Abstract
Myocardial bridging is a congenital anomaly in which a segment of a coronary artery takes a “tunneled” intramuscular course under a “bridge” of overlying myocardium. This causes vessel compression in systole, resulting in hemodynamic changes that may be associated with angina, myocardial ischemia, acute coronary syndrome, left ventricular dysfunction, arrhythmias, and even sudden cardiac death. While described on autopsy for centuries, technological advances such as coronary computed tomography angiography and intravascular ultrasound have contributed greatly to our understanding of the anatomic, hemodynamic, and pathophysiological consequences of systolic compression. Atherosclerosis preferentially develops immediately proximal to the bridged segment, likely due to alterations in shear stress, while the compressed segment itself is often spared. First-line therapy of symptomatic bridging remains medical treatment with beta-blockers and non-dihydropyridine calcium-channel blockers, and nitrates are contraindicated. Surgical myotomy, intracoronary stenting, and coronary artery bypass graft surgery have been used for refractory symptoms, but long-term outcomes remain uncertain. Further research is required to better define the patient population that would derive the greatest benefit from surgical and percutaneous intervention.
Keywords: myocardial bridging, tunneled artery, coronary angiography, ultrasound, anatomy
Myocardial bridging, first described anatomically by Reyman in 1737,1 is a congenital variant of a coronary artery in which a portion of an epicardial coronary artery (most frequently the middle segment of the left anterior descending [LAD] artery) takes an intramuscular course.2 This arrangement of a “tunneled” segment of the artery under the “bridge” of overlying myocardium frequently results in vessel compression during systole. While frequently asymptomatic, this condition in many cases may be responsible for adverse complications including angina, myocardial ischemia,3 acute coronary syndromes,4–6 left ventricular dysfunction and stunning, 7 arrhythmias,8,9 and even sudden cardiac death.10,11
Since the initial angiographic imaging by Portsmann in 1960,12 newer diagnostic modalities such as coronary computed tomographic angiography (CCTA),13 intravascular ultrasound (IVUS),14 intracoronary Doppler,15 and fractional flow reserve (FFR)16 have enabled fuller analysis of the anatomic and hemodynamic consequences of the systolic compression, including pathological effects on coronary flow.17 Despite this increased understanding, treatment options remain limited. Medications such as beta-blockers and calcium-channel blockers remain first-line therapy,18 with surgical myotomy reserved for refractory cases.19,20 While percutaneous coronary intervention in the form of stenting has been used, serious complications such as stent fracture21,22 and coronary perforation have been reported.23–26 This manuscript summarizes our current understanding of the hemodynamic alterations in myocardial bridging, especially as it relates to the observed clinical sequelae, describes the anatomical characteristics on angiography and non-invasive imaging, and explores current treatment options including medical and invasive therapies.
Prevalence
Reported rates of myocardial bridging differ based on the mode of evaluation. Numerous autopsy series have been performed, with rates reported from 5%–86%,18 with a mean of 25%. The largest study by Risse et al27 involving 1056 patients found an intramyocardial coronary artery course in 26% of patients. These rates are much higher than angiographically reported bridging, which typically detects systolic compression at rates from 0.5%–12%,28 although detection can increase up to 40% if provocation tests are used.18 More recent studies involving CCTA13,29 find bridged coronary segments at rates similar to the autopsy series. Certain populations, namely hypertrophic cardiomyopathy patients30,31 and heart transplant patients,32 have rates much higher than the general population.
The large discrepancy in reported prevalence clearly reflects the difference between an anatomical finding of a “tunneled” artery on autopsy or CCTA, and resulting vessel compression on angiography. Pathologic series include thin bridges or even myocardial loops with little hemodynamic consequence. Angiographic significance, on the other hand, depends on multiple factors including bridge length, depth/thickness, orientation of myocardial fibers to the artery, coronary smooth muscle tone, the presence of a proximal fixed coronary obstruction, the presence of surrounding fat, the state of myocardial contractility at the time of angiography, and observer experience.17 In addition, it is likely that angiographic bridging is underappreciated when it involves either the circumflex or right coronary arteries. Although there is lack of universal definition criteria for myocardial bridging, diagnosis of this condition is relevant in patients with clear angiographic evidence along with signs or symptoms suggestive of coronary flow limitation.
Morphology/Histology
Although most frequently localized to the middle segment of the LAD artery on coronary angiography, some autopsy series find right coronary artery (RCA) and left circumflex (LCX) artery involvement at similar rates.33 In addition, secondary arteries such as diagonal (18%) and marginal (40%) branches are also commonly involved on histology.18 Typical myocardial bridge depth is reported at 1–10 mm, with length of 10–30 mm.34 There does not appear to be a difference in prevalence of bridging by gender or age.
Myocardial bridges of the LAD artery found on pathology have been characterized by Ferreira35 as falling into two distinct subtypes. The more common “superficial” bridges (approximately 75% of cases) involve the LAD artery situated as usual in the intraventricular groove, but crossed by a muscle bundle perpendicularly or at an acute angle. There is also a “deep” variant in which the LAD artery deviates toward the right ventricle and dives into the intraventricular septum, with an overlying longitudinal muscle bundle arising from the right ventricular apex and crossing the tunneled segment transversely, obliquely, or helically before terminating in the intraventricular septum.35 It is thought that systolic compression of the superficial subtype happens only infrequently, whereas the orientation and degree of overlying bridging in the deep variant results in twisting of the tunneled segment over the course of the cardiac cycle, resulting in hemodynamic compromise during coronary flow.
A subset of myocardial bridging known as the myocardial loop, in which an epicardial vessel (typically artery but occasionally vein) runs through a segment of atrial myocardium, has been described on histology.33 These typically involve the distal LCX as well as distal RCA, are usually 10–15 mm long, and 0.1–0.3 mm thick.36 Because of the minimal compression exerted by atrial muscle as well as the shorter and thinner size of loops compared to ventricular tunnels, these loops have not been described angiographically, and almost certainly are without clinical consequence.
