10.1152/ajplegacy.1975.229.2.350. [DOI] [PubMed] [Google Scholar]
- Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC, Munzberg H, Hutson SM, Gettys TW, Schwartz MW, Morrison CD. FGF21 is an endocrine signal of protein restriction. J Clin Invest. 2014;124:3913–3922. doi: 10.1172/JCI74915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, Werner CD, Phan GQ, Kammula US, Kebebew E, Pacak K, Chen KY, Celi FS. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Flier JS, Lechan RM. Leptin prevents fasting-induced suppression of prothyrotropin-releasing hormone messenger ribonucleic acid in neurons of the hypothalamic paraventricular nucleus. Endocrinology. 1997;138:2569–2576. doi: 10.1210/endo.138.6.5209. [DOI] [PubMed] [Google Scholar]
- Legradi G, Emerson CH, Ahima RS, Rand WM, Flier JS, Lechan RM. Arcuate nucleus ablation prevents fasting-induced suppression of ProTRH mRNA in the hypothalamic paraventricular nucleus. Neuroendocrinology. 1998;68:89–97. doi: 10.1159/000054354. [DOI] [PubMed] [Google Scholar]
- Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med. 1995;332:621–628. doi: 10.1056/NEJM199503093321001. [DOI] [PubMed] [Google Scholar]
- Levine JA. Non-exercise activity thermogenesis (NEAT) Nutr Rev. 2004;62:S82–S97. doi: 10.1111/j.1753-4887.2004.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Lidell ME, Betz MJ, Dahlqvist LO, Heglind M, Elander L, Slawik M, Mussack T, Nilsson D, Romu T, Nuutila P, Virtanen KA, Beuschlein F, Persson A, Borga M, Enerback S. Evidence for two types of brown adipose tissue in humans. Nat Med. 2013;19:631–634. doi: 10.1038/nm.3017. [DOI] [PubMed] [Google Scholar]
- Loewy AD. Viruses as transneuronal tracers for defining neural circuits. Neurosci Biobehav Rev. 1998;22:679–684. doi: 10.1016/s0149-7634(98)00006-2. [DOI] [PubMed] [Google Scholar]
- Lopez M, Varela L, Vazquez MJ, Rodriguez-Cuenca S, Gonzalez CR, Velagapudi VR, Morgan DA, Schoenmakers E, Agassandian K, Lage R, Martinez de Morentin PB, Tovar S, Nogueiras R, Carling D, Lelliott C, Gallego R, Oresic M, Chatterjee K, Saha AK, Rahmouni K, Dieguez C, Vidal-Puig A. Hypothalamic AMPK and fatty acid metabolism mediate thyroid regulation of energy balance. Nat Med. 2010;16:1001–1008. doi: 10.1038/nm.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ. Glucoprivation in the ventrolateral medulla decreases brown adipose tissue sympathetic nerve activity by decreasing the activity of neurons in raphe pallidus. Am J Physiol Regul Integr Comp Physiol. 2012;302:R224–R232. doi: 10.1152/ajpregu.00449.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Excitatory amino acid receptors in the dorsomedial hypothalamus mediate prostaglandin-evoked thermogenesis in brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2004;286:R320–R325. doi: 10.1152/ajpregu.00515.2003. [DOI] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Hypoxic activation of arterial chemoreceptors inhibits sympathetic outflow to brown adipose tissue in rats. J Physiol. 2005;566:559–573. doi: 10.1113/jphysiol.2005.086322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden C, Tupone D, Cano G, Morrison S. Î}\^{I} {± 2 Adrenergic receptor-mediated inhibition of thermogenesis. J Neurosci. 2013;33:2017–2028. doi: 10.1523/JNEUROSCI.4701-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan KR, Naber MC, Ameka MK, Anderegg MD, Mangelsdorf DJ, Kliewer SA, Mohammadi M, Potthoff MJ. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes. 2014;63:4057–4063. doi: 10.2337/db14-0595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer J, Barrnett RJ. Obesity following unilateral hypothalamic lesions in rats. Science. 1955;121:599–600. doi: 10.1126/science.121.3147.599. [DOI] [PubMed] [Google Scholar]
- Moriscot A, Rabelo R, Bianco AC. Corticosterone inhibits uncoupling protein gene expression in brown adipose tissue. Am J Physiol. 1993;265:E81–E87. doi: 10.1152/ajpendo.1993.265.1.E81. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Berthoud HR. Neurobiology of nutrition and obesity. Nutr Rev. 2007;65:517–534. doi: 10.1301/nr.2007.dec.517-534. [DOI] [PubMed] [Google Scholar]
