Abstract
Background
Disproportionately high rates of alcohol use disorders are present in many American Indian/Alaska Native (AI/AN) communities, yet little information exists regarding the effectiveness of alcohol treatments in AI/AN populations. Contingency management is an intervention for illicit drug use in which tangible reinforcers (rewards) are provided when patients demonstrate abstinence as assessed by urine drug tests. Contingency management has not been widely studied as an intervention for alcohol problems because until recently, no alcohol biomarker has been available to adequately verify abstinence.
Aims
The HONOR Study is designed to determine whether a culturally-tailored contingency management intervention is an effective intervention for AI/AN adults who suffer from alcohol use disorders.
Methods
Participants include 400 AI/AN alcohol-dependent adults residing in one rural reservation, one urban community, as well as a third site to be decided, in the Western U.S. Participants complete a 4-week lead-in phase prior to randomization, then 12 weeks of either a contingency management intervention for alcohol abstinence, or a control condition where participants receive reinforcers for attending study visits regardless of alcohol use. Participants are then followed for 3-more months post-intervention. The primary study outcome is urinary ethyl glucuronide-confirmed alcohol abstinence; secondary outcomes include self-reported alcohol and drug use, HIV risk behaviors, and self-reported cigarette smoking.
Discussion
This will be the largest randomized, controlled trial of any alcohol for AI/ANs and the largest contingency management study targeting alcohol use disorders, thus providing important information to AI/AN communities and the alcohol treatment field in general.
Keywords: American Indians, Alaska Natives, Alcohol, Treatment, Contingency management, Ethyl glucuronide, Alcohol biomarker
1. Introduction
For complex reasons the need for alcohol treatment is great in many American Indian and Alaska Native (AI/AN) communities. Previous studies have observed higher rates of alcohol use disorders (AUD) in AI/AN communities than in the mainstream U.S. population (10.7% vs. 7.6%) [1]. In another study, nearly twice as many AI/AN adults reported needing alcohol treatment, when compared to others in the U.S. [3] Alarmingly, only 13% of AI/ANs who needed AUD treatment received it in the last year [3]. When AI/AN adults do receive treatment, their completion rate is lower than that of the general population [2–4]. As a result of these disparities, the alcohol mortality rate AI/ANs experience is approximately twice that of other Americans [5]. AI/AN communities are seeking culturally acceptable, feasible, and cost-effective strategies to combat AUDs.
Despite this need, little information exists about the effectiveness of AUD interventions for AI/AN populations. Observational studies support use of “Western” and AI/AN cultural-based AUD interventions in Native communities [6–9]. Surprisingly, there are only three published randomized, controlled trials of AUD interventions in AI/AN adults [10–12]. Two observed reductions in alcohol use associated with pharmacological (naltrexone) [10] and behavioral (motivational interviewing) [11] interventions; while another found no impact on drinking when AI/AN women participated in an online intervention focused on preventing prenatal alcohol exposure [12]. While two of these studies observed reductions in drinking; one was not focused exclusively on AI/ANs and therefore, lacked the statistical power to determine intervention effectiveness for AI/ANs, and the other only included individuals involved in the criminal justice system [11]. Further research is needed to identify effective AUD interventions for AI/AN communities.
Contingency Management (CM) is an addiction intervention where participants receive reinforcers such as vouchers or prizes for providing objective evidence of drug abstinence [13,14]. CM is an effective intervention for illicit drugs, and relative to other psychosocial interventions, CM is the most successful at initiating abstinence [14–16,18–25]. In previous studies CM has demonstrated cost-effectiveness, feasibility, and a long term reductions in substance use that are comparable to cognitive behavioral therapies [15–17]. Though untested in AI/ANs, CM is an effective intervention for illicit drug use in other minority racial and ethnic groups [18–21].
Feasible CM interventions require a biomarker that can detect substance use in the preceding three days. Previous research on CM for AUDs has been limited by lack of such a biomarker. Ethyl glucuronide (EtG) is an alcohol metabolite [22–31] that can be detected in urine for up to five days after drinking and can be evaluated in a clinical setting using a benchtop analyzer [29,32–35]. Our work supports the efficacy and feasibility of an EtG-based CM intervention [36].
1.1. Study aims
In collaboration with three communities, we are conducting the Honoring Our Native Ongoing Recovery (HONOR) study, funded by the National Institutes of Health (R01AA022070), to: 1) determine whether participants randomized to a culturally-tailored CM intervention are more likely to achieve alcohol abstinence, as assessed by EtG urine tests, compared with those assigned to a control group; 2) quantify group differences for other addiction and health outcomes; and 3) identify demographic, clinical and cultural factors that modify the effect of CM on alcohol abstinence.
2. Methods
2.1. Design
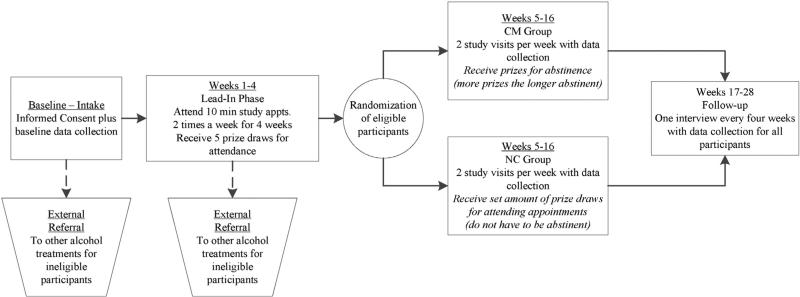
The HONOR Study involves two phases: 1) a qualitative phase focused on identifying cultural adaptations to the CM intervention and 2) an RCT of the adapted CM intervention (Fig. 1). One or more focus groups will be conducted at each of the three study sites to improve the cultural acceptability of the study recruitment and intervention procedures. Up to 20 alcohol treatment providers, individuals with alcohol dependence, and their family members will be recruited from each community to participate in focus groups. Focus group data will be used to modify study recruitment and intervention materials, including the CM reinforcers. After focus groups are completed, 400 AI/AN alcohol dependent adults will be recruited and will complete a four-week lead-in assessment period designed to increase engagement and reduce post-randomization dropout (weeks 1–4). Those who complete the lead-in phase will be randomized to receive 12 weeks (weeks 5–16) of either treatment-as-usual with contingency management (CM group) or treatment-as-usual with reinforcers that are not contingent on alcohol abstinence (Non-contingent [NC] or control group). All randomized participants will be followed for an additional three months to assess post-intervention outcomes (weeks 17–28).
Fig. 1.
Overview of HONOR Study procedures.