Pathophysiology of Ischemia
The degree of myocardial ischemia and resulting symptoms appear on first glance to be out of proportion to the degree of compromise in coronary blood flow by myocardial bridging. As the majority of coronary filling occurs in diastole (with mean flow systolic to diastolic ratios measured in one study of 0.22 and 0.85 in the LAD and the RCA, respectively), systolic compression of the artery should have only a blunted impact on total effective myocardial perfusion.37 However, studies involving multiple imaging modalities have shed light on this apparent paradox.
While accelerated atherosclerosis does develop proximal to the bridged segment, the mechanism of ischemia does not appear to be entirely related to this fixed obstruction. A portion of the effect relates to tachycardic states in which diastolic filling shortens and lessens in importance and disturbances in systolic filling have a greater effect.38 Provocation testing using dobutamine or rapid atrial pacing has relied on this relationship. Using frame-by-frame quantitative coronary angiography (QCA) along with IVUS, it was recognized that vessel compression of bridged arteries extended into diastole and thus did affect the predominant phase of coronary perfusion.39,40 Klues expanded on this finding by combining angiography with intracoronary Doppler flow and pressure measurements in patients with symptomatic bridging, and discovered hemodynamic abnormalities characterized by a persistent decrease in diastolic vessel diameter, increase in blood flow velocities and retrograde flow, and a reduced flow reserve.41 Not only is the diameter of the intramuscular segment smaller compared to the adjacent proximal segment overall, but during diastole there is a persistent reduction of 34%–51% in the bridged segment. 15 In addition, it was found that the greater the systolic narrowing, the greater the reduction in diastolic diameter, associated with a corresponding decrease in flow and flow reserve.
Early pathological analysis of myocardial bridging (later confirmed with IVUS) recognized “sparing” of bridged segments from atherosclerotic lesions.14 Histology identified changes in vessel wall structure and morphology which may explain this “protective” effect. The intima of the tunneled segment is significantly thinner than the proximal segment, and contains a predominance of the “contractile” subtype of smooth muscle cells thought to be negatively associated with the development of atherosclerotic lesions.27 Foam cells, an important component of atherosclerosis, also appeared to be missing in the tunneled segments.42 In addition, the expression of known vasoactive agents endothelial nitric oxide synthase, endothelin-1, and angiotensin-converting enzyme is reduced in the bridged vessel wall.43 These agents have been implicated in the proliferation of smooth muscle cells resulting in increased size of atherosclerotic lesions. Systolic kinking of the bridged segments, coupled with the aforementioned endothelial dysfunction, may also predispose to coronary vasospasm and thrombus formation.44
Conversely, the vessel segment proximal to the bridge appears to develop atherosclerosis at increased rates, approaching 90%.14 Analysis of endothelial cell morphology at the entrance to the tunneled segment reveals “flat, polygonal, and polymorphic” structure, indicative of a low shear stress state, while the endothelial cells within the tunnel maintain a helical orientation, a sign of laminar flow and high shear.45 This suggests a hemodynamic basis for the increased plaque formation proximal to the tunnel, through impairment of endothelial cell function and morphology. Also, in contradistinction to the tunneled segment, expression of vasoactive agents endothelial nitric oxide synthase, endothelin-1, and angiotensin-converting enzyme are all increased in the proximal segment.43 It is uncertain, however, whether the association between vasoactive agent expression and atherosclerosis is causal in nature, or whether it simply reflects an as-yet undiscovered mechanism, perhaps also related to hemodynamic forces.
Diagnostic Testing
Coronary angiography
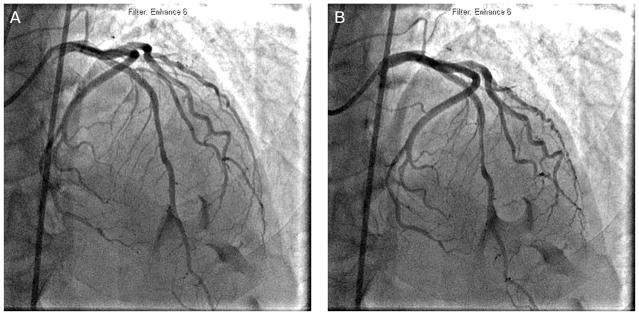
Coronary cineangiography remains the most common technique for diagnosing myocardial bridging. The typical description of bridging on angiography involves a systolic narrowing, or “milking,” of an epicardial artery, with a “step-down” and “step-up” demarcating the impacted area (Figure 1). As previously discussed, atherosclerotic lesions are frequently found immediately proximal to the bridged segment. Indeed, bridging is occasionally identified only after percutaneous coronary intervention of a proximal lesion, after which the higher intravascular flow unmasks the vulnerable segment.46
FIGURE 1.
Coronary angiography of a 58-year-old male who presented with acute coronary syndrome. (A) During diastole, the mid left anterior descending artery had mild disease. (B) Myocardial bridging was observed during systole.
