Drugs don’t work in patients who don’t take them.
—C. Everett Koop, M.D. (1)
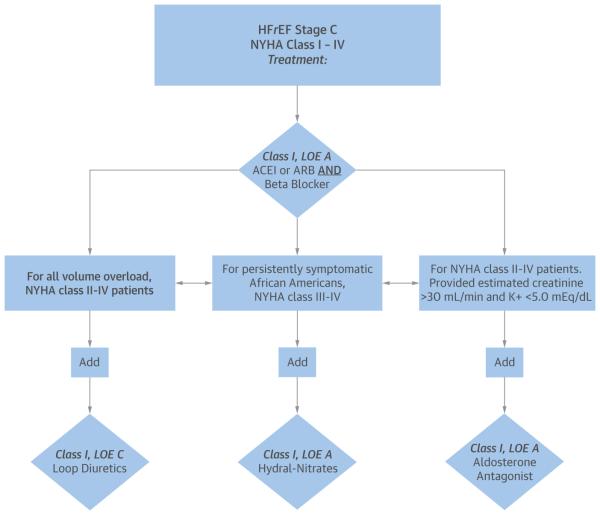
Guideline-directed medical therapy (GDMT) is the mainstay of initial and chronic management of heart failure with reduced ejection fraction (HFrEF). The term GDMT refers to the drug treatments that benefit patients with HFrEF, and it evokes the body of evidence-based literature and the endorsement of several professional societies. The cornerstone of therapy is the initiation of heart failure (HF)-approved beta-blockers and renin-angiotensin inhibitors (RAIs) shown to improve symptoms, cardiac function, and mortality (Figure 1) (2). A patient newly diagnosed with HFrEF requires close follow-up and careful titration of multiple medications as hemodynamics, electrolytes, and symptoms permit. Although the known benefits of GDMT have solidified, a persistent observable gap remains in the provision and receipt of GDMT for both ambulatory and hospitalized patients with HFrEF (3).
FIGURE 1. Guideline-Directed Medical Therapy in Stage C Heart Failure With Reduced Ejection Fraction Patients.
Guideline-directed medical therapy for stage C heart failure with reduced ejection fraction (HFrEF). Reproduced with permission from Yancy et al. (2). ACEI = angiotensin-converting enzyme inhibitor; ARB = angiotensin receptor blocker; K+ = potassium; LOE = Level of Evidence; NYHA = New York Heart Association.
In this issue of the Journal, Roth et al. (4) use the National Cardiovascular Data Registry (NCDR) ICD Registry to identify receipt of GDMT before placement of an implantable cardioverter-defibrillator (ICD) for the primary prevention of sudden cardiac death. The study selected NCDR registry patients with Medicare and Medicare Plan D prescription benefits who had received a primary prevention ICD between 2007 and 2011. The NCDR database was linked to Medicare Part D prescription data to assess adherence to a HF-approved beta-blocker and RAI for the 90 days before ICD implantation. Astonishingly, only 61.1% of patients received a beta-blocker and RAI before ICD implantation and only 28.3% received an adequate supply (defined as ≥80% coverage for the 90 days before ICD implantation). The findings are consistent and expand on a previous study that provided a snapshot of beta-blocker and RAI use at time of ICD placement, in which one-quarter of eligible patients were not on GDMT (5). The new analysis suggests that an even larger portion of patients are not adhering to GDMT before undergoing implantation of a primary prevention ICD, which is recommended by current HFrEF guidelines.
Failure to adequately treat with GDMT before a primary prevention ICD implantation suggests that at least some patients who may have responded to medical therapy with improvements in left ventricular ejection fraction (LVEF) above the range at which they would derive sufficient benefit from the ICD are needlessly receiving a costly and invasive device therapy. The Intervention in Myocarditis and Acute Cardiomyopathy–2 cohort study of patients with nonischemic HFrEF reported >90% beta-blocker and RAI use and noted a ≥10% LVEF improvement for 70% of patients and ≥20% LVEF improvement for 39% of patients by 6 months (6). A single-center Italian study also reported that 67% of patients with nonischemic cardiomyopathy treated with GDMT (87% beta-blocker and 95% RAI usage rates) no longer met criteria for a primary prevention ICD (7).
