Abstract
Evidence for a role of supplemental vitamin D and marine omega-3 fatty acids in preventing cancer and cardiovascular disease (CVD) remains inconclusive and insufficient to inform nutritional recommendations for primary prevention. The VITamin D and Omega-A 3 TriaL (VITAL) is an ongoing nationwide, randomized, double-blind, placebo-controlled clinical trial designed to fill this knowledge gap. The study population consists of 25,874 U.S. adults without cancer or CVD at baseline, who were selected only on age (men aged ≥50 and women aged ≥55), with an oversampling of African Americans (n=5,107). In a 2x2 factorial design, participants were randomized to one of four supplement groups: (1) active vitamin D3 (cholecalciferol; 2000 IU/d) and active marine omega-3 fatty acids (Omacor® fish oil, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], 1 g/d); (2) active vitamin D and omega-3 placebo; (3) vitamin D placebo and active marine omega-3 fatty acids; or (4) vitamin D placebo and omega-3 placebo. The mean length of the randomized treatment period will be 5 years. The randomization was successful, as evidenced by similar distributions of baseline demographic, health, and behavioral characteristics across treatment groups. The similar distribution of known potential confounders across treatment groups strongly suggests that unmeasured or unknown potential confounders are also equally distributed. VITAL is expected to provide important information on the benefit-risk balance of vitamin D and omega-3 fatty acid supplementation when taken for the primary prevention of cancer and CVD.
Keywords: cancer, cardiovascular disease, omega-3 fatty acids, primary prevention, randomized clinical trial, vitamin D
1. Introduction
Vitamin D supplementation has long been prescribed for the prevention and treatment of bone-related disorders (1), and marine omega-3 fatty acid supplementation has been recommended for heart health in patients with coronary heart disease (CHD) who fail to meet target intakes for fatty fish rich in omega-3s (2). Vitamin D and omega-3 supplements have also been increasingly used for the possible prevention of cancer or a first cardiovascular event. Indeed, sales of these supplements have skyrocketed in recent years (3–5). However, whether vitamin D and omega-3 supplements are actually of benefit for the primary prevention of cancer and cardiovascular disease (CVD) is unclear. Results of ecologic, laboratory, and observational studies are promising but inconclusive (1, 6, 7). Appropriately designed randomized trials, which minimize confounding and provide unbiased estimates of the balance of benefits and risks of supplementation, are necessary to resolve the question. No large trials of supplemental vitamin D in doses adequate to produce meaningful changes in 25-hydroxyvitamin D [25(OH)D] levels or designed to assess cancer or CVD as primary prespecified outcomes have been completed. Several randomized trials that included cancer and/or CVD outcomes in secondary or post-hoc analyses have had generally null results, but most have tested low doses of vitamin D, had inadequate statistical power, and/or lacked rigorous endpoint adjudication (1, 8–20). For marine omega-3 fatty acids, some (21–23) though not all (24–28) trials in secondary prevention or high-risk settings have found CVD risk reductions. There are no large trials of omega-3 fatty acids for the prevention of cancer or CVD in general populations unselected for elevated cardiovascular risk.
The ongoing VITamin D and OmegA-3 TriaL (VITAL) is a randomized double-blind, placebo-controlled, 2x2 factorial trial of vitamin D and omega-3 fatty acid supplementation for the primary prevention of cancer and CVD in a nationwide cohort of 25,874 U.S. adults not selected for elevated cardiovascular or cancer risk. To our knowledge, VITAL is one of only three ongoing or planned vitamin D trials with >10,000 participants (the others are D-Health in Australia (29, 30) and VIDAL in the U.K. (31, 32)) and the only large primary prevention trial of omega-3 fatty acids in a population not selected for elevated CVD risk.
In this article, we describe the baseline characteristics of the VITAL cohort and its most relevant subgroups, and evaluate the success of randomization in distributing known potential confounding factors equally among intervention groups. Given the large sample size, if known confounders are similarly distributed among the randomized treatment groups, unmeasured or unknown confounders should also be comparably distributed, suggesting that any observed differences between treatment groups with respect to the outcomes of interest are likely to be attributable to the interventions themselves rather than to confounding. We also describe the baseline characteristics of the 16,956 VITAL participants who provided an optional blood sample (“the blood cohort”) and the 1,054 Boston-area participants who had an in-person clinical evaluation at a local Clinical and Translational Science Center (“the CTSC cohort”).
2. Materials and Methods
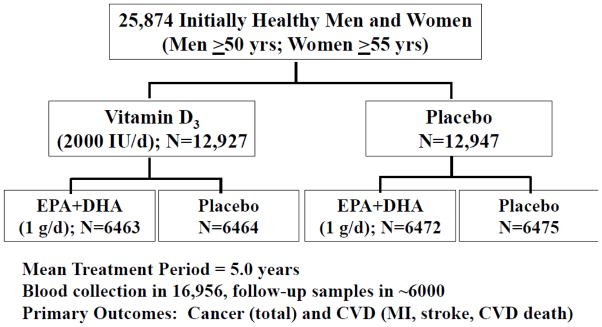
VITAL is an ongoing, randomized, double-blind, placebo-controlled clinical trial designed to assess the role of supplemental vitamin D and marine omega-3 fatty acids in the primary prevention of cancer and CVD. A detailed description of the trial’s design can be found in reference (6). A summary of the design is provided in Figure 1. Study participants are a nationwide sample of 25,874 U.S. adults without cancer or CVD at baseline. By design, approximately equal numbers of men and women were enrolled in the trial (12,793 men aged ≥50 and 13,081 women aged ≥55), and African Americans were oversampled (n=5,107 [the target was 5,000]) to allow an assessment of the effects of vitamin D supplementation in this important subgroup. Participants meeting the eligibility requirements were randomized in a 2x2 factorial design to vitamin D3 (cholecalciferol; 2000 IU/day) and marine omega-3 fatty acids (Omacor® fish oil, eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA], 1 g/d) supplements (or placebos), resulting in four treatment groups: (1) active vitamin D and active marine omega-3 fatty acids (n=6,463); (2) active vitamin D and omega-3 placebo (n=6,464); (3) vitamin D placebo and active marine omega-3 fatty acids (n= 6,472); or (4) vitamin D placebo and omega-3 placebo (n=6,475). Randomization, which was computer generated within sex, race (African American vs. not) and 5-year age groups (age 50–54, 55–59, 60–64, 65–69, 70–74, 75–79, ≥80 years) in blocks of eight, took place from November 2011 to March 2014. Blinded calendar packs containing the assigned pills were centrally mailed to the randomized participants. The taking of the randomized treatments is scheduled to end in late 2017, yielding a mean treatment period of 5 years. Participants receive annual follow-up questionnaires by postal mail to assess treatment compliance, use of non-study drugs or supplements, cancer and vascular risk factors, and occurrence of endpoints. Plasma biomarker measures will also assess compliance in a random sample of participants. The primary aims of the trial are to test whether vitamin D or marine omega-3 fatty acid supplementation reduces risk of total cancer and major CVD events, the latter defined as a composite of myocardial infarction [MI], stroke, and cardiovascular mortality. The secondary aims are to test whether these supplements reduce risk of colorectal cancer, breast cancer, and prostate cancer; total cancer mortality; an expanded composite cardiovascular endpoint that also includes coronary revascularization; and the individual components of the primary CVD endpoint, particularly CVD mortality. Self-reported endpoints are confirmed by medical record review by a committee of physicians blinded to treatment assignment, and deaths are ascertained through the National Death Index-Plus and other sources. VITAL endpoint surveillance includes linkage to the Centers for Medicare and Medicaid Services (CMS) administrative database, which will complement our disease follow-up procedures.
Figure 1.