Intracoronary Doppler
Doppler-tipped guidewires allowed accurate measurement of intracoronary flow velocity for the first time.47 Interrogation of myocardial bridges revealed a characteristic “spike-and-dome” pattern or “fingertip” phenomenon, with abrupt early diastolic flow acceleration, rapid mid-diastolic flow deceleration, and a mid-tolate diastolic plateau.41 Retrograde flow during the systolic period can be detected immediately proximal to the bridged segment, exacerbated by nitroglycerin provocation, especially in deep bridges.48 In addition, coronary flow reserve in these patients is impaired distal to the bridge, with a mean of 2.0 (normal >3.0), despite being normal or mildly reduced (mean 2.7) proximal to the bridge.15
Intravascular ultrasound
On IVUS, the tunneled segment of artery clearly demonstrates systolic compression (which can be either eccentric or concentric) that persists into diastole. There is also a highly specific echolucent “half-moon” appearance throughout the cardiac cycle, the etiology of which is not well understood.14 IVUS can detect vessel compression with coronary provocation testing even in the absence of angiographically significant “milking.”40 In a study of 331 consecutive patients with de novo LAD artery lesions who underwent both angiography and IVUS, IVUS detected bridging in 23% of patients, while angiographic systolic compression was only apparent in 3%.49 It was in early IVUS pullback studies that revealed the predilection for plaque formation proximal to the tunneled segment, but a “sparing” of the bridged vessel from atherosclerosis.14 IVUS still remains an important confirmatory modality when angiographic diagnosis is uncertain, especially when combined with provocation testing with nitroglycerin,48 acetylcholine,50 dobutamine,51 or rapid atrial pacing.52
Fractional flow reserve
FFR assessment has proven to be an important tool in the physiologic assessment of myocardial bridges. In an early series of 12 patients with mid-LAD artery bridging on angiography, Escaned et al measured FFR both at baseline and with dobutamine provocation.16 Hemodynamic alteration due to the myocardial bridging manifested most prominently in a decrease in diastolic FFR (0.88 down to 0.77), whereas mean FFR decreased to a lesser extent (0.90 down to 0.84). It is thought that mean FFR measurements are artifactually elevated by overshooting of systolic pressures, and thus diastolic FFR evaluation should be the technique of choice. Dobutamine provocation appears to be more accurate when compared with adenosine for FFR evaluation of bridging, highlighting the importance of inotropic state in the development of vessel compression.53
Cardiac computed tomography (CT) angiography
CT (initially with electron-beam CT and more recently multislice CT) has become a valuable tool in the analysis of coronary anatomy and patency. Studies using CT to evaluate myocardial bridging have detected intramyocardial segments at much higher rates than by angiography.13,29,54,55 This surely reflects the higher prevalence of anatomical bridged segments than the subset that result in vessel compression. However, given that the information provided by this technique is structural rather than functional in nature, further correlation would still need to be performed to determine clinical relevance. CT-based non-invasive FFR measurement may yet prove useful as a method for combined anatomical/hemodynamic study of myocardial bridges, but such an application has not yet been reported in the literature.56
Other proposed diagnostic techniques
Stress echocardiography has been proposed as a non-invasive functional test for myocardial bridging. Lin et al identified characteristic septal wall motion abnormalities on two-dimensional imaging in 14 patients with IVUS- and FFR-proven bridging. 57 Myocardial perfusion imaging, although useful in the evaluation of ischemia due to fixed stenoses, does not yet appear to have the sensitivity required to adequately identify myocardial bridging.58,59 Further studies will be necessary to improve and validate these techniques before they can be accepted as a method for the diagnosis of bridging.
Special Patient Populations
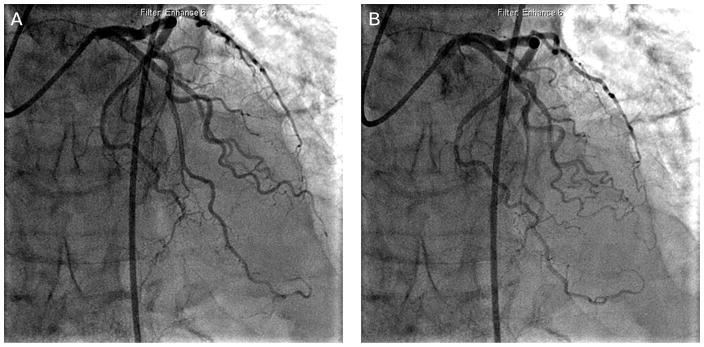
Patients with hypertrophic cardiomyopathy have been found to have a high prevalence of myocardial bridging, with reported rates of up to 80% on angiography (Figure 2).60 This is thought to be a contributing factor in the increased mortality in the pediatric hypertrophic cardiomyopathy population, presumably through ischemic and/or arrhythmic mechanisms. Multiple studies in children with hypertrophic cardiomyopathy have found greater rates of chest pain, ventricular tachycardia, history of resuscitated cardiac arrest, and positive changes on exercise testing in those with concurrent myocardial bridging.61 Interestingly, this relationship does not hold in the adult population. In a series of 425 adult patients with hypertrophic cardiomyopathy with (15%) and without (85%) myocardial bridging, there was no difference in all-cause mortality and cardiac death at 6.8 year follow-up.31 A more recent study examined autopsied hearts in 255 patients who died of sudden cardiac death, and did not find an independent contribution of myocardial bridging to mortality in patients with hypertrophic cardiomyopathy, despite occurring at a higher prevalence.62
FIGURE 2.
Coronary angiography of a 62-year-old male with hypertrophic cardiomyopathy with angina. (A) During diastole, the mid left anterior descending (LAD) artery followed an intramyocardial course. (B) Systolic compression was observed in the mid LAD.
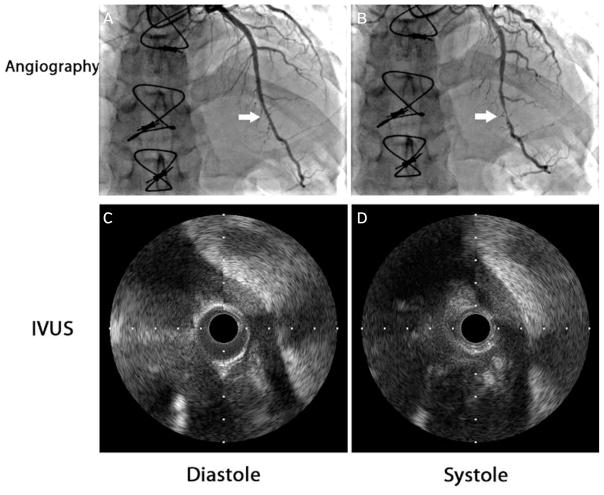
Orthotopic heart transplantation patients have higher reported rates of myocardial bridging (Figure 3). In a series of 64 heart transplant recipients, 33% were found to have angiographically significant bridging.32 This was thought to be related to the increased stiffness and hypertrophy of the myocardium post transplant, resulting in increased rates of systolic vessel compression. While the presence of bridging was previously thought to be a risk factor for early death after cardiac transplantation,63 more recent experience64,65 suggests that this may not be the case.