In the paper by Roth et al. (4), one caveat to the society guideline recommendations during much of the study period between 2007 and 2011 is that they were vague regarding the duration of “optimal medical therapy” before a primary prevention ICD implantation for patients with nonischemic HFrEF, as the evidence was uncertain (8,9). It was not until 2010 that the Heart Failure Society of America guidelines discussed the duration of optimal medical therapy as 3 to 6 months before ICD placement (10). The American College of Cardiology Foundation and the American Heart Association followed with similar recommendations in 2013 (2).
There are important limitations to the study by Roth et al. (4) that deserve further consideration before accepting that GDMT is substantially underused before primary prevention defibrillator placement. One difficulty with administrative data is knowing the true rate of contraindications and intolerance to GDMT. Roth et al. included patients with chronic renal disease, who likely have higher rates of intolerance to RAI, and the comorbidity was associated with an 11% lower relative risk of receiving GDMT before ICD implantation. In the IMPROVE HF (Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting) study, detailed chart review revealed 6% to 8% of patients had contraindications or were intolerant to beta-blockers or RAIs (11). Another limitation of the data is that studies have shown that some patients do not use prescription benefits when purchasing inexpensive generic drugs, and medication use and adherence may not be captured for this group of patients (12). However, medication intolerance and incomplete prescription data are likely only a partial explanation for the large, observed gap in treatment.
Of greater concern is that medical providers may not be prescribing GDMT and titrating doses appropriately in eligible patients with HFrEF. Despite the promotion in professional society guidelines of evidenced-based practice, it is recognized that the dissemination of knowledge to practice progresses at unacceptably slow rates (13). Monitoring and financial incentives for GDMT performance metrics are currently scarce and may reflect the low priority of medical optimization in real-world practice. It is also a substantial missed opportunity that the NCDR ICD registry does not currently capture and provide feedback on the duration and dosing of GDMT before device implantation. The wide regional variation in GDMT described by Roth et al. (4) may suggest either regional variations in physician practice or patient factors, such as socioeconomic status and health literacy, that affect medication adherence.
Medication nonadherence is a vexing challenge for many chronic conditions that require complex daily medication regimens. Among HF patients with Medicare Part D plans, low adherence to HF medications has been observed in about one-quarter to more than one-half of outpatients (14,15). Physicians are notoriously poor in recognizing nonadherence (16). Evidence for new technologies such as electronic medication pack-aging, which transmits adherence feedback to patients and providers, is limited but may be associated with improved adherence (17). Further research is needed to determine whether streamlining feedback from pharmacies to outpatient practices can identify nonadherent patients or facilitate targeted interventions. Research on improving medication adherence, and the resulting implementation of effective strategies, has the potential to reduce health care utilization and save tens of thousands of lives (18).
Roth et al. (4) report an associated 20% higher relative risk of adjusted mortality rate for patients not on GDMT. Although these are observational data, patient factors incompletely accounted for in the statistical model and administrative data may explain the perceived elevated mortality risk. The receipt of inadequate GDMT is unlikely to negate the observed benefits for ICD placement on mortality. Patients treated with inadequate GDMT are undoubtedly at higher risk for sudden cardiac death. The randomized clinical trials showing the benefits of ICD therapy did not require GDMT for 3 to 6 months before implantation and not all patients were on both beta-blockers and RAI therapy at baseline. In the Multicenter Automatic Defibrillator Implantation Trial II, approximately 70% of the patients with a history of myocardial infarction and LVEF <30% were taking angiotensin-converting enzyme inhibitors and beta-blockers (19). In the Sudden Cardiac Death in Heart Failure Trial of patients with New York Heart Association functional class II and III and LVEF <35%, approximately 96% were on an RAI and 69% were on a beta-blocker (20).
These findings in the Journal highlight the notable treatment gap observed in the real-world management of HFrEF and the vital opportunities to improve care. Further research is required to understand the hurdles facing providers and patients in optimally managing patients. A new diagnosis of HF is an opportunity to reverse myocardial dysfunction, improve quality of life, and reduce the need for implanted device therapies such as ICDs through the optimal use of GDMT. Establishing systems of care that streamline guideline-based treatments and improve patient medication adherence are critical to maximizing patient health, improving survival, and preventing unnecessary health care utilization.
Acknowledgments
Dr. Fonarow has received research funding from the National Institutes of Health and the Agency for Healthcare Research and Quality; and he has received consulting fees from Amgen, Bayer, Baxter, Janssen, Medtronic, and Novartis. Dr. Ziaeian is supported by the National Institutes of Health Cardiovascular Scientist Training Program (T32 HL007895); his brother is a clinical specialist for Biotronik. Richard Troughton, MB ChB, PhD, served as Guest Editor for this paper
Footnotes
Editorials published in the Journal of the American College of Cardiology reflect the views of the authors and do not necessarily represent the views of JACC or the American College of Cardiology.