2X2 factorial design of the VITamin D and OmegA-3 TriaL (VITAL)
The analysis plan and power calculations for the trial’s key aims can be found in reference (6), which was written prior to completion of recruitment and assumed a target sample size of 20,000. Updated power calculations using the actual sample size of 25,874 (as well as the actual demographic distribution of the study population) yield estimates that are slightly higher than those reported earlier. Assuming only one treatment (vitamin D or omega-3 fatty acids) is effective, there will be 93% power to detect an observed rate ratio of 0.85 for the primary endpoint of total cancer incidence and 93% power to detect an observed rate ratio of 0.80 for the primary cardiovascular endpoint of major CVD events. These statistics compare favorably to the earlier estimates of 86% and 89% obtained with a target sample size of 20,000.
At trial entry, participants were required to have no history of cancer (except non-melanoma skin cancer), MI, stroke, transient ischemic attack, or coronary revascularization. Other than this requirement, the presence or absence of cancer and vascular risk factors was not a selection factor for enrollment. Participants were also required to agree to limit consumption of supplemental vitamin D and supplemental calcium to no more than 800 IU/day and 1200 mg/day, respectively, and to avoid use of fish-oil supplements during the trial. In addition, they were required to be compliant with pill taking, defined as taking ≥2/3 of the study pills, during a 3-month placebo run-in period prior to randomization. Safety exclusions were renal failure or dialysis, hypercalcemia, hypo- or hyperparathyroidism, severe liver disease (cirrhosis), or chronic granulomatous conditions associated with elevated risk of hypercalcemia, including sarcoidosis, Wegener’s granulomatosis, or chronic active tuberculosis; use of anti-coagulant medications; allergy to soy (which is in the vitamin D placebo) or fish; or other serious illness that could preclude participation.
To date, 20 ancillary studies have received independent funding to evaluate the effect of vitamin D and omega-3 fatty acid supplementation on other outcomes, including diabetes, hypertension, cognitive decline, autoimmune diseases (e.g., thyroid disease, rheumatoid arthritis, and lupus), physical disability and falls, bone health and fractures (33), anemia, macular degeneration, dry eye syndrome, infections, asthma, depression, diabetes-related kidney disease, kidney function in people with hypertension, chronic knee pain symptoms, and atrial fibrillation.
At baseline, 16,956 participants —65.5% of the total study population—provided an optional blood sample. (Approximately 6,000 of these participants will provide a follow-up sample during years 1–4 of the trial.) Most of these samples were collected by the participants’ own healthcare providers or by Examination Management Services, Inc., a nationwide company that provides phlebotomy and specimen collection services in patients’ homes or at local blood-drawing facilities, and were shipped overnight to our laboratory in Boston. The remaining samples were collected as part of an optional health assessment provided to some Boston-area participants at the Clinical and Translational Science Center (CTSC) of Brigham and Women’s Hospital (described below). 25(OH)D and EPA+DHA concentrations will be measured in all samples. Whether the effectiveness of the intervention varies according to baseline nutrient levels (and also according to treatment-induced changes in these levels) will be assessed.
A subcohort of 1,054 participants living within driving distance of Boston, Massachusetts received detailed health assessments at the aforementioned CTSC prior to randomization; these participants also receive 2-year post-randomization assessments. Baseline visits took place between January 2012 and March 2014; follow-up visits began in January 2014 and will be completed in March 2016. During the CTSC visit, participants have a clinical exam, including measurement of height, weight, other anthropometric indices, blood pressure, and physical performance. They also provide fasting blood and urine samples, and undergo 2-hour oral glucose tolerance testing, spirometry, bone mineral density testing, 2D-echocardiography, and structured cognitive and mood assessments. The CTSC visits provide a valuable opportunity for face-to-face contact with a subset of the VITAL study population, allowing for detailed phenotyping and in-person validation of the remote assessment methods used in the main trial and ancillary studies. The CTSC subcohort was randomized separately into the four treatment groups created by the factorial design (259–268 participants per treatment group). Similar to the randomization procedure in the overall cohort, the CTSC randomization was computer generated within sex, race (African American vs. not) and age group (age <65 vs. ≥65 years) in blocks of eight.
The data on baseline characteristics reported here were taken from study questionnaires answered prior to randomization. Demographic characteristics included sex, age, race/ethnicity, geographic region of residence, education, and income. Health history variables included body mass index, obesity, hypertension, ever use of anti-hypertensive medication, current use of cholesterol-lowering medication, diabetes, current use of anti-diabetic medication, parental history of premature MI, and history of cancer in first-degree relatives. Behavioral characteristics included smoking status, weekly energy expenditure in leisure-time physical activities and stair climbing, alcohol use, aspirin use, postmenopausal hormone use (women only), colonoscopy/sigmoidoscopy in past 10 years, prostate-specific antigen test in past 10 years (men only), number of mammograms in past 10 years (women only), current use of multivitamins, current use of supplemental vitamin D, current use of supplemental calcium, and daily or weekly intake of foods related to vitamin D and/or omega-3 fatty acids (as assessed by a modified version of the Harvard Food Frequency Questionnaire (34)). The definitions of these variables are included in the footnotes to Table 1.
Table 1.
Baseline demographic, health, and behavioral characteristics among VITAL participants, according to randomized treatment assignmenta
| Baseline Characteristic | Total cohortb (n=25,874) | Vitamin D and Omega-3 fatty acid (n=6,463) | Vitamin D and Placebo (n=6,464) | Placebo and Omega-3 fatty acid (n=6,472) | Placebo and Placebo (n=6,475) |
|---|---|---|---|---|---|