FIGURE 3.
Coronary angiography of a patient 1 month post orthotopic heart transplantation. (A) During diastole, the distal left anterior descending (LAD) artery was unremarkable. (B) Systolic narrowing was seen on angiography of the mid and distal LAD artery (arrow) as well as the septal arteries. Intravascular ultrasound of a segment of myocardial bridging during diastole (C) and systole (D) demonstrated near-complete obliteration of the lumen during systole.
Prognosis
Although myocardial bridging is generally considered to be a benign condition, it has been proposed as a cause of angina-like chest pain, coronary spasm, myocardial ischemia (as evidenced by changes on electrocardiography and myocardial perfusion stress testing), acute coronary syndromes, left ventricular dysfunction/stunning, arrhythmias (including supraventricular tachycardia and ventricular tachycardia), and even sudden cardiac death. Serious events are uncommon, and it is still controversial and unclear whether myocardial bridging can be directly attributable as the cause of the events. In studies involving patients with myocardial bridging, testing for inducible myocardial ischemia revealed rates that varied from 21%–88%, with the wide range likely related to differences in test method sensitivity and specificity. 58 The ischemia was more closely associated with the degree of systolic compression rather than lesion length or bridge location.
Schwarz has proposed a classification scheme for myocardial bridging based on symptoms as well as non-invasive and invasive measurement of hemodynamic and anatomical parameters in order to guide therapy (Table 1).66 While some data exist on long-term follow-up of patients with myocardial bridging,67 there is a paucity of studies that have established the natural history of the condition in response to intervention to validate such a classification scheme.
Table 1.
Proposed classification scheme and therapeutic strategy for myocardial bridging (adapted from Schwartz et al).66
| Clinical Symptoms | Signs of Ischemia | Initial Treatment Strategy | Secondary Treatment if No Improvement | |
|---|---|---|---|---|
| Type A | Yes | No | Reassurance | – |
| Type B | Yes | Yes, by non-invasive stress testing | Beta-blockersb | Intracoronary hemodynamic evaluationa → surgery or stenting if abnormal |
| Type C | Yes | Yes, by altered intracoronary hemodynamicsa | Beta-blockersb | Surgery or stenting |
By quantitative coronary arteriography, fractional flow reserve, or intracoronary Doppler.
If beta-blockers are contraindicated, non-dihydropyridine calcium-channel blockers can be used.
Treatment
Medical therapy
First-line therapy for patients thought to be experiencing symptoms secondary to myocardial bridging consists of beta-blockers68,69 and non-dihydropyridine calcium-channel blockers.70 However, the evidence to support these interventions is limited, and rationale is based on theoretical improvement in coronary hemodynamics with decreased chronotropy and inotropy. It is thought that the prolongation of diastole with reductions in heart rate (and reduced chronotropy with exercise and provocation) is a major contributor to reduction in bridging sequelae and resultant symptoms. But while beta-blockade has been shown to decrease vessel compression and improve angina symptoms in a limited study of 15 patients with bridging, no studies have ever evaluated clinical endpoints of morbidity or mortality.68
Nitrates are contraindicated in patients with myocardial bridging. Nitroglycerin has been shown to accentuate systolic compression of bridged segments, and indeed is used as an agent for provocation of these lesions.71 This effect likely relates to an increase in vessel wall compliance, as well as a reflex sympathetic increase in contractility.48
Surgery
Surgical options for myocardial bridging include surgical myotomy and coronary artery bypass graft surgery. Surgical myotomy involving resection of the overlying muscle fibers should be limited to patients with refractory symptoms despite medical therapy, especially in those with demonstrated inducible ischemia and those who are at high risk for myocardial infarction, ventricular tachycardia, or resuscitated cardiac arrest. Surgical myotomy has been shown to eliminate symptoms19,20 and increase coronary flow.72 However, risks include dissection into the right ventricle in patients with myocardial bridges that take a deep subendocardial course.73
Coronary artery bypass graft surgery has been reported as a treatment for bridging, and typically involves anastomosis of the left internal mammary artery to the LAD artery.74,75 Published series (ranging from 11–31 patients) comparing surgical myotomy to coronary artery bypass graft surgery for myocardial bridging show excellent symptom relief with both techniques, with no surgical complications reported. 76–78 Despite the risk of graft occlusion in the presence of competitive flow, there were no graft failures noted in follow-up of up to 35 months. Coronary artery bypass grafting can be considered a treatment option for myocardial bridging, especially in extensive and deep bridges.
Percutaneous coronary intervention
The first case of coronary stenting for severe bridging refractory to medical therapy was reported in 1995 by Stables et al.79 Early studies evaluating this option have shown that stenting can resolve hemodynamic abnormalities and symptoms.42 However, multiple studies have since demonstrated high rates of target lesion revascularization with percutaneous coronary intervention. In a study involving 70 patients with myocardial bridging who received stents (primarily drug-eluting stents) for a LAD artery lesion proximal to the bridge, rates of target lesion revascularization at 1 year were much higher (24% vs 3%) in patients whose stents extended into the bridged segment.80 Another study reported a target lesion revascularization rate of 36% (4 out of 11) in a group of patients receiving bare-metal stents specifically for symptomatic myocardial bridging, despite significant improvements in minimal luminal area (from 0.6 mm2 to 1.9 mm2) and cross-sectional area (from 3.3 mm2 to 6.8 mm2) at the time of stenting.67 However, multiple cases of coronary perforation23–26 and stent fracture21,22 in a stented myocardial bridge have been reported, perhaps related to stent oversizing. As with surgical intervention, percutaneous coronary intervention should only be considered as a therapeutic option in patients with bridging refractory to medical therapy, with the expectation that revascularization rates will be high even with drug-eluting stents.