REFERENCES
- 1.Cramer JA. Enhancing patient compliance in the elderly. Role of packaging aids and monitoring. Drugs Aging. 1998;12:7–15. doi: 10.2165/00002512-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Ambrosy AP, Fonarow GC, Butler J, et al. The global health and economic burden of hospitalizations for heart failure: lessons learned from hospitalized heart failure registries. J Am Coll Cardiol. 2014;63:1123–33. doi: 10.1016/j.jacc.2013.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Roth GA, Poole JE, Zaha R, Zhou W, Skinner J, Morden NE. Use of guideline-directed medications for heart failure before cardioverter-defibrillator implantation. J Am Coll Cardiol. 2016;67:1062–9. doi: 10.1016/j.jacc.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller AL, Wang Y, Curtis J, et al. Optimal medical therapy use among patients receiving implantable cardioverter/defibrillators: insights from the National Cardiovascular Data Registry. Arch Intern Med. 2012;172:64–7. doi: 10.1001/archinternmed.2011.466. [DOI] [PubMed] [Google Scholar]
- 6.McNamara DM, Starling RC, Cooper LT, et al. Clinical and demographic predictors of outcomes in recent onset dilated cardiomyopathy: results of the IMAC (Intervention in Myocarditis and Acute Cardiomyopathy)-2 study. J Am Coll Cardiol. 2011;58:1112–8. doi: 10.1016/j.jacc.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zecchin M, Merlo M, Pivetta A, et al. How can optimization of medical treatment avoid unnecessary implantable cardioverter-defibrillator implantations in patients with idiopathic dilated cardiomyopathy presenting with “SCD-HeFT criteria?”. Am J Cardiol. 2012;109:729–35. doi: 10.1016/j.amjcard.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 8.Zipes DP, Camm AJ, Borggrefe M, et al. ACC/ AHA/ESC 2006 guidelines for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death—executive summary: a report of the American College of Cardiology/American Heart Association Task Force and the European Society of Cardiology Committee for Practice Guidelines. J Am Coll Cardiol. 2006;48:1064–8. doi: 10.1016/j.jacc.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 9.Epstein AE, DiMarco JP, Ellenbogen K, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline) J Am Coll Cardiol. 2008;51:e1–62. doi: 10.1016/j.jacc.2008.02.032. [DOI] [PubMed] [Google Scholar]
- 10.Lindenfeld J, Albert NM, Boehmer JP, et al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–194. doi: 10.1016/j.cardfail.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 11.Heywood JT, Fonarow GC, Yancy CW, et al. Comparison of medical therapy dosing in outpatients cared for in cardiology practices with heart failure and reduced ejection fraction with and without device therapy report from IMPROVE HF. Circ Hear Fail. 2010;3:596–605. doi: 10.1161/CIRCHEARTFAILURE.109.912683. [DOI] [PubMed] [Google Scholar]
- 12.Stuart B, Loh FE, Loh EF. Medicare Part D enrollees’ use of out-ofplan discounted generic drugs. J Am Geriatr Soc. 2012;60:387–8. doi: 10.1111/j.1532-5415.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 13.Berwick DM. Disseminating innovations in health care. JAMA. 2003;289:1969–75. doi: 10.1001/jama.289.15.1969. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Wu SH, Fendrick AM, Baicker K. Variation in medication adherence in heart failure. JAMA Intern Med. 2013;173:468–70. doi: 10.1001/jamainternmed.2013.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sueta CA, Rodgers JE, Chang PP, et al. Medication adherence based on part D claims for patients with heart failure after hospitalization (from the Atherosclerosis Risk in Communities Study) Am J Cardiol. 2015;116:413–9. doi: 10.1016/j.amjcard.2015.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 17.Checchi KD, Huybrechts KF, Avorn J, Kesselheim AS. Electronic medication packaging devices and medication adherence: a systematic review. JAMA. 2014;312:1237–47. doi: 10.1001/jama.2014.10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fonarow GC, Yancy CW, Hernandez AF, Peterson ED, Spertus JA, Heidenreich PA. Potential impact of optimal implementation of evidence-based heart failure therapies on mortality. Am Heart J. 2011;161:1024–30. doi: 10.1016/j.ahj.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 19.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 20.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]