- Morrison CD, Laeger T. Protein-dependent regulation of feeding and metabolism. Trends Endocrinol Metab. 2015;26:256–262. doi: 10.1016/j.tem.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison S, Madden C, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, Van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH. Ghrelin. Mol Metab. 2015;4:437–460. doi: 10.1016/j.molmet.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin-sensitive sensory nerves innervate white fat. Am J Physiol Endocrinol Metab. 2013;304:E1338–E1347. doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musatov S, Chen W, Pfaff DW, Mobbs CV, Yang XJ, Clegg DJ, Kaplitt MG, Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K. Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol. 2011;301:207–228. doi: 10.1152/ajpregu.00109.2011. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Morrison SF. A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci U S A. 2010;107:8848–8853. doi: 10.1073/pnas.0913358107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur J Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Nakamura K, Morrison SF. Different populations of prostaglandin EP3 receptor-expressing preoptic neurons project to two fever-mediating sympathoexcitatory brain regions. Neuroscience. 2009;161:614–620. doi: 10.1016/j.neuroscience.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman D, Mukherjee S, Symons D, Jung RT, Lever JD. Neuropeptides in interscapular and perirenal brown adipose tissue in the rat: a plurality of innervation. J Neurocytol. 1988;17:305–311. doi: 10.1007/BF01187853. [DOI] [PubMed] [Google Scholar]
- Nozdrachev AD, Jimenez B, Morales MA, Fateev MM. Neuronal organization and cell interactions of the cat stellate ganglion. Auton Neurosci. 2002;95:43–56. doi: 10.1016/s1566-0702(01)00360-5. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Giles ME, Watson A, Anderson C, Colvill LM, McKinley MJ. The neurochemical characterisation of hypothalamic pathways projecting polysynaptically to brown adipose tissue in the rat. Neuroscience. 2002;110:515–526. doi: 10.1016/s0306-4522(01)00555-3. [DOI] [PubMed] [Google Scholar]
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol Metab. 2015;26:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- Rathner JA, Morrison SF. Rostral ventromedial periaqueductal gray: A source of inhibition of the sympathetic outflow to brown adipose tissue. Brain Res. 2006;1077:99–107. doi: 10.1016/j.brainres.2006.01.035. [DOI] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Munzberg H. Integration of sensory information via central thermoregulatory leptin targets. Physiol Behav. 2013;121:49–55. doi: 10.1016/j.physbeh.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezai-Zadeh K, Yu S, Jiang Y, Laque A, Schwartzenburg C, Morrison CD, Derbenev AV, Zsombok A, Munzberg H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol Metab. 2014;3:681–693. doi: 10.1016/j.molmet.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricardo JA. Hypothalamic pathways involved in metabolic regulatory functions, as identified by track-tracing methods. Adv Metab Disord. 1983;10:1–30. doi: 10.1016/b978-0-12-027310-2.50007-4. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Barnes MJ, Hermann GE. Leptin “gates” thermogenic action of thyrotropin-releasing hormone in the hindbrain. Brain Res. 2009;1295:135–141. doi: 10.1016/j.brainres.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers RC, McDougal DH, Hermann GE. Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study. Brain Res. 2011;1385:47–55. doi: 10.1016/j.brainres.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab. 2002;87:2391–2394. doi: 10.1210/jcem.87.5.8628. [DOI] [PubMed] [Google Scholar]
- Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ, Tyzbir RS. Mechanisms of thermogenesis induced by low protein diets. Metabolism. 1983;32:257–261. doi: 10.1016/0026-0495(83)90190-7. [DOI] [PubMed] [Google Scholar]
- Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc. 2014;90:1279–1297. doi: 10.1111/brv.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu V, Bartness TJ. Short and long sympathetic-sensory feedback loops in white fat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R886–R900. doi: 10.1152/ajpregu.00060.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu V, Garretson JT, Liu Y, Vaughan CH, Bartness TJ. Brown adipose tissue has sympathetic-sensory feedback circuits. J Neurosci. 2015;35:2181–2190. doi: 10.1523/JNEUROSCI.3306-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon J, de Truchis P, Melchior JC. Body composition and nutritional parameters in HIV and AIDS patients. Clin Chem Lab Med. 2002;40:1329–1333. doi: 10.1515/CCLM.2002.229. [DOI] [PubMed] [Google Scholar]
- Scammell TE, Elmquist JK, Griffin JD, Saper CB. Ventromedial preoptic prostaglandin E2 activates fever-producing autonomic pathways. J Neurosci. 1996;16:6246–6254. doi: 10.1523/JNEUROSCI.16-19-06246.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shabalina IG, Nedergaard J. Mitochondrial (’mild’) uncoupling and ROS production: physiologically relevant or not? Biochem Soc Trans. 2011;39:1305–1309. doi: 10.1042/BST0391305. [DOI] [PubMed] [Google Scholar]