Conclusion
Myocardial bridging is a congenital anomaly in which an epicardial coronary artery takes an intramyocardial course. It is present anatomically in approximately 25% of patients based on autopsy and CT, but only results in angiographically detectable systolic compression in less than 10% of patients. Flow alterations from this condition can cause accelerated atherosclerosis in the coronary segment immediately proximal to the bridged segment. The bridged portion itself is “spared” from atherosclerosis, likely through favorable shear forces resulting in increased expression of vasoactive agents as well as morphological changes in endothelial and smooth muscle cells in the area. Hemodynamic effects of bridging include systolic coronary flow reversal proximal to the bridge, as well as a decrease in coronary flow reserve. Clinical consequences range from angina to acute coronary syndrome to sudden cardiac death. First-line therapy involves medical treatment with beta-blockers and non-dihydropyridine calcium-channel blockers, while nitrates are contraindicated due to secondary tachycardia and hypercontractility from reflex sympathetic activation. For refractory symptoms, multiple interventional strategies have been attempted such as surgical myotomy, coronary artery bypass surgery, and stenting. A prospective randomized trial is required to identify the best treatment strategy for patients with myocardial bridging.
Footnotes
Disclosure: The authors have completed and returned the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors report no conflicts of interest regarding the content herein.
References
- 1.Reyman HC. Disertatio de vasis cordis propriis. Bibl Anat. 1737;2:359–379. [Google Scholar]
- 2.Angelni P, Velasco JA, Flamm S. Coronary anomalies: incidence, pathophysiology, and clinical relevance. Circulation. 2002;105:2449–2454. doi: 10.1161/01.cir.0000016175.49835.57. [DOI] [PubMed] [Google Scholar]
- 3.Rossi L, Dander B, Nidasio GP, et al. Myocardial bridges and ischemic heart disease. Eur Heart J. 1980;1:239–245. doi: 10.1093/oxfordjournals.eurheartj.a061125. [DOI] [PubMed] [Google Scholar]
- 4.Tauth J, Sullebarger JT. Myocardial infarction associated with myocardial bridging: case history and review of the literature. Cath Cardiovasc Diagn. 1997;40:364–367. doi: 10.1002/(sici)1097-0304(199704)40:4<364::aid-ccd9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Yano K, Yoshino H, Taniuchi M, et al. Myocardial bridging of the LAD in acute inferior wall myocardial infarction. Clin Cardiol. 2001;24:202–208. doi: 10.1002/clc.4960240306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erbel R, Ge J, Mohlenkamp S. Myocardial bridging: a congenital variant as an anatomic risk factor for myocardial infarction? Circulation. 2009;120:357–359. doi: 10.1161/CIRCULATIONAHA.109.881367. [DOI] [PubMed] [Google Scholar]
- 7.Marchionni N, Chechi T, Falai M, Margheri M, Fumagalli S. Myocardial stunning associated with a myocardial bridge. Int J Cardiol. 2002;82:65–67. doi: 10.1016/s0167-5273(01)00580-0. [DOI] [PubMed] [Google Scholar]
- 8.Feld H, Guadanino V, Hollander G, Greengart A, Lichstein E, Shani J. Exercise-induced ventricular tachycardia in association with a myocardial bridge. Chest. 1991;99:1295–1296. doi: 10.1378/chest.99.5.1295. [DOI] [PubMed] [Google Scholar]
- 9.Den Dulk K, Brugada P, Braat S, Heddle B, Wellens HJ. Myocardial bridging as a cause of paroxysmal atrioventricular block. J Am Coll Cardiol. 1983;1:976–969. doi: 10.1016/s0735-1097(83)80218-6. [DOI] [PubMed] [Google Scholar]
- 10.Tio RA, Van Gelder IC, Boonstra PW, Crijns HJ. Myocardial bridging in a survivor of sudden cardiac near-death: role of intracoronary Doppler flow measurements and angiography during dobutamine stress in the clinical evaluation. Heart. 1997;77:280–282. doi: 10.1136/hrt.77.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler D, Wallace JM. Myocardial bridging in a young patient with sudden death. Clin Cardiol. 1997;20:581–583. doi: 10.1002/clc.4960200614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Portsmann W, Iwig J. Die intramurale Koronarie im Angiogramm. Fortschr Geb Rontgenstr Nuklearmed. 1960;92:129–132. [PubMed] [Google Scholar]
- 13.Konen E, Goitein O, Sternik L, Eshet Y, Shemesh J, Di Segni E. The prevalence and anatomical patterns of intramuscular coronary arteries: a coronary computed tomography angiographic study. J Am Coll Cardiol. 2007;49:587–593. doi: 10.1016/j.jacc.2006.09.039. [DOI] [PubMed] [Google Scholar]
- 14.Ge J, Jeremias A, Rupp A, et al. New signs characteristic of myocardial bridging demonstrated by intracoronary ultrasound and Doppler. Eur Heart J. 1999;20:1707–1716. doi: 10.1053/euhj.1999.1661. [DOI] [PubMed] [Google Scholar]
- 15.Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional characteristics of myocardial bridging: a combined angiographic and intracoronary Doppler flow study. Eur Heart J. 1997;18:434–442. doi: 10.1093/oxfordjournals.eurheartj.a015263. [DOI] [PubMed] [Google Scholar]
- 16.Escaned J, Cortes J, Flores A, et al. Importance of diastolic fractional flow reserve and dobutamine challenge in physiologic assessment of myocardial bridging. J Am Coll Cardiol. 2003;42:226–233. doi: 10.1016/s0735-1097(03)00588-6. [DOI] [PubMed] [Google Scholar]