2.2. Setting
The study will take place at an Urban Indian healthcare clinic in the Northwest, one rural reservation located on the Northern Plains, and a to-be-determined third study site pending approval. To protect their confidentiality, specific names of these sites are not provided. The Urban Indian healthcare clinic provides primary care, disease prevention, mental healthcare, and addiction treatment to AI/AN adults and youth in a city surrounded by a number of rural reservations. The site also hosts numerous cultural events and engages in community outreach, with a focus on health promotion and disease prevention. The agency does not offer intensive outpatient addiction treatment to adults; patients are instead referred to outpatient addiction providers throughout the community.
The Northern Plains site is home to approximately 11,000 tribal members from two AI tribes. Addiction treatment services are offered at two locations on the reservation and both utilize a culturally informed intensive outpatient addiction treatment model. Individuals with addictions at this site also have access to mental and physical health care through Indian Health Services.
Importantly, alcohol possession and consumption are illegal in the reservation participating in this study.
2.3. Participants
We will recruit 400 alcohol-dependent AI/AN individuals through advertisements in outpatient addiction clinics, primary care clinics, social service agencies, and places where adults with alcohol problems are likely to frequent, as well as through radio, newspaper, and online advertising. Interested individuals will contact study staff, who will then explain the study and assess recent alcohol and drug use. Eligible participants (see Section 2.6.2) will be scheduled for an in-person interview where they will provide written, informed consent.
2.4. Ethical oversight
Overall ethical oversight for this study will be provided by the University of Washington Institutional Review Board (IRB). One study site, an Urban Indian healthcare clinic, elected to defer human subject protections oversight to the University of Washington IRB. The third site has provided tribal resolutions supporting the study, and requested that an additional AI/AN organization's IRB review and approve the study procedures. In partnership with the tribal and organizational leadership at each site, we have formulated data ownership, and dissemination agreements to protect partnering communities from inappropriate use of data gathered throughout the study.
2.5. Intervention adaptation
Prior to recruitment for the qualitative and RCT phases of the study we convened a Community Advisory Board (CAB). The CAB includes five community members who are knowledgeable about their respective communities, addiction treatment, and research. These individuals, as well as study staff from each community, attended a two day CAB meeting in Seattle, WA. During the meeting, CAB members reviewed the study design, procedures, and timeline. CAB members recommended changes to recruitment materials, created a study name, modified questionnaires, and provided feedback on the feasibility of the intervention. Subsequent CAB meetings will be held by teleconference or in person throughout the five year study.
2.6. Study procedures
Community members will be hired and trained to perform research procedures, including administering interviews and analyzing biological samples, and delivering the contingency management intervention.
2.6.1. Focus groups
Prior to implementing the RCT at each site, one or more focus groups will be conducted to improve the cultural acceptability of the study recruitment and intervention procedures. Up to 20 alcohol treatment providers, individuals with alcohol dependence, and their family members will be recruited from each community to participate in focus groups. A research coordinator hired from the community will present the study to the focus groups using a short PowerPoint presentation and a video demonstration of the intervention. The research coordinator will then ask a series of questions related to the cultural acceptability of the study recruitment strategy and CM intervention to elicit group member feedback. Focus groups will be audio-recorded and transcribed for analysis. Thematic coding of focus group transcriptions will be conducted by two independent coders and themes identified within each focus group by both coders will inform intervention adaptation within the community in question. Themes identified across all focus groups will inform adaptations across all participating communities. Attempts will be made to assure that recruitment strategies will be consistent across sites, focusing on recruitment from clinics, as well as the community. While focus groups will be used to tailor the intervention to each community, the magnitude (value) and frequency of reinforcers will be equal across sites.
2.6.2. RCT participant eligibility
Eligibility criteria include 1) self-reported AI/AN race or heritage; 2) age 18 years and older; 3) Diagnostic and Statistical Manual, Fourth Edition [37] diagnosis of current alcohol dependence per the Mini International Neuropsychiatric Interview (MINI) (a semi-structured clinical interview) [38]; 4) consumption of 4 or more standard drinks on 5 or more occasions in the last 30 days; 5) ability to read and speak English; and 6) ability to provide written, informed consent. Exclusion criteria, which have been minimized to mimic a “real-world” clinic setting, include 1) number of days of illicit drug or prescription amphetamine or opioid use is greater than number of days of alcohol use in the last 90 days; 2) risk of dangerous alcohol withdrawal, defined as a history of dangerous alcohol withdrawal symptoms in the last 12 months or concern by the patient or healthcare provider about dangerous withdrawal; and 3) any medical or psychiatric condition that would preclude safe study participation. Research assistants will use the MINI to assess individuals that seem confused or psychotic or are known to have a history of psychosis for possible exclusionary psychiatric conditions [37,38]. The Principal Investigators, a clinical psychologist with over 10 years of conducting studies with severely mentally ill adults (M.G.M.), and an internist (D.B.), together with the site Principal Investigator will together make the final determination regarding exclusions due to psychiatric or medical disorders. Excluded individuals will be referred for appropriate treatment.
2.6.3. Lead-in phase
The lead-in phase is a pre-randomization strategy designed to reduce participant drop-out based on strategies used in previous CM studies, including our own work [39]. The lead-in phase allows participants to become acclimatized to study procedures and study investigators to identify individuals who are suited for the CM intervention. During the 4-week lead-in phase, participants will receive reinforcement in the form of 5 prize draws each Monday and Thursday for providing urine samples and study-related data. There is no requirement to provide alcohol-negative urine samples (see Prize draw procedure below) and research assistants will not give feedback to participants regarding their EtG results. Those who provide at least one urine sample each week during weeks 1–4 will receive a $20 gift card. Participants who meet initial eligibility criteria, successfully complete these attendance criteria, and submit at least one alcohol-positive urine sample (supporting a diagnosis of alcohol dependence) during the lead-in phase will progress to randomization. Previous studies have found that 80% of participants complete the lead-in phase and are eligible for randomization [39]. Those who do not progress to randomization will be referred to other alcohol treatment programs.
2.6.4. Randomization
After completing the lead-in phase, eligible participants will be randomized. Secondary eligibility criteria for randomization include providing at least one alcohol-positive urine sample and attending at least 4 study visits during weeks 1–4. Randomization will be stratified by study site and baseline interview EtG test result (positive or negative according to a cutoff level of 150 ng/mL) using the built-in randomization program of Research Electronic Data Capture (REDCap) [40], an electronic data capture tool hosted at the University of Washington and used in this study for collecting and managing data. This randomization model, along with a randomization allocation table created in Microsoft Excel, will be entered into REDCap's randomization module. Participants will be randomized and informed of their group assignment at their final lead-in phase visit (visit 8; week 4), or by phone if they do not attend this visit. All informed participants will be considered part of the intent-to-treat sample.