| Demographic characteristics | |||||
| Sex, % | 25,874 / 100.0 | ||||
| Male | 12,793 / 49.4 | 49.4 | 49.4 | 49.4 | 49.5 |
| Female | 13081 / 50.6 | 50.6 | 50.6 | 50.6 | 50.5 |
| Mean age (SD), years | 67.1 (7.1) | 67.1 (7.1) | 67.1 (7.0) | 67.2 (7.1) | 67.1 (7.1) |
| Age group, years, % | 25,874 / 100.0 | ||||
| 50–54 | 977 / 3.8 | 3.8 | 3.8 | 3.7 | 3.8 |
| 55–64 | 8,871 / 34.3 | 34.2 | 34.3 | 34.3 | 34.3 |
| 65–74 | 12,708 / 49.1 | 49.1 | 49.2 | 49.2 | 49.1 |
| ≥75 | 3,318 / 12.8 | 12.9 | 12.7 | 12.8 | 12.8 |
| Race/ethnicity, %c | 25,307 / 97.8 | ||||
| Non-Hispanic White | 18,047 / 71.3 | 71.3 | 71.2 | 71.6 | 71.1 |
| African American | 5,107 / 20.2 | 20.2 | 20.2 | 20.1 | 20.2 |
| Hispanic (not African American) | 1,013 / 4.0 | 3.9 | 4.3 | 3.9 | 4.0 |
| Asian/Pacific Islander | 389 / 1.5 | 1.6 | 1.4 | 1.5 | 1.6 |
| American Indian/Alaskan Native | 228 / 0.9 | 1.0 | 0.9 | 0.9 | 0.8 |
| Other/unknown | 523 / 2.1 | 2.0 | 2.1 | 1.9 | 2.2 |
| Geographic region, % | 25,873 / 100.0 | ||||
| West (Far west, Southwest, Mtn) | 5,928 / 22.9 | 23.1 | 22.3 | 23.1 | 23.1 |
| Midwest | 5,541 / 21.4 | 22.1 | 20.8 | 21.0 | 21.7 |
| Southeast | 7,242 / 28.0 | 27.6 | 28.3 | 28.1 | 27.9 |
| Northeast | 7,162 / 27.7 | 27.1 | 28.6 | 27.7 | 27.3 |
| Education, % | 25,821 / 99.8 | ||||
| Did not complete high school | 359 / 1.4 | 1.5 | 1.6 | 1.2 | 1.3 |
| High school diploma or GED | 2,945 / 11.4 | 11.7 | 10.8 | 11.6 | 11.5 |
| Attended or graduated from college | 10,853 / 42.0 | 42.0 | 42.4 | 42.0 | 41.8 |
| Post-college | 11,664 / 45.2 | 44.8 | 45.3 | 45.2 | 45.4 |
| Income, % | 23,276 / 90.0 | ||||
| <$15,000 | 1,474 / 6.3 | 6.1 | 6.4 | 6.5 | 6.4 |
| $15,000–49,999 | 7,050 / 30.3 | 30.8 | 30.6 | 29.5 | 30.4 |
| $50,000–89,999 | 6,752 / 29.0 | 29.4 | 28.3 | 29.5 | 28.9 |
| $90,000–120,000 | 3,786 /16.3 | 15.8 | 16.3 | 16.6 | 16.4 |
| >$120,000 | 4214 / 18.1 | 17.9 | 18.5 | 18.0 | 18.0 |
| Health history | |||||
| Mean body mass index (SD), kg/m2 | 28.1 (5.7) | 28.1 (5.7) | 28.1 (5.7) | 28.1 (5.8) | 28.0 (5.8) |
| Obesity, %d | 7,205 / 28.8 | 29.4 | 28.7 | 29.1 | 28.1 |
| Hypertension, %e | 13,721 / 53.6 | 52.2 | 54.3 | 54.3 | 53.7 |
| Ever use of anti-hypertensive medication, % | 13,169 / 51.2 | 49.8 | 52.0 | 51.8 | 51.4 |
| Current use of cholesterol-lowering medication, % | 9,525 / 37.5 | 38.0 | 37.9 | 37.4 | 36.5 |
| Diabetes, %f | 3,402 / 13.2 | 13.8 | 13.3 | 13.1 | 12.8 |
| Current use of antidiabetic medication, % | 2,728 / 10.5 | 10.9 | 10.6 | 10.2 | 10.5 |
| Parental history of premature myocardial infarction, %g | 3,632 / 16.0 | 15.9 | 16.0 | 16.4 | 15.6 |
| History of cancer in first-degree relative | |||||
| Colorectal, % | 3,158 /13.4 | 13.7 | 13.4 | 13.8 | 12.7 |
| Breast cancer, % (women only) | 2,137 / 17.7 | 16.7 | 17.6 | 18.4 | 18.3 |
| Prostate cancer, % (men only) | 1,750 / 15.2 | 15.8 | 14.4 | 15.4 | 15.1 |
| Lung cancer, % | 3,403 / 14.5 | 14.3 | 14.0 | 15.1 | 14.4 |
| Behavioral characteristics, including medication use | |||||
| Smoking, % | 25,488 / 98.5 | ||||
| Current | 1,836 / 7.2 | 7.2 | 7.3 | 7.3 | 7.1 |
| Past | 10,465 / 41.1 | 41.0 | 41.4 | 41.5 | 40.4 |
| Never | 13,187 / 51.7 | 51.9 | 51.3 | 51.3 | 52.5 |
| Leisure-time physical activity and stair climbing, total MET-hours/week, median (interquartile range)h | 15.4 (4.6–31.6) | 15.5 (4.6–31.5) | 15.1 (4.4–31.3) | 15.4 (4.7–31.5) | 15.6 (4.7–32.4) |
| Alcohol use, % | 25365 / 98.0 | ||||
| Never | 7,971 / 31.4 | 30.8 | 31.9 | 31.5 | 31.5 |
| Rarely-<weekly | 1,901 / 7.5 | 7.4 | 7.3 | 7.9 | 7.3 |
| 1–6/week | 8,872 / 35.0 | 36.2 | 34.8 | 33.9 | 35.0 |
| Daily | 6,621 / 26.1 | 25.5 | 26.0 | 26.7 | 26.2 |
| Aspirin use in past month, % | 11,571 / 45.4 | 45.2 | 45.2 | 45.4 | 45.8 |
| Postmenopausal hormone use, % (women only) i | 12,801 / 97.9 | ||||
| Current | 1,481 / 11.6 | 10.7 | 11.7 | 12.3 | 11.6 |
| Past | 5,504 / 43.0 | 43.9 | 42.6 | 42.0 | 43.5 |
| Never | 5,816 / 45.4 | 45.4 | 45.7 | 45.7 | 44.9 |
| Screening behaviors in past 10 years | |||||
| Colonoscopy/sigmoidoscopy, % | 19,327 / 78.4 | 78.7 | 78.4 | 78.9 | 77.7 |
| Prostate-specific antigen test (men only), % | 8,806 / 75.2 | 75.1 | 74.8 | 74.9 | 75.8 |
| Number of mammograms (women only), % | 12,618 / 96.5 | ||||
| 0 | 742 / 5.9 | 6.3 | 6.2 | 4.8 | 6.2 |
| 1–4 | 3,237 / 25.7 | 25.1 | 25.3 | 26.2 | 26.1 |
| ≥5 | 8639 / 68.5 | 68.6 | 68.6 | 69.0 | 67.7 |
| Current use of multivitamins, % | 11,406 / 44.8 | 44.9 | 45.6 | 44.1 | 44.8 |
| Current use of supplemental vitamin D (≤800 IU/dayj), % | 11,030 / 42.6 | 42.3 | 42.7 | 42.7 | 42.8 |
| Current use of supplemental calcium (≤1200 mg/dayk), % | 6,831 / 26.4 | 26.5 | 27.2 | 25.6 | 26.3 |
| Intake of foods related to vitamin D and/or omega-3 fatty acids, mean (SD)l | |||||
| Milk, servings/daym | 0.71 (0.91) | 0.70 (0.90) | 0.71 (0.88) | 0.73 (0.92) | 0.71 (0.92) |
| Other vitamin D-fortified foods, servings/dayn | 0.63 (0.78) | 0.63 (0.82) | 0.63 (0.76) | 0.65 (0.77) | 0.63 (0.77 |
| Dark-meat fish, servings/weeko | 1.05 (1.84) | 1.04 (1.86) | 1.05 (2.01) | 1.06 (1.99) | 1.04 (1.47 |
| Other fish and seafood, servings/weekp | 1.11 (2.32) | 1.11 (2.08) | 1.14 (2.76) | 1.11 (2.60) | 1.09 (1.70 |
P-values for all 4-way comparisons between treatment groups were >0.05.
For categorical variables, this column contains # participants in category / % of nonmissing responses. For continuous variables, this column contains mean (standard deviation) or median (interquartile range) for nonmissing responses. The prevalence of missing responses for all variables ranged from 0 to <5%, except for the following: income (10% missing), parental history of premature myocardial infarction (12.1%), history of cancer in first-degree relative (colorectal, 9.0%; breast, 7.9%; prostate, 10.0%; lung, 9.0%), and PSA test (8.4%).
Race/ethnicity: Non-Hispanic white; African American (whether or not Hispanic); Hispanic (not African American); Asian/Pacific Islander (not Hispanic); American Indian/Alaskan Native (not Hispanic)
Obesity: Body mass index ≥30 kg/m2
Hypertension: Ever diagnosed with high blood pressure or ever use of anti-hypertensive medication
Diabetes: Ever diagnosed with diabetes or current use of anti-diabetic medication
Parental history of premature myocardial infarction: prior to age 60 for father; prior to age 65 for mother
Leisure-time physical activities: walking or hiking; jogging; running; bicycling; aerobic exercise/aerobic dance/exercise machines; lower intensity exercise/yoga/stretching/toning; tennis/squash/racquetball; lap swimming; weight lifting/strength training; other exercise
Virtually all female participants are postmenopausal (>99%).