- 17.Alegria JR, Herrmann J, Holmes DR, Jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J. 2005;26:1159–1168. doi: 10.1093/eurheartj/ehi203. [DOI] [PubMed] [Google Scholar]
- 18.Mohlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106:2616–2622. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 19.Betriu A, Tabau J, Sanz G, Magrina J, Navarro-Lopez F. Relief of angina by periarterial muscle resection of myocardial bridges. Am Heart J. 1980;100:223–226. doi: 10.1016/0002-8703(80)90118-0. [DOI] [PubMed] [Google Scholar]
- 20.Katznelson Y, Petchenko P, Knobel B, Cohen AJ, Kishon Y, Schachner A. Myocardial bridging: surgical technique and operative results. Military Med. 1996;161:248–250. [PubMed] [Google Scholar]
- 21.Tandar A, Whisenant BK, Michaels AD. Stent fracture following stenting of a myocardial bridge: report of two cases. Catheter Cardiovasc Interv. 2008;71:191–196. doi: 10.1002/ccd.21365. [DOI] [PubMed] [Google Scholar]
- 22.Srinivasan M, Prasad A. Metal fatigue in myocardial bridges: stent fracture limits the efficacy of drug-eluting stents. J Invasive Cardiol. 2011;23:E150–E152. [PubMed] [Google Scholar]
- 23.Broderick TM, Kereiakes DJ, Whang DD, Toltzis RJ, Abbottsmith CW. Myocardial bridging may predispose to coronary perforation during rotational atherectomy. J Invasive Cardiol. 1996;8:161–163. [PubMed] [Google Scholar]
- 24.Hering D, Horstkotte D, Schwimmbeck P, Piper C, Bilger J, Schultheiss HP. Acute myocardial infarct caused by a muscle bridge of the anterior interventricular ramus: complicated course with vascular perforation after stent implantation. Z Kardiol. 1997;86:630–638. doi: 10.1007/s003920050103. [DOI] [PubMed] [Google Scholar]
- 25.Li W, Li Y, Sheng L, Gong Y. Myocardial bridge: is the risk of perforation increased? Can J Cardiol. 2008;24:e80–e81. doi: 10.1016/s0828-282x(08)70198-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang M, Kang WC, Moon CI, Han SH, Ahn TH, Shin EK. Coronary artery perforation following implantation of a drug-eluting stent rescued by deployment of a covered stent in symptomatic myocardial bridging. Korean Circ J. 2010;40:148–151. doi: 10.4070/kcj.2010.40.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Risse M, Weiler G. Die koronare Muskelbrücke und ihre Beziehung zu lokaler Koronarsklerose, regionaler Myokardischämie und Koronarspasmus. Eine morphometrische Studie. Z Kardiol. 1985;74:700–705. [PubMed] [Google Scholar]
- 28.Soran O, Pamir G, Erol C, Kocakavak C, Sabah I. The incidence and significance of myocardial bridge in a prospectively defined population of patients undergoing coronary angiography for chest pain. Tokai J Exp Clin Med. 2000;25:57–60. [PubMed] [Google Scholar]
- 29.La Grutta L, Runza G, Lo Re G, et al. Prevalence of myocardial bridging and correlation with coronary atherosclerosis studied with 64-slice CT coronary angiography. Radiol Med. 2009;114:1024–1036. doi: 10.1007/s11547-009-0446-y. [DOI] [PubMed] [Google Scholar]
- 30.Mohiddin SA, Begley D, Shih J, Fananapazir L. Myocardial bridging does not predict sudden death in children with hypertrophic cardiomyopathy but is associated with more severe cardiac disease. J Am Coll Cardiol. 2000;36:2270–2278. doi: 10.1016/s0735-1097(00)00987-6. [DOI] [PubMed] [Google Scholar]
- 31.Sorajja P, Ommen SR, Nishimura RA, Gersh BJ, Tajik AJ, Holmes DR. Myocardial bridging in adult patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003;42:889–894. doi: 10.1016/s0735-1097(03)00854-4. [DOI] [PubMed] [Google Scholar]
- 32.Wymore P, Yedlicka JW, Garcia-Medina V, et al. The incidence of myocardial bridges in heart transplants. Cardiovasc Intervent Radiol. 1989;12:202–206. doi: 10.1007/BF02577154. [DOI] [PubMed] [Google Scholar]
- 33.Polacek P, Kralove H. Relation of myocardial bridges and loops on the coronary arteries to coronary occlusions. Am Heart J. 1961;61:44–52. doi: 10.1016/0002-8703(61)90515-4. [DOI] [PubMed] [Google Scholar]
- 34.Angelini P, Tivellato M, Donis J, Leachman RD. Myocardial bridges: a review. Prog Cardiovasc Dis. 1983;26:75–88. doi: 10.1016/0033-0620(83)90019-1. [DOI] [PubMed] [Google Scholar]
- 35.Ferreira AG, Jr, Trotter SE, Konig B, Decourt LV, Fox K, Olsen EG. Myocardial bridges: morphological and functional aspects. Br Heart J. 1991;66:364–367. doi: 10.1136/hrt.66.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giampalmo A, Bronzini E, Bandini T. Sulla minor compromissione aterosclerotica delle arterie coronarie quando siano (per variante anatomica) in situazione intramiocardica. Giornale Ital Arterioscl. 1964;2:1–14. [Google Scholar]
- 37.Marcus JT, Smeenk HG, Juijer JP, et al. Flow profiles in the left anterior descending and the right coronary artery assessed by MR velocity quantification: effects of through-plane and in-plane motion of the heart. J Comput Assist Tomogr. 1999;23:567–576. doi: 10.1097/00004728-199907000-00017. [DOI] [PubMed] [Google Scholar]
- 38.Bourassa MG, Butnaru A, Lespérance J, Tardif JC. Symptomatic myocardial bridges: overview of ischemic mechanisms and current diagnostic and treatment strategies. J Am Coll Cardiol. 2003;41:351–359. doi: 10.1016/s0735-1097(02)02768-7. [DOI] [PubMed] [Google Scholar]