- Shi YC, Lau J, Lin Z, Zhang H, Zhai L, Sperk G, Heilbronn R, Mietzsch M, Weger S, Huang XF, Enriquez RF, Baldock PA, Zhang L, Sainsbury A, Herzog H, Lin S. Arcuate NPY controls sympathetic output and BAT function via a relay of tyrosine hydroxylase neurons in the PVN. Cell Metab. 2013;17:236–248. doi: 10.1016/j.cmet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Harris L, Berglund E, Liu T, Vong L, Lowell B, Balthasar N, Williams K, Elmquist J. Melanocortin 4 receptors reciprocally regulate sympathetic and parasympathetic pre-ganglionic neurons. Cell. 2013;152:612–619. doi: 10.1016/j.cell.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song CK, Schwartz GJ, Bartness TJ. Anterograde transneuronal viral tract tracing reveals central sensory circuits from white adipose tissue. Am J Physiol Regul Integr Comp Physiol. 2009;296:R501–R511. doi: 10.1152/ajpregu.90786.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci USA. 2000;97:12339–12344. doi: 10.1073/pnas.220409497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton AK, Pei H, Burnett KH, Myers MG, Jr, Rhodes CJ, Olson DP. Control of food intake and energy expenditure by Nos1 neurons of the paraventricular hypothalamus. J Neurosci. 2014;34:15306–15318. doi: 10.1523/JNEUROSCI.0226-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Shimazu T, Maruyama Y. Importance of sympathetic nerves for the stimulatory effect of cold exposure on glucose utilization in brown adipose tissue. Jpn J Physiol. 1992;42:653–664. doi: 10.2170/jjphysiol.42.653. [DOI] [PubMed] [Google Scholar]
- Tanche M, Therminarias A. Participation of stellate ganglions in rewarming thermogenesis in the dog. Arch Sci Physiol (Paris) 1967;21:67–80. [PubMed] [Google Scholar]
- Tsukazaki K, Nikami H, Shimizu Y, Kawada T, Yoshida T, Saito M. Chronic administration of beta-adrenergic agonists can mimic the stimulative effect of cold exposure on protein synthesis in rat brown adipose tissue. J Biochem. 1995;117:96–100. doi: 10.1093/oxfordjournals.jbchem.a124728. [DOI] [PubMed] [Google Scholar]
- Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J Neurosci. 2011;34:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushikubi F, Segi E, Sugimoto Y, Murata T, Matsuoka T, Kobayashi T, Hizaki H, Tuboi K, Katsuyama M, Ichikawa A, Tanaka T, Yoshida N, Narumiya S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- Vaughan CH, Zarebidaki E, Ehlen JC, Bartness TJ. Analysis and measurement of the sympathetic and sensory innervation of white and brown adipose tissue. Methods Enzymol. 2014;537:199–225. doi: 10.1016/B978-0-12-411619-1.00011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, Nuutila P. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Voets T. TRP channels and thermosensation. Handb Exp Pharmacol. 2014;223:729–741. doi: 10.1007/978-3-319-05161-1_1. [DOI] [PubMed] [Google Scholar]
- Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci. 2012;15:1350–1355. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL. Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science. 2005;307:418–420. doi: 10.1126/science.1108177. [DOI] [PubMed] [Google Scholar]
- Xu Y, Nedungadi TP, Zhu L, Sobhani N, Irani BG, Davis KE, Zhang X, Zou F, Gent LM, Hahner LD, Khan SA, Elias CF, Elmquist JK, Clegg DJ. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Kim ER, Zhao R, Myers MG, Jr, Munzberg H, Tong Q. Glutamate release mediates leptin action on energy expenditure. Mol Metab. 2013;2:109–115. doi: 10.1016/j.molmet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Maruyama M, Hosono T, Nagashima K, Fukuda Y, Gerstberger R, Kanosue K. Fos expression induced by warming the preoptic area in rats. Brain Res. 2002;933:109–117. doi: 10.1016/s0006-8993(02)02287-4. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Konishi M, Nagashima K, Saper CB, Kanosue K. Fos activation in hypothalamic neurons during cold or warm exposure: Projections to periaqueductal gray matter. Neuroscience. 2005;133:1039–1046. doi: 10.1016/j.neuroscience.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel Preoptic Pathways for Thermo-regulation. J Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Sunanaga J, Takahashi Y, Mori T, Sakurai T, Kanmura Y, Kuwaki T. Orexin neurons are indispensable for stress-induced thermogenesis in mice. J Physiol. 2010;588:4117–4129. doi: 10.1113/jphysiol.2010.195099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoeller RT, Kabeer N, Albers HE. Cold exposure elevates cellular levels of messenger ribonucleic acid encoding thyrotropin-releasing hormone in paraventricular nucleus despite elevated levels of thyroid hormones. Endocrinology. 1990;127:2955–2962. doi: 10.1210/endo-127-6-2955. [DOI] [PubMed] [Google Scholar]