≤800 IU/day from all supplemental sources of vitamin D combined (individual vitamin D supplements, calcium+vitamin D supplements, medications with vitamin D [e.g., Fosamax Plus D], and multivitamins)
≤1200 mg/day from all supplemental sources of calcium combined
As assessed by a modified version of the Harvard Food Frequency Questionnaire (34).
Milk: Dairy and soy milk
Other vitamin-D fortified foods: vitamin D-fortified cereal, vitamin D-fortified orange juice, yogurt
Dark-meat fish: e.g., mackerel, salmon, sardines, bluefish, swordfish; canned tuna p.
Other fish and seafood: e.g., cod, haddock, halibut; breaded fish cakes, pieces, or fish sticks; shrimp, lobster, scallops
To ascertain the comparability of treatment groups at baseline, we calculated the mean or median values of continuous variables and percentages in each category of discrete variables. Differences for continuous variables were tested by two-sample t-test or analysis of variance for more than two groups for normally distributed data and the nonparametric two-sample Wilcoxon rank-sum or Kruskal-Wallis test comparing multiple groups for data that was not normally distributed. Percentages were compared by chi-square tests. Statistical significance was assessed with two-sided p-values.
3. Results
Table 1 presents the baseline demographic, health history, and behavioral characteristics of the VITAL cohort as a whole and also stratified by randomized treatment assignment. Of the 25,874 participants, 49.4% are men and 50.6% are women. The mean age at baseline was 67.1 years (range, 50–100 years). With respect to race/ethnicity, 71.3% of the 25,307 participants who reported this information are non-Hispanic white, 20.2% are African American, 4.0% are Hispanic, 1.5% are Asian or Pacific Islander, and 0.9% are American Indian or Alaskan Native. The cohort is geographically diverse, with the four major regions in the U.S. well represented. Participants were well educated, with 42% of the cohort reporting their highest level of education as having attended or graduated from college, and 45.2% reporting post-college education. There were no clinically important or statistically significant differences between the treatment groups in these demographic factors. With respect to key risk factors for cancer and/or CVD, 28.8% of participants were obese, 53.6% were hypertensive, 13.2% had diabetes, and 7.2% were current smokers. In addition, 37.5% were taking cholesterol-lowering medication. There were no clinically important or statistically significant differences between the treatment groups in these or other characteristics, including parental history of MI, family history of cancer, exercise frequency, alcohol use, aspirin use, postmenopausal hormone use (in women), cancer screening behaviors, and multivitamin use.
Baseline mean intakes of foods related to vitamin D and/or omega-3 fatty acids, as well as prevalence of use of supplemental vitamin D and calcium in amounts permitted by study guidelines (≤800 IU/d and ≤1200 mg, respectively, from all supplemental sources combined [individual supplements of vitamin D or calcium; combination calcium-vitamin D supplements; medications containing vitamin D or calcium; and multivitamins]) were also nearly identical across the treatment groups. At initial screening, 20.1% of the cohort had been taking >800 IU/d of supplemental vitamin D, and 18.8% had been taking fish oil supplements [data not shown]. However, these individuals agreed to reduce their use of vitamin D and to eliminate their use of fish oil supplements to comply with study guidelines and thus were randomized into the trial.
Table 2 provides the baseline demographic, health, and behavioral characteristics of study participants stratified by sex, and within sex, by the two major racial/ethnic groups represented in the study—non-Hispanic white and African American. As expected given the sex-specific age cutpoints for trial entry (age ≥50 years for men and age ≥55 years for women), female participants were older than male participants (mean age, 68.1 vs. 66.1 years; p<0.001); they were also more likely to be African American (24.1% vs 15.2%.; p<0.001). In both men and women, there were significant differences between whites and African Americans on nearly all characteristics examined. Although African-American men and women were on average 5 years younger than their white counterparts, they had a more unfavorable cardiovascular and cancer risk profile, with higher prevalence of obesity, hypertension, diabetes, current smoking, and low physical activity level. African Americans were also less likely than whites to report baseline use of supplemental vitamin D, supplemental calcium, and multivitamins. In addition, they reported lower dietary intakes of milk but higher intakes of dark-meat fish and other types of seafood.
Table 2.
Baseline demographic, health, and behavioral characteristics among VITAL participants, according to sex and race/ethnicity
| Baseline Characteristica | Men | Women | ||||
|---|---|---|---|---|---|---|
| Totalb (n=12,793) | Non- Hispanic White (n=9,368) | African American (n=1,950) | Totalb (n=13,081) | Non- Hispanic White (n=8,679) | African American (n=3,157) | |
| Demographic characteristics | ||||||
| Mean age (SD), years | 66.1 (7.2) | 66.9 (6.9) | 61.9 (7.3)**** | 68.1 (6.8) | 69.4 (6.4) | 64.3 (6.4)**** |
| Age group, years, % | 12,793/100 | 13,081/100 | ||||
| 50–54 | 977/7.6 | 5.4 | 20.5**** | 0 | 0 | 0 |
| 55–64 | 4,872/38.1 | 35.6 | 49.4 | 3,999/30.6 | 19.8 | 62.7**** |
| 65–74 | 5,558/43.4 | 47.3 | 24.3 | 7,150/54.7 | 62.9 | 30.3 |
| ≥75 | 1,386/10.8 | 11.7 | 5.8 | 1,932/14.8 | 17.3 | 6.9 |
| Geographic region, % | 12,792/99.9 | 13,081/100 | ||||
| West (FarWest/ Southwest/Mtn) | 2,892/22.6 | 22.0 | 13.0**** | 3,036/23.2 | 24.7 | 13.3**** |
| Midwest | 2,577/20.1 | 20.7 | 21.6 | 2,964/22.7 | 22.6 | 25.8 |
| Southeast | 3,579/28.0 | 27.2 | 33.9 | 3,663/28.0 | 25.4 | 36.4 |
| Northeast | 3,744/29.3 | 30.1 | 31.5 | 3,418/26.1 | 27.3 | 24.6 |
| Education, % | 12,785/99.9 | 13,036/99.7 | ||||
| Did not complete high school | 146/1.1 | 0.4 | 3.8**** | 213/1.6 | 0.5 | 3.5**** |