2.7. Study intervention
2.7.1. Contingency management intervention group
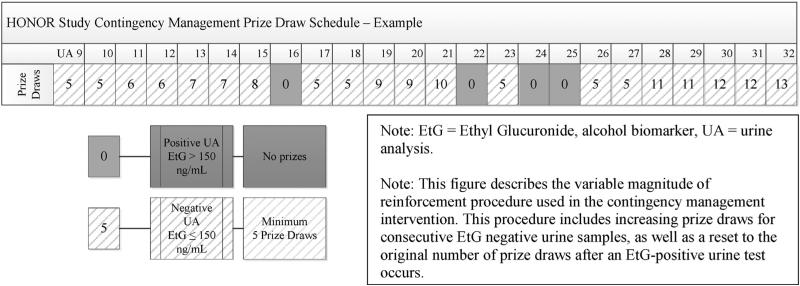
Participants randomized to the CM group will receive an escalating schedule of reinforcement for submitting alcohol-negative urine samples, with a reset condition, an approach that has been used in many CM trials [41–43]. Participants will submit urine samples twice weekly (Monday and Thursday) during intervention visits; alcohol urine tests will be conducted using an on-site analyzer (see Section 2.8.3) that provides results within 20 min. Participants who provide an alcohol-negative urine sample will be allowed to engage in the Variable Magnitude of Reinforcement Procedure (“prize draw”) that is described below. During the first week, participants will be allowed to make 5 prize draws each time they submit an alcohol-negative urine sample, with every additional week of continuous abstinence resulting in an additional prize draw per visit (Fig. 2). The number of prize draws for each alcohol-negative urine sample submitted will continue to increase from 6 (one week of continuous abstinence) to a maximum of 16 (12 weeks of continuous abstinence).
Fig. 2.
HONOR Study contingency management prize draw schedule.
If a participant submits an alcohol-positive urine sample or fails to provide a urine sample, she or he will not receive prize draws at that appointment. Alcohol-positive or missing urine samples will result in a reset to 5 prize draws the next time an alcohol-negative sample is submitted. Participants will return to their previously attained magnitude of reinforcement if they submit two consecutive alcohol-negative samples following a positive or missing test (Fig. 2).
2.7.1.1. Prize draw procedure
At each study visit, the participant will draw 5 or more chips out of a bowl containing 500 chips. Fifty percent of the chips will say “Good Job!” or a similar phrase and are not associated with a prize. Most of the remainder (41.8%) of the chips will say “Small Prize”. Each Small Prize token may be exchanged for a prize worth approximately $1 in value. A small number (8%) of the chips will say “Large Prize”, exchangeable for a prize valued at $20. One of the 500 chips (0.2%) will say “Jumbo Prize” and can be exchanged for an $80 prize. Prizes will be displayed in a locked storage cabinet at study sites. Typical prizes might include toiletries and gift cards ($1 value); gift cards, mp3 and CD players, Native and non-Native jewelry and clothing ($20 value); and DVD players, digital cameras, Native artwork, and ceremonial items ($80 value). The maximum value available to participants who remain continuously abstinent will be approximately $500, with an average estimated payout of $300 per participant. Prizes will be modified for cultural acceptability after focus groups are completed.
2.7.2. Non-contingent control group
Compensation for participants in the Non-contingent (NC) control group will follow an established protocol to isolate the effect of CM interventions in large randomized trials [44,45]. NC participants will receive prize draws simply for submitting urine samples every Monday and Thursday; even if their samples are positive for alcohol. Their level of reinforcement will be “yoked” to that of the CM group, so they will receive a number of prize draws that is equal to the average number of prize draws earned by the CM group in the previous week. This equates the level of reinforcement across groups while allowing us to isolate the effect of CM on alcohol use.
2.8. Measures and materials
2.8.1. Data collection
Collection of study outcomes will occur throughout the study period (weeks 1–16, including baseline assessment, lead-in phase, and intervention) and the follow-up period (weeks 17–28). The primary outcome measure for the trial will be alcohol-negative EtG urine tests, defined as EtG less than or equal to 150 ng/mL, collected at the baseline assessment, at every Monday and Thursday follow-up visit during the intervention period (weeks 1–16), and at each of the 3 monthly follow-up period appointments. Additional outcomes will include alcohol breath tests and self-reported alcohol use assessed at each study visit. Other outcomes will include other drug use, cigarette smoking, HIV-risk behaviors, and physical and mental health. Other clinical and cultural characteristics will be gathered at the baseline interview to determine whether or not they are associated with intervention effectiveness. Major study assessments will occur at baseline and every four weeks (also known as “monthly” interviews) throughout the study.
2.8.2. Demographic measures
During the baseline assessment we will collect demographic characteristics via self-report, including: sex, gender, age, race, ethnicity, housing status, and religious preference will be gathered using items from the Addiction Severity Index, Native American Version [46].
2.8.3. Alcohol and drug biomarkers
At each study visit, urine samples will be collected and analyzed for EtG using DRI EtG semi-quantitative enzyme immunoassay tests with an Indiko Clinical and Specialty Chemistry System (Thermo Scientific, Fremont, CA). Tests will be conducted using EtG 100 ng/mL, 500 ng/mL, 1000 ng/mL, 2000 ng/mL, and Negative calibrators and EtG 100 ng/mL and 375 ng/mL controls. Antibody/Substrate and Enzyme Conjugate reagents will be used. When controls deviate more than 25% from given concentrations, the analyzer will be recalibrated and controls rerun before immunoassays are conducted again. To prevent bacterial hydrolysis, all samples, calibrators, controls, and reagents will be refrigerated at 4 °C until analyses are conducted. The analyzer will be calibrated once per week and samples will be collected and analyzed twice per week. Eight drops of urine from each sample are required for analysis. Dilution will be conducted according to manufacturer guidelines when sample results display an error message indicating high absorbance. The EtG analysis returns a value between 0 and 2000 ng/mL. Equipment will be purchased from the manufacturer to enable on-site EtG testing with immediate results, which will facilitate timely delivery of CM reinforcers. Consistent with our previous studies recent alcohol use will be defined as an EtG> 150 ng/mL. Our previous studies suggest that this cutoff level is not associated with “false positives” resulting from exposure to non-beverage alcohol (e.g., hand sanitizer, mouthwash) [35]. Despite the low risk of false positive tests, participants will be reminded that they should abstain from all alcohol-containing products.
Alcohol breath tests will also be administered to assess alcohol intoxication at each visit, using Alco-Sensor III breathalyzers (Intoximeters, Inc., St. Louis, MO). Criteria for an alcohol-positive breath test will be blood alcohol equal to or greater than 0.01.