- 39.Erbel R, Rupprecht HJ, Ge J, Gerber T, Gunter G, Meyer J. Coronary artery shape and flow changes induced by myocardial bridging. Assessment by intravascular ultrasound. Echocardiography. 1993;10:71–77. [Google Scholar]
- 40.Ge J, Erbel R, Rupprecht HJ, et al. Comparison of intravascular ultrasound and angiography in the assessment of myocardial bridging. Circulation. 1994;89:1725–1732. doi: 10.1161/01.cir.89.4.1725. [DOI] [PubMed] [Google Scholar]
- 41.Klues HG, Schwarz ER, vom Dahl J, et al. Disturbed intracoronary hemodynamics in myocardial bridging. Early normalization by intracoronary stent placement. Circulation. 1997;96:2905–2913. doi: 10.1161/01.cir.96.9.2905. [DOI] [PubMed] [Google Scholar]
- 42.Ishii T, Asuwa N, Masuda S, Ishikawa Y. The effects of a myocardial bridge on coronary atherosclerosis and ischemia. J Pathol. 1998;185:4–9. doi: 10.1002/(SICI)1096-9896(199805)185:1<4::AID-PATH50>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Masuda T, Ishikawa Y, Akasaka Y, Itoh K, Kiguchi H, Ishii T. The effect of myocardial bridging of the coronary artery on vasoactive agents and atherosclerosis localization. J Pathol. 2001;193:408–414. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH792>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 44.Ciampricotti R, el Gamal M. Vasospastic coronary occlusion associated with a myocardial bridge. Cathet Cardiovasc Diagn. 1988;14:118–120. doi: 10.1002/ccd.1810140213. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa Y, Ishii T, Asuwa N, Masuda S. Absence of atherosclerosis evolution in the coronary arterial segment covered by myocardial tissue in cholesterol-fed rabbits. Virchows Arch. 1997;430:163–171. doi: 10.1007/BF01008038. [DOI] [PubMed] [Google Scholar]
- 46.Tobias SL, Videlefsky SW, Misra VK. Physiological significance of a proximal coronary artery stenosis on a distal intramyocardial bridge: coronary flow velocity patterns pre- and post-angioplasty. Cathet Cardiovasc Diagn. 1995;35:127–130. doi: 10.1002/ccd.1810350209. [DOI] [PubMed] [Google Scholar]
- 47.Tio RA, Van Gelder IC, Boonstra PW, Crijns HJ. Myocardial bridging in a survivor of sudden cardiac near-death: role of intracoronary Doppler flow measurements and angiography during dobutamine stress in the clinical evaluation. Heart. 1997;77:280–282. doi: 10.1136/hrt.77.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hongo Y, Tada H, Ito K, Yasumura Y, Miyatake K, Yamagishi M. Augmentation of vessel squeezing at coronary-myocardial bridge by nitroglycerin: study by quantitative coronary angiography and intravascular ultrasound. Am Heart J. 1999;138:345–350. doi: 10.1016/s0002-8703(99)70123-7. [DOI] [PubMed] [Google Scholar]
- 49.Tsujita K, Maehara A, Mintz GS, et al. Comparison of angiographic and intravascular ultrasonic detection of myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 2008;102:1608–1613. doi: 10.1016/j.amjcard.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 50.Im SI, Rha SW, Choi BG, et al. Angiographic and clinical characteristics according to intracoronary acetylcholine dose in patients with myocardial bridge. Cardiology. 2013;125:250–257. doi: 10.1159/000351181. [DOI] [PubMed] [Google Scholar]
- 51.Yoshino S, Cassar A, Matsuo Y, et al. Fractional flow reserve with dobutamine challenge and coronary microvascular endothelial dysfunction in symptomatic myocardial bridging. Circ J. 2014;78:685–692. doi: 10.1253/circj.cj-13-0846. [DOI] [PubMed] [Google Scholar]
- 52.Pichard AD, Casanegra P, Marchant E, Rodriguez JA. Abnormal regional myocardial flow in myocardial bridging of the left anterior descending coronary artery. Am J Cardiol. 1981;47:978–982. doi: 10.1016/0002-9149(81)90201-0. [DOI] [PubMed] [Google Scholar]
- 53.Hakeem A, Cilingiroglu M, Leesar MA. Hemodynamic and intravascular ultrasound assessment of myocardial bridging: fractional flow reserve paradox with dobutamine versus adenosine. Catheter Cardiovasc Interv. 2010;75:229–236. doi: 10.1002/ccd.22237. [DOI] [PubMed] [Google Scholar]
- 54.Kim PJ, Hur G, Kim SY, et al. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: a comparison between computed tomography and invasive coronary angiography. Circulation. 2009;119:1408–1416. doi: 10.1161/CIRCULATIONAHA.108.788901. [DOI] [PubMed] [Google Scholar]
- 55.Wang MH, Sun AJ, Qian JY, et al. Myocardial bridging detection by non-invasive multislice spiral computed tomography: comparison with intravascular ultrasound. Chin Med J (Engl) 2008;121:17–21. [PubMed] [Google Scholar]
- 56.Taylor CA, Fonte TA, Min JK. Computational fluid dynamics applied to cardiac computed tomography for noninvasive quantification of fractional flow reserve: scientific basis. J Am Coll Cardiol. 2013;61:2233–2241. doi: 10.1016/j.jacc.2012.11.083. [DOI] [PubMed] [Google Scholar]
- 57.Lin S, Tremmel JA, Yamada R, et al. A novel stress echocardiography pattern for myocardial bridge with invasive structural and hemodynamic correlation. J Am Heart Assoc. 2013;2:1–11. doi: 10.1161/JAHA.113.000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang K, Wang L, Shi R, et al. The role of myocardial perfusion imaging in evaluating patients with myocardial bridging. J Nucl Cardiol. 2011;18:117–122. doi: 10.1007/s12350-010-9303-6. [DOI] [PubMed] [Google Scholar]