| High school diploma or GED | 1,198/9.4 | 6.4 | 20.6 | 1,747/13.4 | 9.1 | 21.7 |
| Attended or graduated college | 4,816/37.7 | 35.4 | 48.1 | 6,037/46.3 | 46.4 | 47.9 |
| Post-college | 6,625/51.8 | 57.7 | 27.4 | 5,039/38.7 | 44.0 | 26.9 |
| Income, % | 11,693/91.4 | 11,583/88.5 | ||||
| <$15,000 | 541/4.6 | 2.4 | 14.4**** | 933/8.1 | 3.9 | 17.5**** |
| $15,000–49,999 | 2,674/22.9 | 19.1 | 37.0 | 4,376/37.8 | 34.3 | 45.6 |
| $50,000–89,999 | 3,363/28.8 | 29.3 | 26.6 | 3,389/29.3 | 31.9 | 23.9 |
| $90,000–120,000 | 2,209/18.9 | 20.5 | 11.8 | 1,577/13.6 | 15.9 | 8.3 |
| >$120,000 | 2,906/24.9 | 28.7 | 10.1 | 1,308/11.3 | 14.0 | 4.7 |
| Health history | ||||||
| Mean body mass index (SD), kg/m2 | 27.8 (4.7) | 27.5 (4.5) | 29.0 (5.6)**** | 28.4 (6.6) | 27.3 (5.9) | 31.6 (7.2)**** |
| Obesity, % | 3,074/24.8 | 22.6 | 34.9**** | 4,131/32.8 | 26.0 | 52.5**** |
| Hypertension, % | 6,495/51.4 | 48.9 | 62.3**** | 7,226/55.8 | 49.8 | 73.2**** |
| Ever use of anti-hypertensive medication, % | 6,165/48.5 | 46.0 | 59.5**** | 7,004/53.9 | 47.9 | 71.6**** |
| Current use of cholesterol- lowering medication, % | 4,995/39.7 | 42.0 | 29.5**** | 4,530/35.2 | 35.8 | 33.5 |
| Diabetes, % | 1,642/12.9 | 10.7 | 20.6**** | 1,760/13.5 | 9.3 | 24.6**** |
| Current use of anti-diabetic medication, % | 1,308/10.2 | 8.6 | 15.9**** | 1,420/10.9 | 7.5 | 19.9**** |
| Parental history of premature myocardial infarction, % | 1,664/14.7 | 15.5 | 12.1**** | 1,968/17.3 | 17.1 | 18.0 |
| History of cancer in first- degree relative | ||||||
| Colorectal, % | 1,474/12.7 | 13.2 | 10.4** | 1,684/14.1 | 14.3 | 14.5 |
| Breast cancer, % (women only) | N/A | N/A | N/A | 2,137/17.7 | 17.7 | 18.4 |
| Prostate cancer, % (men only) | 1,750/15.2 | 15.1 | 17.4* | N/A | N/A | N/A |
| Lung cancer, % | 1,539/13.2 | 13.4 | 13.2 | 1,864/15.7 | 15.8 | 15.5 |
| Behavioral characteristics, including medication use | ||||||
| Smoking, % | 12,598/98.5 | 12,890/98.5 | ||||
| Current | 937/7.4 | 5.4 | 17.5**** | 899/7.0 | 5.1 | 12.3**** |
| Past | 5,487/43.6 | 44.6 | 37.4 | 4,978/38.6 | 41.3 | 33.5 |
| Never | 6,174/49.0 | 50.0 | 45.0 | 7,013/54.4 | 53.7 | 54.3 |
| Leisure-time physical activity and stair climbing, total MET- hours/week, median (interquartile range) | 18.0 (5.8–35.4) 12,664/99.0 |
19.5 (6.5–35.7) | 12.5**** (3.6–30.1) | 13.2 (3.7–28.5) 12,958/99.1 |
15.5 (5.1–30.3) | 7.5*** * (2.1–21.7) |
| Alcohol use, % | 12,521/97.9 | 12,844/98.2 | ||||
| Never | 3,084/24.6 | 22.1 | 35.3**** | 4,887/38.0 | 31.7 | 52.6**** |
| Rarely-<weekly | 672/5.4 | 4.7 | 7.4 | 1,229/9.6 | 9.0 | 10.5 |
| 1–6/week | 4,354/34.8 | 33.9 | 37.1 | 4,518/35.2 | 37.8 | 29.6 |
| Daily | 4,411/35.2 | 39.2 | 20.2 | 2,210/17.2 | 21.5 | 7.3 |
| Aspirin use in past month, % | 6,335/50.2 | 53.0 | 39.7**** | 5,236/40.6 | 41.4 | 38.4* |
| Postmenopausal hormone use, % (women only) | 12,801/97.9 | |||||
| Current | N/A | N/A | 1,481/11.6 | 12.6 | 8.5**** | |
| Past | N/A | N/A | 5,504/43.0 | 49.3 | 26.8 | |
| Never | N/A | N/A | 5,816/45.4 | 38.1 | 64.6 | |
| Screening behaviors in past 10 years | ||||||
| Colonoscopy/sigmoidoscopy, % | 9,614/79.3 | 82.2 | 68.1**** | 9,713/77.5 | 78.9 | 75.1**** |
| Prostate-specific antigen test (men only), % | 8,806/75.2 | 79.3 | 58.7**** | NA | NA | |
| Number of mammograms (women only), % | 12,618/96.5 | |||||
| 0 | N/A | N/A | 742/5.9 | 5.8 | 5.2**** | |
| 1–4 | N/A | N/A | 3,237/25.7 | 23.1 | 31.2 | |
| ≥5 | N/A | N/A | 8,639/68.5 | 71.2 | 63.6 | |
| Current use of multivitamins, % | 5,664/45.0 | 47.0 | 39.8**** | 5,742/44.6 | 46.6 | 40.3**** |
| Supplemental vitamin D use (≤800 IU/day), % | 4,922/38.5 | 42.1 | 26.6**** | 6,108/46.7 | 54.0 | 29.8**** |
| Supplemental calcium use (≤1200 mg/day), % | 1,865/14.6 | 15.2 | 11.2**** | 4,966/38.0 | 44.1 | 22.5**** |
| Intake of foods related to vitamin D and/or omega-3 fatty acids, mean (SD) | ||||||
| Milk, servings per day | 0.71 (0.90) | 0.75 (0.89) | 0.50 (0.86)**** | 0.72 (0.92) | 0.78 (0.90) | 0.53 (0.93)**** |
| Other vitamin D-fortified foods, servings/day | 0.59 (0.72) | 0.59 (0.68) | 0.57 (0.91) | 0.68 (0.83) | 0.68 (0.75) | 0.67 (0.99) |
| Dark-meat fish, servings/week | 1.06 (2.04) | 0.96 (1.16) | 1.52 (3.85)**** | 1.03 (1.63) | 0.93 (1.09) | 1.33 (2.53)**** |
| Other fish and seafood, servings/week | 1.15 (2.63) | 1.02 (1.09) | 1.74 (5.83)**** | 1.07 (1.98) | 0.96 (1.35) | 1.43 (3.20)**** |
p<0.05,
p<0.01,
p<0.005,
p<0.0001 for non-Hispanic white vs. African American comparison within sex strata.
Variable definitions are provided in footnotes to Table 1.
For categorical variables, this column contains # participants in category / % of nonmissing responses. For continuous variables, this column contains mean (standard deviation) or median (interquartile range) for nonmissing responses.
Table 3 provides the baseline characteristics of the 16,956 participants who contributed an optional baseline blood sample (the “blood cohort”) as well as those of the 1,054 Boston-area participants who had a baseline health examination at a local CTSC (the “CTSC cohort”). As expected, given the high rate of participation in the blood collection, the blood cohort is generally representative of the overall study population, although African Americans were less likely to provide a blood sample than members of other racial/ethnic groups. As might also be expected given the higher level of effort required to travel to the clinic and complete a 6- to 8-hour clinical assessment as compared with completing paper-and-pencil questionnaires at home, the CTSC cohort is somewhat younger and healthier than the overall study population, with lower prevalence of obesity, hypertension, diabetes, current smoking, and physical inactivity, and higher prevalence of compliance with cancer screening recommendations (colonoscopy and mammography). The CTSC cohort is also less diverse with respect to race/ethnicity than the overall study population (84.4% of the former vs. 71.3% of the latter are non-Hispanic white), in part reflecting the race/ethnicity of VITAL participants residing within the clinic’s catchment area (81.9% of whom are non-Hispanic white and 7.4% of whom are African American).
Table 3.