Urine drug tests will be conducted at each study visit using point of care screening cups. Drugs screened for will include: opioids (morphine > 2000 ng/mL), amphetamine (d-amphetamine > 1 000 ng/mL), methamphetamine (d-methamphetamine > 1 000 ng/mL), cocaine (benzoylecgonine > 300 ng/mL), and cannabis (tetrahydrocannabinol > 50 ng/mL).
2.8.4. Self-reported alcohol and drug measures
At each study visit self-reported alcohol use will be assessed by the Alcohol Timeline FollowBack method [47], which measures the frequency and amount of daily drinking for up to a 30 day period. At each study visit we will also assess alcohol cravings with a 10 cm visual analog scale anchored at 0 (no craving) and 100 (most intense craving possible). At the baseline and monthly interviews we will administer a modified version of the Addiction Severity Index, Native American Version (ASI-NAV) [46], to assess self-reported alcohol and drug use, alcohol and drug addiction severity and the impact of alcohol and drug use on psychiatric, legal, medical, and family functioning.
2.8.5. Other outcome measures
The impact of the CM intervention on other outcomes will be assessed. HIV risk behavior (i.e. injection drug use and sexual behaviors) will be assessed at baseline and at each monthly interview with the brief HIV Risk Behavior Scale [48,49]. Physical and mental health-related quality of life will be assessed by the Short-Form-12 Health Survey, a well-established measure [50] that has been used to asses health-related quality of life in AI/AN populations [51]. This measure will also be administered at baseline and at each monthly interview. The daily number of cigarettes smoked will be assessed at each study visit using the Timeline FollowBack method [47].
2.8.6. Baseline clinical measures
Several clinical measures will be administered at the baseline interview to determine their influence on CM effectiveness. Readiness to change alcohol use will be assessed by the Stages of Change Readiness and Treatment Eagerness Scale (SOCRATES) [52], a 19-item instrument in which the participant indicates their level of agreement with statements regarding their alcohol use. Severity of nicotine dependence will be assessed using the Fagerström Test of Nicotine Dependence [53], a short 8-item self-report measure utilizing theoretical concepts of reliance on nicotine. Severity of psychiatric problems related to substance use will be assessed with the ASI-NAV [47]. Given the high comorbidity of Post-Traumatic Stress Disorder in adults with AUDs, we will assess for the presence of this condition using the MINI [40]. We will also assess for potential Fetal Alcohol Spectrum Disorder using the Life History Screen [54], a brief measure that unobtrusively screens participants for adverse life-course outcomes typically found in FASD. Medical comorbidity will be measured using a modified version of the Charleson Deyo Scale [55], a simple self-report questionnaire assessing the presence of chronic medical conditions. Healthcare utilization (i.e. mental health care, addiction treatment, emergency room visits) during the previous year will also be measured via self-report using a modified tool developed from in our previous CM studies. Finally, early life and chronic stress will be assessed by a 25 item measure examining 25 specific adverse events occurring before 18 years of age (e.g. sexual abuse, life-threatening accident, suicide of someone close), used in a psychiatric epidemiology study of 3084 AI adults [56].
2.8.7. Baseline cultural measures
We will also administer the following cultural measures that have been developed specifically for AI/AN populations. Enculturation, or the extent to which someone feels involved and a part of their culture, will be measured by the American Indian Enculturation Scale [57], a 17-item instrument asking participants to rate how much they have participated in certain cultural activities. Historical trauma/loss will be measured by the Historical Loss and Historical Loss Associated Symptoms scales [58]. These scales assess the frequency at which an individual thinks about perceived historical losses (e.g. “loss of our land”, “losing our culture”) and their emotional responses to these losses (e.g. “a loss of sleep”, “rage”) [55]. Perceived discrimination will be measured by Whitbeck's Perceived Discrimination Scale [59], a 10-item scale assessing how often an individual feels they have experienced race-based discrimination in particular situations.
2.8.8. Intervention attrition
Individuals will be considered to have dropout of their respective treatment condition (CM or NC reinforcement) if they have 6 consecutive study absences (roughly equal to three weeks) during the 12-week treatment phase.
2.9. Adverse events
Throughout the study, research staff may be notified of adverse events through participant self-report, study assessments, or via participants’ clinicians. Participants will be evaluated for alcohol withdrawal symptoms at each study visit using the short SHOT (sweating, hallucination, orientation, and tremors) assessment for current alcohol withdrawal [60]. Those who are identified through the SHOT or self-report as experiencing withdrawal symptoms will be immediately referred for a medical evaluation. Because we do not anticipate that a large number of participants will experience severe withdrawal symptoms, we will also report qualitatively on the experience of these individuals. Individuals who endorse elevated psychiatric distress will be referred to their primary care or psychiatric providers for evaluation and treatment. Other adverse and serious adverse events will be reported to the Principal Investigator (M.G.M.) immediately after they occur. Per the Data Safety Monitoring Plan, all serious adverse events will be reported immediately to the Data Safety Monitoring Board, relevant IRBs, and the National Institutes of Health. Quarterly Data Safety Monitoring Plan reports will be reviewed by the Data Safety Monitoring Board until data collection is complete.
3. Analytic plan
3.1. Preliminary data analyses
We will describe each treatment arm and study site in terms of demographic and clinical variables using percentages (categorical variables) and means and standard deviations (continuous variables). We will assess randomization by comparing the baseline distributions of variables in the two treatment arms, using t-tests for continuous factors and chi square tests for categorical factors. For primary and secondary biochemical outcomes, we will create indicator variables for abstinence at each time point. We will also calculate the number of days from baseline to the first negative test and the duration (in days) of the longest period of abstinence. We will follow similar procedures to create descriptive variables for self-reported secondary outcomes.
3.2. Primary analyses
We will perform intent-to-treat analyses for three outcomes comparing treatment groups. The first outcome will be biochemically-identified abstinence based on an EtG test of less than 150 ng/mL at each clinic visit during follow-up (weeks 5–16). The model used to examine this outcome will use GEE for time-varying outcome. The second outcome is a continuous measure of the longest duration of abstinence (defined using the EtG tests), modeled using linear regression. The third outcome is a time to return to alcohol use (defined using EtG tests) from baseline, and will be modeled using Cox proportional hazards regression. Details for the various regression models, including handling of missing data and adjustment schemes, are included below. These analytic approaches are based on previous trials of CM as a treatment for drug dependence [41–43,61,62]. Results will be reported as estimates of risk for the outcome, comparing treatment with control groups; 95% confidence intervals will be calculated based on a two-sided alpha of 0.05.