- 59.Gawor R, Kusmierek J, Płachcinska A, et al. Myocardial perfusion GSPECT imaging in patients with myocardial bridging. J Nucl Cardiol. 2011;18:1059–1065. doi: 10.1007/s12350-011-9406-8. [DOI] [PubMed] [Google Scholar]
- 60.Navarro-Lopez F, Soler J, Magriña J, et al. Systolic compression of coronary artery in hypertrophic cardiomyopathy. Int J Cardiol. 1986;12:309–320. doi: 10.1016/0167-5273(86)90267-6. [DOI] [PubMed] [Google Scholar]
- 61.Yetman AT, McCrindle BW, MacDonald C, Freedom RM, Gow R. Myocardial bridging in children with hypertrophic cardiomyopathy — a risk factor for sudden death. N Engl J Med. 1998;339:1201–1209. doi: 10.1056/NEJM199810223391704. [DOI] [PubMed] [Google Scholar]
- 62.Basso C, Thiene G, Mackey-Bojack S, Frigo AC, Corrado D, Maron BJ. Myocardial bridging, a frequent component of the hypertrophic cardiomyopathy phenotype, lacks systematic association with sudden cardiac death. Eur Heart J. 2009;30:1627–1634. doi: 10.1093/eurheartj/ehp121. [DOI] [PubMed] [Google Scholar]
- 63.Pittaluga J, de Marchena E, Posoda JD, Romanelli R, Morales A. Left anterior descending coronary artery bridge: a cause of early death after cardiac transplantation. Chest. 1997;111:511–513. doi: 10.1378/chest.111.2.511. [DOI] [PubMed] [Google Scholar]
- 64.Singhal AK, McClurken JB, Fisher CA, Macha M, Mohara J, Furukawa S. Successful transplantation of an older donor heart with documented myocardial bridging: a case report. J Heart Lung Transplant. 2005;24:340–342. doi: 10.1016/j.healun.2003.11.402. [DOI] [PubMed] [Google Scholar]
- 65.D’Ancona G, Baglini R, Clemenza F, et al. Left anterior descending coronary artery bridge: contraindication to cardiac transplantation? J Heart Lung Transplant. 2007;26:637–638. doi: 10.1016/j.healun.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 66.Schwarz ER, Gupta R, Haager PK, et al. Myocardial bridging in absence of coronary artery disease: proposal of a new classification based on clinical-angiographic data and long-term follow-up. Cardiology. 2009;112:13–21. doi: 10.1159/000137693. [DOI] [PubMed] [Google Scholar]
- 67.Haager PK, Schwarz ER, vom Dahl J, Klues HG, Reffelmann T, Hanrath P. Long-term angiographic and clinical follow-up in patients with stent implantation for symptomatic myocardial bridging. Heart. 2000;84:403–408. doi: 10.1136/heart.84.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schwarz ER, Klues HG, vom Dahl J, Klein I, Krebs W, Hanrath P. Functional, angiographic, and intracoronary Doppler flow characteristics in symptomatic patients with myocardial bridging: effect of short-term intravenous beta-blocker medication. J Am Coll Cardiol. 1996;27:1637–1645. doi: 10.1016/0735-1097(96)00062-9. [DOI] [PubMed] [Google Scholar]
- 69.Nair CK, Dang B, Heintz MH, Sketch MH. Myocardial bridges: effect of propranolol on systolic compression. Can J Cardiol. 1986;2:218–221. [PubMed] [Google Scholar]
- 70.Alessandri N, Dei Giudici A, De Angelis S, Urciuoli F, Garante MC, Di Matteo A. Efficacy of calcium channel blockers in the treatment of the myocardial bridging: a pilot study. Eur Rev Med Pharmacol Sci. 2012;16:829–834. [PubMed] [Google Scholar]
- 71.Ishimori T, Raizner AE, Chahine RA, Awdeh M, Luchi RJ. Myocardial bridges in man: clinical correlations and angiographic accentuation with nitroglycerin. Cathet Cardiovasc Diagn. 1977;3:59–65. doi: 10.1002/ccd.1810030107. [DOI] [PubMed] [Google Scholar]
- 72.Hill RC, Chitwood WR, Jr, Bashore TM, Sink JD, Cox JL, Wechsler AS. Coronary flow and regional function before and after supraarterial myotomy for myocardial bridging. Ann Thorac Surg. 1981;31:176–181. doi: 10.1016/s0003-4975(10)61539-1. [DOI] [PubMed] [Google Scholar]
- 73.Iversen S, Hake U, Mayer E, Erbel R, Diefenbach C, Oelert H. Surgical treatment of myocardial bridging causing coronary artery obstruction. Scand J Thorac Cardiovasc Surg. 1992;26:107–111. doi: 10.3109/14017439209099063. [DOI] [PubMed] [Google Scholar]
- 74.Attaran S, Moscarelli M, Athanasiou T, Anderson J. Is coronary artery bypass grafting an acceptable alternative to myotomy for the treatment of myocardial bridging? Interact Cardiovasc Thorac Surg. 2013;16:347–349. doi: 10.1093/icvts/ivs459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pratt JW, Michler RE, Pala J, Brown DA. Minimally invasive coronary artery bypass grafting for myocardial muscle bridging. Heart Surg Forum. 1999;2:250–253. [PubMed] [Google Scholar]
- 76.Wan L, Wu Q. Myocardial bridge, surgery, or stenting? Interact Cardiovasc Thorac Surg. 2005;4:517–520. doi: 10.1510/icvts.2005.111930. [DOI] [PubMed] [Google Scholar]
- 77.Wu QY, Xu ZH. Surgical treatment of myocardial bridging: report of 31 cases. Chin Med J. 2007;120:1689–1693. [PubMed] [Google Scholar]
- 78.Huang XH, Wang SY, Xu JP, et al. Surgical outcome and clinical follow-up in patients with symptomatic myocardial bridging. Chin Med J. 2007;120:1563–1566. [PubMed] [Google Scholar]
- 79.Stables RH, Knight CJ, McNeill JG, Sigwart U. Coronary stenting in the management of myocardial ischaemia caused by muscle bridging. Br Heart J. 1995;74:90–92. doi: 10.1136/hrt.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsujita K, Maehara A, Mintz GS, et al. Impact of myocardial bridge on clinical outcome after coronary stent placement. Am J Cardiol. 2009;103:1344–1348. doi: 10.1016/j.amjcard.2009.01.340. [DOI] [PubMed] [Google Scholar]