Baseline demographic, health, and behavioral characteristics among VITAL participants, in overall cohort and according to participation in (a) baseline blood collection and (b) Clinical and Translational Science Center (CTSC) component of study
| Baseline characteristica | Total (n=25,874) | Blood cohort (n=16,956) | CTSC cohort (n=1,054) |
|---|---|---|---|
| Demographic characteristics | |||
| Sex, % | |||
| Male | 49.4 | 49.2 | 51.1 |
| Female | 50.6 | 50.8 | 48.9 |
| Mean age (SD), years | 67.1 (7.1) | 67.8 (7.0) | 64.9 (6.5) |
| Age group, years, % | |||
| 50–54 | 3.8 | 3.1 | 5.7 |
| 55–64 | 34.3 | 30.6 | 47.7 |
| 65–74 | 49.1 | 52.1 | 39.7 |
| ≥75 | 12.8 | 14.2 | 6.9 |
| Race/ethnicity, % | |||
| Non-Hispanic White | 71.3 | 76.6 | 84.4 |
| African American | 20.2 | 15.4 | 8.5 |
| Hispanic (not African American) | 4.0 | 3.6 | 2.8 |
| Asian/Pacific Islander | 1.5 | 1.5 | 1.7 |
| American Indian/Alaskan Native | 0.9 | 0.8 | 0.6 |
| Other/unknown | 2.1 | 2.1 | 1.9 |
| Geographic region, % | |||
| West (Far west, Southwest, Mountain) | 22.9 | 23.2 | 0.0 |
| Midwest | 21.4 | 21.7 | 0.0 |
| Southeast | 28.0 | 26.4 | 0.0 |
| Northeast | 27.7 | 28.7 | 100.0 |
| Education, % | |||
| Did not complete high school | 1.4 | 1.0 | 1.1 |
| High school diploma or GED | 11.4 | 9.5 | 8.2 |
| Attended or graduated college | 42.0 | 41.5 | 38.8 |
| Post-college | 45.2 | 48.0 | 51.9 |
| Income, % | |||
| <$15,000 | 6.3 | 4.7 | 4.1 |
| $15,000–49,999 | 30.3 | 28.3 | 19.0 |
| $50,000–89,999 | 29.0 | 30.2 | 28.3 |
| $90,000–120,000 | 16.3 | 17.3 | 19.6 |
| $120,000 | 18.1 | 19.5 | 29.0 |
| Health history | |||
| Mean body mass index (SD), kg/m2 | 28.1 (5.7) | 27.8 (5.6) | 27.0 (4.7) |
| Obesity, % | 28.8 | 27.0 | 21.8 |
| Hypertension, % | 53.6 | 52.8 | 45.5 |
| Ever use of anti-hypertensive medication, % | 51.2 | 50.6 | 43.5 |
| Current use of cholesterol-lowering medication, % | 37.5 | 39.3 | 34.6 |
| Diabetes, % | 13.2 | 13.0 | 9.6 |
| Current use of anti-diabetic medication,% | 10.5 | 10.4 | 7.5 |
| Parental history of premature myocardial infarction, % | 16.0 | 15.8 | 17.6 |
| History of cancer in first-degree relative | |||
| Colorectal, % | 13.4 | 13.7 | 15.0 |
| Breast cancer, % (women only) | 17.7 | 18.0 | 17.4 |
| Prostate cancer, % (men only) | 15.2 | 15.5 | 12.9 |
| Lung cancer, % | 14.5 | 14.2 | 13.8 |
| Behavioral characteristics, including medication use | |||
| Smoking, % | |||
| Current | 7.2 | 6.0 | 5.4 |
| Past | 41.1 | 42.2 | 43.3 |
| Never | 51.7 | 51.8 | 51.3 |
| Leisure-time physical activity and stair climbing, total MET-hours/week, median (interquartile range) | 15.4 (4.6–31.6) | 16.6 (5.1–32.4) | 21.5 (7.2–37.6) |
| Alcohol use, % | |||
| Never | 31.4 | 29.8 | 21.9 |
| Rarely-<weekly | 7.5 | 7.2 | 6.7 |
| 1–6/week | 35.0 | 35.5 | 40.2 |
| Daily | 26.1 | 27.5 | 31.1 |
| Aspirin use in past month, % | 45.4 | 46.5 | 43.7 |
| Postmenopausal hormone use, % (women only) | |||
| Current | 11.6 | 12.1 | 6.8 |
| Past | 43.0 | 46.5 | 30.6 |
| Never | 45.4 | 41.5 | 62.6 |
| Screening behaviors in past 10 years | |||
| Colonoscopy/sigmoidoscopy, % | 78.4 | 82.9 | 89.4 |
| Prostate-specific antigen test (men only), % | 75.2 | 79.2 | 68.3 |
| Number of mammograms (women only), % | |||
| 0 | 5.9 | 4.6 | 2.8 |
| 1–4 | 25.7 | 23.3 | 17.5 |
| 5 | 68.5 | 72.1 | 79.7 |
| Current use of multivitamins, % | 44.8 | 46.9 | 46.2 |
| Supplemental vitamin D use (≤800 IU/day), % | 42.6 | 46.1 | 45.3 |
| Supplemental calcium use (≤1200 mg/day), % | 26.4 | 28.5 | 25.8 |
| Intake of foods related to vitamin D and/or omega-3 fatty acids, mean (SD) | |||
| Milk, servings/day | 0.71 (0.91) | 0.74 (0.90) | 0.75 (1.02) |
| Other vitamin D-fortified foods, servings/day | 0.63 (0.78) | 0.64 (0.75) | 0.74 (0.93) |
| Dark-meat fish, servings/week | 1.05 (1.84) | 1.03 (1.71) | 1.09 (1.22) |
| Other fish and seafood, servings/week | 1.11 (2.32) | 1.07 (1.76) | 1.27 (1.88) |
| Treatment allocation | |||
| Vitamin D and omega-3 fatty acids | 25.0 | 25.0 | 24.8 |
| Vitamin D and placebo | 25.0 | 24.9 | 24.6 |
| Placebo and omega-3 fatty acids | 25.0 | 25.0 | 25.2 |
| Placebo and placebo | 25.0 | 25.1 | 25.4 |
Variable definitions are provided in the footnotes to Table 1.
4. Discussion
These analyses demonstrate a nearly identical distribution of demographic, health, and behavioral characteristics among the four treatment groups in VITAL. Given the large sample size, randomization was clearly effective in distributing known cancer and cardiovascular risk factors equally among these groups. No statistically significant or clinically important differences were detected. The similar distribution of known potential confounders strongly suggests that unknown confounders are also equally distributed and supports the internal validity of VITAL. Thus, any future observed differences in cancer, cardiovascular, and other endpoints between the treatment groups will, with a high degree of confidence, be attributable to the interventions themselves and not to the effects of uncontrolled confounding.
The characteristics of the blood cohort (i.e., the nearly two-thirds of study participants who provided an optional baseline blood sample) are largely similar to those of the overall VITAL study population, which suggests that results of analyses dependent on availability of baseline biomarker data will apply to the total cohort. The CTSC cohort is slightly younger and healthier than the overall VITAL study population, but the results are still expected to illuminate findings from the main trial and to have internal validity.
The issue of generalizability, also known as external validity, is also important in assessing whether the eventual results of VITAL can justifiably be used to guide decisions regarding the use of vitamin D or marine omega-3 fatty acid supplements for the primary prevention of cancer and CVD. A comparison of selected characteristics of VITAL participants with those of the general population (U.S. Census surveys) or nationally representative samples of midlife and older adults shows that the VITAL study population is a diverse and reasonably representative cohort but, as is true for many clinical trials, the participants are not an exact cross-section of the U.S. population. Compared with U.S. Census data for these age groups, the VITAL cohort has a higher percentage of African Americans (20.2% vs. 10.5% (35)) and a higher level of education (87.2% vs. 51.9% reported college or post-college attendance (36)). These differences are expected given that the trial’s recruitment strategy included targeted mailings to African Americans and to college graduates and members of professional organizations (6). VITAL participants are also a generally healthy and health-conscious group of men and women, especially when compared with their similarly aged peers in terms of selected risk factors for cancer or CVD. For example, the prevalence of obesity among male and female VITAL participants aged ≥60 was 23.4% and 30.9%, respectively, compared with 36.6% and 42.3% of similarly aged respondents in the National Health Interview and Nutritional Examination Survey (NHANES) (37). The prevalence of diabetes in VITAL was lower than in the National Health Interview Survey (NHIS) (38), although the difference was seen only in people aged ≥65 (12.9% vs. 21.2%) and not in those aged 55–64 (14.1% vs. 16.0%). The prevalence of smoking in VITAL was also lower than in NHIS (39). VITAL participants were also more likely to be physically active; to report use of multivitamin supplements and menopausal hormone therapy (women); and to meet federal guidelines for seafood consumption than participants in national surveys (40–43). On the other hand, VITAL participants had a similar prevalence of hypertension and use of cholesterol-lowering medications as NHANES participants (39, 44), as well as comparable milk intake (45).