Analyses will be stratified by site (study center); effect modification by site will be evaluated using tests of differences among the primary endpoint coefficients. If these tests indicate that there is no significant difference among sites, then pooled estimates will be reported.
GEE (time-varying abstinence at each visit) is flexible with respect to estimating unbiased effects in the presence of non-informative missing data. Linear models (longest duration of abstinence) may be biased somewhat towards the null, as participants with more missing data will tend to have shorter durations of abstinence. Cox PH models will account for missing data by censoring participants with missed clinic visits.
Although randomization should remove confounding, adjustment for potential residual confounders and precision variables will be evaluated in sensitivity analyses.
3.2.1. Secondary analyses
Additional analyses will examine other outcomes, including proportion of negative drug tests and self-reported abstinence, using similar models as primary analyses. Variables regarding alcohol and drug use severity, psychiatric and medical comorbidity, and AI-specific cultural factors will be examined as potential determinants of longest abstinence duration; adjustment schemes in these models will follow a nested approach, with baseline model including a priori, known confounders, and subsequent models incorporating additional factors.
3.3. Power
The choice of sample size (N = 400) is based on primary outcome models examining CM-associated reductions in alcohol use and secondary addiction-related outcomes and alcohol-associated health-impairing behaviors across the 12-week intervention and 12-week follow-up periods. Based on prior research of drug dependent individuals [63], we estimate 20% participant loss during the lead in phase, resulting in a randomized sample of N = 320 and a CM effect size of 0.4 on the primary outcome, alcohol urine tests [64,65], with a smaller effect (~0.2) of the CM intervention expected on Specific Aim 2 outcomes. Power analyses are based an intent-to-treat analysis with a maximum of 36 data collection points for each participant (24 data points per week for 12 weeks of intervention; 3 monthly data points during follow-up), assuming a correlation between time points of r = 0.3. Based on these parameters, we will have 90% power to detect an effect size of 0.21. Power analyses for Specific Aim 3, modification of treatment outcome in the CM group (N = 160) based on the same assumptions indicate >90% power to detect an effect size of 0.29.
3.4. Missing data
To minimize missing data, we will diligently collect data when participants are available. We will also utilize the lead-in phase, which has been associated with lower rates of attrition in the randomized sample in CM RCTs [44,17,60]. In addition, we will reduce loss of participants by modifying the study interventions using qualitative procedures to maximize cultural acceptability.
Data that are missing despite these efforts will be handled in a manner consistent with previous large-scale investigations of CM for illicit drug use [41–43], which emphasizes using end-point analyses. Random effects modeling within a general latent variable modeling framework such as GEE allows parameter estimation from the non-missing data in a manner that accounts for bias that may result from missingness. Participants who drop out before study completion will be compared with those who do not; if the proportion of missing information is substantial (over 20%), we will use multiple imputation as a sensitivity analysis [61,66].
4. Implementation
Considerable planning and partnership development have been required to successfully implement this study. Community collaboration has been key to success at every step of this process from initial community engagement, to grant writing to implementation. Using the principles of Community Based Participatory Research as a framework, this project was initiated from community requests, followed by engagement with key stakeholders and public discussions with members of each community. Following the initial request from community partners, we applied for and were awarded a Community Pilot Grant from the University of Washington's Institute for Translational Health Sciences which provided pilot funds for in-person visits to each tribal site to gather input on the proposed intervention prior to the grant submittal. This momentum was sustained through grant review and award by the support of local advocates who met and strategized regularly with researchers to work to keep community leadership informed and begin planning for implementation. Members of the CAB have continued to provide community oversight and advocacy through frequent phone, video and in-person contact to further facilitate community involvement.
Site staff have been integral to successful implementation of this research. The research assistants that are charged with all study related tasks at their site, including recruitment and implementing the intervention, are trusted AI/AN community members. This has ensured cultural adaptations and awareness for study materials and practices, while also earning the trust and buy-in of the community. These community members are aware of customs, such as greeting Elders and other community members respectfully, knowing cultural traditions and ceremonial practices, and creating a safe and understanding environment for AI/AN participants struggling with alcohol problems. These community members also assisted in identifying potential barriers to study participation and attendance, such as high rates of medical comorbidity and limited access to transportation, as well as strategies for overcoming these barriers, such as decreasing the number of weekly study visits from three to two per week, and providing bus tokens or gas cards as reinforcers.
Official tribal approvals were required to begin this research. Each Tribe maintains their own tribal governments and approval processes, many of which consist of elected Tribal Council or Business Council members. To best navigate this process, our local advocates and key stakeholders took the lead on drafting tribal resolutions (i.e., a tribal law) and letters of support to present to Tribal Councils. Extra time was taken to ensure all parties understood the benefits and risks of the study to their community and worked to thoroughly inform leadership of the goals and inter-workings of the intervention. Additional documentation was formed regarding approvals for data and publications in a “Data Ownership and Dissemination Agreement”. This agreement between the UW researchers and local authorizing bodies describes how data will be used by researchers and describe a process for tribal review and approval related to dissemination of study results. These documents protect partner tribes and organizations from the use of data for purposes outside agreed upon study aims and publication of study findings that might bring harm or stigma to their communities.
One further ongoing consideration is finding a sustainable way to finance the CM intervention in these communities in the future if it proves to be successful as a research study. We are working with site staff to consider billing options for the intervention (both urine tests and prizes). One hurdle will be to ensure that the EtG test and Indiko urine analyzer will be approved or waived under the Clinical Laboratory Improvement Amendments.
5. Summary
To our knowledge, the HONOR Study will be the largest RCT ever conducted of an intervention designed to treat alcohol problems in AI/AN adults, as well as the largest CM RCT targeting alcohol use disorders in any population. The financial costs and negative impact of alcohol misuse in American Indian/Alaska Native communities underscore the need for alcohol interventions that are effective, culturally acceptable to these communities, and can be implemented practically. Contingency management has the potential to meet this need as an adaptable and low-cost behavioral intervention for alcohol use. In the present RCT, we are using qualitative research methods to learn from our community partners and are working closely to culturally adapt the CM intervention. We are also testing potential modifiers of intervention effectiveness (i.e. demographic characteristics, alcohol use severity, medical and psychiatric comorbidities, and cultural factors). Most importantly, the study will have clinical and policy implications, should this culturally tailored CM intervention prove successful.
Acknowledgments
The funding for this research is provided by the National Institute on Alcohol Abuse and Alcoholism and the Office of the Director's Office of Behavioral and Social Science Sciences research grant R01AA022070, Principal Investigators McDonell and Buchwald, as well as a small grant from the University of Washington's Institute for Translational Health Sciences, Principal Investigator Buchwald. We would like to thank our community partners for their ongoing collaboration and support throughout all phases of this project.
References
- 1.SAMHSA . The NSDUH report: substance use and substance use disorders among American Indians and Alaska Natives. Substance Abuse and Mental Health Services Administration, Office of Applied Studies; Rockville, MD: Jan 19, 2007. 2007. [Google Scholar]
- 2.Evans E, Spear SE, Huang Y-C, Hser Y-I. Outcomes of drug and alcohol treatment programs among American Indians in California. Am. J. Public Health. 2006;96(5):889–896. doi: 10.2105/AJPH.2004.055871. (%U http://ajph.aphapublications.org/cgi/content/abstract/896/885/889/) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldstein SW, Venner KL, May PA. American Indian/Alaska Native alcohol-related incarceration and treatment. Am. Indian Alsk. Native Ment. Health Res. (Online) 2006;13(3):1–22. doi: 10.5820/aian.1303.2006.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Callaghan RC. Risk factors associated with dropout and readmission among First Nations individuals admitted to an inpatient alcohol and drug detoxification program. CMAJ. 2003;169(1):23–27. [PMC free article] [PubMed] [Google Scholar]
- 5.Naimi TS, Cobb N, Boyd D, Jarman DW, Espey D, Snesrud P, Chavez P. Alcohol-attributable deaths and years of potential life lost among American Indians and Alaska Natives — United States, 2001–2005. MMWR. 2008;57(34):938–941. [PubMed] [Google Scholar]
- 6.Boyd-Ball AJ. A culturally responsive, family-enhanced intervention model. Alcohol. Clin. Exp. Res. 2003;27(8):1356–1360. doi: 10.1097/01.ALC.0000080166.14054.7C. [DOI] [PubMed] [Google Scholar]
- 7.Gossage JP, Barton L, Foster L, Etsitty L, LoneTree C, Leonard C, May PA. Sweat lodge ceremonies for jail-based treatment. J. Psychoactive Drugs. 2003;35(1):33–42. doi: 10.1080/02791072.2003.10399991. [DOI] [PubMed] [Google Scholar]
- 8.Allen J, Mohatt GV, Rasmus SM, Hazel KL, Thomas L, Lindley S. The tools to understand: community as co-researcher on culture-specific protective factors for Alaska Natives. J. Prev. Interv. Community. 2006;32(1–2):41–59. doi: 10.1300/J005v32n01_04. [DOI] [PubMed] [Google Scholar]
- 9.Ringwalt C, Bliss K. The cultural tailoring of a substance use prevention curriculum for American Indian youth. J. Drug Educ. 2006;36(2):159–177. doi: 10.2190/369L-9JJ9-81FG-VUGV. [DOI] [PubMed] [Google Scholar]
- 10.O'Malley SS, Robin RW, Levenson AL, GreyWolf I, Chance LE, Hodgkinson CA, Romano D, Robinson J, Meandzija B, Stillner V, Wu R, Goldman D. Naltrexone alone and with sertraline for the treatment of alcohol dependence in Alaska natives and non-natives residing in rural settings: a randomized controlled trial. Alcohol. Clin. Exp. Res. 2008;32(7):1271–1283. doi: 10.1111/j.1530-0277.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodall WG, Delaney HD, Kunitz SJ, Westerberg VS, Zhao H. A randomized trial of a DWI intervention program for first offenders: intervention outcomes and interactions with antisocial personality disorder among a primarily American-Indian sample. Alcohol. Clin. Exp. Res. 2007;31(6):974–987. doi: 10.1111/j.1530-0277.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- 12.Montag AC, Brodine SK, Alcaraz JE, Clapp JD, Allison MA, Calac DJ, Hull AD, Gorman JR, Jones KL, Chambers CD. Preventing alcohol-exposed pregnancy among an American Indian/Alaska Native population: effect of a screening, brief intervention, and referral to treatment intervention. Alcohol. Clin. Exp. Res. 2015;39(1):126–135. doi: 10.1111/acer.12607. [DOI] [PubMed] [Google Scholar]
- 13.Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction. 2006;101(2):192–203. doi: 10.1111/j.1360-0443.2006.01311.x. [DOI] [PubMed] [Google Scholar]
- 14.Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction. 2006;101(11):1546–1560. doi: 10.1111/j.1360-0443.2006.01581.x. [DOI] [PubMed] [Google Scholar]
- 15.Olmstead TA, Petry NM. The cost-effectiveness of prize-based and voucher-based contingency management in a population of cocaine- or opioid-dependent outpatients. Drug Alcohol Depend. 2009;102(1–3):108–115. doi: 10.1016/j.drugalcdep.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murphy SM, McDonell MG, McPherson S, Srebnik D, Angelo F, Roll JM, Ries RK. An economic evaluation of a contingency-management intervention for stimulant use among community mental health patients with serious mental illness. Drug Alcohol Depend. doi: 10.1016/j.drugalcdep.2015.05.004. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W. A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction (Abingdon) 2006;101(2):267–274. doi: 10.1111/j.1360-0443.2006.01312.x. [DOI] [PubMed] [Google Scholar]
- 18.Montgomery L, Petry NM, Carroll KM. Moderating effects of race in clinical trial participation and outcomes among marijuana-dependent young adults. Drug Alcohol Depend. 2012;126(3):333–339. doi: 10.1016/j.drugalcdep.2012.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barry D, Sullivan B, Petry NM. Comparable efficacy of contingency management for cocaine dependence among African American, Hispanic, and white methadone maintenance clients. Psychol. Addict. Behav. 2009;23(1):168–174. doi: 10.1037/a0014575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bride BE, Humble MN. Increasing retention of African-American women on welfare in outpatient substance user treatment using low-magnitude incentives. Subst. Use Misuse. 2008;43(8–9):1016–1026. doi: 10.1080/10826080801914154. [DOI] [PubMed] [Google Scholar]
- 21.Hser YI, Li J, Jiang H, Zhang R, Du J, Zhang C, Zhang B, Evans E, Wu F, Chang YJ, Peng C, Huang D, Stitzer ML, Roll J, Zhao M. Effects of a randomized contingency management intervention on opiate abstinence and retention in methadone maintenance treatment in China. Addiction. 2011;106(10):1801–1809. doi: 10.1111/j.1360-0443.2011.03490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wurst FM, Kempter C, Metzger J, Seidl S, Alt A. Ethyl glucuronide: a marker of recent alcohol consumption with clinical and forensic implications. Alcohol. 2000;20(2):111–116. doi: 10.1016/s0741-8329(99)00076-2. [DOI] [PubMed] [Google Scholar]