Although the men and women who were eligible and willing to be randomized into VITAL appear to be somewhat healthier than the general U.S. population of midlife and older adults, there is little biological basis to expect that the balance of benefits and risks of vitamin D or omega-3 fatty acid supplementation would be materially different in these individuals than in other populations with similar racial/ethnic diversity. Nevertheless, the large size of the study will allow stratification by potential effect modifiers, including baseline blood levels of the nutrients under study, to assess whether treatment effects vary according to these characteristics. VITAL is the only large vitamin D trial to include a high percentage of African American or black participants, but ongoing (29) or planned (31) large randomized trials of vitamin D in other countries may provide evidence regarding the generalizability of VITAL findings to less diverse populations. Testing the effect of vitamin D supplementation in a diverse study population, and specifically in African Americans, is critically important, as African Americans are at higher risk of vitamin D deficiency and also for certain cancers (46) and cardiovascular events (47), as well as mortality from cancer (46) and CVD (47).
With adequate duration of treatment and follow-up, VITAL will provide important and relevant information about the balance of benefits and risks of vitamin D and marine omega-3 fatty acid supplementation for the primary prevention of cancer and CVD in a diverse population of midlife and older adults.
Acknowledgments
We are indebted to the 25,874 VITAL participants and to the entire VITAL staff for their dedicated and conscientious collaboration. VITAL is supported by grants U01 CA138962 and R01 CA138962, which include support from the National Cancer Institute, National Heart, Lung and Blood Institute, Office of Dietary Supplements, National Institute of Neurological Disorders and Stroke, and the National Center for Complementary and Integrative Health. The ancillary studies are supported by grants from multiple Institutes, including the National Heart, Lung and Blood Institute; the National Institute of Diabetes and Digestive and Kidney Diseases; the National Institute on Aging; the National Institute of Arthritis and Musculoskeletal and Skin Diseases; the National Institute of Mental Health; and the National Eye Institute. Pharmavite LLC of Northridge, California (vitamin D) and Pronova BioPharma (BASF) of Norway (Omacor® fish oil) donated the study agents, matching placebos, and packaging in the form of calendar packs. Dr. Buring’s spouse is on the Scientific Advisory Board of Pharmavite LLC. VITAL has been approved by the Institutional Review Board of Partners Healthcare/Brigham and Women’s Hospital, and the study agents have received Investigational New Drug Approval from the U.S. Food and Drug Administration. Voting members of the Data and Safety Monitoring Board for VITAL include Lawrence S. Cohen, MD; Theodore Colton, ScD; Mark A. Espeland, PhD; Craig Henderson, MD; Alice H. Lichtenstein, ScD; Rebecca A. Silliman, MD, PhD; and Nanette Wenger, MD (chair). Ex-officio members include Josephine Boyington, PhD, MPH; Rebecca Costello, PhD; Cindy Davis, PhD; Peter Greenwald, MD; Gabriela Riscuta, MD; and Harold Seifried, PhD. VITAL is registered at clinicaltrials.gov (NCT01169259). The VITAL website is www.vitalstudy.org.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
JoAnn E. Manson, Email: jmanson@partners.org.
I-Min Lee, Email: ilee@partners.org.
Nancy R. Cook, Email: ncook@partners.org.
William G. Christen, Email: wchristen@partners.org.
Vadim Y. Bubes, Email: vbubes@partners.org.
David S. Gordon, Email: dgordon@partners.org.
Trisha Copeland, Email: pcopeland2@partners.org.
Georgina Friedenberg, Email: gfriedenberg@partners.org.
Denise M. D’Agostino, Email: ddagostino@partners.org.
Claire Y. Ridge, Email: cridge@partners.org.
Jean G. MacFadyen, Email: jmacfadyen@partners.org.
Kate Kalan, Email: katekalan@gmail.com.
Julie E. Buring, Email: jburing@partners.org.
References
- 1.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 2.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 3.Greider K. Has vitamin D been oversold? [Accessed October 16, 2015];AARP Bulletin. 2012 Jul 5; Available at: http://www.aarp.org/health/drugs-supplements/info-07-2012/how-much-vitamin-d-is-enough.1.html.
- 4.Kupferschmidt K. Uncertain verdict as vitamin D goes on trial. Science. 2012;337(6101):1476–8. doi: 10.1126/science.337.6101.1476. [DOI] [PubMed] [Google Scholar]
- 5.Bailey RL, Gahche JJ, Miller PE, Thomas PR, Dwyer JT. Why US adults use dietary supplements. JAMA Intern Med. 2013;173(5):355–61. doi: 10.1001/jamainternmed.2013.2299. [DOI] [PubMed] [Google Scholar]
- 6.Manson JE, Bassuk SS, Lee IM, Cook NR, Albert MA, Gordon D, et al. The VITamin D and OmegA-3 TriaL (VITAL): Rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33(1):159–71. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manson JE, Bassuk SS. Vitamin D research and clinical practice: at a crossroads. JAMA. 2015;313(13):1311–2. doi: 10.1001/jama.2015.1353. [DOI] [PubMed] [Google Scholar]
- 8.Trivedi DP, Doll R, Khaw KT. Effect of four monthly oral vitamin D3 (cholecalciferol) supplementation on fractures and mortality in men and women living in the community: randomised double blind controlled trial. BMJ. 2003;326(7387):469. doi: 10.1136/bmj.326.7387.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lappe JM, Travers-Gustafson D, Davies KM, Recker RR, Heaney RP. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am J Clin Nutr. 2007;85(6):1586–91. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- 10.Lappe JM, Heaney RP. Calcium supplementation: Results may not be generalisable. BMJ. 2008;336(7641):403. doi: 10.1136/bmj.39493.476667.1F. author reply 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O'Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354(7):684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 12.Jackson RD, LaCroix AZ, Gass M, Wallace RB, Robbins J, Lewis CE, et al. Calcium plus vitamin D supplementation and the risk of fractures. N Engl J Med. 2006;354(7):669–83. doi: 10.1056/NEJMoa055218. [DOI] [PubMed] [Google Scholar]
- 13.Chlebowski RT, Johnson KC, Kooperberg C, Pettinger M, Wactawski-Wende J, Rohan T, et al. Calcium plus vitamin D supplementation and the risk of breast cancer. J Natl Cancer Inst. 2008;100(22):1581–91. doi: 10.1093/jnci/djn360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avenell A, MacLennan GS, Jenkinson DJ, McPherson GC, McDonald AM, Pant PR, et al. Long- term follow-up for mortality and cancer in a randomized placebo-controlled trial of vitamin D3 and/or calcium (RECORD trial) J Clin Endocrinol Metab. 2012;97(2):614–22. doi: 10.1210/jc.2011-1309. [DOI] [PubMed] [Google Scholar]
- 15.Chung M, Balk EM, Brendel M, Ip S, Lau J, Lee J, et al. Evidence Report No. 183. Rockville MD: Agency for Healthcare Research and Quality; 2009. [Accessed July 20, 2015]. Vitamin D and Calcium: A Systematic Review of Health Outcomes. (Prepared by the Tufts Evidence-based Practice Center under Contract No. HHSA 290-2007-10055-I). AHRQ Publication No. 09-E015. Available at: http://www.ahrq.gov/downloads/pub/evidence/pdf/vitadcal/vitadcal.pdf. [Google Scholar]