- 23.Wurst FM, Kempter C, Seidl S, Alt A. Ethyl glucuronide—a marker of alcohol consumption and a relapse marker with clinical and forensic implications. Alcohol Alcohol. 1999;34(1):71–77. doi: 10.1093/alcalc/34.1.71. [DOI] [PubMed] [Google Scholar]
- 24.Wurst FM, Seidl S, Ladewig D, Muller-Spahn F, Alt A. Ethyl glucuronide: on the time course of excretion in urine during detoxification. Addict. Biol. 2002;7(4):427–434. doi: 10.1080/1355621021000006035. [DOI] [PubMed] [Google Scholar]
- 25.Wurst FM, Skipper GE, Weinmann W. Addiction. Vol. 98. Suppl. 2: 2003. Ethyl glucuronide—the direct ethanol metabolite on the threshold from science to routine use; pp. 51–61. [DOI] [PubMed] [Google Scholar]
- 26.Wurst FM, Tabakoff B, Alling C, Aradottir S, Wiesbeck GA, Muller-Spahn F, Pragst F, Johnson B, Javors M, Ait-Daoud N, Skipper GE, Spies C, Nachbar Y, Lesch O, Ramskogler K, Hartmann S, Wolfersdorf M, Dresen S, Weinmann W, Hines L, Kaiser A, Lu RB, Ko HC, Huang SY, Wang TJ, Wu YS, Whitfield J, Snell LD, Wu C, Hoffman PL. World Health Organization/International Society for Biomedical Research on Alcoholism study on state and trait markers of alcohol use and dependence: back to the future. Alcohol. Clin. Exp. Res. 2005;29(7):1268–1275. doi: 10.1097/01.alc.0000171483.93724.96. [DOI] [PubMed] [Google Scholar]
- 27.Wurst FM, Vogel R, Jachau K, Varga A, Alling C, Alt A, Skipper GE. Ethyl glucuronide discloses recent covert alcohol use not detected by standard testing in forensic psychiatric inpatients. Alcohol. Clin. Exp. Res. 2003;27(3):471–476. doi: 10.1097/01.ALC.0000057942.57330.E2. [DOI] [PubMed] [Google Scholar]
- 28.Skipper GE, Weinmann W, Thierauf A, Schaefer P, Wiesbeck G, Allen JP, Miller M, Wurst FM. Ethyl glucuronide: a biomarker to identify alcohol use by health professionals recovering from substance use disorders. Alcohol Alcohol. 2004;39(5):445–449. doi: 10.1093/alcalc/agh078. [DOI] [PubMed] [Google Scholar]
- 29.Helander A, Bottcher M, Fehr C, Dahmen N, Beck O. Detection times for urinary ethyl glucuronide and ethyl sulfate in heavy drinkers during alcohol detoxification. Alcohol Alcohol. 2009;44(1):55–61. doi: 10.1093/alcalc/agn084. [DOI] [PubMed] [Google Scholar]
- 30.Kip MJ, Spies CD, Neumann T, Nachbar Y, Alling C, Aradottir S, Weinmann W, Wurst FM. The usefulness of direct ethanol metabolites in assessing alcohol intake in nonintoxicated male patients in an emergency room setting. Alcohol. Clin. Exp. Res. 2008;32(7):1284–1291. doi: 10.1111/j.1530-0277.2008.00696.x. [DOI] [PubMed] [Google Scholar]
- 31.McDonell MG, Srebnik D, Angelo F, Sugar AM, Howell D, Rainey C, Roll J, Short R, Ries R. Evaluation of ethyl glucuronide immunoassay urinalysis in five alcohol-dependent outpatients. Am. J. Addict. 2011;20(5):482–484. doi: 10.1111/j.1521-0391.2011.00157.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wurst FM, Metzger J. The ethanol conjugate ethyl glucuronide is a useful marker of recent alcohol consumption. Alcohol. Clin. Exp. Res. 2002;26(7):1114–1119. doi: 10.1111/j.1530-0277.2002.tb02646.x. [DOI] [PubMed] [Google Scholar]
- 33.Litten RZ, Bradley AM, Moss HB. Alcohol biomarkers in applied settings: recent advances and future research opportunities. Alcohol. Clin. Exp. Res. 2010;34(6):955–967. doi: 10.1111/j.1530-0277.2010.01170.x. [DOI] [PubMed] [Google Scholar]
- 34.Leickly E, McDonell M, Vilardaga R, Angelo F, Lowe J, McPherson S, Srebnik D, Roll J, Ries R. High levels of agreement between clinic-based ethyl glucuronide immunoassays and laboratory-based mass spectrometry. Am. J. Drug Alcohol Abuse. doi: 10.3109/00952990.2015.1011743. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lowe J, McDonell M, Leickly E, Angelo F, Vilardaga R, McPherson S, Srebnik D, Roll J, Ries R. Determining ethyl glucuronide cutoffs when detecting self-reported alcohol use in addiction treatment patients. Alcohol. Clin. Exp. Res. doi: 10.1111/acer.12699. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McDonell MG, Howell DN, McPherson S, Cameron JM, Srebnik D, Roll JM, Ries RK. Voucher-based reinforcement for alcohol abstinence using the ethyl-glucuronide alcohol biomarker. J. Appl. Behav. Anal. 2012;45(1):161–165. doi: 10.1901/jaba.2012.45-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Association AP . DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. 4th ed. text revision ed. AMERICAN PSYCHIATRIC PRESS INC (DC); Washington, DC: 2000. [Google Scholar]
- 38.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl. 20):22–33. [PubMed] [Google Scholar]
- 39.Donlin WD, Knealing TW, Needham M, Wong CJ, Silverman K. Attendance rates in a workplace predict subsequent outcome of employment-based reinforcement of cocaine abstinence in methadone patients. J. Appl. Behav. Anal. 2008;41(4):499–516. doi: 10.1901/jaba.2008.41-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petry NM, Peirce JM, Stitzer ML, Blaine J, Roll JM, Cohen A, Obert J, Killeen T, Saladin ME, Cowell M, Kirby KC, Sterling R, Royer-Malvestuto C, Hamilton J, Booth RE, Macdonald M, Liebert M, Rader L, Burns R, DiMaria J, Copersino M, Stabile PQ, Kolodner K, Li R. Effect of prize-based incentives on outcomes in stimulant abusers in outpatient psychosocial treatment programs: a national drug abuse treatment clinical trials network study. Arch. Gen. Psychiatry. 2005;62(10):1148–1156. doi: 10.1001/archpsyc.62.10.1148. [DOI] [PubMed] [Google Scholar]
- 42.Peirce JM, Petry NM, Stitzer ML, Blaine J, Kellogg S, Satterfield F, Schwartz M, Krasnansky J, Pencer E, Silva-Vazquez L, Kirby KC, Royer-Malvestuto C, Roll JM, Cohen A, Copersino ML, Kolodner K, Li R. Effects of lower-cost incentives on stimulant abstinence in methadone maintenance treatment: a national drug abuse treatment clinical trials network study. Arch. Gen. Psychiatry. 2006;63(2):201–208. doi: 10.1001/archpsyc.63.2.201. [DOI] [PubMed] [Google Scholar]
- 43.Roll JM, Petry NM, Stitzer ML, Brecht ML, Peirce JM, McCann MJ, Blaine J, MacDonald M, DiMaria J, Lucero L, Kellogg S. Contingency management for the treatment of methamphetamine use disorders. Am. J. Psychiatry. 2006;163(11):1993–1999. doi: 10.1176/ajp.2006.163.11.1993. [DOI] [PubMed] [Google Scholar]
- 44.Kosten T, Oliveto A, Feingold A, Poling J, Sevarino K, McCance-Katz E, Stine S, Gonzalez G, Gonsai K. Desipramine and contingency management for cocaine and opiate dependence in buprenorphine maintained patients. Drug Alcohol Depend. 2003;70(3):315–325. doi: 10.1016/s0376-8716(03)00032-2. [DOI] [PubMed] [Google Scholar]
- 45.Oliveto A, Poling J, Sevarino KA, Gonsai KR, McCance-Katz EF, Stine SM, Kosten TR. Efficacy of dose and contingency management procedures in LAAM-maintained cocaine-dependent patients. Drug Alcohol Depend. 2005;79(2):157–165. doi: 10.1016/j.drugalcdep.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 46.Carise D, Wicks K, McLellan AT, Olton P. Addiction Severity Index 5th Edition — North Dakota State Adaptation for Use With Native Americans. Treatment Research Institute at University of Pennsylvania; Philadelphia, PA: 1998. [Google Scholar]
- 47.Sobell LC, Sobell MB. Alcohol timeline followback (TLFB), Handbook of Psychiatric Measures. American Psychiatric Association; Washington DC: 2000. pp. 477–479. [Google Scholar]
- 48.Barry D, Weinstock J, Petry NM. Ethnic differences in HIV risk behaviors among methadone-maintained women receiving contingency management for cocaine use disorders. Drug Alcohol Depend. 2008;98(1–2):144–153. doi: 10.1016/j.drugalcdep.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. AIDS. 1991;5(2):181–185. doi: 10.1097/00002030-199102000-00008. [DOI] [PubMed] [Google Scholar]
- 50.Ware J, Jr., Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med. Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Quandt SA, Graham CN, Bell RA, Snively BM, Golden SL, Stafford JM, Arcury TA. Ethnic disparities in health-related quality of life among older rural adults with diabetes. Ethn. Dis. 2007;17(3):471–476. [PMC free article] [PubMed] [Google Scholar]
- 52.Miller WR, Tonigan JS. Assessing drinkers' motivation for change: the stages of change readiness and treatment eagerness scale (SOCRATES) Psychol. Addict. Behav. 1996;10(2):81–89. [Google Scholar]
- 53.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br. J. Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 54.Grant T, Novick Brown N, Graham J, Whitney N, Dubovsky D, Nelson L. Screening in treatment programs for Fetal Alcohol Spectrum Disorders that could affect therapeutic progress. Int. J. Alcohol Drug Res. 2013;2(3):37–49. [Google Scholar]
- 55.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J. Chronic. Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 56.Jiang L, Beals J, Whitesell NR, Roubideaux Y, Manson SM, Team A-S. Stress burden and diabetes in two American Indian reservation communities. Diabetes Care. 2008;31(3):427–429. doi: 10.2337/dc07-2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Winderowd C, Montgomery D, Stumblingbear G, Harless D, Hicks K. Development of the American Indian enculturation scale to assist counseling practice. Am. Indian Alsk. Native Ment. Health Res. 2008;15(2):1–14. doi: 10.5820/aian.1502.2008.1. [DOI] [PubMed] [Google Scholar]
- 58.Whitbeck LB, Adams GW, Hoyt DR, Chen X. Conceptualizing and measuring historical trauma among American Indian people. Am. J. Community Psychol. 2004;33(3–4):119–130. doi: 10.1023/b:ajcp.0000027000.77357.31. [DOI] [PubMed] [Google Scholar]
- 59.Whitbeck LB, Chen XJ, Hoyt DR, Adams GW. Discrimination, historical loss and enculturation: culturally specific risk and resiliency factors for alcohol abuse among American Indians. J. Stud. Alcohol. 2004;65(4):409–418. doi: 10.15288/jsa.2004.65.409. [DOI] [PubMed] [Google Scholar]
- 60.Gray S, Borgundvaag B, Sirvastava A, Randall I, Kahan M. Feasibility and reliability of the SHOT: a short scale for measuring pretreatment severity of alcohol withdrawal in the emergency department. Acad. Emerg. Med. 2010;17(10):1048–1054. doi: 10.1111/j.1553-2712.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 61.Mcdonell M, Srebnik D, Angelo F, McPherson S, Lowe J, Sugar A, Short R, Roll J, Ries R. A randomized controlled trial of contingency management for psycho-stimulant use in community mental health outpatients with co-occurring serious mental illness. Am. J. Psychiatry. 2013;170:94–101. doi: 10.1176/appi.ajp.2012.11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McPherson S, Barbosa-Leiker C, Burns GL, Howell D, Roll J. Missing data in substance abuse treatment research: current methods and modern approaches. Exp. Clin. Psychopharmacol. 2012 doi: 10.1037/a0027146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silverman K, Wong CJ, Needham M, Diemer KN, Knealing T, Crone-Todd D, Fingerhood M, Nuzzo P, Kolodner K. A randomized trial of employment-based reinforcement of cocaine abstinence in injection drug users. J. Appl. Behav. Anal. 2007;40(3):387–410. doi: 10.1901/jaba.2007.40-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Higgins ST, Delaney DD, Budney AJ, Bickel WK, Hughes JR, Foerg F, Fenwick JW. A behavioral approach to achieving initial cocaine abstinence. Am. J. Psychiatry. 1991;148(9):1218–1224. doi: 10.1176/ajp.148.9.1218. [DOI] [PubMed] [Google Scholar]
- 65.Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ. Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch. Gen. Psychiatry. 1994;51(7):568–576. doi: 10.1001/archpsyc.1994.03950070060011. [DOI] [PubMed] [Google Scholar]
- 66.Enders CK. Applied Missing Data Analysis. Guilford Press; New York: 2010. [Google Scholar]