- 16.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96(7):1931–42. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 17.Keum N, Giovannucci E. Vitamin D supplements and cancer incidence and mortality: a meta-analysis. Br J Cancer. 2014;111(5):976–80. doi: 10.1038/bjc.2014.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JP. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ. 2014;348:g2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Welsh P, Sattar N. Vitamin D and chronic disease prevention. BMJ. 2014;348:g2280. doi: 10.1136/bmj.g2280. [DOI] [PubMed] [Google Scholar]
- 20.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, et al. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;6:CD007469. doi: 10.1002/14651858.CD007469.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial. Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico. Lancet. 1999;354(9177):447–55. [PubMed] [Google Scholar]
- 22.Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, Ishikawa Y, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090–8. doi: 10.1016/S0140-6736(07)60527-3. [DOI] [PubMed] [Google Scholar]
- 23.GISSI-HF Investigators. Effect of n-3 polyunsaturated fatty acids in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372(9645):1223–30. doi: 10.1016/S0140-6736(08)61239-8. [DOI] [PubMed] [Google Scholar]
- 24.Roncaglioni MC, Tombesi M, Avanzini F, Barlera S, Caimi V, Longoni P, et al. n-3 fatty acids in patients with multiple cardiovascular risk factors. N Engl J Med. 2013;368(19):1800–8. doi: 10.1056/NEJMoa1205409. [DOI] [PubMed] [Google Scholar]
- 25.Bosch J, Gerstein HC, Dagenais GR, Diaz R, Dyal L, Jung H, et al. n-3 fatty acids and cardiovascular outcomes in patients with dysglycemia. N Engl J Med. 2012;367(4):309–18. doi: 10.1056/NEJMoa1203859. [DOI] [PubMed] [Google Scholar]
- 26.Kromhout D, Giltay EJ, Geleijnse JM for the Alpha Omega Trial Group. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015–26. doi: 10.1056/NEJMoa1003603. [DOI] [PubMed] [Google Scholar]
- 27.Galan P, Kesse-Guyot E, Czernichow S, Briancon S, Blacher J, Hercberg S. Effects of B vitamins and omega 3 fatty acids on cardiovascular diseases: a randomised placebo controlled trial. BMJ. 2010;341:c6273. doi: 10.1136/bmj.c6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauch B, Schiele R, Schneider S, Diller F, Victor N, Gohlke H, et al. OMEGA, a randomized, placebo-controlled trial to test the effect of highly purified omega-3 fatty acids on top of modern guideline-adjusted therapy after myocardial infarction. Circulation. 2010;122(21):2152–9. doi: 10.1161/CIRCULATIONAHA.110.948562. [DOI] [PubMed] [Google Scholar]
- 29.Neale R. D-Health Trial. [Accessed August 13, 2015];Australian New Zealand Clinical Trials Registry identifier: ACTRN12613000743763. Available at: https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364534&isClinicalTrial=False.
- 30.Neale R. [Accessed August 13, 2015];D-Health website. http://dhealth.qimrberghofer.edu.au/
- 31.Peto J. [Accessed July 20, 2015];Vitamin D and Longevity (ViDAL) Trial: Randomised Feasibility Study. ISRCTN46328341. Available at: http://www.controlled-trials.com/ISRCTN46328341.
- 32.Peto J. [Accessed August 13, 2015];ViDAL website. http://vidal.lshtm.ac.uk/
- 33.LeBoff MS, Yue AY, Copeland T, Cook NR, Buring JE, Manson JE. VITAL-Bone Health: rationale and design of two ancillary studies evaluating the effects of vitamin D and/or omega-3 fatty acid supplements on incident fractures and bone health outcomes in the VITamin D and OmegA-3 TriaL (VITAL) Contemp Clin Trials. 2015;41:259–68. doi: 10.1016/j.cct.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135(10):1114–26. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services, Centers for Disease Control and Prevention (CDC), National Center for Health Statistics (NCHS) Bridged-Race Population Estimates, U.S. July 1 resident population by state, county, age, sex, bridged-race, and Hispanic origin. [on June 9, 2015];Compiled from 1990–1999 bridged-race intercensal population estimates (released by NCHS on 7/26/2004); revised bridged-race 2000–2009 intercensal population estimates (released by NCHS on 10/26/2012); and bridged-race Vintage 2013 (2010–2013) postcensal population estimates (released by NCHS on 6/26/2014) Available on CDC WONDER Online Database. Accessed at http://wonder.cdc.gov/bridged-race-v2013.html.
- 36.U.S. Census. Current Population Survey, Annual Social and Economic Supplement, 2012. [on June 16, 2015];Internet release date. 2013 Accessed at http://www.census.gov/population/age/data/2012.html.
- 37.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–7. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 38.Centers for Disease Control and Prevention. [Accessed July 17, 2015];Early Release of Selected Estimates Based on Data from the January-September 2013 National Health Interview Survey. 2014 Mar 27; Available at: www.cdc.gov/nchs/nhis/released201403.htm.
- 39.National Center for Health Statistics. Health, United States, 2014: With Special Feature on Adults Aged 55–64. Hyattsville MD: National Center for Health Statistics; 2015. [Accessed July 20, 2015]. Available at: http://www.cdc.gov/nchs/data/hus/hus14.pdf. [PubMed] [Google Scholar]
- 40.Harris CD, Watson KB, Carlson SA, Fulton JE, Dorn JM, Elam-Evans L. Adult participation in aerobic and muscle-strengthening physical activities--United States, 2011. MMWR. 2013;62(17):326–30. [PMC free article] [PubMed] [Google Scholar]
- 41.Nicastro HL, Bailey RL, Dodd KW. Using 2 assessment methods may better describe dietary supplement intakes in the United States. J Nutr. 2015;145(7):1630–4. doi: 10.3945/jn.115.211466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sprague BL, Trentham-Dietz A, Cronin KA. A sustained decline in postmenopausal hormone use: results from the National Health and Nutrition Examination Survey, 1999-2010. Obstet Gynecol. 2012;120(3):595–603. doi: 10.1097/AOG.0b013e318265df42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.U.S. Department of Agriculture. [Accessed July 20, 2015];Scientific Report of the 2015 Dietary Guidelines Advisory Committee. 2015 Available at: http://www.health.gov/dietaryguidelines/2015-scientific-report/
- 44.Gu Q, Paulose-Ram R, Burt VL, Kit BK. NCHS data brief. 177. Hyattsville MD: National Center for Health Statistics; 2014. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. [PubMed] [Google Scholar]
- 45.Sebastian RS, Goldman JD, Wilkinson Enns C, LaComb RP. Fluid Milk Consumption in the United States: What We Eat in America, NHANES 2005–2006. [Accessed July 20, 2015];Food Surveys Research Group Dietary Data Brief. 2010 Sep;(3) Available at http://www.ars.usda.gov/SP2UserFiles/Place/80400530/pdf/DBrief/3_milk_consumption_0506.pdf.
- 46.American Cancer Society. Cancer Facts & Figures for African Americans 2013–2014. Atlanta: American Cancer Society; 2013. [Accessed July 20, 2015]. Available at: http://www.cancer.org/acs/groups/content/@epidemiologysurveilance/documents/document/acspc-036921.pdf. [Google Scholar]
- 47.Harris SS. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr. 2011;93(5):1175S–8S. doi: 10.3945/ajcn.110.003491. [DOI] [PubMed] [Google Scholar]